Abstract
Objective/Background
There is a high risk of relapse in stage IIIB/IIIC melanoma. The utility of 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography integrated with computed tomography (FDG-PET/CT) in these patients to evaluate response to treatment or for surveillance after treatment is currently not well defined.
Methods
Prospective data from 2 centers identified 97 patients with stage IIIB/IIIC extremity melanoma undergoing isolated limb infusion (ILI) who had whole body FDG-PET/CT scans before and every 3 months after treatment. Clinical response was determined at 3 months by Response Evaluation Criteria In Solid Tumors.
Results
Complete response (CR) after ILI occurred in 33% (32/97) of patients. FDG-PET/CT accurately identified 59% of patients who were CRs (19/32), whereas 41% (13/32) had residual metabolic activity in the extremity that was histologically negative for melanoma. The 3-year disease-free rate was 62.2% (95% CI: 40.1%–96.4%) for those patients who were CRs by both clinical/pathologic examination and FDG-PET/CT (n = 19) compared to only 29.4% (95% CI: 9.9%–87.2%) of those CRs who still had residual FDG-PET/CT activity (n = 13). FDG-PET/CT was utilized for surveillance of disease recurrence outside the regional field of treatment. Fifty-two percent (51/97) of patients developed disease outside the extremity at a median time of 212 days from pre-ILI FDG-PET/CT. In 47% (29/62) of these cases, the recurrence was resected.
Conclusions
Although FDG-PET/CT does not appear to accurately identify patients who appear to be CRs to ILI, it does appear to identify a subgroup of patients whose regional progression-free survival is markedly worse. However, FDG-PET/CT appears to be an excellent method for surveillance in stage IIIB/IIIC patients after ILI with ability to identify surgically resectable recurrent disease in these high-risk patients.
Keywords: melanoma, PET/CT, regional chemotherapy
Patients with in-transit melanoma (American Joint Committee on Cancer stage IIIB/C extremity melanoma) are commonly treated with isolated limb infusion (ILI), which delivers a high dose of regional chemotherapy to an isolated extremity in an attempt to control local disease.1 ILI with a melphalan dose corrected for ideal body weight has a complete response rate of approximately 30%.2 Close follow-up is critical as this population is at high risk of recurrence both regionally and systemically, which often occurs in an unpredictable pattern; the 5-year risk of relapses at any site was recently reported to be 71% for stage IIIB and 85% for stage IIIC melanoma patients.3 Few effective systemic treatment options exist for patients with multifocal in-transit recurrences and while complete surgical resection of metastases including regional lymph nodes and distant disease has been associated with improved survival, the surgical excision of in-transit disease becomes increasingly difficult as the number and size of lesions increases.3–14
Positron emission tomography integrated with computed tomography (PET/CT) imaging, employing 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (FDG) has gained popularity as a sensitive diagnostic tool to detect melanoma metastases that may ultimately be surgically resected. Multiple studies have reported higher sensitivity and specificity for detecting oncologic metastases with FDG PET as opposed to independently acquired CT scan alone, whereas the combination of functional and morphologic imaging with use of dual-modality FDG-PET/CT is superior to either alone.15–22 Reinhardt et al demonstrated that the accuracy of FDG-PET/CT for N-restaging was 100% (95% CI: 0.98 to 1.0), the sensitivity was 100% (95% CI: 0.98 to 1) and the specificity was 100% (95% CI: 0.98 to 1); for M-restaging accuracy was 98.5% (95% CI: 0.955 to 1), sensitivity was 100% (95% CI: 0.98 to 1), and specificity was 95.8% (95% CI: 0.909 to 1) in patients with cutaneous melanoma of various stages undergoing restaging after initial therapy.23 However, few studies have examined directly the clinical utility of FDG-PET/CT to specifically identify metastases that are amenable to surgical resection.24 In addition, the performance of FDG-PET/CT in evaluating responses to chemotherapy in melanoma and whether responses as assessed by FDG-PET/CT equate with clinical and pathologic responses is currently unknown.25–28 The aims of the study were to (1) compare how response to ILI as assessed by FDG-PET/CT correlates with clinical and pathologic response to ILI and (2) evaluate the use FDG-PET/CT as a surveillance tool to detect systemic recurrence that were surgically resected in this population of patients at high risk of developing progressive regional and systemic disease outside of the regional field of treatment.
METHODS
Patients/Intervention
A prospective database identified patients undergoing ILI at 2 institutions who had pre-ILI FDG-PET/CT scans (within 30 days of treatment) and had FDG-PET/CT scans at 3-month intervals for the first year and 6-month intervals thereafter following initial treatment. Most patients received ILI with melphalan plus dactinomycin (n = 69), whereas some patients were treated as part of phase I or II trials consisting of ILI with melphalan plus systemic adh-1 (n = 22),29 or ILI with melphalan plus systemic sorafenib (n = 6).30 ILI was performed as previously described,31 with a rapid infusion of chemotherapy (2 to 5 minutes) into the arterial portion of the circuit when the extremity had reached at least 37Ì. All patients had measurable cutaneous disease before therapy. Clinical response is determined by physical examination at one time point 3 months after ILI using Response Evaluation Criteria In Solid Tumors modified for cutaneous lesions. For patients having persistent clinical lesions at the 3-month time point by physical examination or persistent activity on FDG-PET/CT in the absence of physical examination findings, fine needle aspiration or punch biopsy was performed to confirm either persistent disease or absence of tumor but persistent pigment-laden macrophages. Patients with no clinically visible lesions at 3 months or patients with persistent pigment (but no viable tumor in pathologic specimen) were considered to be complete responders to treatment. Patients who had out of field disease (above planned tourniquet placement) on pre-ILI FDG-PET/CT scans (within 30 days of treatment) had disease surgically resected before ILI, at the time of ILI, or in the postoperative period (3 to 7 days after ILI). After the initial 3-month evaluation, patients were initially followed every 3 months for 1 year and then every 6 months to determine progression-free survival.
FDG-PET/CT Imaging
At Duke University, all patients (n = 63) were examined on a dedicated PET/CT system (Discovery ST, General Electric, Milwaukee, WI) using imaging protocols previously described.32 Diagnostic CT scans (with intravenous and oral contrast) were obtained as part of the PET/CT study in 42% of the studies. Patients received FDG intravenously on a sliding scale according to weight (0.18 mCi/kg or 6.7 MBq/kg; minimum dose 10 mCi (370 MBq); maximum 25 mCi (925 MBq). PET images were acquired using 2-dimensional acquisition mode, with acquisition time based on patient weight :2 minutes per bed position if less than 150 lbs (68 kg); 2.5 minutes per bed position if 150 to 199 lbs (68–90 kg); 3 minutes per bed position if 200 to 250 lbs (91–114 kg); 4 minutes per bed position if more than 250 lbs (114 kg). The CT protocol included the following parameters: tube current of 30 to 340 mA utilizing automated tube-current modulation, 140 kVp, 0.5 second per rotation, and pitch 1.375. The CT axial images were reconstructed in a 512 × 512 matrix of 50-cm field of view, with a 3.27-mm center-to-center spacing to match the PET and a thickness of 3.75 mm. The diagnostic CT scan was also utilized for PET attenuation correction, and additional corrections for scatter, random events, and dead time were performed in reconstructing 128 × 128 matrix images, also covering a 50-cm field of view using ordered-subset expectation maximization (OSEM) reconstruction.33
At Moffitt Cancer Center, scans were performed on either of 2 sister Discovery VCT PET/CT systems (GE Medical Systems). Each patient (n = 34) received a net (allowing for dose retained in syringe) injected dose of 11.18 mCi (437 MBq)18F-FDG (SD = 2.03, range = 8.6–18.5 mCi) of 18F-FDG. With clock synchronization, dose and uptake calculations accommodated for physical decay. Each patient was scanned, on the basis of patient weight, for 2 to 5 minutes in each of 6 to 7 bed positions, typically from the base of skull through mid-thighs; the same acquisition time was targeted for subsequent scans. Whole body uptake time interval was targeted for at least 90 minutes after injection, with subsequent lower extremity acquisition about 20 minutes later.
A fused 64-slice CT scan of the same field of view, typically; row -0- vertex, rotation time 0.5 second per rotation, slice thickness 3.75 mm, field of view 50 to 70 mm, tube voltage 120 kV, and tube electric current 110 to 120 mA, using integrated concurrent 64-slice CT acquisition technology. No enteric or intravenous contrast material was administered. Using the standard clinical software provided by the manufacturer, PET images were reconstructed after compensation for random coincidences, scattered radiation, and CT-based attenuation correction, The performance and the acquisition conditions of the PET scanners were as follows: field of view, 70; matrix size, 128 × 128; slice thickness, 3.3 mm; acquisition time, 21 to 24 minutes; reconstruction method iterative high definition view point (Iteration, subset), 20/2 iterations; with # 7 Gaussian smoothing filter.
Equipment performance verification steps complied with instructions of the manufacturer for calibration, attenuation correction, coincidences correction, and detector variation correction, image reconstruction including smoothing, decay correction, and clock synchronization for dose calibration.
After interpretation by a radiologist, patients were considered to be a complete responder to treatment as assessed by FDG-PET/CT if “complete resolution of FDG activity in the treated extremity” or “no evidence of FDG activity in the treated extremity” was reported. FDG avid sites on PET/CT were confirmed in appropriate cases with fine needle aspiration, CT guided biopsy, and subsequent surgical resection when feasible. This study was approved by the institutional review boards at the participating centers, and informed consent was obtained from all subjects.
Statistics
Descriptive statistics were used to summarize baseline demographics and response. In field response (below tourniquet) was defined as complete response (CR) or partial response with no response defined as either stable disease or progressive disease. Time to in-field response was defined as time from ILI date to in-field disease progression and patients without a progression were censored at the date of last clinical follow-up. Overall survival was defined as time from ILI procedure to death due to any cause. Log-rank tests were used to compare 2 or more distributions of time to in-field response and overall survival. Time-to-event plots were based on the Kaplan-Meier product limit estimator. Confidence intervals for proportions were calculated using the Exact Binomial method. All statistical analyses were performed using SAS (SAS Institute, Cary, NC), and plots were created using S+ (TIBCO Spotfire).
RESULTS
Ninety-seven patients at 2 centers treated from 2004 to 2010 had data available for analysis. Patient characteristics are listed in the table. Stage at the time of ILI is included in the table. Pre-ILI FDG-PET/CT scans identified disease above planned tourniquet placement in 10 patients. These patients underwent surgical resection of the regional lymph node basin (n = 7) or resections of lung metastases (n = 3) around the time of ILI with melphalan. In these patients, stage at the time of ILI was updated as applicable to reflect changes in stage that were the results of these resections. For example, a patient with known stage IIIB disease before the pre-ILI FDG-PET/CT scan that was found to have a lung metastasis and subsequently underwent resection around the time of ILI with melphalan was appropriately classified as stage IV disease for the purposes of our analysis. All other stage IIIC patients included in this study by definition had prior lymph node involvement, and nearly all had undergone regional lymphadenectomy at a previous time point (>30 days before ILI).
TABLE 1.
Patient Characteristics
| No. | % | |
|---|---|---|
| Total | 97 | |
| Age | Median 67 (33–68) | |
| Sex | ||
| Male | 48 | 49 |
| Female | 49 | 51 |
| Stage | ||
| Unknown | 4 | 4 |
| IIIB | 61 | 63 |
| IIIC | 29 | 30 |
| IV | 3 | 3 |
| Stage IIIC/IV resected peri-ILI | 10 | 10 |
| M-ILI + ADH-1 trial | 22 | 23 |
| M-ILI + sorafenib trial | 6 | 6 |
| Previous regional chemotherapy | 4 | 4 |
At the 3-month time point after ILI, 33% (32/97) of patients had clinical/pathologic CR, 21% (20/97) had a partial response, 10% (10/97) had stable disease, 34% (34/97) had progressive disease, and 1 patient was lost to follow-up. The median time between the pre-treatment scan and first scan post-ILI was 117 days (range 45 to 265). Of patients who were classified as CRs (n = 32), 11 had persistent pigmented lesions by clinical examination whereas 21 had complete disappearance of lesions by physical examination. An example of a patient who had complete resolution of lesions on FDG-PET/CT but persistence by physical examination of pigmented lesions ultimately determined to be melanin pigmented cells and is shown in Figure 1. Interestingly, in the 32 patients who were CR by clinical/pathologic examination, FDG-PET/CT demonstrated CR in only 59% (19/32) as outlined in Figure 2. The remaining 13 patients (41%) had remaining FDG activity but no physical or pathologic evidence of disease. An example of a complete responder with persistent FDG activity at 12 weeks post-ILI with no visible disease and biopsy of the FDG avid area consistent with pigment-laden macrophages is shown in Figure 3. Conversely, FDG-PET/CT demonstrated persistence of disease in 92% (59/64) of patients who were determined clinically or pathologically to have a partial response, stable disease, or progressive disease. In some cases (5%, 3/59), complete response was observed on physical examination but persistent FDG avid site biopsies were consistent with melanoma as depicted in Figure 4. An additional 5 of the 64 patients (8%) were classified as CRs by FDG-PET/CT but had persistent disease on physical examination and/or pathologic examination; an example is shown in Figure 5. Collectively, examples as outlined by Figures 1, 3, 4, and 5 demonstrate the complexity of utilizing FDG-PET/CT for assessing response to ILI for in-transit melanoma.
FIGURE 1.
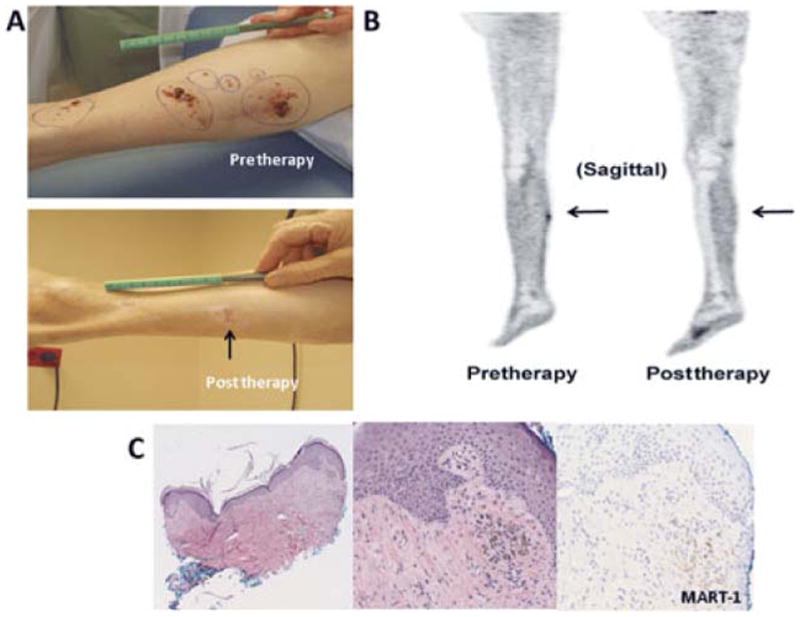
An example of a patient who was CR by FDG-PET/CT with persistent superficial skin lesions on physical examination ultimately found to be pigment only on histology; the patient was classified as a CR. A, Photographs demonstrate the persistence of a pigmented lesion (arrow) 12 weeks post-ILI. B, Sagittal FDG-PET/CT images pre-ILI (left) showing increased FDG uptake within the right calf and at 12 weeks post-ILI (right) showing resolution of the hypermetabolic soft tissue nodule within the right calf. C, Punch biopsy of the pigmented lesion at 12 weeks post-ILI. Left, a low-power magnification shows pigmented cells in the dermis (4X), the middle and right photographs taken in high power (20X) highlight the MART-1 negative pigmented cells supporting the interpretation of melanin-laden macrophages.
FIGURE 2.
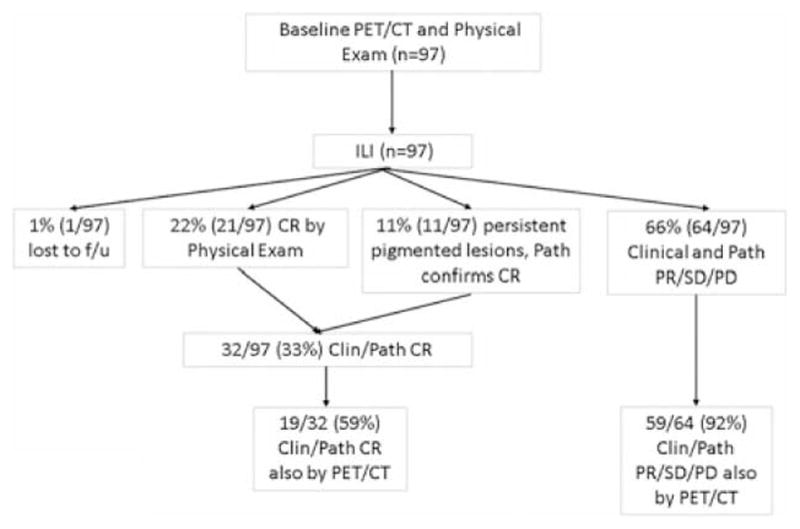
Flow diagram of patients examining in-field response. Clin indicates clinical; Path, pathologic.
FIGURE 3.
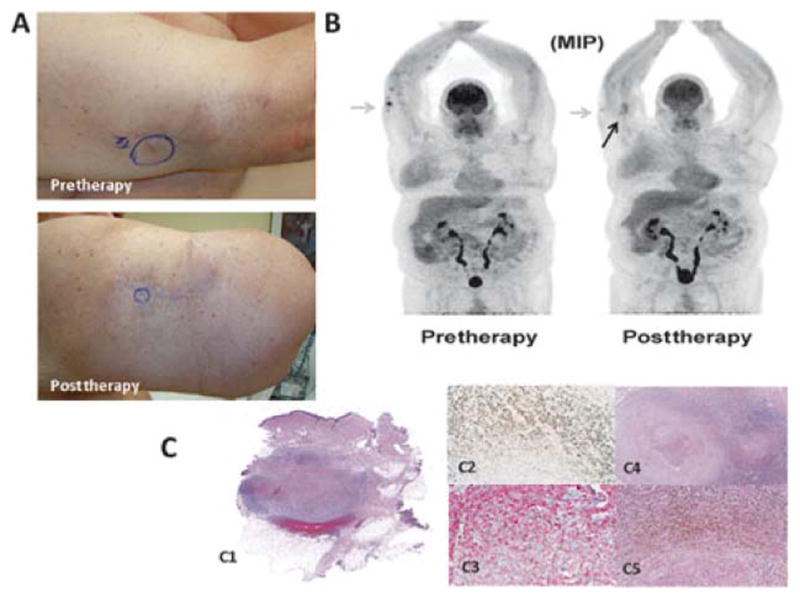
An example of a patient who had palpable but decreased in size nodules on physical examination and persistent FDG activity ultimately determined to be fibrotic nodules in-filtrated with pigment-laden macrophages and no tumor; this patient was classified as a CR. A, Photographs demonstrate absence of lesions at 12 weeks post-ILI but a persistent palpable nodule. B, FDG-PET/CT maximum intensity projection images pre-ILI (left) showing hypermetabolic superficial soft tissue nodules along the right forearm and at 12 weeks post-ILI (right) showing a presence but decrease in number and metabolic activity of right upper extremity nodules (an example is highlighted by the gray arrows). The black arrow points to fat necrosis that can be seen on FDG-PET/CT after treatment. C, Wide local excision of the palpable lesion at 12 weeks post-ILI. On the left (C1), a scan of the whole section mount to demonstrate the location of the nodule, at right there is a medium (C2, 2X) and high (C3, 10X) power view of the pigmented cells. Immunohistochemical stains performed in this specimen show that the cells are negative for MART-1 (C4, 10X) and positive for CD163 (C5, 10X), supporting the interpretation of melanin-laden macrophages.
FIGURE 4.
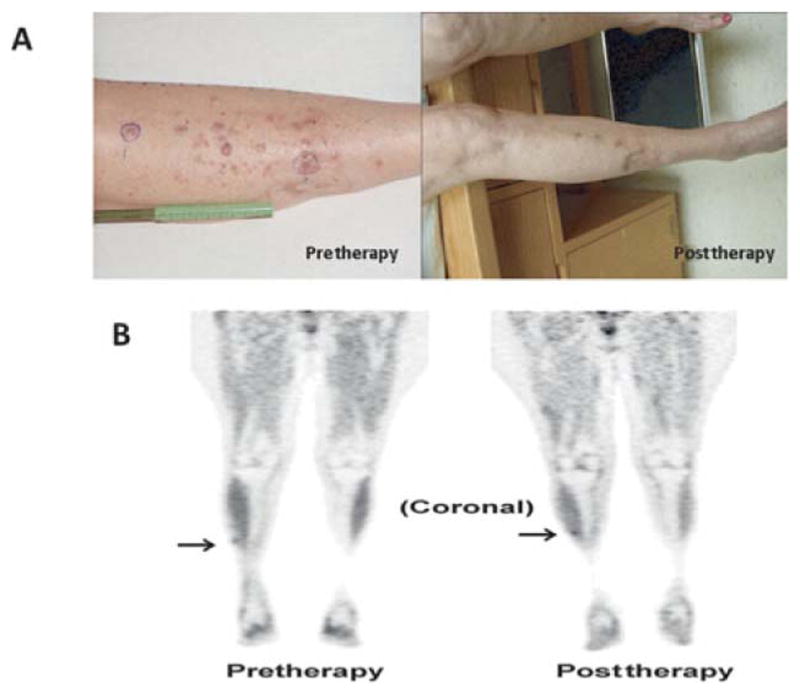
An example of a patient who had a CR by physical examination but had persistence of FDG activity on PET/CT determined by histology to be melanoma; this patient is classified as a partial responder. A, Photographs demonstrate resolution of lesions at 12 weeks post-ILI. B, Coronal FDG-PET/CT images pre-ILI (left) showing mild focal FDG uptake in the right anterior leg (arrow) and at 12 weeks post-ILI (right) showing unchanged low-level FDG activity in the right anterolateral shin. Biopsy of this FDG spot using needle localization was consistent with melanoma.
FIGURE 5.
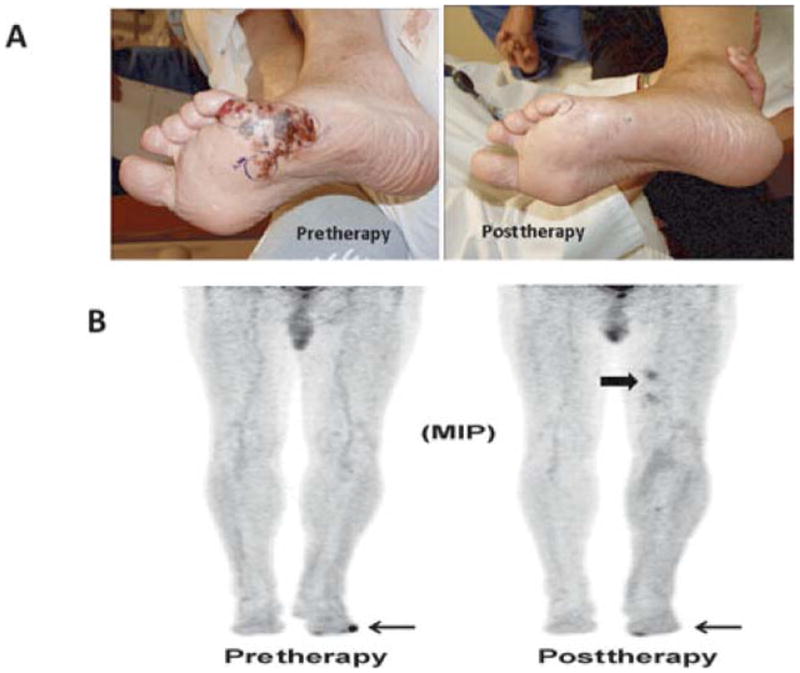
An example of a patient who had a partial response by physical examination but complete resolution of FDG activity on PET/CT; this patient was classified as a partial responder. A, Photographs demonstrate a partial response of lesions at 12 weeks post-ILI. Biopsy of this clinical evident lesion was consistent with melanoma. B, Maximum intensity projection FDG-PET/CT images pre-ILI (left) showing a hypermetabolic focus in the lateral aspect of the left foot (arrow) and at 12 weeks post-ILI (right) showing interval resolution of the hyper-metabolic focus of activity along the lateral aspect of the left foot. Note there is some activity on the medial portion of the foot near the great toe, which was unrelated to the melanoma. Also noted is myonecrosis not infrequently seen after ILI in the upper thigh (block arrow), which is considered a false-positive FDG-PET/CT finding.
Time to progression in the extremity after complete response to ILI is important in determining the effectiveness of the treatment. The median time to progression in the extremity for all patients in this study after achieving a CR was 2.66 years. Interestingly the 3-year disease-free rate was 62.2% (95% CI: 40.1% to 96.4%) for those patient who were CRs by both clinical/pathologic examination and FDG-PET/CT (n = 19) compared to only 29.4% (95% CI: 9.9% to 87.2%) of those complete responders who still had residual FDG-PET/CT activity (n = 13) (Fig. 6).
FIGURE 6.
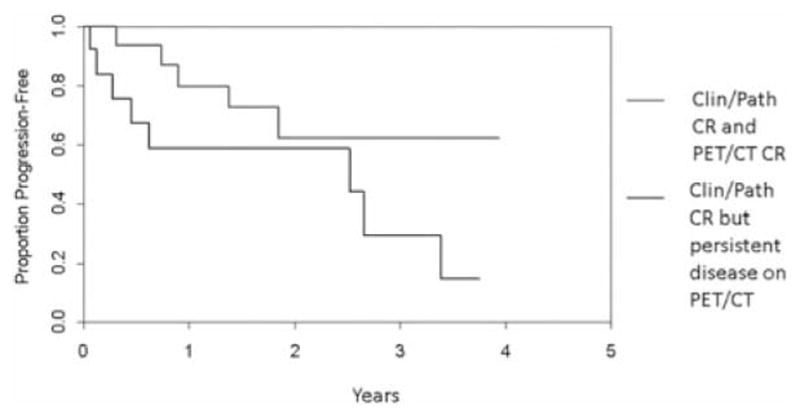
Time to progression for those achieving a clinical/pathologic CR and a CR via FDG-PET/CT compared to those achieving a clinical/pathologic CR only.
Figure 7 details the results of post-ILI FDG-PET/CT scans in this study population. Disease outside the field was identified in 51 patients at a median time after ILI of 212 days (range 34 to 1013); 11 patients had multiple sites of recurrence, whereas 40 initially had a single site of recurrence. Regional lymph nodes were the most common site accounting for 56% (35/62) of recurrences, whereas lung metastases accounted for 23% (14/62), and 21% (13/62) were metastatic to other sites. These sites included subcutaneous disease above the tourniquet (n = 2), liver (n = 4), osseous lesions (n = 2), brain (n = 1), cervical lymph nodes (n = 1), and abdomen (n = 3). Of the disease identified, 63% (22/35) underwent regional lymphadenectomy, 36% (5/14) underwent lung resections, and 15% (2/13) underwent complete surgical resections of other metastatic disease. Of the 51 patients who were initially found to have distant disease, FDG-PET/CT identified a second site out of field in 23 patients at a median time of 468 days (range 82 to 944). Two of these patients had multiple sites of recurrence (Fig. 7). Surgical resections were performed in 50% (3/6) of these patients for involved regional lymph nodes, and 21% (4/19) of these patients underwent complete surgical resections of other metastases.
FIGURE 7.
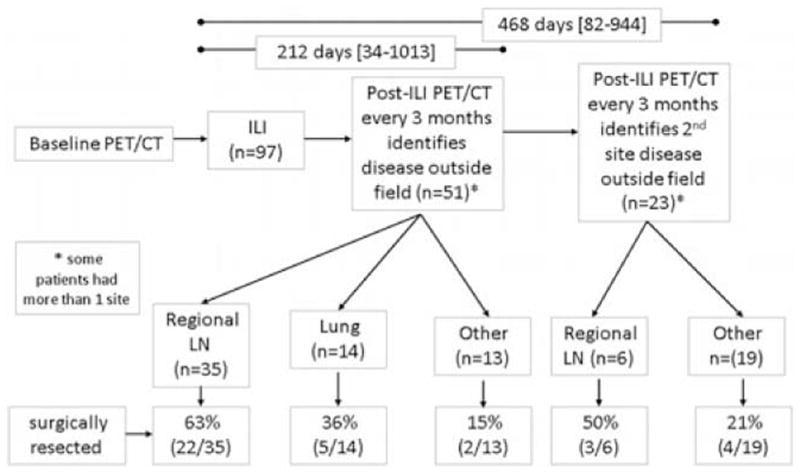
Flow diagram of patients examining identification of systemic disease. LN indicates lymph node.
The 3-year survival rate for all 97 patients in the study was 49% (95% CI: 40% to 61%). This falls within the range expected given this study population included those with stage IIIB disease (n = 61), stage IIIC disease (n = 29), and stage IV disease (n = 3). The association of overall survival with response was assessed with a landmark analysis using 3 months as the landmark time. This analysis shows that patients achieving a CR after ILI appeared to have a survival benefit compared to patients not achieving a CR as shown in Figure 8 (P < 0.001). For patients achieving a CR, the median survival is not yet available (>50% of patients have died over nearly 5-year follow-up) whereas the median survival for those achieving a partial response (n = 38) was 1.55 years and progressive disease (n = 62) was 1.82 years. The difference in median survival among the 2 types of CRs (those with persistent FDG activity and those with no residual FDG activity) was not statistically evaluated because of small sample size and low number of events (deaths) in the groups.
FIGURE 8.
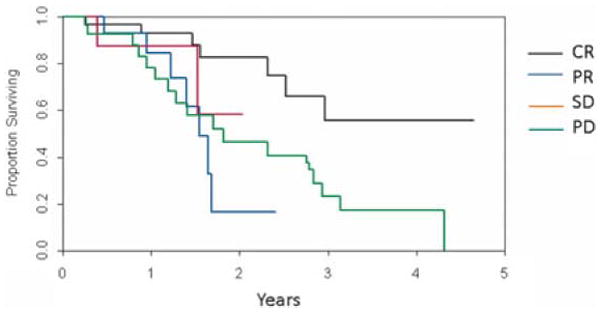
Survival after ILI according to clinical/pathologic response.
DISCUSSION
Patients with American Joint Committee on Cancer IIIB/IIIC extremity melanoma are at high risk of recurrence both regionally and systemically. Currently, there is no uniform consensus or guidelines for surveillance of these patients. The National Comprehensive Care Network guidelines state to consider FDG-PET/CT scans every 6 to 12 months for stage IIIB-IV.34 There is increasing evidence to suggest a more definitive role for FDG-PET/CT in managing patients with advanced stages of melanoma. Conservative estimates of the sensitivity for FDG-PET/CT are 87% to 91% with a specificity of 92% to 98% in detecting metastases in patients with stage III or IV melanoma.20,35 Results of FDG-PET/CT imaging have been found to change treatment plans in approximately 37% to 53.8% of patients with advanced melanoma undergoing restaging for recurrence lending further support to the clinical utility of FDG-PET/CT scans.15,20,23 We currently utilize a strategy in patients treated with ILI for in-transit disease of obtaining FDG-PET/CT scans before therapy (within 30 days), at 3-month intervals during the first year after therapy, and then every 6 months in subsequent years until 5 years posttreatment at which point if there is no evidence of disease, FDG-PET/CT scans are obtained yearly. As such, we are able to evaluate the diagnostic performance of FDG-PET/CT for both detection of distant metastases and for assessing response to regional chemotherapy.
Given the high sensitivity, specificity, and accuracy of FDG-PET/CT to detect distant metastases in patients with stage IIIB//IIIC/IV, we specifically examined how often findings on FDG-PET/CT were amenable to a surgical intervention. Using surveillance imaging with FDG-PET/CT at the intervals specified following ILI, FDG-PET/CT lead to surgical resection in 47% (29/62) of distant (outside the extremity) recurrences following ILI. With limited systemic therapy options, complete surgical resection of metastatic disease remains a critical component of the management of these patients as surgical excision can be associated with improved survival.4–6,24,36,37 Previous data from Duke University in 2505 patients undergoing full lymph node resections for metastatic melanoma found the estimated overall survival rates at 5, 10, 15, and 20 years were 43%, 35%, 28%, and 23%; suggesting that some patients can achieve exceptionally long-term survival after resection of metastatic nodal disease.8 Additional data from Duke University in 1720 melanoma patients with pulmonary metastases found that pulmonary metastasectomy was associated with a survival advantage of 12 months for patients with a disease-free interval longer than 5 years (19 vs 7 months, P < 0.01) and of 10 months for patients without extra thoracic metastasis (18 vs 8 months, P < 0.01).38 Resection of properly staged and evaluated patients with limited pulmonary metastases also appeared to convey a significant survival benefit in 86 patients undergoing lung resections at Moffitt Cancer Center.39 The ability of FDG-PET/CT to allow us to more optimally select patients with small volume metastatic disease (both nodal and distant) who might be candidates for surgical resection remains important as surgical resection continues to be an important treatment option in this group of patients.40
Less data is available on the diagnostic performance of FDG-PET/CT to assess response to chemotherapy. In-transit disease is unique in that pathologic confirmation and physical examination can readily and safely identify complete responses to chemotherapy. In contrast, responses to chemotherapy at visceral sites are reliant on interpretations of FDG-PET/CT that may include measures such as comparisons of FDG uptake or standard uptake values before and after treatment25 followed by subsequent retrospective correlations with outcome. In 25 patients with stage IV melanoma undergoing treatment with systemic chemotherapy, Strobel et al25,27 assessed response by FDG-PET/CT with radiographic criteria and found that FDG-PET/CT responders as assessed by the specific criteria (n = 10) had a median survival of 18 months compared to 11 months in nonresponders as assessed by the same radiologic criteria (n = 15) (P = 0.072). In addition, responders on FDG-PET/CT also had remarkably better 1-year overall survival of 80% compared to non-responders (40%) (P = 0.048). In our study, response as assessed by FDG-PET/CT also provided valuable prognostic information as the median time to progression was longer in patients who were complete responders by FDG-PET/CT compared to those who were nonresponders by FDG-PET/CT but had complete responses by physical examination and pathologic examination. At present, the small number of patients in the 2 groups precluded finding this difference to be significant, and future studies are needed to validate this finding.
Although FDG-PET/CT may provide prognostic value, the ability to assess response to ILI with FDG-PET/CT may be more limited. Melanoma cells present with high uptake of FDG which allows for the high sensitivity and specificity of FDG-PET/CT.41,42 There is a single case report of a patient who had complete resolution of in-transit metastasis 5 weeks after ILI determined clinically and confirmed by FDG-PET/CT.26 However, FDG-PET/CT may not be as sensitive at detecting small volume or microscopic disease (≤5 mm) and therefore has limited utility in patients with stage I/II cutaneous melanoma or those with and microscopic lymph node metastases.43,44 In addition, Reinhardt et al23 showed the detection rate of nonvisceral metastases was about 3% less than the detection rate of visceral metastases. In-transit melanoma includes a broad spectrum of disease presentations that range from multiple small lesions (<1 cm) to large bulky lesions. As such, evaluating responses in patients who have smaller foci of disease burden with FDG-PET/CT may be problematic not only with regard to the absolute size of the lesion but also with regard to being able to separate 2 small lesions that are very close to each other. In addition, post-ILI changes including fat necrosis and myonecrosis (Figs. 3 and 5) may be seen on post-ILI images further complicating ability to assess tumor response. These complexities were evident in our study especially in cases where lesions or FDG activity persisted. Although FDG-PET/CT did identify CR in 59% (19/32) of the true CRs in this study, an additional 13/32 (41%) of CRs were identified by physical examination and pathologic examination of tissue. The multifocal nature of disease, with lesions present throughout the entire extremity also makes applying FDG-PET/CT criteria for response to each individual lesion difficult. The treatment effects after ILI are thus assessed by FDG-PET/CT, physical examination, and pathologic examination when available.
Interestingly, a majority of recurrences (56%) occurred in the regional lymph node basin even though approximately 30% of patients in this study had previous lymph node dissections. This is not necessarily surprising as after complete nodal dissection, recurrence in the nodal basin is estimated to occur in 16% to 30% of patients.45,46 In addition, for patients with in-transit disease, a reported 47% (14/30) will have positive sentinel nodes when the intransit lesion is mapped including patients who had previous lymph node evaluation.47 The study by Yao et al,47 and results from this study suggesting a high rate of nodal recurrence, has led to the practice of some investigators in this study performing sentinel lymph node biopsies in patients presenting with in-transit disease. How this practice might affect earlier and more sensitive detection of nodal metastases is currently being studied. Certainly, patients presenting with in-transit disease who have a positive sentinel node would be candidates for ILI plus regional lymphadenectomy or hyperthermic isolated limb perfusion, which can include a lymph node dissection.
The CR rate of 32% (32/97) seen among treated patients in this study is similar to the CR rate of 29% reported in a US multi-center review of ILI.48 As such, the group of patients in this study is likely representative of our larger collective experience with ILI. Whether any form of regional treatment is associated with improved survival is currently unknown. In a large, multicenter World Health Organization/European Organization for Research and Treatment of Cancer trial, improvement in locoregional recurrence after adjuvant hyperthermic isolated limb perfusion (another form of melphalan-based regional chemotherapy) did not improve overall survival and there was no impact on prevention of systemic disease.49 However, there does appear to be a survival benefit for those who do achieve a CR to regional treatment. After ILI, Kroon et al50 reported that median survival was 53 months in those with CR compared with 25 months in those with progressive disease. Recent data from Duke University also suggest a survival benefit after obtaining a response to regional chemotherapy.2 When all responders were pooled in the Duke study, there was not a statistically significant difference in overall survival based upon the type of regional therapy that individual patients received.2 In this study with patients from 2 centers, those achieving a CR had a statistically significant improvement in survival. Whether a CR assessed by FDG-PET/CT criteria alone will correlate with an equal or greater survival benefit compared to clinical and pathologic CRs who have persistent FDG-PET/CT activity remains to be determined.
CONCLUSIONS
Although assessment of FDG-PET/CT to evaluate a CR after treatment with ILI only matched assessment by physical examination and/or pathologic examination in 59% (19/32) cases, a CR on FDG-PET/CT may provide prognostic value regarding the duration of a complete response and possibly survival. In addition, FDG-PET/CT performed at regular intervals after ILI for extremity melanoma was a valuable diagnostic tool. Particularly the diagnostic performance to detect distant metastases that were amenable to surgical resection was beneficial; surgical resection was performed successfully in 47% (29/62) of initial distant recurrences.
Footnotes
Disclosure: Dr. Tyler received support for the ADH-1 trial from Adherex Technologies, Inc. Bayer Healthcare Pharmaceuticals provided study drug (sorafenib, Nexavar) for the phase I trial of systemic sorafenib and regional melphalan, and Dr. Tyler receives clinical trial support from Merck, Schering-Plough.
References
- 1.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 2.Raymond AK, Beasley GM, Broadwater G, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Amer Coll Surg. 2011;213:306–316. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romano E, Scordo M, Dusza SW, et al. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28:3042–4047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbe C, Paul A, Koher-Spath H, et al. Prospective Evaluation of a follow-up schedule in cutaneous melanoma patients: recommendations for an effective follow-up strategy. J Clin Oncol. 2003;21:520–529. doi: 10.1200/JCO.2003.01.091. [DOI] [PubMed] [Google Scholar]
- 5.Ollila D. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7:919–924. doi: 10.1016/S1470-2045(06)70938-X. [DOI] [PubMed] [Google Scholar]
- 6.Meyer T, Merkel S, Goehl J, et al. Surgical therapy for distant metastases of malignant melanoma. Cancer. 2000;89:1983–1991. doi: 10.1002/1097-0142(20001101)89:9<1983::aid-cncr15>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Morton DL, Waneck L, Nizze JA, et al. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;4:491–499. doi: 10.1097/00000658-199110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White RR, Stanley WE, Johnson JL, et al. Long-term survival in 2,505 patients with melanoma with regional lymph node metastasis. Ann Surg. 2002;235:879–887. doi: 10.1097/00000658-200206000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher S. Elective, therapeutic, and delayed lymph node dissection for malignant melanoma of the head and neck: analysis of 1444 patients from 1970 to 1998. Laryngoscope. 2002;112:99–110. doi: 10.1097/00005537-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Badgwell B, Xing Y, Gershenwald JE, et al. Pelvic lymph node dissection is beneficial in subsets of patients with node-positive melanoma. Ann Surg Oncol. 2007;14:2867–2875. doi: 10.1245/s10434-007-9512-7. [DOI] [PubMed] [Google Scholar]
- 11.Chan AD, Essner R, Wanek LA, et al. Judging the therapeutic value of lymph node dissections for melanoma. J Am Coll Surg. 2002;191:16–22. doi: 10.1016/s1072-7515(00)00313-6. [DOI] [PubMed] [Google Scholar]
- 12.Kretschmer L, Hilgers R, Möhrle M, et al. Patients with lymphatic metastasis of cutaneous malignant melanoma benefit from sentinel lymphonodectomy and early excision of their nodal disease. Eur J Cancer. 2004;40:212–218. doi: 10.1016/j.ejca.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Young SE, Martinez SR, Faries MB, et al. Can surgical therapy alone achieve long-term cure of melanoma metastatic to regional nodes? Cancer. 2006;12:207–211. doi: 10.1097/00130404-200605000-00009. [DOI] [PubMed] [Google Scholar]
- 14.McLoughlin JM, Zager JS, Sondak VK, et al. Treatment options for limited or symptomatic metastatic melanoma. Cancer Control. 2008;15:239–247. doi: 10.1177/107327480801500307. [DOI] [PubMed] [Google Scholar]
- 15.Brady MS, Akhurst T, Spanknebel K, et al. Utility of preoperative [(18)]f fluorodeoxyglucose-positron emission tomography scanning in high-risk melanoma patients. Ann Surg Onc. 2006;13:525–532. doi: 10.1245/ASO.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Tyler DS, Onaitis M, Kherani A, et al. positron emission tomography scanning in malignant melanoma. Cancer. 2000;89:1019–1025. [PubMed] [Google Scholar]
- 17.Swetter SM, Carroll LA, Johnson DL, et al. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646–653. doi: 10.1007/BF02574480. [DOI] [PubMed] [Google Scholar]
- 18.Finkelstein SE, Carrasquillo J, Hoffman JM, et al. A prospective analysis of positron emission tomography and conventional imaging for detection of stage IV metastatic melanoma in patients undergoing metastasectomy. Ann Surg Oncol. 2004;11:731–738. doi: 10.1245/ASO.2004.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoder H, Larson SM, Yeung HW. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med. 2004;45:72s–81s. [PubMed] [Google Scholar]
- 20.Aukema TS, Valdes Omos RA, Wouters MW, et al. Utility of preoperative 18F-FDG PET/CT and brain MRI in melanoma patients with palpable lymph node metastases. Ann Surg Oncol. 2010;17:2773–2778. doi: 10.1245/s10434-010-1088-y. [DOI] [PubMed] [Google Scholar]
- 21.Macapinlac H. FDG PET and PET/CT imaging in lymphoma and melanoma. Cancer J. 2004;10:262–270. doi: 10.1097/00130404-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Antoch G, Vogt FM, Freudenberg LS, et al. Whole-body dual modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290:3199–3206. doi: 10.1001/jama.290.24.3199. [DOI] [PubMed] [Google Scholar]
- 23.Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18 F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–1186. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 24.Dalrymple-Hay MJ, Rome PD, Kennedy C, et al. Pulmonary metastatic melanoma—the survival benefit associated with positron emission tomography screening. Eur J Cardiothorac Surg. 2002;21:611–614. doi: 10.1016/s1010-7940(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 25.Strobel K, Dummer R, Steinert HS, et al. Chemotherapy response assessment in stage IV melanoma patients—comparison of 18 F-FDG-PET/CT, CT, brain MRI, and tumormarker S-100B. Eur J Nucl Med Mol Imaging. 2008;35:1786–1795. doi: 10.1007/s00259-008-0806-1. [DOI] [PubMed] [Google Scholar]
- 26.Ryan ER, Hill AD, Skehan SJ. FDG PET/CT demonstrates the effectiveness of isolated limb infusion for malignant melanoma. Clin Nucl Med. 2006;31:707–708. doi: 10.1097/01.rlu.0000242602.56590.c5. [DOI] [PubMed] [Google Scholar]
- 27.Strobel K, Skalsky J, Steinert HC, et al. S-100B and FDG-PET/CT in therapy response assessment of melanoma patients. Dematology. 2007;215:191–201. doi: 10.1159/000106575. [DOI] [PubMed] [Google Scholar]
- 28.Hofman MS, Constantinidou A, Acland K, et al. Assessing response to chemotherapy in metastatic melanoma with FDG PET: early experience. Nucl Med Commun. 2007;12:902–906. doi: 10.1097/MNM.0b013e3282f1b97b. [DOI] [PubMed] [Google Scholar]
- 29.Beasley GM, McMahon N, Sanders G, et al. A phase 1 study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. Cancer. 2009;115:4766–4774. doi: 10.1002/cncr.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon N, Beasley G, Sanders G, et al. A phase I study of systemic sorafenib in combination with isolated limb infusion with melphalan (ILI-M) in patients (pts) with locally advanced in-transit melanoma. J Clin Oncol. 2009;27(suppl):abstr 9065. [Google Scholar]
- 31.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 32.Wong TZ, Paulson EK, Nelson RC, et al. Practical approach to diagnostic CT combined with PET imaging. AJR Am J Roentgenol. 2007;188:622–629. doi: 10.2214/AJR.06.0813. [DOI] [PubMed] [Google Scholar]
- 33.Chin BB, Green ED, Turkington TG, et al. Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol. 2008;11:118–122. doi: 10.1007/s11307-008-0177-9. [DOI] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology, Version 1. Fort Washington, PA: NCCN; 2011. [Google Scholar]
- 35.Akcali C, Zincirkeser S, Erbagcy Z, et al. Detection of metastases in patients with cutaneous melanoma using FDG-PET/CT. J Int Med Res. 2007;35:547–553. doi: 10.1177/147323000703500415. [DOI] [PubMed] [Google Scholar]
- 36.Sanki A, Scolyer R, Thompson JF. Surgery for melanoma metastases of the gastrointestinal tract: indications and results. Eur J Surg Onc. 2009;35:313–319. doi: 10.1016/j.ejso.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Wong SL, Coit DG. Role of surgery in patients with stage IV melanoma. Curr Opin Oncol. 2004;2:155–160. doi: 10.1097/00001622-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RP, Hanish S, Haney JC, et al. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 2007;133:104–110. doi: 10.1016/j.jtcvs.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 39.Andrews S, Robinson L, Cantor A, et al. Survival after surgical resection of isolated pulmonary metastases from malignant melanoma. Cancer Control. 2006;13:218–223. doi: 10.1177/107327480601300309. [DOI] [PubMed] [Google Scholar]
- 40.Wasif N, Bagaria SP, Ray P, et al. Does metastasectomy improve survival in patients with stage IV melanoma? A cancer registry analysis of outcomes. J Surg Onc. 2011;104:111–115. doi: 10.1002/jso.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kern K. [14C] deoxygluocse uptake and imaging in malignant melanoma. J Surg Res. 1991;50:643–647. doi: 10.1016/0022-4804(91)90056-r. [DOI] [PubMed] [Google Scholar]
- 42.Wahl RL, Hutchins GD, Buchsbaum DJ, et al. 18F-2-deoxy-2-fluoro-D-glucose uptake into human tumor xenografts: feasibility studies for cancer imaging with positron-emission tomography. Cancer. 1991;47:1544–1550. doi: 10.1002/1097-0142(19910315)67:6<1544::aid-cncr2820670614>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 43.Mijnhout GS, Hoekstra OS, van Tulder MW, et al. Systematic review of the diagnostic accuracy of [18F] fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer. 2001;91:1530–1542. [PubMed] [Google Scholar]
- 44.Wagner JD, Schauwecker D, Hutchins G, et al. I, Initial assessment of positron emission tomography for the detection of subclinical regional lymphatic metastasis in melanoma. J Surg Oncol. 1997;64:181–189. doi: 10.1002/(sici)1096-9098(199703)64:3<181::aid-jso2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Nathansohn N, Schacter J, Gutman H, et al. Patterns of recurrence in patients with melanoma after radical lymph node dissection. Arch Surg. 2005;140:1172–1177. doi: 10.1001/archsurg.140.12.1172. [DOI] [PubMed] [Google Scholar]
- 46.Lee RJ, Gibbs JF, Proulx GM, et al. Nodal basin recurrence following lymph node dissection for melanoma: implications for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2000;62:467–474. doi: 10.1016/s0360-3016(99)00431-9. [DOI] [PubMed] [Google Scholar]
- 47.Yao KA, Hsueh EC, Essner R, et al. Is sentinel lymph node mapping indicated for isolated local and in-transit recurrent melanoma? Ann Surg. 2003;238:743–747. doi: 10.1097/01.sla.0000094440.50547.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715–717. [DOI] [PubMed] [Google Scholar]
- 49.Koops S, Vaglini M, Suciu S, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol. 1998;16:2906–2912. doi: 10.1200/JCO.1998.16.9.2906. [DOI] [PubMed] [Google Scholar]
- 50.Kroon HM, Moncrieff M, Kam PC, et al. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15:3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]


