Abstract
Background
We assessed vaccine effectiveness (VE) for RotaTeq (RV5; 3 doses) and Rotarix (RV1; 2 doses) at reducing rotavirus acute gastroenteritis (AGE) inpatient and emergency department (ED) visits in US children.
Methods
We enrolled children <5 years of age hospitalized or visiting the ED with AGE symptoms from November 2009–June 2010 and from November 2010–June 2011 at 7 medical institutions. Fecal specimens were tested for rotavirus by enzyme immunoassay and genotyped. Vaccination among laboratory-confirmed rotavirus cases was compared with rotavirus-negative AGE controls. Regression models calculated VE estimates for each vaccine, age, ethnicity, genotype, and clinical setting.
Results
RV5-specific analyses included 359 rotavirus cases and 1811 rotavirus-negative AGE controls. RV1-specific analyses included 60 rotavirus cases and 155 rotavirus-negative AGE controls. RV5 and RV1 were 84% (95% confidence interval [CI], 78%–88%) and 70% (95% CI, 39%–86%) effective, respectively, against rotavirus-associated ED visits and hospitalizations combined. By clinical setting, RV5 VE against ED and inpatient rotavirus-associated visits was 81% (95% CI, 70%–84%) and 86% (95% CI, 74%–91%), respectively. RV1 was 78% (95% CI, 46%–91%) effective against ED rotavirus disease; study power was insufficient to evaluate inpatient RV1 VE. No waning of immunity was evident during the first 4 years of life for RV5, nor during the first 2 years of life for RV1. RV5 provided genotype-specific protection against each of the predominant strains (G1P[8], G2P[4], G3P[8], G12P[8]), while RV1 VE was statistically significant for the most common genotype, G3P[8].
Conclusions
Both RV5 and RV1 significantly protected against medically attended rotavirus gastroenteritis in this real-world assessment.
Keywords: rotavirus, vaccine, RotaTeq, New Vaccine Surveillance Network
Prior to rotavirus vaccine licensure in the United States, rotavirus infected nearly every US child early in life, accounting for up to 70% of winter hospitalizations due to acute gastroenteritis (AGE), with >$1 billion in healthcare and societal costs each year [1, 2]. Rotavirus vaccines were recommended by the US Advisory Committee on Immunization Practices (ACIP) [3] for routine immunization in 2006 (RotaTeq [RV5], Merck and Co, Whitehouse Station, New Jersey) and 2008 (Rotarix [RV1], GlaxoSmithKline Biologicals, Rixensart, Belgium), leading to dramatic declines in childhood rotavirus gastroenteritis [4–8].
RV5 contains 5 reassortant rotaviruses derived from human and bovine parent strains that express human outer capsid proteins of 5 common circulating strains (G1, G2, G3, G4, and P[8]). Three oral doses of live, attenuated RV5 vaccine are administered to infants at ages 2, 4, and 6 months. RV1 contains the live, attenuated monovalent G1P[8] human rotavirus strain; 2 doses of RV1 are given orally at ages 2 and 4 months. While both vaccines were found to be highly effective in prelicensure studies [9–12], there currently are no published studies demonstrating the performance of both vaccines during routine concurrent field use in US childhood populations.
Using a large prospective, geographically diverse rotavirus surveillance network in the United States, we assessed RV5 and RV1 vaccine effectiveness (VE) in preventing rotavirus AGE hospitalization and emergency department (ED) visits among US children <5 years of age during 2 consecutive rotavirus seasons.
METHODS
Definition and Enrollment of Subjects
Details of New Vaccine Surveillance Network (NVSN) surveillance methods have been previously published [13–15]. Surveillance sites included Children’s Hospital and Research Center Oakland (Oakland, California), Seattle Children’s Hospital (Seattle, Washington), Children’s Mercy Hospitals and Clinics (Kansas City, Missouri), Texas Children’s Hospital (Houston), Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio), Vanderbilt University Medical Center (Nashville, Tennessee), and the University of Rochester Medical Center (Rochester, New York) and are hereafter referred to as “Oakland,” “Seattle,” “Kansas City,” “Houston,” “Cincinnati,” “Nashville,” and “Rochester.” Institutional review board approvals were obtained from the Centers for Disease Control and Prevention (CDC) and from each study site.
Children <5 years of age were enrolled if they were hospitalized or visited the ED from 1 November 2009 through 30 June 2010 (hereafter “2010”) and from 1 November 2010 through 30 June 2011 (hereafter “2011”) with signs of diarrhea (≥3 episodes within 24 hours) and/or vomiting (≥1 episode within 24 hours) and had informed consent obtained from a parent or guardian. Children were ineligible if they had a history of immune deficiency, were previously enrolled for the same AGE episode within 3 days, or were transferred from another hospital. Children enrolled in the ED but subsequently hospitalized for the illness were categorized as inpatients. Race and ethnicity were reported by the child’s parent or guardian. Oakland participated during the 2011 season and the 6 other surveillance sites participated during both 2010 and 2011 seasons.
Data Collection and Laboratory Testing
Demographic and clinical information were collected for each enrolled child. Fecal samples were obtained within 14 days of symptom onset, with >95% of specimens obtained within 7 days. Testing for rotavirus was performed using the commercial enzyme immunoassay (EIA) Rotaclone (Meridian Bioscience, Inc) at each surveillance site. Rotavirus strains were characterized by genotyping using reverse transcription polymerase chain reaction (RT-PCR) and nucleotide sequencing at the CDC [16–19]. EIA-negative results with negative PCR results were confirmed as rotavirus negative in our analytical dataset.
Cases and Controls
Cases were children hospitalized or visiting the ED with AGE symptoms whose fecal specimens tested positive for rotavirus. The primary control group included children with AGE whose fecal specimens tested negative for rotavirus (“rotavirus-negative AGE controls”).
Vaccine Effectiveness Analyses
Demographic and socioeconomic data for cases and control groups were compared by using Wilcoxon rank-sum tests for continuous variables and χ2 tests for categorical variables.
VE for the prevention of rotavirus-associated inpatient admissions and ED visits during 2 rotavirus seasons, 2010 and 2011, were independently assessed for RV5 and RV1. Rotavirus immunization status was verified by contacting the subjects’ primary care providers and through regional immunization information systems. Vaccine doses were defined as valid if given ≥14 days before onset of symptoms for the cases and rotavirus-negative AGE controls. Additionally, to ensure vaccine age eligibility following licensure, subjects were required to be born on or after 1 April 2006 for RV5 analyses and on or after 1 August 2008 for RV1 analyses. Finally, we restricted analyses to children who had reached the ACIP-recommended age for completion of the vaccine series to avoid residual confounding by age at the time of last dose (ie, >8 months of age) [3].
The adjusted odds ratios and 95% confidence intervals (CIs) were calculated by logistic regression and were adjusted for month/year of birth, month/year of symptom onset, and surveillance site. VE was calculated using the following formula: [VE = (1 − odds ratio) × 100]. All tests were 2-sided and P values <.05 were considered significant.
In addition to the primary VE analysis for RV5 and RV1 in concurrent use, we evaluated VE by vaccine dose number, season, clinical setting, age, predominant rotavirus genotype, and ethnicity. Subjects recorded as having 3 RV1 doses (representing <1% of RV1-vaccinated subjects), or those having mixed doses of both RV5 and RV1 (representing approximately 5% of vaccinated subjects) were excluded from our analyses. Owing to low RV1 vaccine coverage (<5%) in Houston, Seattle, and Nashville, these 3 surveillance sites were not included in RV1 analyses.
Alternate Models
We conducted an alternate analysis whereby we restricted RV5 VE estimates to the same 4 surveillance sites having RV1 vaccine coverage ≥5%, with little difference in results from those which we describe (data not presented). Additionally, we adjusted our data for insurance status and clinical setting, which modified the final estimates for both vaccines by 0%–2% (data not presented). Lastly, we conducted conditional logistic regression models in which rotavirus cases were matched to rotavirus-negative AGE controls based on ±30 days of date of birth, and ±30 days of date of symptom onset.
RESULTS
Characteristics of Cases and Controls
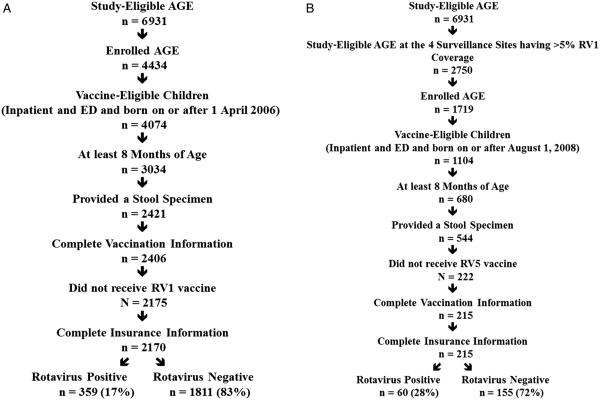
RV5-specific VE analyses included 359 rotavirus cases and 1811 rotavirus-negative AGE controls (Figure 1A). RV1-specific VE analyses included 60 rotavirus cases and 155 rotavirus-negative AGE controls (Figure 1B).
Figure 1.
A, RV5 analysis subject inclusion flowchart. B, RV1 analysis subject inclusion flowchart. Abbreviations: AGE, acute gastroenteritis; ED, emergency department; RV1, Rotarix; RV5, RotaTeq.
The median ages of cases in both the RV5 and RV1 groups were older than the respective rotavirus-negative AGE controls (P < .001; Table 1). For the RV5 analyses, statistically significant differences between cases and rotavirus-negative AGE controls were observed by age, clinical setting, season, and NVSN surveillance site. For the RV1 analysis, differences between cases and rotavirus-negative AGE controls were seen only by season (Table 1).
Table 1.
Description of New Vaccine Surveillance Network Cases and Acute Gastroenteritis Control Subjects in RV5 and RV1 Vaccine Effectiveness Analytical Datasets
| Variables | RV5 Analysis |
RV1 Analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 359) |
AGE Controls (n = 1811) |
Cases (n = 60) |
AGE Controls (n = 155) |
|||||||
| No. | % | No. | % | P Value | No. | % | No. | % | P Value | |
| Age, mo, median (range) | 26 | (8–59) | 20 | (8–60) | <.001 | 18 | (9–30) | 15 | (8–33) | <.001 |
| Sex | .835 | .322 | ||||||||
| Male | 198 | 55.1 | 988 | 54.6 | 37 | 61.7 | 84 | 54.2 | ||
| Female | 161 | 44.9 | 823 | 45.4 | 23 | 38.3 | 71 | 45.8 | ||
| Race | .837 | .322 | ||||||||
| White | 231 | 64.4 | 1201 | 66.3 | 25 | 41.7 | 54 | 34.8 | ||
| Black | 88 | 24.5 | 434 | 23.7 | 27 | 45.0 | 74 | 47.7 | ||
| Other | 40 | 11.1 | 176 | 9.7 | 8 | 13.3 | 27 | 17.5 | ||
| Ethnicity | .138 | .591 | ||||||||
| Hispanic | 160 | 44.6 | 908 | 50.1 | 12 | 20.0 | 30 | 19.4 | ||
| Non-Hispanic | 199 | 55.4 | 904 | 49.8 | 48 | 80.0 | 125 | 80.8 | ||
| Other/unknown | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | ||
| Insurance | .158 | .659 | ||||||||
| Private | 93 | 25.9 | 404 | 22.5 | 12 | 20.0 | 27 | 17.4 | ||
| Public/none | 266 | 74.1 | 1404 | 77.5 | 48 | 80.0 | 128 | 82.6 | ||
| Clinical setting | <.001 | .161 | ||||||||
| Inpatient | 130 | 36.2 | 372 | 20.5 | 16 | 26.7 | 28 | 18.1 | ||
| ED | 229 | 63.8 | 1439 | 79.5 | 44 | 73.3 | 127 | 81.9 | ||
| Season | <.001 | .002 | ||||||||
| 2010 | 111 | 30.9 | 924 | 51.0 | 7 | 11.7 | 59 | 38.1 | ||
| 2011 | 248 | 69.1 | 887 | 49.0 | 53 | 88.3 | 96 | 61.9 | ||
| NVSN site | .002 | .734 | ||||||||
| Oakland | 31 | 8.6 | 62 | 3.4 | 7 | 11.7 | 11 | 7.1 | ||
| Seattle | 26 | 7.2 | 137 | 7.6 | Φ | Φ | ||||
| Kansas City | 44 | 12.3 | 228 | 12.6 | 27 | 45.0 | 77 | 49.7 | ||
| Houston | 190 | 52.9 | 1044 | 57.7 | Φ | Φ | ||||
| Nashville | 24 | 6.7 | 101 | 5.6 | Φ | Φ | ||||
| Cincinnati | 35 | 9.8 | 181 | 9.9 | 20 | 33.3 | 51 | 32.9 | ||
| Rochester | 9 | 2.5 | 58 | 3.2 | 6 | 10.0 | 16 | 10.3 | ||
Abbreviations: AGE, acute gastroenteritis; ED, emergency department; NVSN, New Vaccine Surveillance Network; RV1, Rotarix; RV5, RotaTeq.
Φ = Insufficient RV1 coverage/subjects (see text).
Rotavirus Vaccine Effectiveness
Over the study period, the VE for ≥1 dose of any rotavirus vaccine was 80% (95% CI, 74%–85%) against rotavirus hospitalizations and ED visits. A complete 3-dose course of RV5 demonstrated a VE of 84% (95% CI, 78%–88%) and a complete 2-dose course of RV1 had a VE of 70% (95% CI, 39%–86%) in preventing rotavirus-associated hospitalizations and ED visits over the study period (Table 2). For those receiving less than the complete course of RV5, the VE estimates were 70% (95% CI, 50%–82%) for 1 dose and 78% (95% CI, 65%–86%) for 2 doses, respectively. The single-dose RV1 VE estimate was not statistically significant (57% [95% CI, −45% to 87%]; Table 2).
Table 2.
Stratified Vaccine Effectiveness and 95% Confidence Intervals for RV5 and RV1, Using the Rotavirus-Negative Acute Gastroenteritis Control Group
| Stratum | RV5 |
RV1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases/Controls | VE (95% CI)a | Cases/Controls | VE (95% CI)a | |||||||
| Dose No. | ||||||||||
| Dose 1 | 233/537 | 70% (50%–82%) | 46/83 | 57% (−45% to 87%) | ||||||
| Dose 2 | 239/638 | 78% (65%–86%) | 56/140 | 70% (39% to 86%) | ||||||
| Dose 3 | 307/1445 | 84% (78%–88%) | NA | NA | ||||||
| Season | ||||||||||
| 2010 (% fully vaccinated) | 111/924 (23%/53%) | 82% (69%–89%) | 7/59 (0%/46%) | Φ | ||||||
| 2011 (% fully vaccinated) | 248/887 (29%/62%) | 84% (77%–89%) | 53/96 (26%/47%) | 64% (23%–83%) | ||||||
| Clinical setting | ||||||||||
| Inpatient | 130/372 | 86% (74%–91%) | 22/34 | 32% (−156% to 82%) | ||||||
| ED | 229/1439 | 81% (70%–84%) | 38/121 | 78% (46%–91%) | ||||||
| Age | ||||||||||
| 1 y | 34/402 | 85% (63%–94%) | 7/54 | 56% (−59% to 100%) | ||||||
| 2 y | 121/681 | 89% (82%–93%) | 46/79 | 86% (60%–95%) | ||||||
| 3 y | 91/414 | 83% (69%–90%) | 7/22 | Φ | ||||||
| 4 y | 86/231 | 79% (56%–90%) | 0/0 | Φ | ||||||
| Predominant genotype | ||||||||||
| G1P[8] | 15/NA | 89% (41%–98%) | 1/NA | Φ | ||||||
| G2P[4] | 82/NA | 87% (77%–93%) | 7/NA | Φ | ||||||
| G3P[8] | 196/NA | 87% (81%–91%) | 44/NA | 74% (40%–89%) | ||||||
| G12P[8] | 36/NA | 83% (57%–93%) | 4/NA | Φ | ||||||
| Ethnicity | ||||||||||
| Hispanic | 160/908 | 85% (76%–90%) | 12/30 | 59% (−100% to 91%) | ||||||
| Non-Hispanic | 199/902 | 83% (75%–88%) | 48/125 | 76% (44%–90%) | ||||||
Abbreviations: CI, confidence interval; ED, emergency department; NA, not applicable; RV1, Rotarix; RV5, RotaTeq; VE, vaccine effectiveness.
Exact odds ratio and 95% CI.
Φ = Insufficient RV1 coverage/subjects (see text).
Similar VE for RV5 was observed during each of the 2010 and 2011 seasons, with VE estimates of 82% (95% CI, 69%–89%) and 84% (95% CI, 77%–89%), respectively. Comparison across both years for RV1 was not possible, since RV1 coverage was too low to generate a VE estimate for 2010. VE for RV1 in 2011 was 64% (95% CI, 23%–83%; Table 2).
Vaccine Effectiveness Against Rotavirus-Associated Hospitalization and ED Visits
RV5 vaccination was 86% (95% CI, 74%–91%) effective in preventing hospitalizations due to rotavirus. Inpatient RV1 VE was 32% (95% CI, −156% to 82%), a statistically nonsignificant result based on 22 cases and with wide confidence intervals. However, RV5 and RV1 vaccines were similarly effective in preventing rotavirus-associated ED visits (81% [95% CI, 70%–84%], and 78% [95% CI, 46%–91%], respectively; Table 2).
Stratified Analyses of Vaccine Effectiveness Against Rotavirus-Associated Hospitalization and ED Visits
Through the fourth year of life, RV5 vaccination demonstrated statistically significant effectiveness in preventing rotavirus hospitalizations and ED visits. VE estimates for the first, second, third, and fourth year of life were 85% (95% CI, 63%–94%), 89% (95% CI, 82%–93%), 83% (95% CI, 69%–90%), and 79% (95% CI, 56%–90%), respectively. RV1 VE was significant for the second year of life (86% [95% CI, 60%–95%]), but not for the first year (56% [95% CI, −59% to 100%]); VE for the first year was again based on only 7 cases, resulting in wide confidence intervals (Table 2).
The genotype-specific RV5 VE estimates against our 4 predominant circulating rotavirus strains were 89% (95% CI, 41%–98%) for G1P[8]; 87% (95% CI, 77%–93%) for G2P[4]; 87% (95% CI, 81%–91%) for our most common strain, G3P[8]; and 83% (95% CI, 57%–93%) for G12P[8], a previously uncommon strain (Figure 2). RV1 had a significant VE for our most common genotype, G3P[8] (74% [CI, 40%–89%]; Figure 2).
Figure 2.
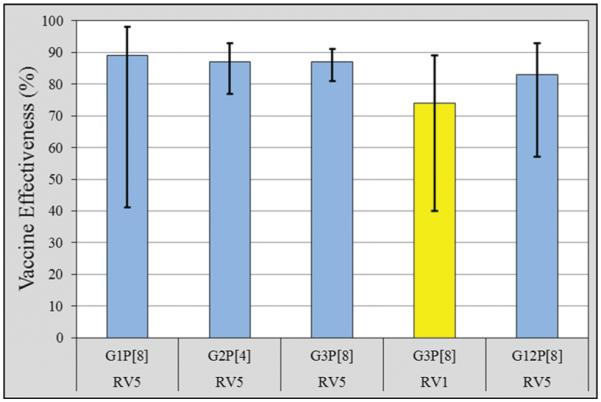
Vaccine effectiveness and 95% confidence intervals by predominant rotavirus genotype, for RV5 (blue) and RV1 (yellow). Abbreviations: RV1, Rotarix; RV5, RotaTeq.
We found no clear difference in VE by subject ethnicity. RV5 VE for Hispanic and non-Hispanic subjects was 85% (95% CI, 76%–90%) and 83% (95% CI, 75%–88%), respectively. Protection afforded by RV1 was statistically significant for non-Hispanic subjects (76% [95% CI, 44%–90%]).
Alternate Analyses
Results from our matched analyses in the inpatient and ED clinical settings without age restrictions were comparable to those adjusted analyses presented as our principal model. In that matched analysis for RV5, with 400 rotavirus cases and 1800 AGE test-negative controls, we found a 3-dose VE of 85% (95% CI, 79%–89%). For the 2-dose course of RV1 (74 cases matched to 255 AGE test-negative controls), the matched analysis VE was 68% (95% CI, 34%–85%).
CONCLUSIONS
In this geographically-diverse “real-world” assessment of concurrent RV5 and RV1 rotavirus vaccine field performance, we found that the administration of complete courses of RV5 or RV1 was associated with protection against medically attended rotavirus infections over our study period, with VE of 84% (95% CI, 78%–88%) and 70% (95% CI, 39%–86%), respectively. Although VE point estimates differed between vaccines, confidence intervals overlapped, suggesting no statistical difference in vaccine performance. No waning of immunity was evident during the first 4 years of life for RV5-immunized children, nor was it observed during the first 2 years of life for RV1-immunized children. Both a single dose and 2 doses of RV5 were statistically effective; the single dose estimate for RV1 was not statistically significant. RV5 provided statistically significant genotype-specific protection against each of the 4 major circulating rotavirus strains (G1P[8], G2P[4], G3P[8], G12P[8]), and RV1 had a statistically significant VE for the most common genotype, G3P[8]. We provide the first data indicating high effectiveness of RV5 against rotavirus caused by the G12 genotype, a strain that was previously uncommon but has emerged over the past decade. Despite much higher rotavirus positive caseloads in 2011, RV5 VE was sustained across the full study period; study power limitations allowed a season-specific RV1 estimate for only 2011.
Our VE estimate for a complete course of RV5 against rotavirus-associated hospitalizations and ED visits was comparable to those published previously for US children. Using similar models, RV5-specific VE calculated by Staat et al was 88% (95% CI, 47%–97%) during the period 2007–2009 [20], and in 2 studies by Boom et al, RV5 VE was 89% (95% CI, 70%–96%) in 2008 [21] and was 80% (95% CI, 45%–93%) in 2009 [22]. A VE assessment using medical institutions and immunization information systems for 3 states participating in the Emerging Infections Program Network by Cortese et al reported a complete-course RV5 VE of 89% (CI, 81%–94%) during the period 2007–2009 [23] among children aged 8 months or older. Our findings extend beyond previous work by demonstrating sustained RV5 VE for the first 4 years of life, which is reassuring regarding the long-term impact of the vaccine program.
To our knowledge, we report the first estimates of postlicensure RV1 VE among US children. In the ED setting, both RV5 and RV1 vaccines performed with similar effectiveness (81% [95% CI, 70%–84%] and 78% [95% CI, 46%–91%], respectively), which is encouraging as approximately two-thirds of our rotavirus-positive cases sought care in an ED, and it is in this clinical setting that rotavirus has a large burden of disease upon US medical care [13]. However, our estimate of RV1 VE did not achieve statistical significance in the inpatient setting, at least partially due to sample size and the relatively low uptake of RV1 in the communities under surveillance, which diminished our study power. The epidemiologic meaning of our lower RV1 inpatient VE estimate is unclear, as large clinical trials in inpatient settings in several high-income countries have consistently found RV1 to have high efficacy [10, 12]. Further investigation of the inpatient subjects in our RV1 analyses revealed that 4 (18%) were premature infants (half of these received complete RV1 vaccinations) and averaged 18.3 months of age at hospitalization. These 4 children spent an average 3.4 days as inpatients, and none were admitted to the intensive care unit. Genotypes G3P[8], G2P[4], G12P[6], and G12P[8] were detected in these RV1 inpatient specimens. Similar VE point estimates were observed between 1 dose of RV5 and a full course of RV1, although our RV1 data alone are insufficient to suggest changes to dose recommendations for rotavirus vaccines.
Our VE estimates of RV5 and RV1 are consistent with those reported from postlicensure evaluations in other high-income countries. In Queensland, Australia, where RV5 coverage was 73% for 3 doses, this vaccine was 89%–94% effective in preventing rotavirus hospitalizations [24]. In Israel, where both RV5 and RV1 were licensed in 2007, children receiving ≥1 dose of either rotavirus vaccine had a VE of 89% (95% CI, 52%–98%) against rotavirus hospitalizations [25]. In Spain, where both RV5 and RV1 have been available since 2006, Castilla et al estimated VE against rotavirus-associated hospitalizations for a complete course of RV5 (81% [95% CI, 68%–89%]) and RV1 (75% [95% CI, 60%–85%]) [26].
Limitations to our study exist. First, RV1 was introduced to the United States in 2008 and, by the time of our assessment, sample sizes for the RV1-vaccinated population remained relatively small. Further evaluation of RV1 performance, particularly among infants and older children, is warranted by our results. Second, unvaccinated controls may be selectively less representative of the source population of cases as the proportion of overall rotavirus vaccine coverage increases. We did not find significant differences between cases and rotavirus-negative AGE controls by major factors, such as race, ethnicity, and insurance status, which—if present—would have potentially introduced bias as these factors might affect the likelihood of hospitalizations or ED visits. Typically, one could expect younger children to be more likely to be hospitalized. Our study design minimized these potential biases by using controls that were sampled from the same clinical settings and had characteristics observed to be similar to our case subjects. However, we did note age differences between cases and controls and employed several epidemiologic methods to reduce this potential confounding; we restricted eligible subjects to those 2 months older than the date of their last possible ACIP-approved dose, adjusted for year and month of birth in our regression analyses, stratified the VE results by age group, and saw no discernible differences in VE estimates when applying an alternate matched model. We note that indirect protective effects from rotavirus vaccination have been suggested in observational studies among US children, including among the population we studied [8], and our estimates could potentially be affected by indirect protective effects among unimmunized children. Finally, we believe it is unlikely that differences in inpatient enrollment practices inherent to individual medical institutions influenced our results, because our RV5 findings are consistent with previous estimates from Cincinnati, Nashville, Rochester, and Houston surveillance sites using similar methodologies [21–23].
In conclusion, our assessment of a diverse sample of US children <5 years of age subject to “real-world” conditions found significant VE for complete courses of both RV5 and RV1 rotavirus vaccines. No indication of waning over time was observed at the detectable limits for either vaccine, nor was there any significant difference in vaccine performance by predominant circulating strains, including the G12 genotype, which is not homotypically covered by these vaccines. Each of these rotavirus vaccines in concurrent use performed well in preventing medically attended rotavirus AGE among young children in the United States.
Acknowledgments
Financial support. This work was funded by a cooperative agreement by the CDC.
M. A. S. served as a consultant for Merck and Co and GlaxoSmithKline Biologicals, grant funding from Glaxo-SmithKline Biologicals, and received payment for lectures from Merck and Co and GlaxoSmithKline Biologicals; E. J. K. has received payment from Medscape for a rotavirus presentation; S. H. J. owns private stock in Merck and Co; C. J. B. has served as a consultant for Novartis Vaccines and Diagnostics; M. M. has had laboratory service agreements with Merck and Co and GlaxoSmithKline Biologicals; J. C. has served as a consultant for and has submitted grant applications to Luminex Molecular Diagnostics.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Widdowson MA, Meltzer MI, Xang X, et al. Cost effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007;119:684–97. doi: 10.1542/peds.2006-2876. [DOI] [PubMed] [Google Scholar]
- 2.Widdowson MA, Meltzer M. Update on cost-effectiveness of rotavirus vaccination in the United States. Advisory Committee for Immunization Practices; Atlanta, GA: 2008. [Google Scholar]
- 3.Centers for Disease Control and Prevention Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58:1–26. [PubMed] [Google Scholar]
- 4.Tate JE, Panozzo CA, Payne DC, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124:465–71. doi: 10.1542/peds.2008-3528. [DOI] [PubMed] [Google Scholar]
- 5.Tate JE, Mutuc JD, Panozzo CA, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30(1 suppl):S30–4. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 6.Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010;29:489–94. doi: 10.1097/INF.0b013e3181d95b53. [DOI] [PubMed] [Google Scholar]
- 7.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus 21 vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30(1 suppl):S56–60. doi: 10.1097/INF.0b013e3181fefdc0. [DOI] [PubMed] [Google Scholar]
- 8.Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis. 2011;53:245–53. doi: 10.1093/cid/cir307. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 10.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 11.Vesikari T, Karvonen A, Puustinen L, et al. Efficacy of RIX 4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23:937–43. doi: 10.1097/01.inf.0000141722.10130.50. [DOI] [PubMed] [Google Scholar]
- 12.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomized, double-blind controlled study. Lancet. 2007;370:1757–63. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 13.Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008;122:1235–43. doi: 10.1542/peds.2007-3378. [DOI] [PubMed] [Google Scholar]
- 14.Payne DC, Szilagyi P, Staat MA, et al. Secular variation in US rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J; 2009;28:948–53. doi: 10.1097/INF.0b013e3181a6ad6e. [DOI] [PubMed] [Google Scholar]
- 15.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 16.Das BK, Gentsch JR, Cicirello HG, et al. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32:1820–2. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin DD, Nakagomi T, Hoshino Y, et al. Characterization of non-typeable rotavirus strains from the United States: identification of a new rotavirus reassortant (P2A[6], G12) and rare P3[9] strains related to bovine rotaviruses. Virology. 2002;294:256–69. doi: 10.1006/viro.2001.1333. [DOI] [PubMed] [Google Scholar]
- 18.Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–73. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gouvea V, Glass RI, Woods P, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–82. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128:e267–75. doi: 10.1542/peds.2010-3722. [DOI] [PubMed] [Google Scholar]
- 21.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2009;125:e199–207. doi: 10.1542/peds.2009-1021. [DOI] [PubMed] [Google Scholar]
- 22.Boom JA, Tate JE, Sahni LC, et al. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban US pediatric hospital. Pediatr Infect Dis J. 2010;29:1–3. doi: 10.1097/INF.0b013e3181ed18ab. [DOI] [PubMed] [Google Scholar]
- 23.Cortese MM, LeBlanc J, White KE, et al. Leveraging state immunization information systems to measure the effectiveness of rotavirus vaccines. Pediatrics. 2011;128:e1474–81. doi: 10.1542/peds.2011-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field EJ, Vally H, Grimwood K, et al. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics. 2010;126:e506–12. doi: 10.1542/peds.2010-0443. [DOI] [PubMed] [Google Scholar]
- 25.Muhsen K, Shulman L, Kasem E, et al. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis-associated hospitalizations in Israel: a case-control study. Hum Vaccin. 2010;6:450–4. doi: 10.4161/hv.6.6.11759. [DOI] [PubMed] [Google Scholar]
- 26.Castilla J, Baristain X, Martinez-Artola V, et al. Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine. 2012;30:539–43. doi: 10.1016/j.vaccine.2011.11.071. [DOI] [PubMed] [Google Scholar]