Abstract
6-hydroxy-2,2′,4,4′-tetrabromodiphenyl ether (6-OH-BDE-47) is both a polybrominated diphenyl ether (PBDE) flame retardant metabolite and a marine natural product. It has been identified both as a neurotoxicant in cell-based studies and as a developmental toxicant in zebrafish. Hydroxylated PBDE metabolites are also considered thyroid hormone disruptors due to their structural similarity to endogenous thyroid hormones. The purpose of this study was to evaluate the effects of 6-OH-BDE-47 on a developmental pathway regulated by thyroid hormones in zebrafish. Morphological measurements of development (head trunk angle, otic vesicle length, and eye pigmentation) were recorded in embryos at 30 hours post fertilization (hpf) and detailed craniofacial morphology was examined in 4 day old larvae using cartilage staining. Exposure to 6-OH-BDE-47 resulted in severe developmental delays. A 100 nM concentration resulted in a 26% decrease in head trunk angle, a 54% increase in otic vesicle length, and a 42% decrease in eye pigmentation. Similarly, altered developmental morphology was observed following: Thyroid Receptor β morpholino knockdown;, exposure to the thyroid hormone triiodothyronine (T3) and to thyroid disrupting chemicals (TDC; iopanoic acid and propylthiouracil). The threshold for lower jaw deformities and craniofacial cartilage malformations was at doses greater than 50 nM. Of interest, these developmental delays and effects were rescued by microinjection of TRβ mRNA during the 1–2 cell stage. These data indicate that OH-BDEs can adversely affect early life development of zebrafish and suggest they may be impacting thyroid hormone regulation in vivo through downregulation of the thyroid hormone receptor.
Keywords: PBDE, OH-BDE, zebrafish, metabolite, development, Thyroid Receptor
1.1 Introduction
Hydroxylated polybrominated diphenyl ethers (OH-BDEs) may be produced from either natural (e.g. marine algae) or anthropogenic sources (Nomiyama et al., 2011; Wan et al., 2009). In mammals, OH-BDEs are formed by oxidative metabolism of polybrominated diphenyl ether (PBDE) flame retardants by cytochrome p450s, particularly CYP2B6 (Erratico et al., 2011; Feo et al., 2013). Both PBDEs and OH-BDEs persist in the environment, where they bioaccumule, and their universal occurrence in environmental media and human tissues (Chen et al., 2013; Kelly et al., 2008; Sun et al., 2013) are well established.
PBDEs affect estrogen, androgen, and thyroid hormone regulation in vitro (Kojima et al., 2009; Meerts et al., 2001; Ren et al., 2013), and in vivo. For example, rodents showed reduced circulating thyroid hormone levels, as well as altered reproductive and metabolic functioning following exposure to specific BDE congeners or the commercial mixtures (Stoker et al., 2004; Szabo et al., 2009; Zhou et al. 2002). Some investigators hypothesized that endocrine effects of PBDEs observed in vivo result from exposure to the OH-metabolites, rather than the parent compounds (Dingemans et al., 2008; Dingemans et al. 2011). OH-BDEs share a strong structural resemblance to endogenous thyroid hormones and in vitro studies show disruption of thyroid hormone signaling by competitive binding to serum thyroid transporter proteins and nuclear receptors (Hamers et al., 2008; Meerts et al., 2000; Ren et al., 2013). OH-BDEs inhibit the activity of thyroid sulfotransferase and deiodinase enzymes, critical for maintaining thyroid hormone levels in peripheral tissues (Butt and Stapleton, 2013; Butt et al., 2011). However, epidemiological studies in humans have observed conflicting associations between PBDE serum levels and thyroid hormone levels (Abdelouahab et al., 2013; Chevrier et al., 2011; Stapleton et al., 2011; Zota et al., 2011). Sources of such differences may be related to the specific population characteristics (i.e. age, pregnancy), methods used to measure thyroid hormone levels, or differences in metabolism. Alternatively, PBDE metabolites may be responsible for driving some of the observed associations; but metabolites are infrequently measured in epidemiological studies.
The PBDE metabolite, 6-OH-BDE-47, is both a naturally produced chemical and a result of in vivo metabolism of PBDEs. 6-OH-BDE-47 disrupts thyroid hormone and causes developmental toxicity in zebrafish (Liu et al., 2015; Usenko et al., 2012; van Boxtel et al., 2008). When the relative acute toxicity of various BDE-47 isomers was assessed in zebrafish, 6-OH-BDE-47 proved the most potent isomer tested (Usenko et al., 2012). We evaluated overt toxicity of eleven halogenated phenolic compounds (HPC) including chlorinated and brominated phenols, and also found 6-OH-BDE-47 to be the most acutely toxic compound in zebrafish embryos (see supporting information Table S1 and Figure S1).
Because 6-OH-BDE-47 has been detected in maternal serum and umbilical cord blood, concern for human developmental exposures has followed (Chen et al., 2013; Stapleton et al., 2011; Zhao et al., 2013; Zota et al., 2011). Fetuses and infants are undergoing rapid development and therefore may be more sensitive to chemical exposures. Furthermore, the maintenance of thyroid homeostasis is during pregnancy and early neurodevelopmental periods (Howdeshell, 2002) underscore the need for assessing developmental impacts of OH-BDEs.
Based on previous work from our laboratory (Dong et al., 2014, 2013) providing evidence of altered deiodinase and thyroid receptor expression after exposure to 6-OH-BDE-47, we sought to further study these pathways by determining their role in developmental morphology including larval cartilage formation. The objectives of the present study were to examine how early-life exposure to 6-OH-BDE-47 affects developmental morphology relative to native thyroid hormones and thyroid disrupting chemicals in embryo-larval zebrafish. Secondly, we sought to determine whether co-exposure with thyroid hormones or overexpression of the thyroid receptor would recover the observed developmental delays and adverse effects observed after 6-OH-BDE-47 exposures.
2.1 Materials & Methods
2.1.1 Fish Husbandry
Adult wild-type (Tropical 5D) zebrafish were used. We obtained these from a population in Dr. David Volz’s laboratory, University of South Carolina, Columbia, SC, USA. Adult fish were housed at 28 ± 0.5°C on a 14:10 light/dark photoperiod in a recirculating AHAB system (Aquatic Habitats) and fed brine shrimp and Ziegler’s Adult Zebrafish Complete Diet (Aquatic Ecosystems, Apopka, FL). Embryos were collected from breeder tanks by 2 hours post-fertilization (hpf) and maintained in embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) within incubators (at 28°C) under identical conditions as adults. Adult care and reproductive techniques were non-invasive and approved by the Duke University Institutional Animal Care & Use Committee.
2.1.2 Chemicals & Exposure Solutions
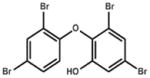
6-OH-BDE-47 was purchased neat from Accustandard (New Haven, CT) and were >99.5% purity. Triiodothyronine (T3) and thyroxine (T4) were purchased from Sigma-Aldrich (St. Louis, MO) and were > 97% purity. Iopanoic acid (IOP) (purity of > 98%) was purchased from TCI Chemicals (Portland, OR). Propylthiouracil (PTU) was purchased from Sigma Aldrich (purity of >97%). Methyl cellulose and alcian blue powder were also purchased from Sigma Aldrich. Dimethyl sulfoxide (DMSO) was purchased from EMD Millipore (>99.9% purity). Chemical information for the other eleven halogenated phenols tested in the overt toxicity assay can be found in the supplemental information. Concentrated stocks of all exposure chemicals were prepared in DMSO in amber vials. Exposure solutions were prepared from the concentrated stocks via serial dilution with embryo media water. All resultant exposure media contained ≤0.4% DMSO. A summary of the chemical properties and concentration ranges tested can be found in Table 1 and Table S2, respectively.
Table 1.
Chemical Properties of compounds used in this study. Log Kow values were estimated using EPA’s EPISuite software.
| Thyroid Disrupting Agents and Native Thyroid Hormones | |||
|---|---|---|---|
| Chemical (CAS #) | Structure | Log Kow (MW g/mol) | Name |
| 6-OH-BDE -47 (n/a) |
|
6.29 (501.8) | 6-hydroxy, 2,2′,4,4′ tetrabromodiphenyl ether |
| PTU (0000051-52-5) |
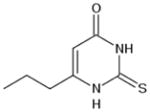
|
0.98 (170.2) | 6-propyl-2-thiouracil |
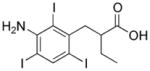
| IOP (000096-83-3) |
|
5.78 (570.9) | Iopanoic Acid |
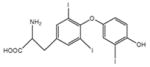
| T3 (00005-48-9) |
|
2.96 (651.0) | Triiodothyronine |
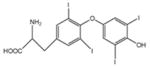
| T4 (000051-48-9) |
|
4.12 (776.88) | Thyroxine |
2.1.3 6 dpf Overt Toxicity
To assess the overt-toxicity of 6-OH-BDE-47, we conducted a 6 dpf overt toxicity assay in embryo-larval zebrafish (ten other halogenatic phenolic compounds were also evaluated for overt toxicity, see Table S1). Zebrafish embryos (sphere to 30% epiboly stage) (according to Kimmel et al., 1995) were placed in a 96-well plate (1 embryo/well insert; Laboratory Supply Distributors, Millville, NJ) containing 500 μL glass inserts (0.4% DMSO). The glass inserts were baked at 450°C for 4 hours prior to use to reduce possible chemical contamination. Each well contained 250 μL embryo medium dosed with 1 μL of a concentrated 6-OH-BDE-47 stock solution (Final concentration = 1 nM - 10 μM). Each plate contained a range of log-concentrations of 6-OH-BDE-47 (1 nM-10 μM) and included a vehicle control and control receiving only embryo media (n = 12–14 fish/dose, 5 doses/plate, 4 plates). Due to difficulties renewing media in the glass wells without damaging embryos, exposures were static.
Embryos were evaluated daily for abnormalities, hatching success, and lethality. Abnormalities were assessed by observing embryos under a dissecting microscope and recording spinal deformities, craniofacial abnormalities, edema of the pericardial/abdominal regions, and changes in pigmentation. Death was defined as the absence of a heartbeat or coagulation of the egg. Percent mortality and LC50 were calculated (Table S1, Figure S1).
2.1.4 Chemical Effects on Larval Morphology at 30 hpf
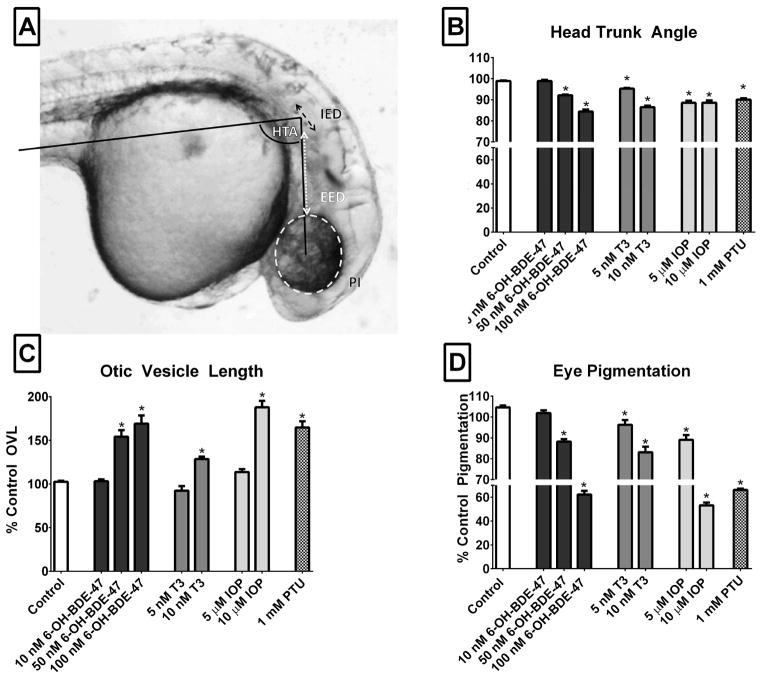
Developmental morphometrics (for detailed description, see (Kimmel et al., 1995)) are robust staging tools for embryonic development and include the head-trunk-angle (HTA), otic-vesicle length (OVL), and eye pigmentation (Figure 1A). These morphometric analyses were regarded as sensitive endpoints for thyroid disruption in zebrafish studies employing morpholino knockdown of deiodinase enzymes (Heijlen et al., 2014; Walpita et al., 2010). The HTA, OVL, and pigmentation enabled evaluation of developmental delays induced by 6-OH-BDE-47, thyroid disrupting chemicals, and native hormones. Briefly, thirty zebrafish embryos (4–5 hpf) were dosed with either 6-OH-BDE-47 (10–250 nM), IOP (5–10 μM), PTU (1 mM), or T3 (5–10 nM) by dissolving a determined amount of stock solution into 15 mL of embryo medium ([Final DMSO] ≤0.1%). IOP and PTU were selected as positive controls due to their established thyroid disrupting properties (Bouzaffour et al., 2010; Schmidt and Braunbeck, 2011). Range finding experiments and previous work in zebrafish were used to identify appropriate concentrations for these thyroid disrupting agents (5 μM and 10 μM IOP and 1mM PTU) (Bouzaffour et al., 2010; Schmidt and Braunbeck, 2011). Similarly, concentrations of native thyroid hormones were based on range finding experiments and previous studies using zebrafish (Brown, 1997; Liu and Chan, 2002; Walpita et al., 2007).
Control embryos received clean DMSO (<0.1%). The dosed embryos were housed in glass petri dishes (Pyrex, 100mm by 20mm) in an incubator until time of use. At 30 hpf, embryos were euthanized in 300 mg/L MS-222 and manually dechorionated with watchmaker’s forceps. Dechorionated embryos were then transferred to microscope slides, embedded in 3% methyl cellulose, positioned in lateral recumbency, and imaged for the morphometrics described above. Rationale for this time point was based on previous work demonstrating sensitivity of 30 hpf embryos to developmental delays mediated by thyroid hormones (Walpita et al., 2009, 2007).
Embryo images were captured using a Nikon Eclipse E600 light microscope equipped with a Nikon DXM 1200 digital camera and NIS Elements imaging software (Nikon, Melville, NY, USA). Image J (NIH, Bethesda, MD) was used to quantify the HTA, OVL, and eye pigmentation in each embryo image (illustrated in Figure 1A) (Heijlen et al., 2014; Kimmel et al., 1995; Walpita et al., 2010, 2009) Briefly, the HTA is formed by a line between the head and body axis (parallel to the notochord in the midtrunk region at somite 5). This angle increases between 20 hpf and 70 hpf as a result of body straightening. OVL was calculated by dividing the distance between the ipsilateral eye and inner ear (EED) by the diameter of the otic vesicle (IED, at its widest point) such that the highest OVL corresponds to the least developed embryo. Pigmentation was quantified in the eye of embryos by using integrated density as a count of the pixel area of the eye, compared to the background in each image. During developmental stages evaluated in this study, eye pigmentation is due to the density of melanin in retinal pigment epithelium and overlying choroid (Dong et al., 2014). All exposure experiments were performed in duplicate or triplicate, and represent n > 30 embryos.
Figure 1.
Various anatomical features used to establish morphometrics are illustrated in embryo image in Panel A. The HTA is formed by a line between the ear and the eye and by a line parallel to the notochord extending caudally to somite 5. The otic vesicle length was calculated using eye-ear-distance (EED- dashed white line) and inner ear diameter (IED- dashed black line) at widest point; OVL=EED/IED. The eye region is also highlighted to show area used for pigmentation measurement. Values for each parameter are shown for each experimental group (Panel B- HTA, Panel C-OVL, Panel D-eye pigmentation). Increases in OVL, decreases in pigmentation, and decreases in HTA are all indicative of developmental delays. Data are normalized to control values and presented as mean ±SEM (n>30/treatment) with statistical differences from controls denoted by an asterisk (One-way ANOVA, Dunnet’s post-hoc p<0.05)
2.1.5 Co-exposure to 6-OH-BDE-47 and THs
To determine whether observed developmental delays were mediated through decreased thyroid hormone levels in peripheral tissues, we conducted co-exposure experiments with T3 (5 nM and 30 nM) or T4 (30 nM) in the presence of 100 nM 6-OH-BDE-47. This concentration of the metabolite was identified in previous experiments to result in severe developmental delays but not lethality. Embryo exposures were conducted in glass petri dishes containing 30 embryos (4–5 hpf). Embryos were dosed simultaneously with 100 nM 6-OH-BDE-47 and T3 or T4 and then at 30 hpf were evaluated for effects on HTA, OVL, and eye pigmentation as described in section 2.1.4. In this way we could determine whether co-exposures with thyroid hormones recovered the developmental delays induced by 6-OH-BDE-47 exposures.
Follow up experiments using the same methodology were designed to determine whether cessation of exposure and/or hormone replacement ameliorated developmental effects. Briefly, embryos were exposed to 6-OH-BDE-47 during early development (4–24 hpf) and then removed from treatment, rinsed in triplicate, and transferred either to clean water or media containing 5 nM or 30 nM T3 or 30 nM T4 (24 hpf – 30 hpf). HTA, OVL, and eye pigmentation were examined at 30 hpf (data not shown). Table S2 contains a summary of exposure conditions.
2.1.6 Thyroid Receptor Morpholino Microinjections
Since multiple mechanisms for thyroid disruption have been demonstrated, we chose to investigate effects on the thyroid receptor to determine if downregulation of TRβ was driving the observed phenotypes following exposure to 6-OH-BDE-47. Thyroid hormone receptor translation blocking (GCAGTATGTCAGAGCAAGCAGACAA, THR-MO) and 5-bp mismatch control (GgAGaATGTCtGAGCtAGCtGACAA; Control-MO) morpholinos were designed with Gene Tools, LLC. Morpholinos were diluted in sterile dH2O to a stock concentration of 100 mM and further diluted to 10 mM. One nL TRβ-MO or Control-MO morpholino was injected into the 1–2 cell stage embryo (1 hpf) as previously described (Dong et al., 2014). At 30 hpf, fish were euthanized, dechorionated, and imaged for developmental morphology (HTA, OVL, and eye pigmentation) as described in section 2.1.4. Morphological evaluations were conducted in duplicate (n=20).
2.1.7 TRβ mRNA overexpression
To evaluate the potential of recovering the observed developmental delays from TRβ knockdown, we evaluated the ability of TRβ mRNA overexpression to rescue the phenotype. TRβ mRNA was synthesized with SP6 polymerase and capped using a G(5′)ppp(5′)A RNA cap structure analog (New England Biolabs) as described previously (Dong et al., 2014). Embryos received microinjections of ~ 3 nL TRβ mRNA (~ 265 ng/μL) during the 1–2 cell stage. Phenol red (0.05%) was used to track injections as described previously (Dong et al., 2014).
Approximately 3 hours after injection, embryos that showed normal development were placed in dosing solutions (100 nM or 250 nM 6-OH-BDE-47) within glass petri dishes. TRβ mRNA injections were also performed in unexposed embryos (Co-TRβ mRNA) to monitor for potential adverse effects of overexpression of the receptor during early development. In addition, we evaluated recovery of TRβ morpholino knockdown by also administering TRβ mRNA injections. For these experiments, embryos were first injected with TRβ-MO and then separately injected with TRβ mRNA (TRβ-MO + TRβ mRNA). In each experiment, embryos were euthanized, dechorionated, and imaged for developmental morphology at 30 hpf (HTA, OVL, and eye pigmentation) as described in section 2.1.4. Injection exposure experiments were conducted in duplicate (Co-TRβ mRNA, 250 nM 6-OH-BDE-47 +TRβ mRNA, n=20) or triplicate (100 nM 6-OH-BDE-47 + TRβ mRNA and Co-Mo, n=30).
2.1.8 Whole-mount Cartilage Staining Using Alcian Blue in 4 dpf Larvae
To examine effects of 6-OH-BDE-47 exposure on developmental morphology of the larval cartilage skeleton, alcian blue dye was used to stain cartilaginous structures in 96 hpf larvae (Walker and Kimmel, 2007). This age was chosen based on previous work showing that craniofacial cartilage development in zebrafish is sensitive to thyroid hormones during the embryo-to-larva transition (Liu and Chan, 2002; Strecker et al., 2013). Briefly, thirty embryos received a static exposure to 50 nM or 100 nM 6-OH-BDE-47 (15 mL embryo media) from 4–96 hpf in glass petri dishes. At 96 hpf larvae were euthanized in MS-222 fixed in 10% neutral buffered formalin overnight at -20°C and resultant intact individuals were stained overnight in fresh alcian blue solution at room temperature in one well of a 12-well plate. Alizarin red staining for bone was also performed, but we were unable to detect any bone formation at this early age.
Following staining, specimens were washed in 95% ethanol for 30 minutes on a shaker table and rehydrated by passage through graded solutions of 75%, 50%, and 25% ethanol in 1x phosphate buffered saline (PBS). To aid imaging, soft tissues were then digested by trypsin (10 mg/mL) in 30% saturated sodium tetraborate solution at 4°C for several hours until cartilage was clearly visible. Specimens were then washed in 0.5% KOH three times, bleached with 3% H2O2 to remove pigment and transferred through a graded series of increasing ratios of 0.5% KOH:glycerol (3:1, 1:1, 1:3 0:1). Once in 100% glycerol, tissues were stored at 4°C until time of imaging. Larvae were placed on depression slides, oriented in dorsal recumbency, and imaged for craniofacial morphology using a Nikon SMZ-1500 Stereoscope.
Craniofacial development was examined in 20 larvae per treatment. We determined forward protrusion of Meckel’s (l1) and ceratohyal (l2) cartilage structures (illustrated in Figure 2) according to previous methods (Mukhi and Patiño, 2007). Briefly, the cartilage complexes form a U- or V-shape that approximate an isosceles triangle. Thus, by measuring the side and base of each cartilage complex of the pharyngeal skeleton, the forward protrusion length was estimated using the Pythagorean theorem (L= square root (s2 - b2/4) as performed previously (Mukhi and Patiño, 2007).
Figure 2.
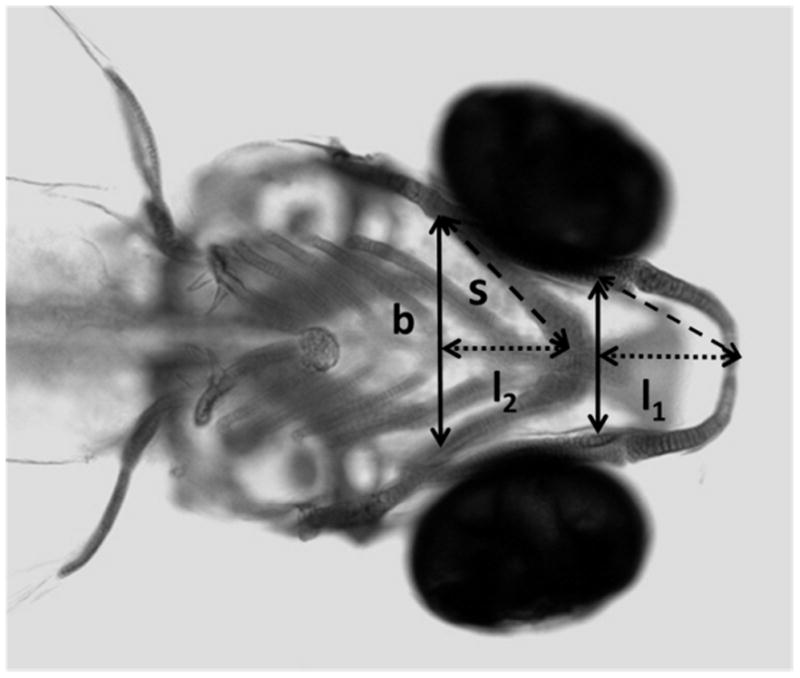
Photomicrograph of head region showing the forward protrusion (length) of the Meckel’s (l1) and the ceratohyal (l2) cartilage complexes in a 4dpf larval zebrafish stained with Alcian Blue. The sides and base (b) were measured using Image J Analysis Software, and the length (l) was calculated using the Pythagorean theorem, l=(s2-b2/4)1/2
2.1.9 Statistical Analysis
Graphpad Prism 6 software was used for statistical analysis (v 6.01 Graphpad Software Inc). For the overt toxicity data, dose-response survival curves were analyzed using a log-rank test and statistical significance was determined using a Bonferroni correction for multiple curves. Morphological data are normalized as percent relative to control (mean ± SEM; n > 30 across 2–3 experiments) and were analyzed using a one-way ANOVA with Dunnet’s post-hoc test. No differences between experimental replicates were observed for any test. For morpholino and mRNA injection experiments, data were analyzed using a two-way ANOVA to check for effects of injection and dose, followed by least squared means (n= 20–30 across 2–3 experiments). Tukey’s post-hoc test was used to determine significant differences between groups. A p-value <0.05 was considered statistically significant.
3.1 Results
3.1.1 Overt Toxicity Assessment at 6 dpf
A concentration-dependent increase in percent mortality was observed with exposure to 6-OH-BDE-47 from 4 hpf to 6 dpf (Figure S1). Importantly, of 11 halogenated phenolic compounds tested, 6-OH-BDE-47 had the lowest LC50 value at 134 nM (for dose response curves of all tested chemicals, see Figure S2). 6-OH-BDE-47 was also more toxic than the parent compound, BDE-47 (LC50> 10 μM) and the other hydroxylated isomers of BDE-47 (Figure S3). We observed no mortality in the DMSO or embryo water controls. Mortality (to 6dpf) was the same in both the 6-OH-BDE-47 exposed and co-exposed (6-OH-BDE-47 and T3 or T4) experiments (data not shown)).
3.1.2 Chemical Effects on Larval Morphology
Altered phenotypes were observed following 6-OH-BDE-47 treatments, including spinal curvature, pericardial edema, craniofacial abnormalities, reduced pigmentation, and failure of the swim bladder to inflate. Pronounced developmental delays were also observed, particularly with increasing concentrations of 6-OH-BDE-47. Exposure to 100 nM and 250 nM 6-OH-BDE-47 significantly delayed development, decreased yolk sac absorption, and reduced pigmentation in the embryos. In the 100 nM 6-OH-BDE-47 exposure, the average HTA decreased 26%, the average OVL increased by 54%, and the average eye pigmentation was reduced by 42% relative to DMSO controls (Figure 1B–D). In addition, different exposure durations were also examined to look for 6-OH-BDE-47 effects on development. No significant differences for OVL, HTA, or eye pigmentation were observed between exposures to 6-OH-BDE-47 from 4–24 hpf (20 hour exposure; with recovery in clean media until morphometric analysis at 30hpf) and a longer exposure from 4–30 hpf (26 h exposure; data not shown).
Positive controls, including the endogenous thyroid hormone T3, PTU, and IOP induced similar morphological delays (Figure 1B–D), including significant decreases in eye pigmentation and HTA, and increases in OVL. The 10 nM T3 exposure reduced the average pigmentation by 21%, decreased the HTA by 12%, and increased the OVL by 28%. Exposure to 10 μM IOP reduced eye pigmentation by 51%, decreased HTA by 13%, and increased the OVL by 80%.
3.1.3 Co-exposures of 6-OH-BDE-47 with Thyroid Hormones
No significant differences in HTA, OVL, or eye pigmentation were observed between co-exposed embryos (6-OH-BDE-47 and T3 or T4) and embryos receiving only 6-OH-BDE-47 treatment. Additionally, no significant differences in morphometric endpoints were detected between embryos receiving a 26 h exposure versus those receiving shorter exposure periods (20 hpf) or with recovery in TH supplemented media.
3.1.4 TRβ Morpholino Knockdown
In the TRβ morpholino knockdown embryos, the average HTA decreased 12%, the average OVL increased by 20%, and the average eye pigmentation was reduced by 27% relative to DMSO controls (Figure S4). These developmental delays are consistent with phenotypes from exposure to 6-OH-BDE-47 and thyroid disrupting agents. TRβ knockdown was rescued by subsequent injection with TRβ mRNA, and no significant differences in developmental morphology were observed between non-injected embryos, embryos receiving control mismatched TRβ morpholino (Co-Mo), or TRβMO + TRβ mRNA, indicating normal development.
3.1.5 Phenotype Rescue with TRβ mRNA overexpression
To further examine the potential role of TRβ downregulation in 6-OH-BDE-47-mediated effects, we evaluated the ability of TRβ mRNA overexpression to rescue the phenotype. There were three sets of controls: embryos receiving no injections (Control-NI; Figure 3A), embryos that received only TRβ mRNA injections (Co + TRβ mRNA; Figure 3B) and embryos that received an injection control morpholino (Co-Mo; Figure 3C). There were no significant differences in HTA, OVL, or PI between non-injected control embryos and Co-Mo injected embryos (data not shown). There was a slight but significant increase in HTA in the Co- TRβ mRNA injected embryos (7%) relative to the Co-Mo injected embryos, but no significant differences in OVL or PI, indicating no adverse developmental effects from TRβ mRNA microinjections. Overall, TRβ mRNA injections alone did not adversely affect zebrafish development.
Figure 3.
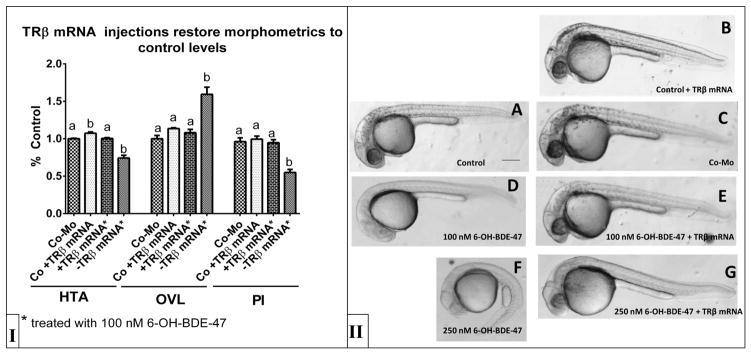
TR β mRNA injections rescue developmental delays (Panel I) caused by exposure to increasing concentrations of 6-OH-BDE-47. Representative 30 hpf zebrafish embryos (Panel II) that were treated with 100 nM 6-OH-BDE-47 (D,E), 250 nM 6-OH-BDE-47 (F,G) or control (A,B,C) are shown. Noninjected embryos are control (A), 100 nM 6-OH-BDE-47 (D), and 250 nM 6-OH-BDE-47 (F). Embryos injected with control morpholino are in Panel B. Embryos injected with TRB are control (C), 100 nM (E) or 250 nM 6-OH-BDE-47 (G). Injection with TR βduring 6-OH-BDE-47 exposure (B,E,G) restored normal development, as indicated by restored morphometric values. Quantification of developmental morphometrics are presented as mean ±SEM (n>20/treatment) and statistical differences are denoted by bars with different letters (One-way ANOVA, Dunnet’s post hoc p<0.05).
Interestingly, exposed embryos (100 nM 6-OH-BDE-47) that also received TRβ mRNA injections recovered the developmental delays observed in embryos treated with 100 nM 6-OH-BDE-47 alone (Figure 3D, E). These TRβ mRNA rescued embryos recovered pigmentation and evidence from morphometric evaluations indicated rescue of normal development (Figure 3E), unlike the embryos exposed to 6-OH-BDE-47 only (Figure 3D). Of note, TRβ mRNA injections with exposure to 250 nM 6-OH-BDE-47 (greater than the LC50) provided partial rescue of developmental delays (Figure 3F,G). Unfortunately morphometrics (HTA, OVL) could not be calculated in those animals receiving the highest concentration (250 nM) as few embryos had appreciable otic vesicle development precluding this aspect of staging. However, eye pigmentation was partially restored (Figure 3G) when compared to similarly aged control individuals.
3.1.6 Effects on Larval Cartilage Development
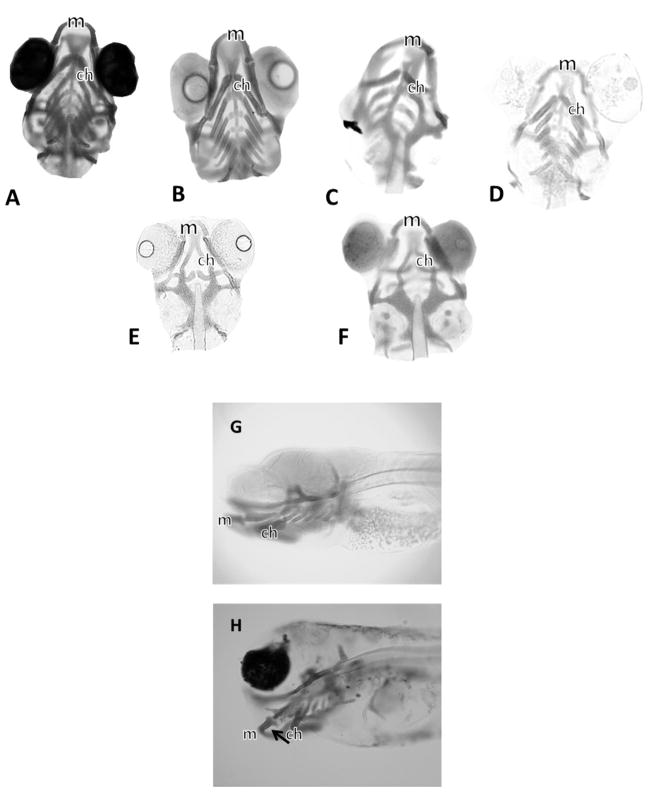
Given the developmental delays observed in 6-OH-BDE-47 exposed embryos, we further examined effects on cartilage skeletal development in larval stages (Figure 4). Qualitatively, fish treated with 6-OH-BDE-47 had reduced head cartilage formation at 4 dpf relative to control animals (Figure 4C–F). Specifically, the forward protrusion (length) of the Meckel’s (m, l1) and ceratohyal (ch, l2) cartilage complexes were reduced by 23% and 21%, respectively, indicating alterations in the lower mandible (Figure 5). In some fish, malformations affected the entire pharyngeal skeleton, for example the angles between individual ceratohyals were malformed and Meckel’s cartilage was smaller and misshapen (Figure 4C–F).
Figure 4.
6-OH-BDE-47 affects cartilage development in zebrafish (Danio rerio) larvae at 96hpf. Compare control larvae (A,G), to that of 50 nM 6-OH-BDE-47 (B), and 100 nM 6-OH-BDE-47 treated larvae (C–F, H). Photomicrographs of head region are shown with varying orientations including ventral in A–D, dorsal in E–F, and lateral G–H. Panel A and G show normal development. In panel B, slight malformation is seen with broadening of right and left portions of Meckel’s (m) cartilage and the position and angle of the ceratohyals (ch) are altered (B,C,D). Panels E and F show malformations of the entire larval pharyngeal skeleton, with Meckel’s cartilage being smaller and misshapen. Note that the angle between the paired ceratohyals is markedly altered. In H, note the severe lower jaw deformities. Note absence of eyes (panel C) and variable eye pigmentation. This is an artifact of handling arising from the repeated staining, bleaching, and rinsing steps involved with these fragile specimens.
Figure 5.
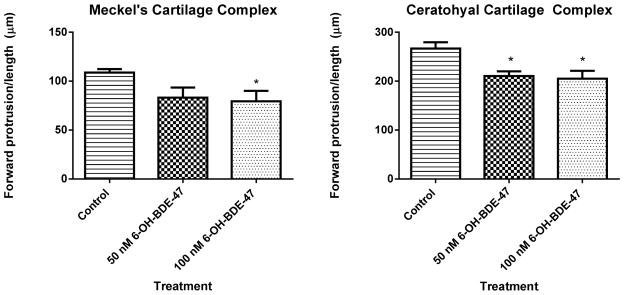
Quantification of forward protrusion of cartilage complexes in 4dpf larval zebrafish. Treatment with 50 nM or 100 nM 6-OH-BDE-47 signficantly reduced the length of the Meckel’s and ceratohyal cartilage forward protrusions. These craniofacial abnormalities (see Methods section above) can be mediated by disruption of thyroid hormones, critical for pharyngeal cartilage and craniofacial development.
4.1 Discussion
In this study, we used specific developmental staging metrics (HTA, OVL, eye pigmentation) to describe the developmental delays observed from exposure to 6-OH-BDE-47 or TDCs (Heijlen et al., 2014; Kimmel et al., 1995; Walpita et al., 2010, 2009). Additionally, positive control chemicals with known modes of action (IOP, PTU) were utilized to further examine their impacts on developmental morphology via thyroid hormone disruption Propylthiouracil targets thyroid peroxidase, and may inhibit DI 2 in fish species (Orozco et al. 2000; Sanders et al., 1997; Visser et al.1983). Iopanoic acid targets DI 1 and DI 2, inhibiting peripheral conversion of T4 to T3 in target tissues (Bouzaffour et al., 2010). Perturbations of the thyroid system during development can elicit neurological and physiological impairments in amphibians, mammals, and fish, implicating the importance of studying chemical impacts on these early life stages (Brown, 1997; McMenamin and Parichy, 2013; Morvan-Dubois et al., 2013; Porazzi et al., 2009).
The present work demonstrated that exposure to 6-OH-BDE-47 resulted in dramatic developmental delays, and, at higher doses, lethality in zebrafish embryos. We initially hypothesized that these delays were a result of thyroid disruption, specifically, that 6-OH-BDE 47 exposure was reducing circulating TH levels via inhibition of deiodinase activity (and thereby limiting peripheral T3 levels). Indeed, studies employing morpholino knockdown of DI 1, 2, and 3 in embryonic zebrafish have shown delays in morphological development similar to those observed in the present study (e.g., decreased HTA, increased OVL, and decreased pigmentation) (Heijlen et al., 2014; Walpita et al., 2010, 2009). To test this hypothesis, T3 and 6-OH-BDE-47 co-exposures were conducted to evaluate potential for recovery with external TH supplementation. However, co-exposure to 6-OH-BDE-47 with either T3 or T4 did not rescue developmental delays, suggesting that DI inhibition was not occurring and tissue T4 and T3 levels were not being impacted. However, in these co-exposure experiments, the concentrations of OH-BDEs were higher than the TH concentrations. TH concentrations could not be increased further due to resultant toxicity (exposure to ≥50 nM T3 proved lethal). Treatment with exogenous THs during development appears to have mixed effects, with some studies reporting developmental toxicity and others reporting accelerated pigment formation and growth (Brown, 1997; Heijlen et al., 2014; Liu and Chan, 2002; Walpita et al., 2007). These discrepancies are likely attributable to differences in developmental stage of exposure, route of exposure, and species (Brown, 1997; Jegstrup and Rosenkilde, 2003; Liu and Chan, 2002), but more work is needed to resolve these issues.
While rescue experiments with T3 and T4 did not restore normal morphological development following 6-OH-BDE-47 exposures, overexpression of the thyroid receptor beta proved successful in restoring normal developmental features. The thyroid nuclear receptor is encoded by two genes, TRα and TRβ. TR isoform expression varies dependent upon the tissue, developmental stage, and species (reviewed in Darras et al., 2011; Nelson and Habibi, 2009) TRα is expressed earlier and at higher levels than TRβ, but both are present at the midblastula stage and subsequent stages examined in this study (Essner et al., 1997; Liu et al., 2000; Power et al., 2001)
Multiple studies evaluating PBDE/OH-BDE toxicity have observed reduced transcription of thyroid receptors. For example, fathead minnows exposed to BDE-47 via the diet showed reduced transcription of both TRα and TRβ in a tissue and sex specific manner (Lema et al., 2008). Another study in zebrafish embryos using 200 μg/L 6-OH-BDE-47 (~398 nM) observed a 2 fold reduction in TRα and 3 fold reduction in TRβ mRNA expression (Zheng et al. 2012). Additional work from our research group has also demonstrated downregulation of TRβ mRNA expression from 6-OH-BDE-47 exposure using both RT-PCR and whole-mount in-situ hybridization in zebrafish (Dong et al., 2014). In addition, studies in TRβ knockout rodents have observed auditory/inner ear deficits and problems with regulation of the HPT axis (Abel et al., 1999; Forrest et al., 1996; Gauthier et al., 1999). For these reasons, we further examined the role of TRβ in mediating the developmental delays observed from 6-OH-BDE-47 exposures. Given the structural similarity between 6-OH-BDE-47 and thyroid hormones, 6-OH-BDE-47 could be acting as a T3 mimic, causing the appearance of surplus ligand and subsequent downregulation of the nuclear receptor.
Morpholino knockdown of the thyroid receptor beta during early development induced similar developmental delays to those observed from exposure to 6-OH-BDE-47 (Figure S4, S5). These delays could be rescued by subsequent injection with TRβ mRNA, indicating the importance of the receptor during early development. Previous studies examining the impacts of TRα knockdown in zebrafish found effects on cranial neural crest migration, proliferation, survival, and differentiation (Bohnsack and Kahana, 2013). This same study also found that TRα knockdown induced malformations of Meckel’s and ceratohyal cartilages, similar to results reported herein. However, it is important to note that in the present report we only examined TRβ and so can make no direct comparisons relating to TRα in our results.
If exposure to 6-OH-BDE-47 downregulates expression of TRβ, this would lead to reduced binding of T3 and reduced transcription of essential growth and developmental pathways. This provides an explanation for why T3 / T4 co-exposures failed to rescue the toxicity. Alternatively, 6-OH-BDE-47 may downregulate TRs independently of T3 mimicry by altering local hormone action. Changes in nuclear receptor expression could also occur through antagonism of other TRs, interference with the ability of TRs to bind to TREs, or interfering with co-activator recruitment following TH binding, or through other unknown mechanisms (Darras et al., 2014). Select PBDEs were able to promote dissociation of the thyroid receptor from the thyroid response element (targeting the DNA Binding Domain) in one study (Ibhazehiebo et al., 2011). More recently, Ren et al. (2013) demonstrated that binding affinity of OH-BDEs for human TRα and TRβ increased with the degree of bromination, likely due to increased hydrophobic interactions in the ligand binding pocket. Ren et al. also demonstrated that 6-OH-BDE-47, 5-OH-BDE-47, and other OH-BDEs were human TRβ agonists. Additional studies are necessary to define OH-BDE-thyroid receptor interactions, enhance our understanding of the mechanism of action, and explore the role of TRα.
In addition to impacts on the thyroid nuclear receptor, 6-MeO-BDE-47 and 6-OH-BDE-47 have been shown to interfere with multiple other nuclear hormone signaling pathways (including aryl hydrocarbon receptor, estrogen receptor, mineralocorticoid receptor, glucocorticoid receptor, and thyroid hormone receptor (Liu et al., 2015)). 6-OH-BDE-47 has also been shown to impact oxidative phosphorylation and energy metabolism in-vitro (van Boxtel et al., 2008), and more recently OH-BDE mixtures found in marine environments have been shown to exhibit strong synergistic toxicity, creating concerns for environmental exposures (Legradi et al., 2014). Therefore, it is possible that other mechanisms independent of endocrine disruption may also be contributing to the observed developmental delays from 6-OH-BDE-47 exposure.
Growing evidence of disrupted thyroid homeostasis by flame retardants (and their metabolites) exists through interactions with nuclear receptors (reviewed in Ren & Guo, 2013). With regard to the thyroid nuclear receptor, the in vitro effects are inconsistent, with differences in activity reported even for the same compound (effects across studies summarized in Table S3). Some reports regard OH-BDEs/PBDEs as thyroid receptor antagonists, and others as receptor agonists. Much of this may be explained on the basis of differing compounds evaluated, cell lines used, and assay conditions (Kitamura et al., 2008; Kojima et al., 2009; Li et al., 2010; Ren et al., 2013; Ren & Guo, 2013; Schriks et al. 2007; Zhang et al., 2014). Furthermore, a recent report examining tetrabromobisphenol-A (TBBPA) toxicity in amphibians observed differential toxicity depending on the developmental stage of the organism at testing, with TBBPA acting as an antagonist during periods of elevated endogenous TH levels and an agonist during other periods (Zhang et al., 2014). In both fish and amphibians, thyroid hormone surges occur as part of normal development (Liu and Chan, 2002; Miwa et al., 1988; Tata, 2006), therefore, testing different developmental stages (with different levels of endogenous THs) could also contribute to the reported differences in activity.
In conclusion, 6-OH-BDE-47 exposures adversely impacts early life development of zebrafish. These effects may be resulting from altered thyroid hormone regulation in vivo through downregulation of the thyroid hormone receptor. Early life exposures to OH-BDEs are important to consider because plasma levels of PBDEs in US pregnant women are several fold higher than those in other countries, and PBDEs can cross the placenta and can be transferred to the developing fetus (Zhao et al. 2013). Although concentrations used in this study are higher than would be anticipated in the environment and in humans, 6-OH-BDE-47 was measured in maternal serum and umbilical cord blood at concentrations ranging between 78–336 pM and, in some cases, at higher levels in cord blood than in serum (Chen et al., 2013; Stapleton et al., 2011). In cell-based studies, 6-OH-BDE-47 impacts multiple aspects of neurogenesis, including cytotoxicity, proliferation, and neuronal/oligodendrocyte differentiation of adult mice neural stem progenitor cells (Li et al., 2013). Studies by Dingemans et al, also demonstrated increased toxicity of 6-OH-BDE-47 relative to BDE-47, and found impaired calcium homeostasis and disrupted neurotransmitter release (Dingemans et al., 2011, 2010, 2008). These observations raise concern for maternal exposure to PBDEs/OH-BDEs and resultant endocrine disruption during pregnancy and early fetal development.
Supplementary Material
Acknowledgments
The funding for this research was provided by a grant from the National Institutes of Environmental Health Sciences [P42ES010356]. We would also like to acknowledge Dr. Erin Kollitz for reviewing the manuscript and providing helpful comments.
Abbreviations
- HPC
Halogenated Phenolic Compound
- dpf
Days Post Fertilization
- CNC
Cranial Neural Crest
- DI
Deiodinase Enzyme
- DMSO
Dimethyl Sulfoxide
- FR
Flame Retardant
- hpf
Hours Post Fertilization
- IOP
Iopanoic Acid
- MO
Morpholino
- OH-BDE
Hydroxylated Polybrominated Diphenyl Ether
- PBDE
Polybrominated Diphenyl Ether
- TH
Thyroid Hormone
- TDC
Thyroid Disrupting Chemical
- PTU
Propylthiouracil
- T4
Thyroxine
- T3
Triiodothyronine
- TR
Thyroid receptor
Footnotes
4.2 Supplementary Data Description
Supplemental information regarding the physicochemical information and overt toxicity screen of 11 other HPCs, detailed dose response information, morpholino knockdown experiments, and a table summarizing PBDE effects on thyroid receptor can be found in the supplemental material. In addition, discussion of other minor endpoints (pigmentation, cartilage analysis) is also found in the supplemental information.
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelouahab N, Langlois MF, Lavoie L, Corbin F, Pasquier JC, Takser L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 2013;178:701–13. doi: 10.1093/aje/kwt141. [DOI] [PubMed] [Google Scholar]
- Abel ED, Boers ME, Pazos-Moura C, Moura E, Kaulbach H, Zakaria M, Lowell B, Radovick S, Liberman MC, Wondisford F. Divergent roles for thyroid hormone receptor beta isoforms in the endocrine axis and auditory system. J Clin Invest. 1999;104:291–300. doi: 10.1172/JCI6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack BL, Kahana A. Thyroid hormone and retinoic acid interact to regulate zebrafish craniofacial neural crest development. Dev Biol. 2013;373:300–9. doi: 10.1016/j.ydbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzaffour M, Rampon C, Ramaugé M, Courtin F, Vriz S. Implication of type 3 deiodinase induction in zebrafish fin regeneration. Gen Comp Endocrinol. 2010;168:88–94. doi: 10.1016/j.ygcen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci U S A. 1997;94:13011–6. doi: 10.1073/pnas.94.24.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt C, Stapleton HM. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chem Res Toxicol. 2013;26(11):1692–1702. doi: 10.1021/tx400342k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol Sci. 2011;124(2):339–47. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Park JS, Linderholm L, Rhee A, Petreas M, DeFranco Ea, Dietrich KN, Ho SM. Hydroxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ Sci Technol. 2013;47:3902–8. doi: 10.1021/es3046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Sjödin A, Eskenazi B. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol. 2011;174:1166–74. doi: 10.1093/aje/kwr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras VM, Houbrechts AM, Van Herck SLJ. Intracellular thyroid hormone metabolism as a local regulator of nuclear thyroid hormone receptor-mediated impact on vertebrate development. Biochim Biophys Acta. 2014;1849:130–141. doi: 10.1016/j.bbagrm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Darras VM, Van Herck SLJ, Heijlen M, De Groef B. Thyroid hormone receptors in two model species for vertebrate embryonic development: chicken and zebrafish. J Thyroid Res. 2011;2011:402320. doi: 10.4061/2011/402320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, de Groot A, van Kleef RGDM, Bergman A, van den Berg M, Vijverberg HPM, Westerink RHS. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ Health Perspect. 2008;116:637–43. doi: 10.1289/ehp.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, van den Berg M, Bergman A, Westerink RHS. Calcium-related processes involved in the inhibition of depolarization-evoked calcium increase by hydroxylated PBDEs in PC12 cells. Toxicol Sci. 2010;114:302–9. doi: 10.1093/toxsci/kfp310. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, van den Berg M, Westerink RHS. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119:900–7. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Macaulay LJ, Ferguson PLL, Hinton DE, Stapleton HM, Kwok KW, Hinton DE, Ferguson PLL, Stapleton HM. The PBDE metabolite 6-OH-BDE 47 affects melanin pigmentation and THRβ MRNA expression in the eye of zebrafish embryos. Endocr. Disruptors. 2014:e969072. doi: 10.4161/23273739.2014.969072. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Macaulay LJ, Kwok KWH, Hinton DE, Stapleton HM. Using whole mount in situ hybridization to examine thyroid hormone deiodinase expression in embryonic and larval zebrafish: a tool for examining OH-BDE toxicity to early life stages. Aquat Toxicol. 2013;132–133:190–9. doi: 10.1016/j.aquatox.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erratico Ca, Moffatt SC, Bandiera SM. Comparative oxidative metabolism of BDE-47 and BDE-99 by rat hepatic microsomes. Toxicol Sci. 2011;123:37–47. doi: 10.1093/toxsci/kfr155. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Breuer JJ, Essner RD, Fahrenkrug SC, Hackett PB. The zebrafish thyroid hormone receptor alpha 1 is expressed during early embryogenesis and can function in transcriptional repression. Differentiation. 1997;62:107–17. doi: 10.1046/j.1432-0436.1997.6230107.x. [DOI] [PubMed] [Google Scholar]
- Feo ML, Gross MS, McGarrigle BP, Eljarrat E, Barceló D, Aga DS, Olson JR. Biotransformation of BDE-47 to Potentially Toxic Metabolites Is Predominantly Mediated by Human CYP2B6. Environ Health Perspect. 2013;121:440–446. doi: 10.1289/ehp.1205446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R, Curran T. Thyroid hormone receptor beta is essential for development of auditory function. Nat Genet. 1996;13:354–7. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 1999;18:623–31. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJM, Brouwer A, Bergman A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) Mol Nutr Food Res. 2008;52:284–98. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- Heijlen M, Houbrechts AM, Bagci E, Van Herck SLJ, Kersseboom S, Esguerra CV, Blust R, Visser TJ, Knapen D, Darras VM. Knockdown of type 3 iodothyronine deiodinase severely perturbs both embryonic and early larval development in zebrafish. Endocrinology. 2014;155:1547–1559. doi: 10.1210/en.2013-1660. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110(Suppl):337–48. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl. Environ Health Perspect. 2011;119:168–175. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegstrup IM, Rosenkilde P. Regulation of post-larval development in the European eel: thyroid hormone level, progress of pigmentation and changes in behaviour. J Fish Biol. 2003;63:168–175. doi: 10.1046/j.1095-8649.2003.00138.x. [DOI] [Google Scholar]
- Kelly BC, Blair JD, Gobas FAPC, Ikonomou MG. Hydroxylated and methoxylated polybrominated diphenyl ethers in a Canadian Arctic marine food web. Environ Sci Technol. 2008;42:7069–77. doi: 10.1021/es801275d. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Shinohara S, Iwase E, Sugihara K, Uramaru N, Shigematsu H, Fujimoto N, Ohta S. Affinity for thyroid hormone and estrogen receptors of hydroxylated polybrominated diphenyl ethers. J Heal Sci. 2008;54:607–614. [Google Scholar]
- Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117:1210–8. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi J, Dahlberg AK, Cenijn P, Marsh G, Asplund L, Bergman A, Legler J. Disruption of Oxidative Phosphorylation (OXPHOS) by Hydroxylated Polybrominated Diphenyl Ethers (OH-PBDEs) Present in the Marine Environment. Environ Sci Technol. 2014;48:14703–11. doi: 10.1021/es5039744. [DOI] [PubMed] [Google Scholar]
- Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ Health Perspect. 2008;116:1694–9. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Xie Q, Li X, Li N, Chi P, Chen J, Wang Z, Hao C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-beta: in vitro and in silico investigations. Environ Health Perspect. 2010;118:602–6. doi: 10.1289/ehp.0901457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang W, Pan YW, Xu L, Xia Z. A hydroxylated metabolite of flame-retardant PBDE-47 decreases the survival, proliferation, and neuronal differentiation of primary cultured adult neural stem cells and interferes with signaling of ERK5 MAP kinase and neurotrophin 3. Toxicol Sci. 2013;134:111–24. doi: 10.1093/toxsci/kft083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Tang S, Zheng X, Zhu Y, Ma Z, Liu C, Hecker MM, Saunders DMV, Giesy JP, Zhang X, Yu H. Bioaccumulation, biotransformation and toxicity of BDE-47, 6-OH-BDE-47 and 6-MeO-BDE-47 in early life-stages of zebrafish (Danio rerio) Environ Sci Technol. 2015;49:1823–33. doi: 10.1021/es503833q. [DOI] [PubMed] [Google Scholar]
- Liu YW, Chan WK. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation. 2002;70:36–45. doi: 10.1046/j.1432-0436.2002.700104.x. [DOI] [PubMed] [Google Scholar]
- Liu YW, Lo LJ, Chan WK. Temporal expression and T3 induction of thyroid hormone receptors alpha1 and beta1 during early embryonic and larval development in zebrafish, Danio rerio. Mol Cell Endocrinol. 2000;159:187–95. doi: 10.1016/s0303-7207(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–52. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin SK, Parichy DM. Metamorphosis in teleosts. Curr Top Dev Biol. 2013;103:127–65. doi: 10.1016/B978-0-12-385979-2.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts Ia, Letcher RJ, Hoving S, Marsh G, Bergman a, Lemmen JG, van der Burg B, Brouwer a. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Miwa S, Tagawa M, Inui Y, Hirano T. Thyroxine surge in metamorphosing flounder larvae. Gen Comp Endocrinol. 1988;70:158–163. doi: 10.1016/0016-6480(88)90105-0. [DOI] [PubMed] [Google Scholar]
- Morvan-Dubois G, Fini JB, Demeneix BA. Is thyroid hormone signaling relevant for vertebrate embryogenesis? Curr Top Dev Biol. 2013;103:365–96. doi: 10.1016/B978-0-12-385979-2.00013-7. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Patiño R. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol Sci. 2007;96:246–54. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- Nelson ER, Habibi HR. Thyroid receptor subtypes: structure and function in fish. Gen Comp Endocrinol. 2009;161:90–6. doi: 10.1016/j.ygcen.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Nomiyama K, Eguchi A, Mizukawa H, Ochiai M, Murata S, Someya M, Isobe T, Yamada TK, Tanabe S. Anthropogenic and naturally occurring polybrominated phenolic compounds in the blood of cetaceans stranded along Japanese coastal waters. Environ Pollut. 2011;159:3364–73. doi: 10.1016/j.envpol.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Orozco A, Linser P, Valverde-R C. Kinetic characterization of outer-ring deiodinase activity (ORD) in the liver, gill and retina of the killifish Fundulus heteroclitus. Comp Biochem Physiol Part B Biochem Mol Biol. 2000;126:283–290. doi: 10.1016/S0305-0491(00)00186-3. [DOI] [PubMed] [Google Scholar]
- Porazzi P, Calebiro D, Benato F, Tiso N, Persani L. Thyroid gland development and function in the zebrafish model. Mol Cell Endocrinol. 2009;312:14–23. doi: 10.1016/j.mce.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Power DM, Llewellyn L, Faustino M, Nowell Ma, Björnsson BT, Einarsdottir IE, Canario aV, Sweeney GE. Thyroid hormones in growth and development of fish. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:447–59. doi: 10.1016/s1532-0456(01)00271-x. [DOI] [PubMed] [Google Scholar]
- Ren XM, Guo LH. Molecular toxicology of polybrominated diphenyl ethers: nuclear hormone receptor mediated pathways. Environ Sci Process Impacts. 2013;15:702–8. doi: 10.1039/c3em00023k. [DOI] [PubMed] [Google Scholar]
- Ren XM, Guo LH, Gao Y, Zhang BT, Wan B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol Appl Pharmacol. 2013;268:256–263. doi: 10.1016/j.taap.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Sanders JP, Van der Geyten S, Kaptein E, Darras VM, Kühn ER, Leonard JL, Visser TJ. Characterization of a propylthiouracil-insensitive type I iodothyronine deiodinase. Endocrinology. 1997;138:5153–60. doi: 10.1210/endo.138.12.5581. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Braunbeck T. Alterations along the Hypothalamic-Pituitary-Thyroid Axis of the Zebrafish (Danio rerio) after Exposure to Propylthiouracil. J Thyroid Res. 2011;376243:1–17. doi: 10.4061/2011/376243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriks M, Roessig JM, Murk AJ, Furlow JD. Thyroid hormone receptor isoform selectivity of thyroid hormone disrupting compounds quantified with an in vitro reporter gene assay. Environ Toxicol Pharmacol. 2007;23:302–7. doi: 10.1016/j.etap.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119:1454–9. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78:144–55. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- Strecker R, Weigt S, Braunbeck T. Cartilage and bone malformations in the head of zebrafish (Danio rerio) embryos following exposure to disulfiram and acetic acid hydrazide. Toxicol Appl Pharmacol. 2013;268:221–31. doi: 10.1016/j.taap.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Sun J, Liu J, Liu Q, Ruan T, Yu M, Wang Y, Wang T, Jiang G. Hydroxylated polybrominated diphenyl ethers (OH-PBDEs) in biosolids from municipal wastewater treatment plants in China. Chemosphere. 2013;90:2388–95. doi: 10.1016/j.chemosphere.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata JR. Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol. 2006;246:10–20. doi: 10.1016/j.mce.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Hopkins DC, Trumble SJ, Bruce ED. Hydroxylated PBDEs induce developmental arrest in zebrafish. Toxicol Appl Pharmacol. 2012;262:43–51. doi: 10.1016/j.taap.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Van Boxtel AL, Kamstra JH, Cenijn PH, Pieterse B, Wagner JM, Antink M, Krab K, van der Burg B, Marsh G, Brouwer A, Legler J. Microarray analysis reveals a mechanism of phenolic polybrominated diphenylether toxicity in zebrafish. Environ Sci Technol. 2008;42:1773–9. doi: 10.1021/es0720863. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaplan MM, Leonard JL, Larsen PR. Evidence for two pathways of iodothyronine 5′-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983;71:992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23–8. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Crawford AD, Darras VM. Combined antisense knockdown of type 1 and type 2 iodothyronine deiodinases disrupts embryonic development in zebrafish (Danio rerio) Gen Comp Endocrinol. 2010;166:134–41. doi: 10.1016/j.ygcen.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Crawford AD, Janssens EDR, Van der Geyten S, Darras VM. Type 2 iodothyronine deiodinase is essential for thyroid hormone-dependent embryonic development and pigmentation in zebrafish. Endocrinology. 2009;150:530–9. doi: 10.1210/en.2008-0457. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Van der Geyten S, Rurangwa E, Darras VM. The effect of 3,5,3′-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors. Gen Comp Endocrinol. 2007;152:206–14. doi: 10.1016/j.ygcen.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Wan Y, Wiseman S, Chang H, Zhang X, Jones PD, Hecker M, Kannan K, Tanabe S, Hu J, Lam MHW, Giesy JP. Origin of hydroxylated brominated diphenyl ethers: natural compounds or man-made flame retardants? Environ Sci Technol. 2009;43:7536–42. doi: 10.1021/es901357u. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Xu W, Lou QQ, Li YY, Zhao YX, Wei WJ, Qin ZF, Wang HL, Li JZ. Tetrabromobisphenol A disrupts vertebrate development via thyroid hormone signaling pathway in a developmental stage-dependent manner. Environ Sci Technol. 2014;48:8227–34. doi: 10.1021/es502366g. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ruan X, Li Y, Yan M, Qin Z. Polybrominated diphenyl ethers (PBDEs) in aborted human fetuses and placental transfer during the first trimester of pregnancy. Environ Sci Technol. 2013;47:5939–46. doi: 10.1021/es305349x. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhu Y, Liu C, Liu H, Giesy JP, Hecker M, Lam MHW, Yu H. Accumulation and Biotransformation of BDE-47 by Zebrafish Larvae and Teratogenicity and Expression of Genes along the Hypothalamus-Pituitary-Thyroid Axis. Environ Sci Technol. 2012;46:12943–51. doi: 10.1021/es303289n. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MMJ, Crofton KKMA. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66:105–16. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45:7896–905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.