Summary
Multipotent human mesenchymal stromal cells (hMSCs) harbor immunomodulatory properties that are therapeutically relevant. One of the most clinically important populations of leukocytes is the interleukin-17A (IL-17A)-secreting T (Th17) lymphocytes. However, mechanisms of hMSC and Th17 cell interactions are incompletely resolved. We found that, along with Th1 responses, hMSCs strongly suppressed Th17 responses and this required both IL-25—also known as IL-17E—as well as programmed death ligand-1 (PD-L1), a potent cell surface ligand for tolerance induction. Knockdown of IL-25 expression in hMSCs abrogated Th17 suppression in vitro and in vivo. However, IL-25 alone was insufficient to significantly suppress Th17 responses, which also required surface PD-L1 expression. Critically, IL-25 upregulated PD-L1 surface expression through the signaling pathways of JNK and STAT3, with STAT3 found to constitutively occupy the proximal region of the PD-L1 promoter. Our findings demonstrate the complexities of hMSC-mediated Th17 suppression, and highlight the IL-25/STAT3/PD-L1 axis as a candidate therapeutic target.
Graphical Abstract
Highlights
-
•
hMSC-secreted IL-25 suppress Th17 responses in vitro and in vivo
-
•
IL-25 alone is insufficient to significantly suppress Th17 responses
-
•
IL-25 upregulates PD-L1 expression in hMSCs to suppress Th17 cells
-
•
IL-25-mediated PD-L1 expression can be driven by STAT3
In this article, Yen and colleagues demonstrate that multipotent human mesenchymal stromal cells (hMSCs) suppress interleukin (IL)-17A-secreting T cell (Th17) responses through expression of IL-25, a paracrine factor, and programmed death ligand-1 (PD-L1), a cell surface ligand. The requirement of both factors is explained by IL-25 modulation of PD-L1 expression via JNK and STAT3 to orchestrate an overall effect of suppressing Th17 responses.
Introduction
Multipotent human mesenchymal stromal cells (hMSCs) are somatic progenitors that can be isolated from bone marrow (BM) (Friedenstein, 1976; Pittenger et al., 1999) and many other sites, such as adipose tissue, umbilical cord blood, and placenta (Erices et al., 2000; Yen et al., 2005; Zuk et al., 2001). Previous studies have indicated that hMSCs can differentiate into the paraxial mesodermal lineages of osteoblasts, chondrocytes, and adipocytes, as well as other non-mesodermal lineages, given the right environmental cues (Dominici et al., 2006; Engler et al., 2006). As such, hMSCs have been widely applied in many clinical trials for regenerative medicine (Giordano et al., 2007; Hare et al., 2012). Moreover, hMSCs have been found to have strong immunomodulatory properties that have tremendous therapeutic potential, as evidenced by the numerous clinical trials for immune-related diseases using these versatile progenitor cells (Gebler et al., 2012; Le Blanc et al., 2008; Tan et al., 2012). The hMSCs modulate diverse populations of leukocytes, with the best studied being that toward T lymphocytes, suppressing T effector functions (Bartholomew et al., 2002; Di Nicola et al., 2002; Uccelli et al., 2008). The molecular basis appears to involve both paracrine factors—especially in the human system—including tumor growth factor-β (TGF-β), indoleamine 2,3-dioxygenase (IDO), and prostaglandin E2 (PGE2), as well as cell surface molecules that engage leukocyte surface receptors (Uccelli et al., 2008; Chen et al., 2011). The hMSCs also influence the diversity of CD4 T helper (Th) subset phenotypes, potently skewing Th1 into Th2 cell responses (Aggarwal and Pittenger, 2005; Aksu et al., 2008) and potentiating induction of regulatory T cells (Tregs), an immunomodulatory population of T cells (Chang et al., 2006; Maccario et al., 2005; Selmani et al., 2008).
One population of T lymphocytes that has moved into greater prominence are interleukin (IL)-17A-secreting T cells (Dong, 2008). Known also as Th17 cells, this T helper cell subpopulation is important in mediating host responses toward microbial infections, as well as participating in the pathogenesis of many autoimmune and chronic inflammatory diseases that had been long believed to be caused by Th1 cells (Miossec and Kolls, 2012). While some studies have shown that hMSCs attenuate Th17-mediated immunity (Ghannam et al., 2010; González et al., 2009; Xu et al., 2012), others have found that hMSCs actually enhance Th17 responses (Darlington et al., 2010; Tso et al., 2010). These discrepant reports are likely due to an incomplete understanding currently of the mechanisms involved in hMSC-Th17 lymphocyte interactions, which have important implications in the clinical use of hMSCs given the role of Th17 cells in human diseases (Korn et al., 2009). We therefore set out to examine the nature of hMSC-Th17 interactions and elucidate the mechanisms involved. We found that hMSCs suppress Th17 responses through both paracrine and cell-cell contact mechanisms, involving IL-25—also known as IL17E—as well as PD-L1, a ligand of the PD-1 family. Our data demonstrate that hMSCs constitutively secrete IL-25 to upregulate the cell surface expression of PD-L1 through JNK and STAT3, with STAT3 involved in the transcriptional control of PD-L1.
Results
hMSCs Inhibit Th17 Responses
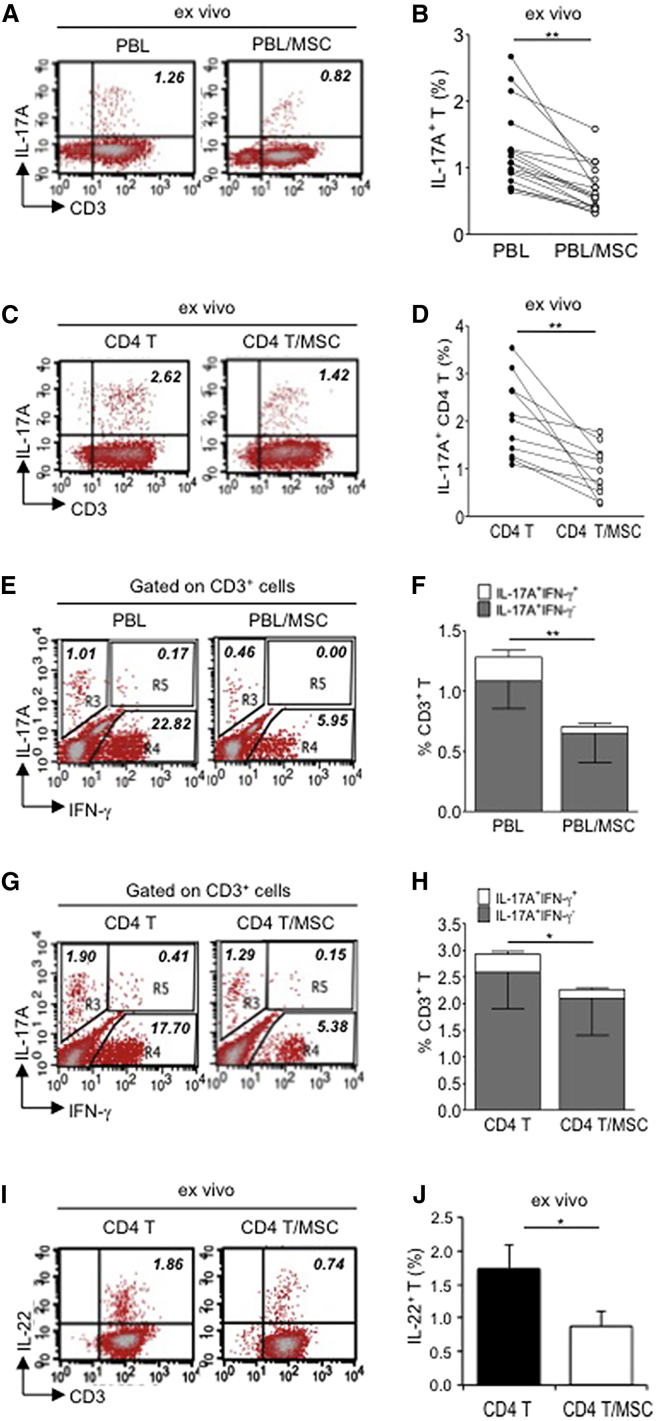
Since there have been discrepant reports on MSC-Th17 interactions, we first set out to answer whether hMSCs enhance or suppress Th17 cell expansion. To determine this, we used placenta-derived hMSCs that we have demonstrated previously to be trilineage multipotent progenitors and immunomodulatory, similar to BMMSCs (Yen et al., 2005, 2013; Chang et al., 2006). We then co-cultured these hMSCs with human peripheral blood leukocytes (PBLs) or purified CD4 T cells in steady state for 3 days. Approximately 1%–3% of non-primed T cells became IL-17A producers after phorbol 12-myristate 13-acetate (PMA)/ionomycin treatment for 6 hr, and we found that, when hMSCs were present, the frequency of IL-17A-expressing T cells was strongly decreased by 60%–65% in PBLs (Figure 1A, representative data; Figure 1B, pooled data) or CD4 T cells (Figure 1C, representative data; Figure 1D, pooled data). To further confirm this phenomenon, we performed in vitro stimulation of PBLs or T cells with anti-CD3/CD28 beads plus ionomycin to activate the Th17 effector phenotype (Santarlasci et al., 2012). We found that the frequency of in-vitro-expanded IL-17A-expressing PBLs (Figure S1A, representative data; Figure S1B, pooled data) and T cells (Figure S1C, representative data; Figure S1D, pooled data) was significantly reduced, as well when hMSCs were present.
Figure 1.
Multipotent Human Mesenchymal Stromal Cells (hMSCs) Suppress Th17 Responses
(A–D) Human peripheral blood CD3+ leukocytes (PBLs) (A, representative data; B, pooled data of 17 PBL donors co-cultured with all three hMSC donors) or CD3+ CD4 T cells (C, representative data; D, pooled data of 11 PBL donors co-cultured with all three hMSC donors) were co-cultured without (left) or with (right) hMSCs ex vivo, followed by PMA/ionomycin stimulation for 6 hr.
(E–H) IL-17A production in ex-vivo-cultured CD3+ T cells was assessed by intracellular staining. IL-17A and IFN-γ production in CD3+ PBLs (E, representative data; F, pooled data) or CD3+ CD4 T cells (G, representative data; H, pooled data) without and with co-culture of hMSCs was analyzed by flow cytometry. Representative intracellular staining is shown for IL-17A+ IFN-γ−- CD3+ T cells (R3 region) and IL-17A+IFN-γ+ (R5 region) CD3+ T cells, and pooled data from PBLs (n = 4) or CD4 T cells (n = 4) co-cultured with two hMSC donors (donors A and B) are provided in (F) and (H), respectively. Gray bars represent the percentages of IL-17A+ IFN-γ−- CD3+ T cells, whereas white bars represent the percentages of IL-17A+IFN-γ+ T cells.
(I and J) IL-22 production in four donors of CD3+ CD4 T cells (I, representative data; J, pooled data) without and with co-culture of two donors of hMSCs (donors A and B) was assessed by intracellular staining. Cell percentages are denoted in the dotplot quadrant of interest. Data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01.
It is known that the lineages of Tregs and Th17 are linked, with one lineage chosen over another to maintain immune homeostasis (Weaver and Hatton, 2009). Concomitantly, we found that, after co-culture with hMSCs, the frequency of FOXP3-expressing natural Tregs in PBLs and CD4 cells was increased (Figures S1E and S1F). hMSCs not only suppress IL-17A cells, but also prominently suppress IL-17A/IFN-γ double producer cells, which are the dominant subtype of Th17 cells at inflammatory sites (Annunziato et al., 2007; Zielinski et al., 2012). Previous reports have shown that hMSCs strongly suppress IFN-γ production—a prototypical Th1 cytokine—in PBLs and T cells (Aksu et al., 2008; Aggarwal and Pittenger, 2005), and we also found this to be true (Figures 1E and 1G, respectively). Additionally, we found that hMSCs substantially suppressed IFN-γ/IL-17A-expressing T cells (for PBLs: Figure 1E, representative data and Figure 1F, pooled data; for CD4 cells: Figure 1G, representative data and Figure 1H, pooled data). Th17 cells also are known to produce IL-22 (Dong, 2008), and co-culture of CD4 T cells with hMSCs also significantly decreased IL-22 production (Figure 1I, representative data; Figure 1J, pooled data). These results, therefore, demonstrate that hMSCs effectively suppress Th17 responses.
hMSCs Constitutively Express IL-25
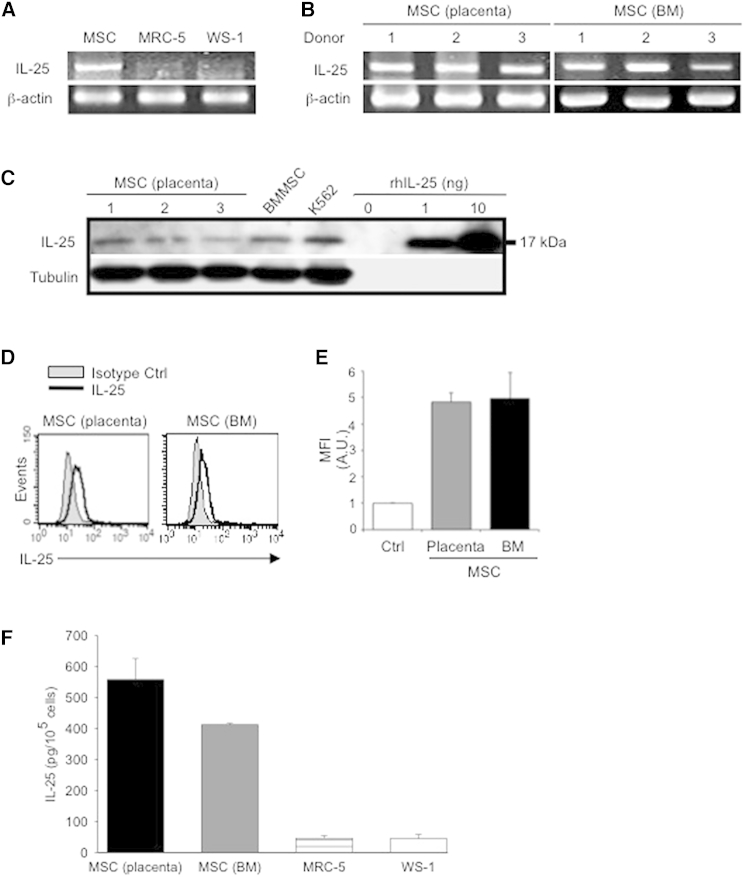
In the human system, MSC-T cell interactions have predominantly involved paracrine factors (Kim et al., 2013); therefore, to identify possible candidate secreted factors capable of suppressing Th17 responses, we performed mass spectrometry (MS) analysis on hMSC-conditioned medium. Surprisingly, MS/MS studies revealed that IL-25, also known as IL17E and a potent suppressor of Th17 responses (Kleinschek et al., 2007; Zaph et al., 2008), was highly secreted by hMSCs. To reconfirm MS/MS results, we examined for transcripts of IL-25 in various sources of hMSCs against other stromal cell types, such as fibroblasts. IL-25 mRNA could be detected in hMSCs, but not in human fibroblast cell lines MRC-5 or WS-1 (Figure 2A). We also detected the expression of IL-25 mRNA in three different donors each of placenta-derived hMSCs and human BMMSCs (Figure 2B), suggesting the reliability of IL-25 expression across the different sources of hMSCs. To ascertain protein production of IL-25, we performed western blot for overall protein production (Figure 2C) and intracellular flow cytometric analysis to detect IL-25 (Figure 2D), and we detected protein expression in both assays. Moreover, we found that hMSCs expressed IL-25 protein that could be detected in a secreted form in the conditioned medium, as detected by ELISA (Figure 2E). These findings indicate that hMSCs constitutively produce IL-25.
Figure 2.
hMSCs Constitutively Express IL-25
(A–D) Gene expression of IL-25 in placenta-derived hMSCs and fibroblast cell lines (MRC-5 and WS-1) (A), as well as three donors each of placental (left) and bone marrow (BM; right) hMSCs (B), was assessed by RT-PCR. Protein expression of IL-25 was determined by western blotting (C, K562 cell line and indicated amounts of recombinant human IL-25 [rhIL-25] as positive controls; tubulin as internal control) and intracellular staining for flow cytometric analysis (D; left, placental hMSC donor A; right, BM hMSC donor A).
(E) Filled histograms represent isotype control; unfilled histograms represent IL-25 antibody staining, with pooled data (three donors each of placental and BM hMSCs). Ctrl, isotype control; MFI, mean fluorescence intensity; A.U., arbitrary units.
(F) Secreted IL-25 by placental hMSCs (donor C, black bar), BM hMSCs (donor B, gray bar), MRC-5 (striped bar), or WS-1 (white bar) was assessed by collected conditioned medium of each cell type and analyzed by ELISA. Data are shown as mean ± SD of technical triplicates.
Silencing of hMSC-Derived IL-25 Reverses Suppression of Th17 Responses In Vitro and In Vivo, but Exogenous IL-25 Alone Is Not Sufficient to Repress Th17 Responses
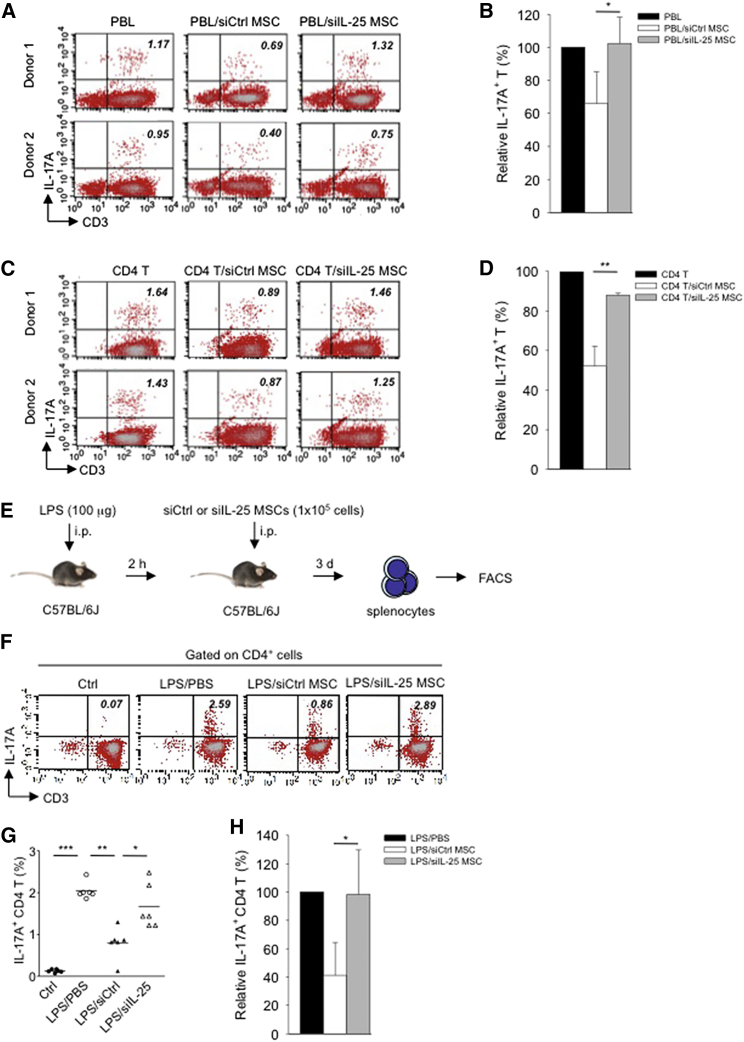
To assess whether hMSC-secreted IL-25 is involved in suppressing Th17 responses, we silenced IL-25 (siIL-25) expression in hMSCs by RNA interference. After confirming the efficiency of knockdown (Figures S2A and S2B), we found that silencing of IL-25 secretion with small interfering RNA (siRNA) specific for the gene in hMSCs almost completely reversed the suppressive effects toward Th17 cells compared with control silenced hMSCs (siCtrl) (Figure 3A). An average decrease of 35% in IL-17A-expressing T cells was seen with co-culture of siCtrl hMSCs; this was completely abrogated when siIL-25 hMSCs were applied (Figure 3B). A similar trend was seen when siCtrl or siIL-25 hMSCs were co-cultured with purified CD4 lymphocytes (Figure 3C). Th17 lymphocytes were decreased to an average of 52% of baseline when siCtrl hMSCs were used in the co-culture, compared to 87% when siIL-25 hMSCs were applied (Figure 3D). We further confirmed the capacity of hMSC-derived IL-25 for Th17 suppression under in vivo inflammatory conditions by adoptive transfer of either siCtrl hMSCs or siIL-25 hMSCs into lipopolysaccharide (LPS)-treated C57BL/6J mice (Figure 3E). We found that in vivo transfer of siCtrl hMSCs suppressed the population of IL-17A-expressing CD4 T cells in the spleen, whereas siIL-25 hMSCs failed to achieve that (Figures 3F and 3G). With transfer of siCtrl hMSCs, IL-17A-expressing T cells were decreased to an average of 41% of baseline, but transfer of siIL-25 hMSCs nearly completely abrogated these effects with IL-17A-expressing T cells back at an average level of 98% (Figure 3H). These data demonstrate that IL-25 secretion by hMSCs is involved in suppressing Th17 responses in vitro and in vivo.
Figure 3.
IL-25 Silencing in hMSCs Reverses Th17 Responses In Vitro and In Vivo
(A–D) Freshly isolated human PBLs (A) or CD4 T cells (C) were co-cultured without (left) or with either siCtrl hMSCs (middle) or siIL-25 hMSCs (right) for 3 days, followed by PMA/ionomycin stimulation for 6 hr. IL-17A production in CD3+ T cells was assessed by intracellular staining. Numbers in the top right quadrants represent the percentages of IL-17A-producing CD3+ T cells. Pooled data from PBLs (n = 3) or CD4 T cells (n = 3) and two hMSC donors (donors A and B) are provided in (B) and (D), respectively. Data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01.
(E) Experimental strategy for establishing in vivo inflammatory conditions in wild-type C57BL/6J mice with expansion of Th17 cells and adoptive transfer of hMSCs is shown.
(F) On day 3 after LPS (100 μg/mouse) challenge, IL-17A production in activated CD4 T cells in splenocytes from control mice, PBS-treated mice, siCtrl-hMSC-treated mice, or siIL-25-hMSC-treated mice was assessed by intracellular staining.
(G and H) Calculated (G) and relative (H) mean percentage of IL-17A-expressing CD4 T cells among control mice, PBS-treated mice, siCtrl-hMSC-treated mice, or siIL-25-hMSC-treated mice (n = 6). Data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005.
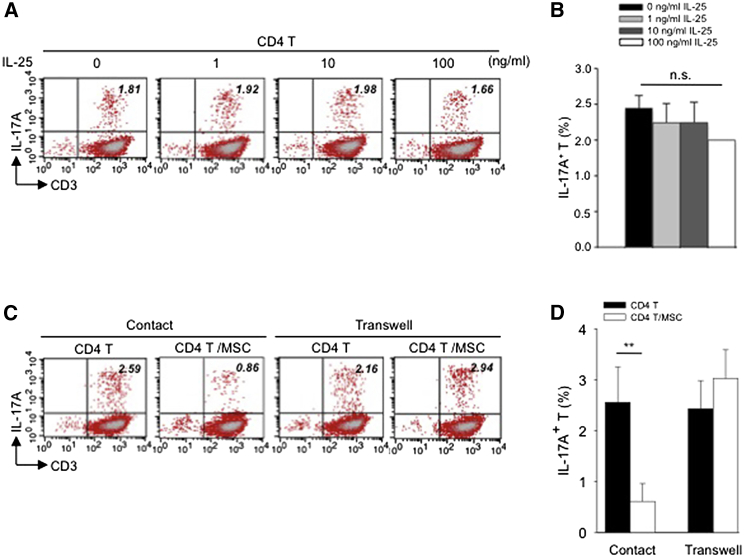
To further ascertain the role of IL-25 in suppressing Th17 responses, we treated CD4 T cells with recombinant human IL-25 (rhIL-25) for 18 hr prior to PMA/ionomycin stimulation and examined for levels of IL-17A in CD4 cells. To our surprise, we found that the addition of IL-25 singly to CD4 T cells failed to suppress Th17 responses to a significant extent (Figure 4A, representative data; Figure 4B, pooled data). Moreover, when hMSCs were separated from CD4 T cells by Transwell membrane, suppressive effects toward Th17 cells in CD4 cells were lost (Figure 4C, representative data; Figure 4D pooled data). This indicates that a membrane-bound factor is likely involved in IL-25-mediated effects.
Figure 4.
Exogenous IL-25 Alone Is Insufficient to Significantly Suppress Th17 Responses, with Cell Contact Required as well for hMSC-Mediated Inhibition of Th17 Responses
(A) Human CD4 T cells were treated with indicated doses of rhIL-25 for 18 hr, followed by PMA/ionomycin stimulation for 6 hr. IL-17A production in CD3+ T cells was assessed by intracellular staining. Numbers in the top right quadrants represent the percentages of IL-17A-producing CD3+ T cells.
(B) Pooled data of five PBL donors are shown.
(C) Human CD4 T cells (n = 4) were co-cultured without or with hMSCs (two donors, B and C) in the absence or presence of transwell barriers.
(D) Pooled data from healthy donors are shown. Data are shown as mean ± SD. ∗∗p < 0.01; n.s., not significant.
hMSC-Secreted IL-25 Suppresses Th17 Responses by Upregulating Surface Expression of PD-L1
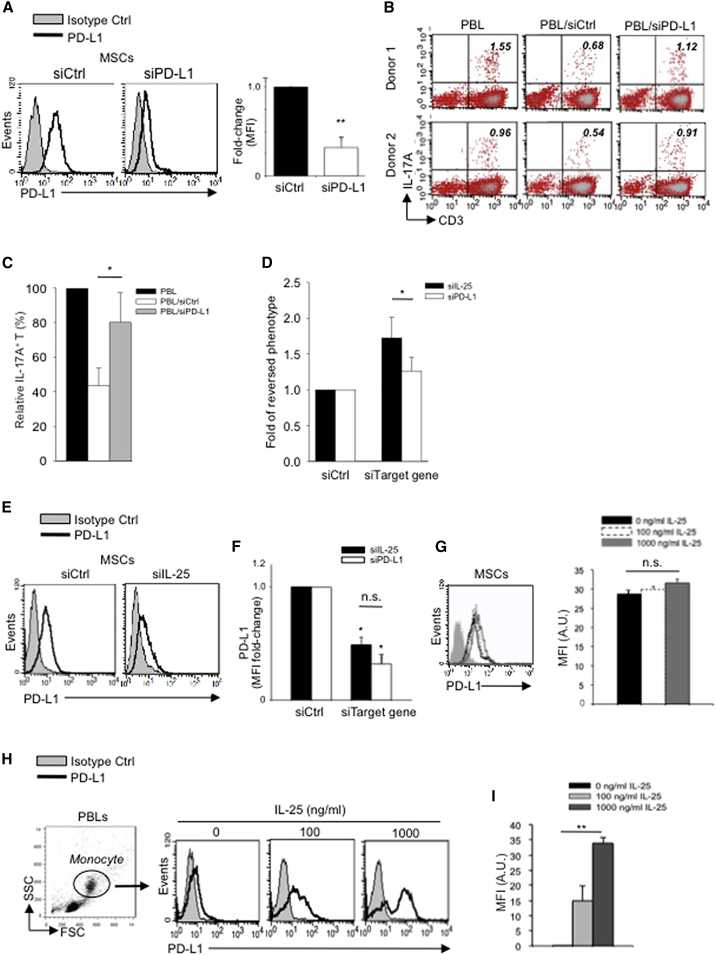
It has been reported that PD-L1 ligand, which is constitutively expressed on hMSC cell surfaces (Chang et al., 2006; Stagg et al., 2006), is a strong inhibitor of IL-17A production in human T cells (Brown et al., 2003; Hirahara et al., 2012). Hence, we considered the possibility that hMSC-secreted IL-25 effects on Th17 responses may be mediated through interacting with this MSC-cell-surface molecule. To ascertain previous reports of the suppressive effects of PD-L1 on Th17 cells, we performed knockdown of PD-L1 expression on hMSCs with siPD-L1 (Figure 5A). In line with previous reports, we found that PD-L1 knockdown in hMSCs reversed the suppression of Th17 cells (Figures 5B and 5C), but to a significantly lesser degree than that with siIL-25 (Figure 5D). To assess the role of PD-L1 in IL-25-dependent suppression of Th17 responses, we asked whether IL-25 is involved in the expression of PD-L1 on hMSCs. We found that, when IL-25 was silenced in hMSCs, surface expression of PD-L1 was strongly reduced, to a degree similar to knockdown with siRNA specific for itself (Figure 5E, representative data; Figure 5F, pooled data of siIL-25 versus siPD-L1), suggesting that IL-25 may induce PD-L1 expression. The receptor for IL-25 is IL-25R (Lee et al., 2001), and we searched for expression of IL-25R in hMSCs; western blotting revealed that hMSCs constitutively express this receptor (Figure S3A). When IL-25R expression was silenced on hMSCs with siIL-25R (Figure S3B), we found that hMSC-mediated suppression of Th17 response was significantly abrogated (Figures S3C and S3D). Thus, hMSC-secreted IL-25 requires interaction with its receptor IL-25R on hMSCs to lead to the suppression of Th17 responses.
Figure 5.
IL-25 Induces PD-L1 Surface Expression on hMSCs and Human Monocytes
(A) PD-L1 in siCtrl MSCs (left) and siPD-L1 MSCs (right) was analyzed by surface staining.
(B) Freshly isolated human PBLs were co-cultured without (left) or with siCtrl MSCs (middle) or siPD-L1 MSCs (right) for 3 days, followed by PMA/ionomycin stimulation for 6 hr. IL-17A production in CD3+ T cells was assessed by intracellular staining. Representative data are shown with numbers in the top right quadrants representing the percentages of IL-17A-producing CD3+ T cells.
(C) Pooled data from PBLs (n = 4) and two hMSC donors (donors A and B) are shown.
(D) Folds of reversed phenotypes of siIL-25 and siPD-L1 are shown.
(E) PD-L1 expression on siCtrl hMSCs (left) and siIL-25 hMSCs (right) was assessed by cell surface staining. Filled histograms represent isotype control; unfilled histograms represent PD-L1 antibody staining.
(F) Pooled data of PD-L1 expression (indicated by fold change in MFI) on siIL-25 hMSCs and siPD-L1 hMSCs (all three donors) are shown. PD-L1 expression levels were compared between hMSCs silenced for the target gene (IL-25 or PD-L1) and the respective siCtrl.
(G) hMSCs were treated with the indicated doses of rhIL-25 for 18 hr and assessed for cell surface PD-L1 expression by cell surface staining. Pooled data (all three donors) are shown in chart to the right with bars representing MFI.
(H) Human PBLs were treated with the indicated doses of rhIL-25 for 18 hr and assessed for cell surface PD-L1 expression on monocytes, gated using FSC and SSC, by flow cytometric analysis.
(I) Pooled data (ten PBL donors) are shown with bars representing MFI. ∗p < 0.05, ∗∗p < 0.01; n.s., not significant.
To further ascertain interactions of IL-25 on PD-L1 expression, we added rhIL-25 directly to hMSCs and assayed for further upregulation of PD-L1. We found that exogenous rhIL-25 can further upregulate surface expression of PD-L1 on hMSCs, but not to a significant extent (Figure 5G), which may be due to the fact that PD-L1 is constitutively expressed at a high level on hMSCs and thereby masking further effects of rhIL-25. Thus, to clarify the significance of IL-25 on PD-L1 expression, we used human primary monocytes from PBLs since these cells are known to respond to IL-25 as well as express low levels of PD-L1 at baseline (Caruso et al., 2009b). We found that, when human PBLs were treated with rhIL-25, PD-L1 expression was dramatically and significantly increased in monocytes in a dose-dependent manner (Figure 5H, representative data; Figure 5I, pooled data). Thus, these findings demonstrate that IL-25 is involved in regulation of PD-L1 surface expression in both hMSCs and human monocytes.
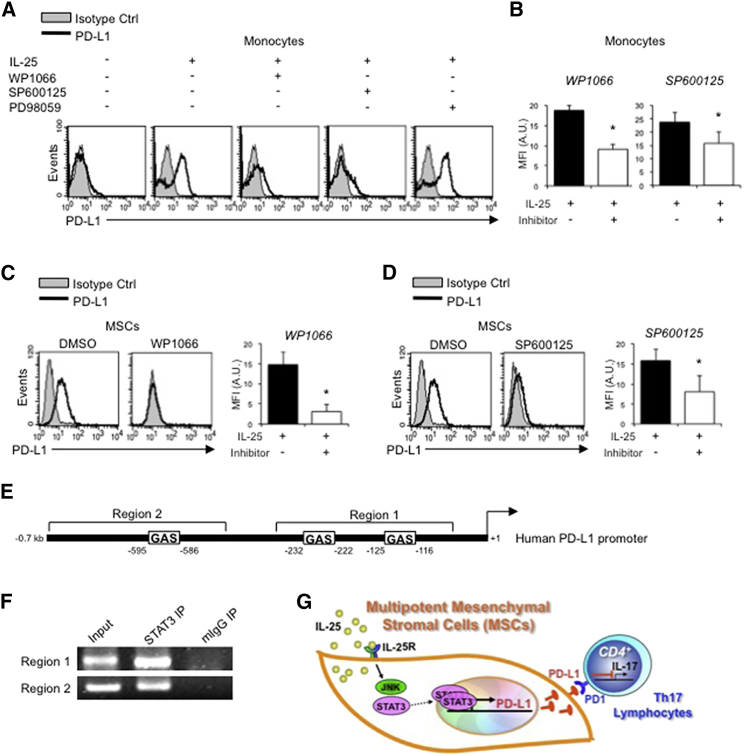
IL-25-Induced Upregulation of PD-L1 Is Mediated through JNK and STAT3, with STAT3 Involved in Transcriptional Control of PD-L1
We next sought to explore the signaling pathways by which IL-25 mediates expression of PD-L1. We first used monocytes from human PBLs, in which the expression of PD-L1 is inducible rather than constitutive, to answer this question. In human primary monocytes, WP1066, a STAT3 inhibitor, or SP600125, a JNK inhibitor, substantially abolished IL-25-mediated induction of PD-L1 (Figure 6A, representative data; Figure 6B, pooled data). In contrast, PD98059, a MEK1/2 inhibitor, showed minimal effect, while LY294002, a PI3K inhibitor, and Akt inhibitor III only partially affected IL-25-induced expression of PD-L1 (Figures S4A and S4B). In hMSCs, which constitutively express high levels of PD-L1, we also found that inhibition of STAT3 with WP1066 (Figure 6C) or JNK with SP600125 (Figure 6D) strongly reduced PD-L1 expression.
Figure 6.
IL-25-Mediated PD-L1 Expression in Human Monocytes and hMSCs Is Mediated through JNK and STAT3, with STAT3 Involved in Transcriptional Control of PD-L1
(A) Human PBLs were pretreated with inhibitors of STAT3 (WP1066; 2.5 μM), JNK (SP600125; 25 μM), or MEK1 (PD98059; 20 μM) prior to 100 ng/ml rhIL-25 for 18 hr, with subsequent flow cytometric analysis for PD-L1 surface expression on monocytes, gated using FSC and SSC. Filled histograms represent isotype control; unfilled histograms represent PD-L1 antibody staining.
(B–D) Pooled data (three donors) are shown (B) with bars representing MFI. hMSCs were treated with inhibitors of STAT3 (C; WP1066, 2.5 μM) and JNK (D; SP600125, 25 μM) for 6 hr, and subsequently assessed by flow cytometric analysis for PD-L1 surface expression. Pooled data (all three donors) for each respective inhibitor are provided (left charts) with bars representing MFI.
(E) Putative GAS elements (STAT-binding sites) in the proximal promoter region of human PD-L1 gene (700 bp region upstream from the transcription start site), as determined with TFSearch web-based software.
(F) Binding of STAT3 or IgG (negative control) in hMSCs was analyzed by chromatin immunoprecipitation (ChIP) with promoter-specific primers for region 1 and region 2. The input samples (positive control) represent 1% starting chromatin.
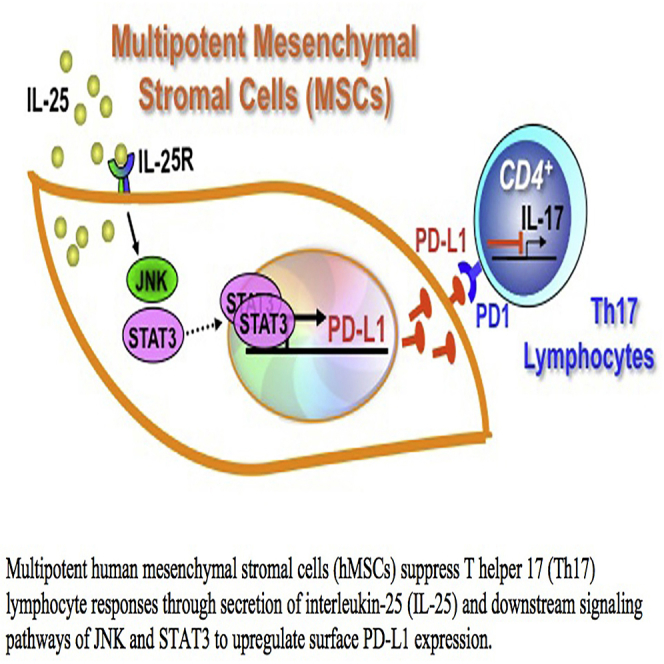
(G) Schematic shows a model of hMSC-mediated suppression of Th17 responses involving the IL-25/STAT3/PD-L1 axis.
Since STAT3 is also a transcription factor, we reasoned that this molecule may not only be involved in the signal pathway of IL-25-mediated PD-L1 expression, but also play a role in transcriptional control of PD-L1. To answer this question, we first analyzed the promoter of human PD-L1 gene between nucleotide −700 and nucleotide +1 for putative STAT3-binding elements. Based on software prediction, three putative GAS elements (STAT3-binding sites) between 595 and 116 bp upstream of the transcriptional start site were found (Figure 6E), raising the possibility that STAT3 may directly bind to the promoter of PD-L1. To test this possibility, we performed chromatin immunoprecipitation (ChIP) to determine whether STAT3 binds to the PD-L1 promoter in hMSCs, and we found that STAT3 was constitutively recruited to the GAS elements on the PD-L1 promoter (Figure 6F). Thus, our data demonstrate that, in hMSCs, IL-25 mediates cell surface expression of PD-L1 through JNK and STAT3, with the latter involved in the transcriptional control of PD-L1 (Figure 6G).
Discussion
hMSCs are known to be broadly immunomodulatory and these effects are therapeutically relevant (Caplan and Correa, 2011; Le Blanc and Mougiakakos, 2012; Uccelli et al., 2008). Th17 cells are now known to be involved in the pathogenesis of a number of autoimmune and chronic inflammatory diseases (Miossec and Kolls, 2012); hMSC interactions with this important population of leukocytes, however, have been shown to be discrepant (Darlington et al., 2010; Ghannam et al., 2010; González et al., 2009; Tso et al., 2010; Xu et al., 2012). Our data reveal that the effects of hMSCs on Th17 cells are suppressive and require both a paracrine factor, IL-25, as well as a cell surface molecule, PD-L1. Moreover, expression of PD-L1 in hMSCs is linked to IL-25 through IL-25R and further downstream through JNK and STAT3, the latter of which is involved in the transcriptional control of PD-L1. Th17 cells have been recognized as a contributor to transplant rejection through unknown mechanisms (Antonysamy et al., 1999; Faust et al., 2009). Our data may shed some light on the mechanisms behind the strong therapeutic effects of hMSC therapy on related diseases (Bassi et al., 2012; Sun et al., 2009; Zhou et al., 2011) and implicate a role for IL-25 agonists in ameliorating autoimmune/inflammatory diseases as well as transplant rejection.
IL-25 (IL-17E) is a member of the IL-17 family (Iwakura et al., 2011). However, unlike IL-17A or F, the better-known members of this IL family that have direct roles in autoimmune and chronic inflammatory diseases, IL-25 actually appears to protect against IL-17A/Th17 and Th1 states (Caruso et al., 2009a). IL-25-deficient mice, in addition to promoting Th1 responses, have a higher amount of IL-17A-expressing T cells and IFN-γ-expressing T cells in Th17-mediated experimental autoimmune encephalomyelitis (EAE), a model of human multiple sclerosis (Kleinschek et al., 2007). Interestingly, accumulating data demonstrate that IL-25 has another role in the immune system by promoting Th2 responses, preventing helminth infections (Fallon et al., 2006) as well as eosinophilic airway inflammation (Kim et al., 2002). To date, reported sources of IL-25 include immune cells, such as T cells, macrophages, monocyte-derived dendritic cells, mast cells, eosinophils, and basophils, as well as non-immune cells, such as epithelial and endothelial cells (Monteleone et al., 2010). We found IL-25 to be highly and constitutively expressed by diverse sources of hMSCs, but not fibroblasts. Moreover, our data show that IL-25 is directly responsible for hMSC suppression of allogeneic Th17 responses, including decreasing the highly pathogenic IL-17A/IFN-γ+ cells, further demonstrating that IL-25 is broadly protective against Th17 and Th1 responses. It is interesting to speculate on other possible biological roles of IL-25 in hMSCs given its high constitutive expression. Further studies are ongoing to evaluate whether this cytokine plays a role in hMSC proliferation and/or differentiation.
One of the striking findings of this study is that IL-25 directly upregulates the surface molecule PD-L1 in both leukocyte—monocytes—and non-leukocyte populations—hMSCs. PD-L1 is strongly immunosuppressive, being an inhibitor of autologous T cell activation in several autoimmune diseases (Keir et al., 2008), and blockade of its receptor, PD-1, on T cells can be very effective against cancer immunosuppression, as recently demonstrated (Topalian et al., 2012). Recently, a report showed that mouse MSCs suppress Th17 responses through this pathway (Luz-Crawford et al., 2012). However, data in this report showed that blockage of the PD-L1/PD-1 pathway only partially reversed mouse MSC suppression of Th17 responses, implicating other factors in this process. Our data also demonstrate that silencing of PD-L1 results in partial reversal of hMSC suppression of Th17 responses, while silencing of IL-25 results in a significantly higher and nearly complete reversal of hMSC-mediated Th17 suppression (Figure 5D). In addition, the degree of knockdown of PD-L1 expression was similar whether the siRNA specific for IL-25 or PD-L1 was used (Figure 5F). Moreover, we found evidence that IL-25 can directly affect the transcription of PD-L1 in both hMSCs and human leukocytes through STAT3, which helps to resolve the question of PD-L1 transcriptional control (Sumpter and Thomson, 2011; Wölfle et al., 2011). Critically, mouse MSCs do not express PD-L1 in steady state, whereas hMSCs constitutively express a high level of PD-L1 (Stagg et al., 2006). It is important to note that while data from mouse systems are clearly important, in MSC immunobiology, at times the results from mouse and human systems have been conflicting (Eliopoulos et al., 2005; Le Blanc et al., 2008), as was the case with PD-L1 expression. Based on the clinical response to hMSC therapy on various immune-related diseases, it appears that hMSCs exert strong immunomodulatory effects (Le Blanc and Mougiakakos, 2012), which is not always evident with mouse studies (Eliopoulos et al., 2005). Thus, to elucidate mechanisms involved in hMSC therapeutic applications, in vitro studies using hMSCs are still critical to conduct.
In summary, our findings demonstrate that hMSCs suppress Th17 responses, which require both the secreted factor IL-25 and IL-25-mediated upregulation of surface PD-L1. The downstream signaling pathways of JNK and STAT3 are involved in IL-25 regulation of PD-L1, with STAT3 implicated in the transcriptional control of PD-L1. In addition to the known roles of Th17 cells in autoimmune and chronic inflammatory diseases, recent studies have shown the importance of Th17 cells in enhancing the efficacy of checkpoint immunotherapy (Lutz et al., 2014). Modulation of IL-25, therefore, may have strong clinical implications since this cytokine can modulate PD-L1/PD-1 interactions and Th17 cells as well. Our findings provide a better understanding of the crosstalk between hMSCs and Th17 cells, as well as highlight the IL-25/STAT3/PD-L1 axis as a candidate therapeutic target for relevant diseases.
Experimental Procedures
Cell Culture
The hMSCs from BM and placenta were isolated and expanded according to previously published protocols (Pittenger et al., 1999; Yen et al., 2005). Briefly, placenta MSCs were isolated from term human placentas (38- to 40-week gestation; three donors designated A, B, and C) obtained with informed consent as approved by the institutional review board. Placental tissue was mechanically and enzymatically digested (0.25% trypsin-EDTA; Gibco, Invitrogen) and cultured in DMEM-low glucose (Gibco, Invitrogen), 10% fetal bovine serum (FBS; HyClone), 2 mM L-glutamine (Gibco, Invitrogen), and 100 U/ml penicillin-streptomycin (Gibco, Invitrogen). BMMSCs were obtained commercially (Cambrex, two donors designated A and B; and Promocell, one donor designated C). All hMSCs used were placental-derived unless otherwise indicated. Human PBLs were isolated from the buffy coat of healthy donor blood samples (Taiwan Blood Services Foundation, Taipei Blood Center), obtained with informed consent approved according to the procedures of the institutional review board, and cultured as previously reported (Chang et al., 2006; Yen et al., 2013). CD4 T cells were purified from PBL using human CD4 MicroBeads (Miltenyi Biotec) according to the manufacturer’s protocols. Purity was assessed by flow cytometric analysis (>98% positive for CD4). Human fibroblast cell lines MRC-5 and WS-1 were obtained from American Type Culture Collection (ATCC) and cultured according to the suggested protocols.
MSC-Leukocyte Co-culture Experiments
The hMSCs were plated at 3.5 × 104 cells per well in six-well plates and incubated at 37°C for 24 hr prior to co-culture with human PBL or CD4 cells. For co-cultures, 1 × 105 human PBL or CD4 cells were added to hMSC-containing wells without or with stimulation by magnetic anti-CD3/CD28-coated Dynabeads (Gibco, Invitrogen), according to the manufacturer’s instructions. After 3 days, cells were stimulated by PMA (50 ng/ml; Sigma-Aldrich) plus ionomycin (1 μg/ml; Sigma-Aldrich) in the presence of monensin (eBioscience) for 6 hr, followed by assessment of IL-17A expression in T cells using intracellular staining. For transwell cultures, human PBL or CD4 cells were plated in the upper compartment of transwell plates (0.4-μm pore size; BD Falcon), while hMSCs were plated in the lower compartment. Human recombinant IL-25 (rhIL-25; PeproTech) and various inhibitors (WP1066/InSolution STAT3 inhibitor III and Akt inhibitor III from Millipore; SP600125 JNK inhibitor and LY294002 PI3 kinase inhibitor from Cell Signaling Technology; and PD98059/MEK1/2 inhibitor from Cell Signaling Technology) were added to various experiments at the indicated doses after establishing toxicity profiles for monocytes and hMSCs.
Flow Cytometry
Cells were stained with antibodies as indicated: anti-human IL-25-PE (R&D Systems, IC1258P), mouse IgG1 isotype control-PE (R&D Systems, IC002P), anti-human CD3-PE/Cy5 (BioLegend, 300310), anti-human CD4-PE (BioLegend, 357404), anti-human IL-17A-PE (eBioscience, 12-7179), anti-human IFN-γ-FITC (BioLegend, 502506), anti-human IL-22-PE (eBioscience, 12-7229), anti-human FOXP3-Alexa Fluor 488 (BD Pharmingen, 561181), anti-human CD274 (B1-H1)-PE (eBioscience, 12-5983), mouse IgG1 isotype control-PE (eBioscience, 12-4714), anti-human IL-25R-PE (R&D Systems, FAB1207P), mouse IgG2b isotype control-PE (R&D Systems, IC0041P), anti-mouse CD4-APC (eBioscience, 17-0041), anti-mouse CD3e-PE/Cy5 (eBioscience, 15-0031), and anti-mouse/rat IL-17A-PE (eBioscience, 12-7177). Data were collected on BD FACSCalibur (BD Biosciences) instruments and analyzed with Cell Quest Pro software (BD Biosciences).
MS
MS/MS experiments were performed as previously reported (Chang et al., 2010). Briefly, MS/MS was performed with an LTQ-Fourier transform (FT) ion cyclotron resonance (ICR) mass spectrometer (Thermo Electron) equipped with a nanoelectrospray ion source (New Objective), an Agilent 1100 series binary high-performance liquid chromatography (HPLC) pump (Agilent Technologies), and a Famos autosampler (LC Packings). A minimum threshold of 1,000 counts was used as the cutoff for MS/MS sequential isolation by the LTQ, with singly charged ions rejected for MS/MS sequencing.
RT-PCR
Total RNA was prepared from cells using TRIzol reagent (Gibco, Invitrogen) according to the manufacturer’s instructions. The first-strand cDNA was synthesized from the RNA using Improm-II reverse transcriptase (Promega). For PCR, cDNA was subjected to PCR using the following primer sets: IL-25, forward 5′-TTCCTACAGGTGGTTGCATTC-3′, reverse 5′-CGCCTGTAGAAGACAGTCTGG-3′ (Furuta et al., 2011); β-actin, forward 5′-TGGCACCAC ACCTTCTACAATGAGC-3′, reverse 5′-GCACAGCTTCTCCTTAATGTCACGC −3′.
ELISA
The human IL-25 ELISA kit was obtained from PeproTech and performed according to the manufacturer’s instructions. The detection range was 0–2,000 pg/ml.
RNA Interference
Human IL-25, IL-25R, or PD-L1 expression in hMSCs was silenced using Stealth RNA interference (RNAi) duplex oligonucleotides (Gibco, Invitrogen) according to the manufacturer’s instructions, with non-target siRNA (medium GC duplex) used as control. Transfection of RNAi was done using Lipofectamine RNAiMAX (Gibco, Invitrogen) according to the manufacturer’s instructions. Knockdown efficiency was confirmed by flow cytometry.
hMSC Adoptive Transfer
All animal work was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee. Wild-type C57BL/6J mice were purchased from the National Laboratory Animal Center of Taiwan. Induction of Th17 cells in vivo was performed similarly as previously reported (Shi et al., 2013). Briefly, LPS (100 μg; Escherichia coli 00041:B4; Sigma-Aldrich) was injected intraperitoneally into 8- to 12-week-old mice, followed 2 hr later by transfer of hMSCs (1 × 105 cells/mouse) after non-target or IL-25 RNAi transfection (siCtrl or siIL-25, respectively). Mice were sacrificed on day 3 with harvesting of splenocytes for assessment of IL-17A+ expression in CD4 T cells.
ChIP
ChIP assay was performed using the EZ-Zyme chromatin prep kit (Millipore) and EZ-ChIP kit (Millipore), according to the manufacturer’s protocols. The digested chromatin was used for multiple immunoprecipitations with anti-STAT3 (124H6) mouse monoclonal antibody (Cell Signaling Technology, 9139) and normal mouse IgG. One percent of the reaction was removed as input chromatin. PCR detection was performed using the primer sets specific for the putative STAT3-binding sites in the human CD274 promoter region. The sequences of the primer sets were forward 5′-AGGTGCGTTCAGATGTTGGC-3′ and reverse 5′-TGCCCAAGGCAGCAAATCCAG-3′, amplifying the segment from −337 to −118 bp, and forward 5′-TGACACCATCGTCTGTCATC-3′ and 5′-GTCAGCAGCAGACCCATATG-3′, amplifying the segment from −803 to −477 bp.
Immunoblot Analyses
Total cell lysates were prepared by lysing cells in lysis buffer (300 mM NaCl, 50 mM HEPES [pH 7.6], 1.5 mM MgCl2, 10% glycerol, 1% Triton X-100, 10 mM NaPyrPO4, 1 mM EGTA, 0.1 mM EDTA, 1 mM DTT, 1 mM PMSF, and 1 mM Na4VO3) at 4°C for 15 min. Lysates were first clarified by centrifugation at 12,000 × g for 20 min. Equal amounts of samples were resolved in 7% SDS-PAGE, followed by transferring to nitrocellulose (GE Healthcare) and blotting with anti-IL-25R antibody (GeneTex, 97C691).
Statistical Analyses
Student’s t test (two-tailed) was performed for statistical analysis between two groups, and ANOVA was performed for statistical analyses of multiple groups. Statistical significance was set at p < 0.05. All data were expressed as mean ± SD.
Author Contributions
W.-B.W. designed and performed experiments, analyzed the data, and wrote the manuscript. M.-L.Y. and K.-J.L. designed experiments, provided reagents, analyzed the data, and edited the manuscript. H.-K.S. provided reagents, analyzed the data, and edited the manuscript. P.-J.H., M.-H.L., P.-M.C., P.-R.S., Chein-Hung Chen, and Chung-Hsuan Chen performed experiments and analyzed data. B.L.Y. designed experiments, analyzed data, provided overall supervision, and wrote the manuscript.
Acknowledgments
This work was supported in part by funding from the NHRI (CS-104-PP-06 to B.L.Y. and CA-104-SP-03 to K.-J.L.) and the Taiwan Ministry of Science & Technology (MoST-103-2314-B-400-018 and MoST-103-2321-B-400-020 to B.L.Y.).
Published: August 27, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.07.013.
Contributor Information
Ko-Jiunn Liu, Email: kojiunn@nhri.org.tw.
B. Linju Yen, Email: blyen@nhri.org.tw.
Supplemental Information
References
- Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Aksu A.E., Horibe E., Sacks J., Ikeguchi R., Breitinger J., Scozio M., Unadkat J., Feili-Hariri M. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin. Immunol. 2008;127:348–358. doi: 10.1016/j.clim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonysamy M.A., Fanslow W.C., Fu F., Li W., Qian S., Troutt A.B., Thomson A.W. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J. Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., McIntosh K., Patil S., Hardy W., Devine S., Ucker D., Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Bassi E.J., Moraes-Vieira P.M., Moreira-Sá C.S., Almeida D.C., Vieira L.M., Cunha C.S., Hiyane M.I., Basso A.S., Pacheco-Silva A., Câmara N.O. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–2545. doi: 10.2337/db11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Dorfman D.M., Ma F.R., Sullivan E.L., Munoz O., Wood C.R., Greenfield E.A., Freeman G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R., Sarra M., Stolfi C., Rizzo A., Fina D., Fantini M.C., Pallone F., MacDonald T.T., Monteleone G. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136:2270–2279. doi: 10.1053/j.gastro.2009.02.049. [DOI] [PubMed] [Google Scholar]
- Caruso R., Stolfi C., Sarra M., Rizzo A., Fantini M.C., Pallone F., MacDonald T.T., Monteleone G. Inhibition of monocyte-derived inflammatory cytokines by IL-25 occurs via p38 Map kinase-dependent induction of Socs-3. Blood. 2009;113:3512–3519. doi: 10.1182/blood-2008-08-172767. [DOI] [PubMed] [Google Scholar]
- Chang C.J., Yen M.L., Chen Y.C., Chien C.C., Huang H.I., Bai C.H., Yen B.L. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- Chang W.C., Chou C.K., Tsou C.C., Li S.H., Chen C.H., Zhuo Y.X., Hsu W.L., Chen C.H. Comparative proteomic analysis of proteins involved in the tumorigenic process of seminal vesicle carcinoma in transgenic mice. Int. J. Proteomics. 2010;2010:726968. doi: 10.1155/2010/726968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.M., Yen M.L., Liu K.J., Sytwu H.K., Yen B.L. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J. Biomed. Sci. 2011;18:49–59. doi: 10.1186/1423-0127-18-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington P.J., Boivin M.N., Renoux C., François M., Galipeau J., Freedman M.S., Atkins H.L., Cohen J.A., Solchaga L., Bar-Or A. Reciprocal Th1 and Th17 regulation by mesenchymal stem cells: Implication for multiple sclerosis. Ann. Neurol. 2010;68:540–545. doi: 10.1002/ana.22065. [DOI] [PubMed] [Google Scholar]
- Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P.D., Matteucci P., Grisanti S., Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N., Stagg J., Lejeune L., Pommey S., Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Erices A., Conget P., Minguell J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Fallon P.G., Ballantyne S.J., Mangan N.E., Barlow J.L., Dasvarma A., Hewett D.R., McIlgorm A., Jolin H.E., McKenzie A.N. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust S.M., Lu G., Marini B.L., Zou W., Gordon D., Iwakura Y., Laouar Y., Bishop D.K. Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J. Immunol. 2009;183:7297–7306. doi: 10.4049/jimmunol.0902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein A.J. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Furuta S., Jeng Y.M., Zhou L., Huang L., Kuhn I., Bissell M.J., Lee W.H. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci. Transl. Med. 2011;3:78ra31. doi: 10.1126/scitranslmed.3001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebler A., Zabel O., Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol. Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Ghannam S., Pène J., Moquet-Torcy G., Jorgensen C., Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- Giordano A., Galderisi U., Marino I.R. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J. Cell. Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- González M.A., Gonzalez-Rey E., Rico L., Büscher D., Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- Hare J.M., Fishman J.E., Gerstenblith G., DiFede Velazquez D.L., Zambrano J.P., Suncion V.Y., Tracy M., Ghersin E., Johnston P.V., Brinker J.A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K., Ghoreschi K., Yang X.P., Takahashi H., Laurence A., Vahedi G., Sciumè G., Hall A.O., Dupont C.D., Francisco L.M. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Ishigame H., Saijo S., Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.R., Manoukian R., Yeh R., Silbiger S.M., Danilenko D.M., Scully S., Sun J., DeRose M.L., Stolina M., Chang D. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- Kim N., Im K.I., Lim J.Y., Jeon E.J., Nam Y.S., Kim E.J., Cho S.G. Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: experiments and practice. Ann. Hematol. 2013;92:1295–1308. doi: 10.1007/s00277-013-1796-z. [DOI] [PubMed] [Google Scholar]
- Kleinschek M.A., Owyang A.M., Joyce-Shaikh B., Langrish C.L., Chen Y., Gorman D.M., Blumenschein W.M., McClanahan T., Brombacher F., Hurst S.D. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee J., Ho W.H., Maruoka M., Corpuz R.T., Baldwin D.T., Foster J.S., Goddard A.D., Yansura D.G., Vandlen R.L., Wood W.I., Gurney A.L. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- Lutz E.R., Wu A.A., Bigelow E., Sharma R., Mo G., Soares K., Solt S., Dorman A., Wamwea A., Yager A. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Crawford P., Noël D., Fernandez X., Khoury M., Figueroa F., Carrión F., Jorgensen C., Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS ONE. 2012;7:e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccario R., Podestà M., Moretta A., Cometa A., Comoli P., Montagna D., Daudt L., Ibatici A., Piaggio G., Pozzi S. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- Miossec P., Kolls J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Pallone F., Macdonald T.T. Interleukin-25: a two-edged sword in the control of immune-inflammatory responses. Cytokine Growth Factor Rev. 2010;21:471–475. doi: 10.1016/j.cytogfr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Santarlasci V., Maggi L., Capone M., Querci V., Beltrame L., Cavalieri D., D’Aiuto E., Cimaz R., Nebbioso A., Liotta F. Rarity of human T helper 17 cells is due to retinoic acid orphan receptor-dependent mechanisms that limit their expansion. Immunity. 2012;36:201–214. doi: 10.1016/j.immuni.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Selmani Z., Naji A., Zidi I., Favier B., Gaiffe E., Obert L., Borg C., Saas P., Tiberghien P., Rouas-Freiss N. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- Shi G., Vistica B.P., Nugent L.F., Tan C., Wawrousek E.F., Klinman D.M., Gery I. Differential involvement of Th1 and Th17 in pathogenic autoimmune processes triggered by different TLR ligands. J. Immunol. 2013;191:415–423. doi: 10.4049/jimmunol.1201732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J., Pommey S., Eliopoulos N., Galipeau J. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- Sumpter T.L., Thomson A.W. The STATus of PD-L1 (B7-H1) on tolerogenic APCs. Eur. J. Immunol. 2011;41:286–290. doi: 10.1002/eji.201041353. [DOI] [PubMed] [Google Scholar]
- Sun L., Akiyama K., Zhang H., Yamaza T., Hou Y., Zhao S., Xu T., Le A., Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Wu W., Xu X., Liao L., Zheng F., Messinger S., Sun X., Chen J., Yang S., Cai J. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso G.H., Law H.K., Tu W., Chan G.C., Lau Y.L. Phagocytosis of apoptotic cells modulates mesenchymal stem cells osteogenic differentiation to enhance IL-17 and RANKL expression on CD4+ T cells. Stem Cells. 2010;28:939–954. doi: 10.1002/stem.406. [DOI] [PubMed] [Google Scholar]
- Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Hatton R.D. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- Wölfle S.J., Strebovsky J., Bartz H., Sähr A., Arnold C., Kaiser C., Dalpke A.H., Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur. J. Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- Xu J., Wang D., Liu D., Fan Z., Zhang H., Liu O., Ding G., Gao R., Zhang C., Ding Y. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen B.L., Huang H.I., Chien C.C., Jui H.Y., Ko B.S., Yao M., Shun C.T., Yen M.L., Lee M.C., Chen Y.C. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- Yen B.L., Yen M.L., Hsu P.J., Liu K.J., Wang C.J., Bai C.H., Sytwu H.K. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports. 2013;1:139–151. doi: 10.1016/j.stemcr.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C., Du Y., Saenz S.A., Nair M.G., Perrigoue J.G., Taylor B.C., Troy A.E., Kobuley D.E., Kastelein R.A., Cua D.J. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Yuan J., Zhou Y., Ghawji M., Jr., Deng Y.P., Lee A.J., Lee A.J., Nair U., Kang A.H., Brand D.D., Yoo T.J. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin. Immunol. 2011;141:328–337. doi: 10.1016/j.clim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Zielinski C.E., Mele F., Aschenbrenner D., Jarrossay D., Ronchi F., Gattorno M., Monticelli S., Lanzavecchia A., Sallusto F. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.