Abstract
Calcium ion plays an important role in the hair cell's mechanoelectrical transduction process; in particular, Ca2+ controls adaptation to protracted mechanical stimuli. Because calmodulin is a ubiquitous intracellular receptor for Ca2+ and has been shown to accumulate at the tips of stereocilia, we determined its concentration and identified the proteins with which it interacts in the hair bundle. By performing quantitative immunoblot analysis on isolated bundles, we ascertained that the average concentration of calmodulin within each stereocilium is approximately 70 microM. Extraction experiments disclosed that, in the presence of 20 microM Ca2+, 50% of the calmodulin is bound to detergent-soluble receptors. To distinguish these receptors, we developed an assay that utilizes calmodulin crosslinked to alkaline phosphatase. This technique is approximately 100-fold more sensitive than calmodulin-binding assays that employ 125I- or biotin-labeled calmodulin. When used with chemiluminescence detection in a blot-overlay assay, the calmodulin-alkaline phosphatase conjugate identified hair-bundle proteins of molecular masses 25, 35, 145, 175, 240, and 350 kDa. We examined the subcellular distribution of these receptors; all but the 240-kDa molecule are soluble in a nonionic detergent. The relatively high concentration of calmodulin and the presence of several calmodulin-binding proteins provide evidence for a role of calmodulin in hair bundles.
Full text
PDF

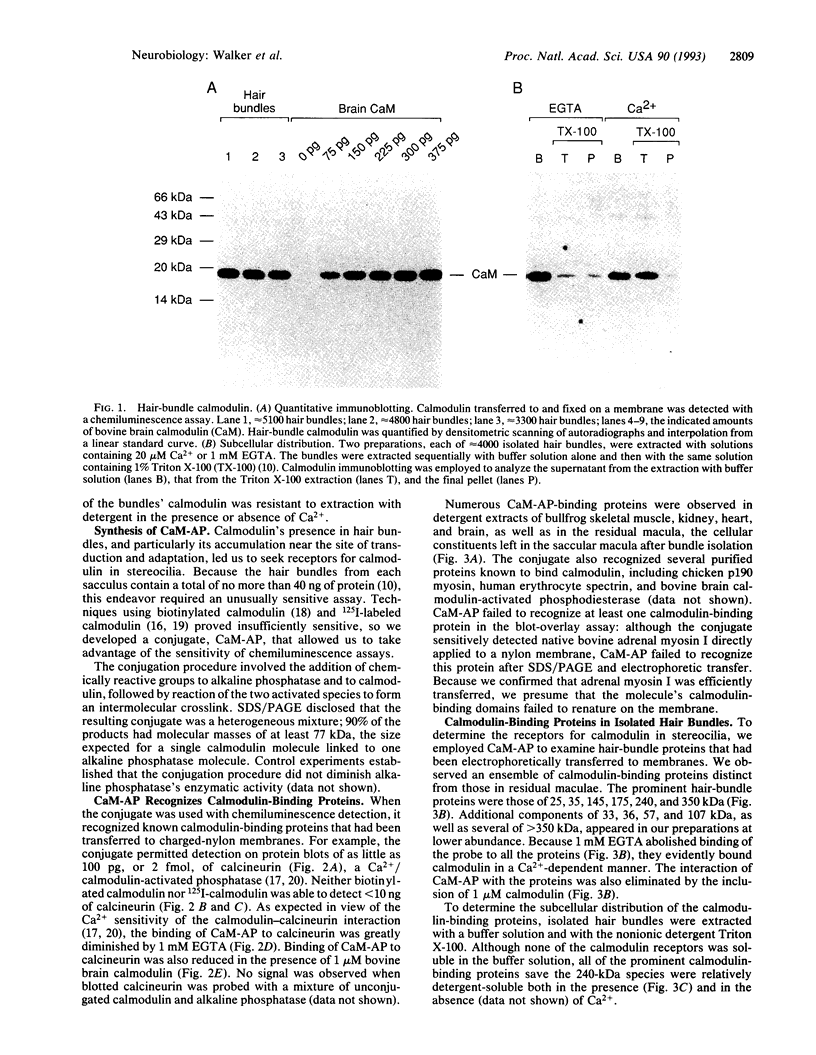
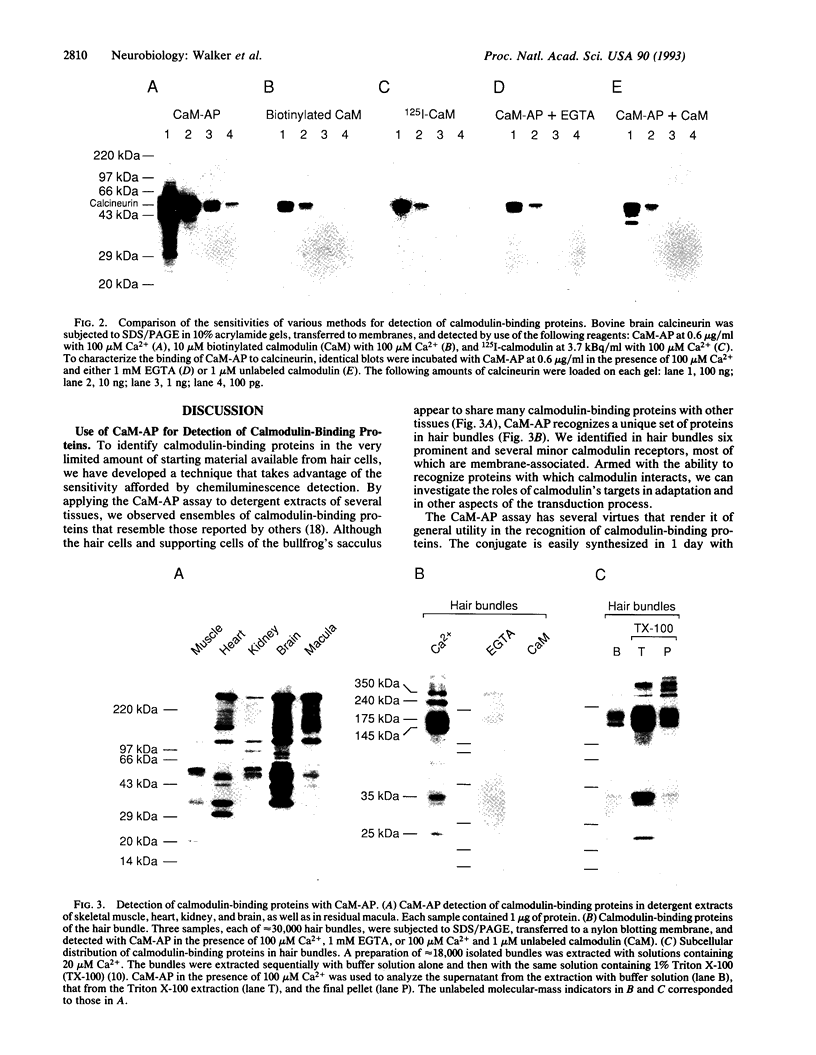

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assad J. A., Hacohen N., Corey D. P. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2918–2922. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley M. L., Polli J. W., Pennypacker K. R., Kincaid R. L. Identification of calmodulin-binding proteins. Methods Enzymol. 1990;184:451–467. [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Gillespie P. G., Hudspeth A. J. Chemiluminescence detection of proteins from single cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2563–2567. doi: 10.1073/pnas.88.6.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Hudspeth A. J. High-purity isolation of bullfrog hair bundles and subcellular and topological localization of constituent proteins. J Cell Biol. 1991 Feb;112(4):625–640. doi: 10.1083/jcb.112.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Weber K. Detection of calmodulin-binding polypeptides separated in SDS-polyacrylamide gels by a sensitive [125I]calmodulin gel overlay assay. Methods Enzymol. 1983;102:204–210. doi: 10.1016/s0076-6879(83)02021-2. [DOI] [PubMed] [Google Scholar]
- Hubbard M. J., Klee C. B. Calmodulin binding by calcineurin. Ligand-induced renaturation of protein immobilized on nitrocellulose. J Biol Chem. 1987 Nov 5;262(31):15062–15070. [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Jacobs R. A., Hudspeth A. J. Ultrastructural correlates of mechanoelectrical transduction in hair cells of the bullfrog's internal ear. Cold Spring Harb Symp Quant Biol. 1990;55:547–561. doi: 10.1101/sqb.1990.055.01.053. [DOI] [PubMed] [Google Scholar]
- Jaramillo F., Hudspeth A. J. Localization of the hair cell's transduction channels at the hair bundle's top by iontophoretic application of a channel blocker. Neuron. 1991 Sep;7(3):409–420. doi: 10.1016/0896-6273(91)90293-9. [DOI] [PubMed] [Google Scholar]
- Kellermann O. K., Ferenci T. Maltose-binding protein from Escherichia coli. Methods Enzymol. 1982;90(Pt E):459–463. doi: 10.1016/s0076-6879(82)90171-9. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Krinks M. H., Manalan A. S., Cohen P., Stewart A. A. Isolation and characterization of bovine brain calcineurin: a calmodulin-stimulated protein phosphatase. Methods Enzymol. 1983;102:227–244. doi: 10.1016/s0076-6879(83)02024-8. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- Nagao S., Yamazaki A., Bitensky M. W. Calmodulin and calmodulin binding proteins in amphibian rod outer segments. Biochemistry. 1987 Mar 24;26(6):1659–1665. doi: 10.1021/bi00380a026. [DOI] [PubMed] [Google Scholar]
- Neugebauer D. C., Thurm U. Chemical dissection of stereovilli from fish inner ear reveals differences from intestinal microvilli. J Neurocytol. 1984 Oct;13(5):797–808. doi: 10.1007/BF01148494. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. M., Barres B. A., Corey D. P. "Bundle blot" purification and initial protein characterization of hair cell stereocilia. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4973–4977. doi: 10.1073/pnas.86.13.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter G. R., Means A. R. Use of the 125I-labeled protein gel overlay technique to study calmodulin-binding proteins. Methods Enzymol. 1987;139:433–444. doi: 10.1016/0076-6879(87)39104-9. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Wolchok S. R. Conditions for reproducible detection of calmodulin and S100 beta in immunoblots. Biochem Biophys Res Commun. 1984 Nov 14;124(3):752–759. doi: 10.1016/0006-291x(84)91022-2. [DOI] [PubMed] [Google Scholar]






