Abstract
Analyses of mutations in genes coding for subunits of RNA polymerase always throw more light on the intricate events that regulate the expression of gene(s). Lon protease of Escherichia coli is implicated in the turnover of RcsA (positive regulator of genes involved in capsular polysaccharide synthesis) and SulA (cell division inhibitor induced upon DNA damage). Failure to degrade RcsA and SulA makes lon mutant cells to overproduce capsular polysaccharides and to become sensitive to DNA damaging agents. Earlier reports on suppressors for these characteristic lon phenotypes related the role of cochaperon DnaJ and tmRNA. Here, we report the isolation and characterization of two novel mutations in rpoB gene capable of modulating the expression of cps genes in Δlon strains of E. coli in concert with HNS. clpA, clpB, clpY, and clpQ mutations do not affect this capsule expression suppressor (Ces) phenotype. These mutant RNA polymerases affect rcsA transcription, but per se are not defective either at rcsA or at cps promoters. The results combined with bioinformatics analyses indicate that the weaker interaction between the enzyme and DNA::RNA hybrid during transcription might play a vital role in the lower level expression of rcsA. These results might have relevance to pathogenesis in related bacteria.
Keywords: Ces, HNS, rcsA, rpoB, Δlon
Introduction
Studies pertaining to transcriptional regulatory mechanism that govern capsule genetics do gain medical importance as capsular polysaccharides are one of the virulence determining factors in most of the pathogenic bacteria. Capsular polysaccharides serve as an insulating coat for most of the pathogenic bacteria which in turn helps the bacteria to overcome the immune reactions that are elicited in the Human body (Clarke 2010). In the case of Escherichia coli the group I capsule is made of colanic acid (composed of glucose, galactose, and glucuronic acid). The genes responsible for capsule synthesis (∼19 genes) are organized as a very large cluster/operon. The genetic regulation of capsular polysaccharide synthesis in E. coli has been extensively studied (reviewed by Gottesman 1989; Gottesman and Maurizi 1992). Capsular polysaccharides are overproduced in the absence of Lon, which is one of the major protease found in E. coli. RcsA, (which is the actual substrate for Lon) along with RcsB activates cps genes that in turn lead to synthesis of capsule resulting in mucoid phenotype of lon mutant (Gottesman et al. 1985; reviewed by Majdalani and Gottesman 2006). The other prominent phenotype of lon mutant is the extreme sensitivity to DNA damaging agents and this stems from accumulation of another well-studied substrate of Lon viz. SulA, the cell division inhibitor which gets induced upon DNA damage. Thus, overproduction of capsule and sensitivity to DNA damaging agents are regarded as the hallmark phenotypes of lon mutants (reviewed by Gottesman and Maurizi 1992). Search for Alternate Lon-like proteases (Alp) led to the understanding of a cascade of events that regulate elicitation of Alp phenotype in lon mutants. Extensive study on the elicitation of Alp phenotype in lon mutants clearly reveal that in Δlon mutants presence of ssrA::cat coupled with a multicopy KanR plasmid/pUC4K or a novel mutation in dnaJ (faa) could suppress the above said characteristic phenotypes of lon mutants to appreciable degree; Also, when lon mutants bear both faa and ssrA::cat mutations, the effect was found to be even stronger (Trempy and Gottesman 1989; Kirby et al. 1994; Trempy et al. 1994; Munavar et al. 2005) (see also Discussion). Recently from this Laboratory it has been reported that mutations in rpoB and gyrA can alleviate the sensitivity selectively toward the Mitomycin C (MMC) in an SOS-deficient E. coli strains such as lexA3 ind− and recA (Shanmughapriya and Munavar 2012). Furthermore, earlier reports also suggest that rpoB mutations show pleiotropic phenotype in E. coli (Jin and Gross 1989; Zhou and Jin 1998; Zhou et al. 2013). Taking the above points into consideration, in this investigation we sought for rpoB (rif) mutations capable of suppressing either one or both the phenotypes of lon mutant. In this effort, starting from a Δlon strain harboring cps::lac transcription fusion, two such rif alleles were isolated; among the two alleles, one of them is novel, hitherto unreported and confers slow movement to the RNA polymerase, the other one is identical to rpoB2 reported by Yanofsky (1981) and confers fast movement. These two rif alleles are capable of suppressing only the overproduction of capsular polysaccharides by 50–70% compared to the parental (Δlon) strain (the sensitivity to DNA damaging agents of Δlon strain is not at all affected by these two rif alleles). It has been shown that this suppression by both the rif alleles do not stem from the global transcription defect and it arises due to selective inhibition of rcsA/cpsB transcription. The suppressor phenotype by these rpoB alleles are herein after referred as Ces referring to Capsule expression suppressor. Results reported herein show that functions like ClpA, ClpB, ClpY, and ClpQ do not play a role in this Ces-mediated suppression and mutant RNA polymerase enzymes per se are not defective in initiating transcription at rcsA/cps promoters. Our results also substantiate major role for HNS in elicitation of Ces phenotype by these rif alleles. It is proposed that the ability of DNA sequences present upstream of rcsA to get bended and positions of mutations in rpoB mutants have impact on binding affinity of RNAP with template DNA and is of great importance.
Many of the pathogenic bacteria like Salmonella enterica, Vibrio cholerae, Klebsiella pneumoniae, Erwinia amylovora, Proteus mirabilis, and Pseudomonas aeruginosa posses homologous signaling pathway that regulate the virulence factors in these nonenteric bacteria. We have shown here that the mutation in one of the widely conserved genes (rpoB) among bacteria can selectively modulate/suppress capsule overproduction in concert with HNS. Our study would be highly beneficial to understand the regulation of expression of genes involved in capsule synthesis in other pathogens, considering E. coli as a model system.
Experimental Procedures
Media composition, chemicals, fine chemicals, genetic, and molecular techniques used in this study
The media (conventional Luria-Bertani and Minimal media) composition used in this entire study is essentially as described in Miller (1992). Materials used for media, buffer, solutions, most of the Antibiotics, Mac-Conkey Lactose Agar and other fine chemicals were purchased from Hi-Media, India. Streptomycin was purchased from Sarabhai Chemicals, India. Methyl methane sulfonate (MMS) was purchased from Sisco Research Laboratories Pvt. Ltd, India and the final concentration of each of them is quoted wherever appropriate. The primers used in this study were obtained from Synergy Scientific, India. All the Genetic techniques were according to Miller (1992) (with minor modifications) and molecular techniques employed in this study are as per Sambrook and Russel (2001).
Bacterial strains, phages, plasmids, and primers used in this study
Table1 gives the list of bacterial strains, phages, plasmids, and primers used in this study. All the bacterial strains are the derivatives of E. coli K-12 and the Genetic nomenclature is according to Demerec et al. (1966) and Berlyn (1998).
Table 1.
List of Escherichia coli strains, phages, plasmids, and primers used in this study
| Strain | Relevant genotype | Source/reference/construction |
|---|---|---|
| SG20780 | F− Δ(argF-lac)169 lon510 cpsB10::lac rpsL150 | S. Gottesman, NIH, USA |
| SG20781 | F− Δ(argF-lac)169 lon+ cpsB10::lac rpsL150 | S. Gottesman, NIH, USA |
| CAG18618 | F− λ− rph-1 thiC3178::Tn10kan | Lab Collection |
| HR318 | F− λ− rph-1 btuB::Tn10 rpoB8 | R. Harinarayanan, CDFD, India |
| HR318K | Same as HR318 but has thiC3178::Tn10kan | This Study HR318 X P1/(CAG18618) |
| MMR6 | Same as SG20780 but has thiC3178::Tn10kan rpoB12 | This Study |
| MMR23 | Same as SG20780 but has thiC3178::Tn10kan rpoB77 | This Study |
| MMR8 | Same as SG20780 but has thiC3178::Tn10kan rpoB8 | This Study SG20780 X P1/(HR318K) |
| KL226 | HfrC(PO2A) relA1 spoT1 | Laboratory Collection |
| SMM12 | Same as KL226 but has thiC3178::Tn10kan rpoB12 | This Study KL226 X P1/(MMR6) |
| SMM23 | Same as KL226 but has thiC3178::Tn10kan rpoB77 | This Study KL226 X P1/(MMR23) |
| SMM8 | Same as KL226 but has thiC3178::Tn10kan rpoB8 | This Study KL226 X P1/(HR318K) |
| AB1157 | F− thr-1, araC14, leuB6(Am), Δ(gpt-proA)62, lacY1, glnX44(AS), hisG4(Oc), rpoS396(Am), rpsL31(StrR), argE3(Oc), thi-1 | Laboratory Collection |
| DM49 | Same as AB1157 but has lexA3 Ind− | Laboratory Collection |
| DM49RN | Same as DM49 but has zfa723::Tn10 gyrA87 argE+ rpoB87 | Shanmughapriya and Munavar (2012) |
| SM49AK | Same as DM49RN but has clpA::kan | Shanmugapriya (2013) |
| SM49BK | Same as DM49RN but has clpB::kan | Shanmugapriya (2013) |
| SM49QC | Same as DM49RN but has clpQ::cat | Shanmugapriya (2013) |
| SM49YC | Same as DM49RN but has clpY::cat | Shanmugapriya (2013) |
| MMR6A | Same as MMR6 but has clpA::kan | This Study MMR6 X P1/(SM49AK) |
| MMR6B | Same as MMR6 but has clpB::kan | This Study MMR6 X P1/(SM49BK) |
| MMR6Q | Same as MMR6 but has clpQ::cat | This Study MMR6 X P1/(SM49QC) |
| MMR6Y | Same as MMR6 but has clpY::cat | This Study MMR6 X P1/(SM49YC) |
| MMR23A | Same as MMR23 but has clpA::kan | This Study MMR23 X P1/(SM49AK) |
| MMR23B | Same as MMR23 but has clpB::kan | This Study MMR23 X P1/(SM49BK) |
| MMR23Q | Same as MMR23 but has clpQ::cat | This Study MMR23 X P1/(SM49QC) |
| MMR23Y | Same as MMR23 but has clpY::cat | This Study MMR23 X P1/(SM49YC) |
| SMM780A | Same as SG20780 but has clpA::kan | This Study SG20780 X P1/(SM49AK) |
| SMM780B | Same as SG20780 but has clpB::kan | This Study SG20780 X P1/(SM49BK) |
| SMM780Q | Same as SG20780 but has clpQ::cat | This Study SG20780 X P1/(SM49QC) |
| SMM780Y | Same as SG20780 but has clpY::cat | This Study SG20780 X P1/(SM49YC) |
| SMM781A | Same as SG20781 but has clpA::kan | This Study SG20781 X P1/(SM49AK) |
| W3110 | F−λ−IN(rrnD-rrnE)1 rph-1 | Laboratory Collection |
| ZK819 | Same as W3110 but has Δ(argF-lac)169 rpoS819 bgl– hns::kan | S. Mahadevan, IISc,India |
| SMM780H | Same as SG20780 but has hns::kan | This Study SG20780 X P1(ZK819) |
| SMM781H | Same as SG20781 but has hns::kan | This Study SG20781 X P1(ZK819) |
| MMR6H | Same as MMR6 but has hns::kan | This Study MMR6 X P1(ZK819) |
| MMR23H | Same as MMR23 but has hns::kan | This Study MMR23 X P1(ZK819) |
| SMM780R | Same as SG20780 but bearing pHYD535 | This Study |
| SMM781R | Same as SG20781 but bearing pHYD535 | This Study |
| MMR6R | Same as MMR6 but bearing pHYD535 | This Study |
| MMR23R | Same as MMR23 but bearing pHYD535 | This Study |
| SMM780RC | Same as SG20780 but bearing pCL1920 | This Study |
| SMM781RC | Same as SG20781 but bearing pCL1920 | This Study |
| MMR6RC | Same as MMR6 but bearing pCL1920 | This Study |
| MMR23RC | Same as MMR23 but bearing pCL1920 | This Study |
| SMM780T | Same as SG20780 but bearing pλTR1 | This Study |
| SMM781T | Same as SG20781 but bearing pλTR1 | This Study |
| MMR6T | Same as MMR6 but bearing pλTR1 | This Study |
| MMR23T | Same as MMR23 but bearing pλTR1 | This Study |
| SMM780TC | Same as SG20780 but bearing pKK232-8 | This Study |
| SMM781TC | Same as SG20781 but bearing pKK232-8 | This Study |
| MMR6TC | Same as MMR6 but bearing pKK232-8 | This Study |
| MMR23TC | Same as MMR23 but bearing pKK232-8 | This Study |
| Phage | Relevant genotype | Source/reference |
|---|---|---|
| P1 | vir | Laboratory Collection; originally obtained from N Willets, U.K. |
| Plasmid | ||
| pHYD535 | Derivative of pCL1920 bearing wild-type rpoBC+ alleles cloned under lac promoter | R. Harinarayanan, CDFD, India |
| pCL1920 | pSC101 ori, SpecR | R. Harinarayanan,CDFD, India |
| pλTR1 | Derivative of pKK232-8 bearing λTR1 site cloned under tac promoter | A. R. Rahmouni, CNRS, France |
| pKK232-8 | pBR322 ori, AmpR, CamR | A. R. Rahmouni, CNRS, France |
| Primer | Sequence | Source/reference |
|---|---|---|
| rif For | 5′-CGTCGTATCCGTTCCGTTGG-3′ | Shanmughapriya and Munavar (2012) |
| rif Rev | 5′-GGCAACAGCACGTTCCATACC-3′ | Shanmughapriya and Munavar (2012) |
| cpsB RT For | 5′-GATCTCACCATGCTGCAA-3′ | This Study |
| cpsB RT Rev | 5′- GTCTTCATCGGCAATCAC-3′ | This Study |
| rcsA RT For | 5′- ACCGTTGA TGACCTTGC-3′ | This Study |
| rcsA RT Rev | 5′- GCCAAGGATATCGTCGAG-3′ | This Study |
| dnaE RT For | 5′-GATGATCGATGGCCTG-3′ | This Study |
| dnaE RT Rev | 5′-GAGATCAGCAACGTCAG-3′ | This Study |
| rpsG RT For | 5′-GTCGCGTCAT TGGTCAG-3′ | This Study |
| rpsG RT Rev | 5′- CGAGAGCTACTTCGAATGCT-3′ | This Study |
| rcsA CL For | 5′-CGGGATCC C TGA TGC TGG CGT TA-3′ | This Study |
| rcsA CL Rev | 5′-ACGCGTCGAC GGCATCAGGACGGTATC-3′ | This Study |
Isolation and genetic characterization of rpoB mutations
Different aliquots of the overnight culture of SG20780 were plated on LB plates containing rifampicin (50 μg/mL) and the plates were incubated at 30°C for 2–3 days. As the intention was to isolate only Cps::Lac− colonies, the cells were plated on LB plates with Rifampicin and the RifR mutants obtained on LB were checked for their phenotype on Mac-Conkey Lactose Agar plates and those colonies that exhibited white/more or less white (Lac−) phenotype were picked and were reconfirmed. Eleven such mutants were isolated out of 367 RifR colonies obtained from 10 independent experiments and they were named as MMR1,2,5,6, MMR21–26, and MMR210. To mobilize the rpoB allele into various strain backgrounds, the strain CAG18618 carrying thiC3178::Tn10kan marker located near rpoB region was used. In order to confirm that the suppression is only due to mutation in rpoB, the rpoB region from all the putative Cps::Lac− RifR mutants were mobilized back to the parental strain SG20780 with a nearby selectable marker thiC3178::Tn10kan through P1 transduction.
Physiological characterization and complementation studies of rif mutants
Growth properties at different temperatures of the selected rif mutants were analyzed by checking their relative viability (by sequential spotting analyses of the serially diluted relevant cultures) and incubating at appropriate temperatures. The dominant and recessive natures of the rif mutations were determined by introducing a plasmid clone pHYD535 (pCL1920 carrying wild-type rpoBC+ operon cloned at HindIII restriction site under lac promoter). The transformation was carried out according to Sambrook and Russel (2001). The transformants were selected on LB Agar plates containing Spectinomycin (50 μg/mL) and checked for the RifR/RifS phenotype by sequentially spotting the appropriate dilutions of the relevant cultures on LB plates with Spectinomycin (50 μg/mL) and IPTG (1 mmol/L) and also on LB plates with Spectinomycin (50 μg/mL), Rifampicin (50 μg/mL) and IPTG (1 mmol/L). The Cps::Lac phenotype was also checked by plating them on Minimal M9 plates with Spectinomycin (50 μg/mL), IPTG (1 mmol/L) and X-gal (20 μg/mL) (see text).
Sequence analyses
Whole genomic DNA from all the 11 rifampicin-resistant mutants was isolated by following Chen and Kuo (1993). Then it was treated with RNase at 37°C for 30 min followed by heat inactivation at 65°C for 15 min. This genomic DNA was then used as template for the amplification of rif region spanning about 777 bp covering all the rif clusters of rpoB gene. The sequences of the primers are given in Table1. The conditions for the PCR amplification are as follows: 5 min at 94°C, 30 sec at 95°C, 40 sec at 50°C, 30 sec at 72°C, 2 min at 72°C for 30 cycles and maintained at 4°C. The PCR products were purified with Fermentas gel purification kit following the protocol given by the manufacturer and the samples were sequenced by Chromous, Biotech., India. With the help of NCBI-BLAST Analyses Tool, sequence analyses was carried out by comparing our sequenced data with that of E. coli K12 substrain MG1655 and screened for the presence of any base change/mutation manually.
Determining the termination efficiency of rpoB mutations
The vector pKK232-8 carries AmpR as selection marker and cat gene (CamR) cloned under IPTG-inducible tac promoter. The derivative of pKK232-8, p λTR1 bears λTR1 region which is at SalI restriction site in between the tac promoter and cat gene. The control plasmid does not contain this λTR1 region. Both the plasmids were transformed into the mutants as well as to the parental strains to compare the termination efficiencies of mutant and wild-type RNAP enzymes. This was carried out by sequentially spotting different dilutions of the selected transformants on LB plates with Ampicillin (100 μg/mL) as well as LB plates with Ampicillin (100 μg/mL) and Chloramphenicol (100 μg/mL) with IPTG (1 mmol/L). The rif allele(s) that renders the relevant transformants, resistant to Chloramphenicol is regarded as the one(s) with faster elongation rate and capable of transcribing past terminator.
Beta-galactosidase assay
Overnight cultures of 0.1 mL of each strain (carrying cps::lac fusion) were subcultured into 5 mL of M9 minimal medium containing glucose as carbon source and grown at 30°C. The cultures were allowed to attain mid-log phase, then the OD (Optical Density) of the cultures were recorded at 600 nm wavelength. When β-gal was analyzed from lac operon, the relevant cultures were grown in minimal M9 medium containing glycerol as the sole carbon source and then the cells were induced with IPTG (Isopropyl-beta D-1-thiogalactopyranoside) (1 mmol/L) for 30 min prior to assay. β-galactosidase assay was carried out as described in Miller (1992).
RT-PCR and densitometry analyses
Total RNA was isolated from each of the cultures grown till mid-log phase using RNA isolation kit from Ambion, Life Technologies, USA. The obtained total RNA was then subjected for DNase treatment (Ambion, Life Technologies, USA). The concentration of the total RNA was normalized by checking their OD at 260 nm. With the help of gene-specific primers, respective mRNAs were converted into cDNA followed by amplification using one-step RT-PCR kit (Invitrogen, Life Technologies, USA). The primer sequences used for the amplification of respective genes are given in Table1. The conditions for the cDNA preparation and PCR amplification are as follows: 30 min at 55°C, 5 min at 94°C, 30 sec at 95°C, 40 sec at 52°C, 30 sec at 72°C, 2 min at 72°C for 25 cycles and maintained at 4°C. Absence of DNA was once again confirmed by performing the same reaction without RT enzyme but with Taq polymerase. The densitometry scanning was performed with the software Image J, freely available at http://rsbweb.nih.gov/ij/download.html.
rcsA cloning
Genomic DNA was isolated manually by following Chen and Kuo (1993) from a wild-type strain MG1655. It was then treated with RNase at 37°C for 30 min followed by heat inactivated at 65°C for 15 min. This preparation was used as template for the PCR amplification of rcsA gene. The sequences of the primers are given in Table1. The conditions for the PCR amplification are as follows: 5 min at 94°C, 30 sec at 95°C, 40 sec at 56°C, 30 sec at 72°C, 2 min at 72°C for 30 cycles and maintained at 4°C. Amplification of ∼1.2 kb fragment was confirmed by running it on 0.8% agarose gel and the clone was constructed by ligating the rcsA+ allele with the pBR322 using BamHI and SalI restriction enzymes. The clone was confirmed by appropriate restriction digestion Analysis.
Bioinformatics analyses
The software Bend.it available at http://hydra.icgeb.trieste.it/dna/index.php was used to predict the bending and curving nature of relevant genes. Approximately, 1000 base pairs upstream to the promoter of each gene were given as input and the resulting output was analyzed accordingly. The red and green colors of peak show the curving and bending natures, respectively. The structural predictions were made by using PYMOL software with the help of already available structures at PDB (PDB ID 4IGC and 2OGJ). The mutant forms were modeled using the mutagenesis option available in PYMOL.
Results
Isolation and characterization of rpoB (rif) mutations capable of suppressing cps::lac expression in Δlon strains
The E. coli strain SG20780 constructed by Gottesman and Co-workers harbors the Δlon-510 mutation and cps::lac transcription fusion (Trisler and Gottesman 1984; Brill et al. 1988). This strain appears red in color on Mac-Conkey Lactose Agar plates or blue on LB/Minimal Agar plates with X-gal due to the overexpression of cps::lac fusion by stabilized RcsA. As SulA is also stabilized due to absence of Lon, the strain SG20780 becomes sensitive to UV, MMS etc., (reviewed by Gottesman and Maurizi 1992). In an attempt to isolate suppressors for these phenotypes, a collection of (367) spontaneous rifampicin-resistant mutants of SG20780 from 10 independent experiments were isolated. Among the 367 rif mutants, 11 rif mutants exhibited more or less white phenotype (Lac−) on Mac-Conkey Lactose Agar plates and one of them was resistant to MMS (see Table S1). However, through genetic analyses it is confirmed that this MMSR phenotype was not due to the rpoB mutation and therefore was not investigated further. In order to confirm that, it is the relevant rpoB mutation present in each of the mutants is responsible for the Lac− phenotype, wild-type rpoB+ allele from CAG18618 strain (that harbors thiC3178::Tn10kan) was mobilized into all the 11 rif mutants by P1 transduction and our results clearly indicate that it is indeed the rpoB mutation in each of the case that elicited this phenotype; this is because, in every cross all the RifR transductants exhibited the expected Lac− phenotype, whereas all the RifS transductants reverted to Lac+ phenotype, as like parent SG20780 (Table S2).
The 11 RifR mutants define only two alleles; both are dominant and one hitherto unreported
Earlier reports convey that most of the rifampicin-resistant mutations were found to be present in the three clusters (I, II, and III) of rpoB gene (Jin and Gross 1988, 1989). Therefore, the 777 bp region of rpoB that covers all the three clusters of rpoB gene was amplified. Sequence analyses of all the 11 rif mutations revealed the presence of only two rif alleles; Among the two, one defines C1576 to T (CAC to TAC) transition changing amino acid His526 to Tyr and this is essentially same as that of rpoB2, isolated and reported by Yanofsky and Horn (1981). The other one harbors C1535 to A (TCT to TAT) transversion changing the amino acid Ser512 to Tyr; this rpoB allele could perhaps be a novel one as such an allele has not been reported by others earlier. These rif alleles are referred to as rpoB12 and rpoB77, respectively. The mutant with rpoB12 allele, as reported earlier, is temperature sensitive at 42°C, but the mutant bearing rpoB77 allele grows well at 42°C. The rpoB12 allele bearing strain becomes sensitive to LB devoid of salt at all temperatures, whereas rpoB77 mutant grows well in LB devoid of salt at 30°C and 37°C, but it becomes sensitive at 42°C (Fig. S1) (the media/salt-dependent temperature-sensitive phenotype of these strains and the defect in macromolecular synthesis in those conditions are being investigated in detail in another line of study). Both the rpoB alleles are dominant over the wild-type rpoB+ allele, as the introduction of the plasmid clone bearing the rpoBC+ alleles namely pHYD535 (Sarkar et al. 2013) (derived from pCL1920) into rpoB mutants, did not change the Cps::Lac− phenotype of the strains. Figure1A and B clearly show the Lac+/Lac− phenotype of all relevant strains.
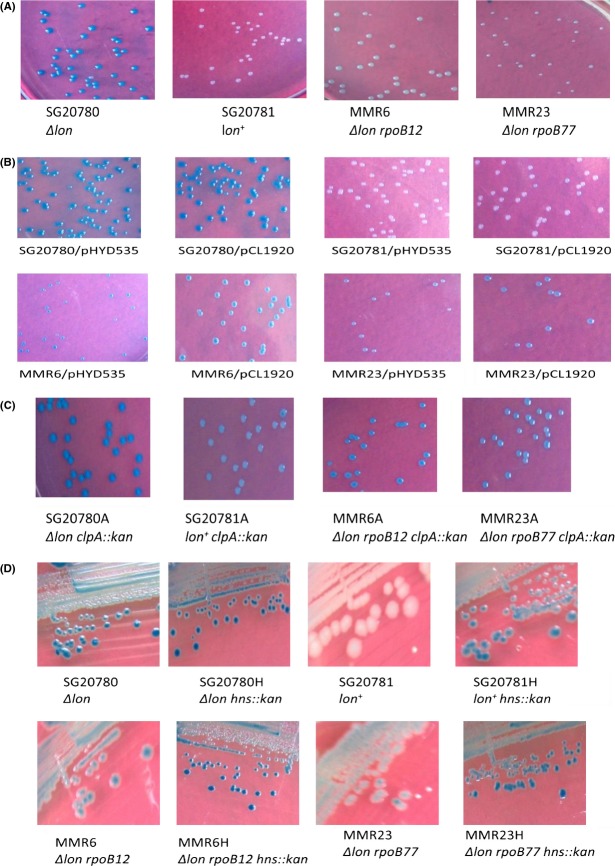
Figure 1.
Pictures of the petri plates showing the Cps::Lac+/− phenotype due to different levels of expression of cps::lac fusion in relevant strains. (A) Growth of lon− cps::lac (SG20780) lon+ cps::lac (SG20781), MMR6 (lon− cps::lac rpoB12), and MMR23 (lon− cps::lac rpoB77) strains. Appropriate dilutions of cells were plated on relevant minimal agar plates containing X-gal (20 μg/mL) and pictures were taken after ∼36 h of incubation at 30°C. (B) Growth of strains as indicated in (A) bearing the rpoBC+ clone (pHYD535) and the control plasmid vector (pCL1920). Cells were pated on similar minimal agar plates but Spectinomycin (50 μg/mL) and IPTG (1 mmol/L) were added as the plasmid confers SpecR phenotype and the rpoBC+ is cloned under lac promoter. Pictures were taken as in (A). The presence of rpoBC+ clone did not change the cps::lac phenotype of any strain indicating the dominant nature of both rif alleles. (C) Growth indicating the Cps::Lac phenotype of the strains as indicated in (A) (SG20780, SG20781, MMR6, and MMR23) bearing clpA::kan insertion. The cells were plated on relevant minimal agar plates containing Kanamycin (50 μg/mL) and X-gal (20 μg/mL).The pictures were taken after ∼44 h incubation at 30°C as the growth rate of clpA::kan derivatives was less compared to that of the original strains. (D) Growth indicating the Cps::Lac phenotype of the strains (SG20780, SG20781, MMR6, and MMR23) as indicated in (A) bearing hns::kan insertion. The cells were streaked on LB plates containing Kanamycin (50 μg/mL) and X-gal (40 μg/mL) as the hns::kan derivatives were relatively very slow grower in minimal agar plates. The pictures were taken after ∼30 h incubation at 30°C.
Evidence that rpoB12 defines a fast moving RNAP, whereas rpoB77 defines slow moving one
λTR1 serves as one of the best site to study the termination efficiency in a Rho-dependent manner. Termination efficiency of different rpoB alleles has been determined mostly through in vitro techniques. One of the in vivo methods to determine termination efficiency is the assay of IPTG induced β-galactosidase enzyme from lac operon in a time-dependent manner which indirectly means the time taken for the formation of functional β-galactosidase enzyme (Jin et al. 1988, 1992). Here, we have used a vector pλTR1 derived from pKK232-8 carrying a selection marker AmpR and a gene coding for Chloramphenicol acetyl transferase enzyme which is cloned under IPTG-inducible tac promoter; In this construct, the region between the tac promoter and cat gene is interrupted by λTR1 site (Guérin et al. 1998). In order to determine the termination efficiency of the rpoB mutations, we compared the viability of the mutants carrying the vector pλTR1 on LB plates containing only Ampicillin (100 μg/mL) and also LB plates with Ampicillin (100 μg/mL) and Chloramphenicol (100 μg/mL) along with IPTG (1 mmol/L). The rpoB12 mutant grew very well compared to wild type confirming the earlier reports that it is able to read through the terminator site and therefore conferring fast movement to the RNAP (Jin et al. 1988; Jin et al. 1989); On the other hand, the rpoB77 mutant did not grow on similar plates showing the inability to read through the terminator site that indirectly means that it confers slow movement to RNAP (see Fig.2). These results clearly reveal that it is not the fast/slow movement of RNAP that is responsible for the elicitation of this Ces phenotype.
Figure 2.
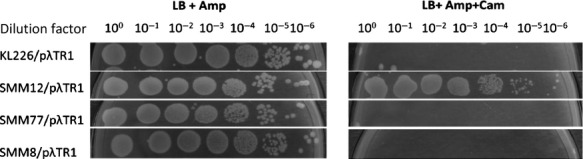
Determination of Termination efficiency of rpoB mutants by sequential spotting analyses of relevant strains. The overnight cultures of the relevant strains were serially diluted and relevant dilutions were spotted on LB Agar plates with Ampicillin (100 μg/mL) as well as on LB plates with Ampicillin (100 μg/mL), Chloramphenicol (100 μg/mL), and IPTG (1 mmol/L). The plates were incubated at least for ∼24 h at 30°C. The ability of relevant strains with appropriate clone to grow both in Ampicillin and Chloramphenicol plates is the indication of fast movement of the RNAP of the relevant strain (transcription past terminator). KL226 is the WT strain, whereas SMM12, SMM77, and SMM8 are the rpoB12, rpoB77, and rpoB8 derivatives of KL226. The fast movement for ces12 is evident from the picture.
Quantification of suppression of cps::lac expression in rpoB mutants
The cps::lac transcriptional fusion not only helps one to infer expression of cps genes qualitatively on relevant plates based on Lac phenotype but also makes even the quantification of cps expression easier by β-galactosidase assay. To quantify the level of expression of cps::lac fusion in rpoB mutants, β-galactosidase in relevant strains were assayed. The Figure3A clearly shows that rpoB12 suppresses the expression of cps::lac by ∼50% and in rpoB77 the suppression is even better (∼70%) compared to the parental strain SG20780 (lon cps::lac rpoB+). One of the semiquantitative methods, reverse transcription-PCR was employed to further confirm our observation. As was expected, the RT-PCR analysis of cpsB gene expression was in agreement with our view that rpoB alleles are indeed able to reduce the transcription level of cpsB and it is very much clear from the Figure4A.
Figure 3.
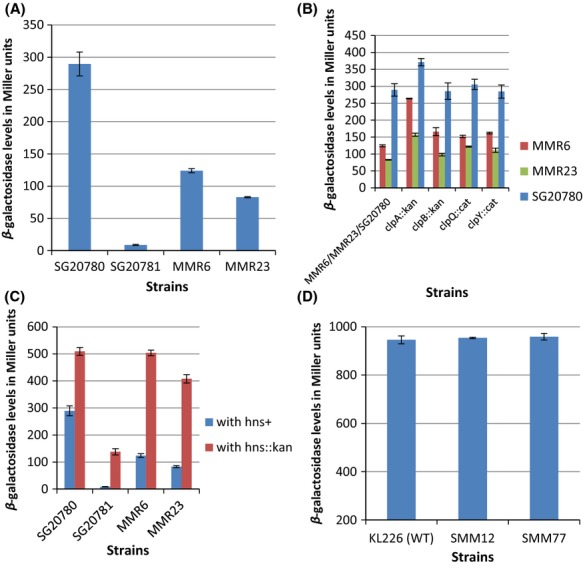
Quantification of cps::lac/lac operon expression through β-galactosidase assay in relevant strains. (A) Level of cps::lac expression in rpoB mutants along with the lon− and lon+ controls, SG20780 and SG20781, respectively. (B) Effect of clpA,clpB, clpQ, and clpY mutations on the cps::lac expression in Δlon rpoB mutants. (C) Effect of hns::kan mutation in modulation of capsule expression in rpoB mutants. (D) Level of IPTG induced β-galactosidase expression from lac operon in relevant strains. In each case the values given are the average of three independent experiments with standard error mean. Whenever the β-galactosidase assay for cps::lac expression was perofrmed, the lon− and lon+ strains were taken as controls. Therefore, the values given for the parental strains (lon− and lon+) are the average of more than three experiments (for more details see text).
Figure 4.
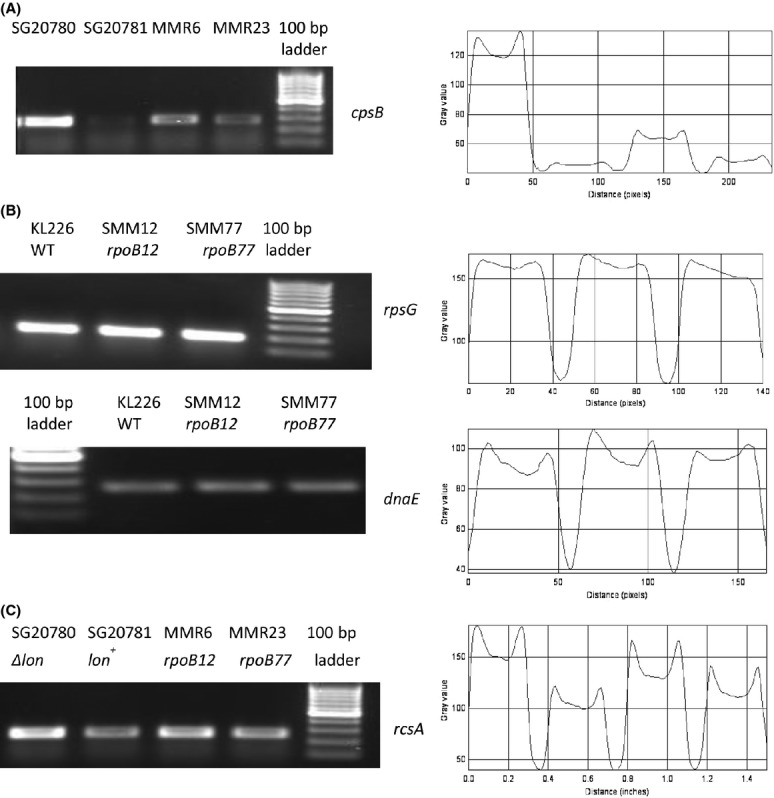
RT-PCR-based transcription profile of indicated genes and densitometry analysis of the gel pictures. (A) Transcription profile of cpsB gene in rpoB mutants with SG20780 (lon−) and SG20781 (lon+) as controls. (B) Transcription profile of house-keeping genes namely rpsG and dnaE in KL226 (WT), SMM12 (rpoB12), and SMM77 (rpoB77) strains. (C) Transcription profile of rcsA in rpoB mutants with the respective controls. In each case, 1 μg of total RNA was used for RT-PCR and gel picture given for each case is the representative of three independent experiments. Shown in the side is the densitometry analysis of the respective gel pictures.
Selective modulation of expression of cps::lac by rpoB alleles
Many reports indicate that mutations in rpoB gene show pleiotropic phenotype in E. coli (Jin and Gross 1989; Zhou and Jin 1998; Zhou et al. 2013). As the mutations are located in the gene that code for one of the subunit of essential transcriptional machinery, it could be possible that the reduction in the level of cps expression might stem from the defect in global transcription by these mutant RNA polymerases with either RpoB12 or RpoB77 β subunit. In order to check this possibility, it is mandatory to check the expression levels of other candidate genes in rpoB mutants. In this respect, it was intended to analyze the expression levels of candidate genes such as lacZ from lac operon, rpsG coding for a ribosomal protein, and dnaE coding the DNA polymerase III α subunit. Therefore, each of the rif alleles (rpoB12/rpoB77) was introduced into a wild-type strain KL226 with a nearby selectable kanamycin-resistant marker (thiC3178::Tn10kan) and the expression level of lac operon was measured through β-galactosidase assay after IPTG induction. The results presented in the Figure3D clearly show that the level of expression of lac operon remains almost equal in all the cases. The expression levels of other candidate genes were analyzed through semiquantitative RT-PCR method. The expression levels of rpsG and dnaE do not vary between wild-type and the rif-mutant strains (see Fig.4B). From these results, it is tenable to conclude that these rif alleles do not interfere with the global transcription but selectively modulate cps expression.
Effect of mutations in clpA, clpB, clpY, and clpQ in elicitation/modulation of Ces phenotype
In E. coli, it has been reported earlier that the substrates of Lon are also recognized by other protease like ClpYQ under certain conditions. Munavar et al. (2005) showed that the Lon substrate SulA is indeed degraded by ClpYQ protease in phenotypically Alp strains. Wu et al. (1999) have also shown that when ClpYQ is present in multicopy, could also degrade RcsA (Wu et al. 1999; Munavar et al. 2005). Therefore, it was necessary to check the possibility of interference of the proteases/components of the same in this suppression phenomenon. Hence, the effect of clpA, clpB, clpQ, clpY mutations on elicitation of Ces phenotype was investigated. To enable the same the insertional mutations of the above said genes were introduced into relevant strains and the level of expression of cps::lac was measured in each strain through β-galactosidase assay. The results presented in the Figure3B provide strong evidence that the studied functions do not play a role in this rpoB-mediated suppression. However, introduction of clpA::kan allele did increase the level of β-galactosidase to some level, but this increase was seen even in the parental strains (see also Fig.1C for the Cps::Lac phenotype). These results give a clue that ClpA might be involved in degradation of either RcsA or Cps::Lac fusion protein, a notion to be vindicated (Wickner et al. 1994; Gottesman et al. 1998).
Transcription profile of rcsA in these rpoB mutants
To further study the molecular details of this suppression, the expression level of rcsA was analyzed, as RcsA is the positive regulator of cps genes (Stout et al. 1991; Ebel and Trempy 1999). It has already been reported that RcsA forms complex with RcsB that in turn regulates cps expression (Stout et al. 1991; reviewed by Majdalani and Gottesman 2006). The expression level of rcsA was analyzed through semiquantitative RT-PCR method and the gel picture (see Fig.4C) of the same which clearly shows that the level of transcription of rcsA indeed goes down in these rpoB mutants compared to the parental strain SG20780. Therefore, the reduction in transcription of rcsA should be playing a vital role in reducing the level of cps expression in these mutants.
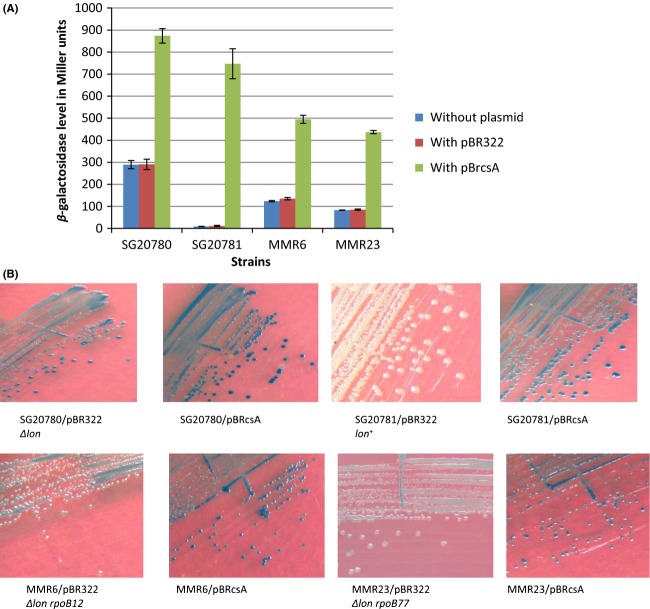
Effect of multicopy RcsA on Ces phenotype
It has been well studied and established that overproduction of RcsA leads to high-level expression of cps::lac in lon mutants (Torres-Cabassa and Gottesman 1987; Kuo et al. 2004). The expression profile of rcsA in the rpoB mutants revealed that there is a decrease in the transcript level of rcsA in rpoB mutants compared to the parental strains. If the reduction in rcsA transcription alone were to be the reason for the elicitation of Ces phenotype, then the introduction of multicopy rcsA+ clone should theoretically abolish the phenotype of Ces in these mutants. The wild-type rcsA allele was cloned in pBR322 under its native promoter and was introduced into the rpoB mutants as well as to the parental strains. The cps::lac levels in these strains were determined through β-galactosidase assay. The Figure5A clearly shows that the beta-galactosidase levels from cps::lac indeed increased as expected both in parental strains and in the mutants; but this increase in level in the case of mutants are not equal to the parental Δlon strain. These results hint the possibility that beyond the role of RcsA there could be some other factors/mechanism might play a role in elicitation of Ces phenotype by rif alleles. Figure5B shows the Cps-Lac phenotype of the relevant strains.
Figure 5.
The cps::lac expression in relevant strains after the introduction of multicopy rcsA+ clone. (A) Effect of multicopy rcsA+ allele in the modulation of cps::lac expression in rpoB mutants along with the parental strains. The values given are the average of three independent experiments with standard error mean. (B) Growth indicating the Cps::Lac phenotype of the relevant strains (SG20780, SG20781, MMR6, and MMR23) bearing rcsA+ clone. The cells were streaked on LB agar plates containing Streptomycin (100 μg/mL), Ampicillin (100 μg/mL), and X-gal (40 μg/mL). The pictures were taken after ∼36 h incubation at 30°C.
Mutant RNA polymerase per se is not defective at rcsA/cps promoters
The one more possibility for the reduced expression levels of cps/rcsA genes could be due to the inability of the mutant RNA polymerases with either RpoB12 or RpoB77 β-subunits to carry out successive transcription at cps/rcsA promoters. It has been already shown that HNS functions as repressor for rcsA and a small RNA dsrA is also involved in the regulation of rcsA transcription by binding to HNS there by relieving the repression by HNS (Sledjeski and Gottesman 1995). These are the known modes by which expression of rcsA is getting regulated in E. coli. If the mutant RNAPs with each of the rpoB mutations per se is not defective in transcription at cps/rcsA promoter, then knocking off the repressor HNS should increase the transcription level of rcsA. On the other hand, if these mutant RNAPs are not efficient in transcribing at cps/rcsA promoter, then even after the absence of functional HNS it should decrease the level of rcsA transcription. The HNS null mutant, hns::kan (Harwani et al. 2012) was introduced into the rpoB mutants as well as to the parental strains. As expected, the cps::lac levels were increased in the parental strains with hns::kan, but surprisingly, the cps::lac levels were higher in rpoB mutants with hns::kan which could be seen from the results presented in the Figure3C. In fact, this is very evident from the phenotype of relevant strains itself (see Fig.1D). From these results, it is tenable to conclude that these rif mutations do not make the RNAP defective at cps/rcsA promoters. Both these mutant enzymes indeed can transcribe the genes cps/rcsA efficiently but probably the interaction of HNS with the mutant RNAPs (with RpoB12/RpoB77 β subunit) leads to the elicitation of Ces phenotype.
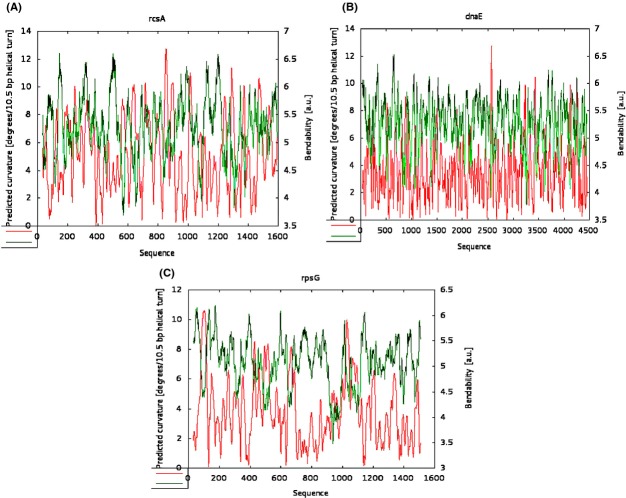
Possible role of bending/curving ability of DNA sequence in the promoter region affected by rpoB alleles
HNS is one of the nucleoid-associated proteins and work with HNS is gaining momentum in recent years (Atlung and Ingmer 1997; Browning et al. 2010; Rimsky and Travers 2011). Extensive studies on HNS have revealed that it binds to DNA sequence preferentially to curved regions and there are also reports indicating that even binding of HNS might bend the DNA region if that particular sequence is amenable for bending (Atlung and Ingmer 1997; Shin et al. 2005; Kahramanoglou et al. 2011). As this study indicates that hns mutation abolishes Ces phenotype, it is conceivable that interaction of HNS with the mutant RNAP should be playing a role in elicitation of Ces phenotype. These results made us to analyze the nature of DNA sequence present in the upstream of rcsA gene, which is shown to be under the control of HNS. The sequence nature for some of the control genes whose expression is not altered by these rpoB alleles was also analyzed. Approximately 1000 base pairs upstream to the promoter of each candidate genes were taken to analyze the bending/curving ability. As could be seen from the Figure6A, it is in agreement with our view that in the case of rcsA, the ability for the DNA sequence to get bended/curved is higher compared to the control genes (Fig.6B and C). In the case of dnaE, even though our results predict that there is a probability to get bended/curved, it is well known that this gene is not under the control of HNS.
Figure 6.
The bioinformatics analyses to predict the bending and curving abilities of relevant genes. The freely available software Bend.it was used to analyze the bending or curving ability of the upstream sequences of the relevant genes. (A) rcsA (B) dnaE and (C) rpsG. The red color indicates the curving ability, whereas the green color indicates the bending ability (refer text for more details).
Discussion
As could be seen from the introduction, E. coli lon mutants overexpress capsular polysaccharides due to stabilization of RcsA and become sensitive to UV/MMS due to stabilization of SulA (reviewed by Gottesman 1989, 1996; Gottesman and Maurizi 1992; Majdalani and Gottesman 2006; Clarke 2010). These two characteristic lon mutant phenotypes are suppressed to appreciable degree in phenotypically Alp strains (∆lon ssrA::cat/pUC4K, lon faa and lon faa ssrA::cat). Although in lon ssrA::cat mutants, the multicopy KanR plasmid pUC4K leads to full suppression of lon phenotypes, per se this Alp Helper Activity (AHA) was found to be innocuous in ∆lon strains bearing ssrA+ allele. That is in this original Alp strain, inactivation of ssrA is mandatory for elicitation of Alp phenotype and AHA only adds to this. However, faa mutation per se can elicit Alp phenotype in ∆lon strains and in such lon faa mutants ssrA has additive effect. faa mutation was perhaps the first identified novel allele of dnaJ implicated in lon suppression (G to A transition affecting codon 232 changing amino acid Gly to Asp). It is now well known that in these phenotypically Alp strains heat-shock induction and expression of ClpYQ protease is implicated in the degradation of SulA and models have been proposed for RcsA inactivation/degradation. In addition, in lon faa mutants, synthesis of SulA also has been shown to be decreased to some degree (Trempy and Gottesman 1989; Kirby et al. 1994; Trempy et al. 1994; Munavar et al. 2005). Mutations in β subunit of RNA polymerase affect a variety of phenotypes in E. coli (Jin and Gross 1989; Zhou and Jin 1998; Zhou et al. 2013). We have also reported that combination of rpoB87 and gyrA87 mutations permit the expression of uvrB by circumventing the super repression posed by lexA3 and this expressed UvrB makes the lexA3 rpoB87 gyrA87 strain to become resistant to MMC (Shanmughapriya and Munavar 2012). In this investigation, rpoB mutations that could suppress either one or both the lon phenotypes were sought for. Two such mutations were isolated (rpoB12 and rpoB77) but both of them could suppress only one of the two hallmark phenotypes of lon, that is, overproduction of capsular polysaccharides. While rpoB77 is a novel hitherto unreported allele rpoB12 is identical to rpoB2 reported earlier by Yanofsky and Horn (1981). Genetic analyses confirmed that both the rpoB mutations could elicit the capsule expression suppressor phenotype (Ces), but to varying degrees. The physiological characterization revealed that rpoB12 makes the cells temperature sensitive, whereas rpoB77 allows the cells to grow well at 42°C. The interesting observation was that the rpoB12 allele render the cells sensitive to LB devoid of salt at all temperatures. However, cells bearing rpoB77 allele become sensitive to LB devoid of salt only at 42°C. Introduction of wild-type rpoB+ allele into these mutants revealed that both rpoB alleles are dominant over the wild-type allele. The quantification of extent of suppression showed that rpoB12 can suppress the cps::lac expression to ∼50%, while rpoB77 suppresses to ∼70%. It should be noted that ClpYQ has been shown to degrade SulA in phenotypically Alp+ strains and both SulA and RcsA when present in multicopy (Wu et al. 1999; Munavar et al. 2005). Therefore, the involvement of components of proteases like ClpA, ClpB, ClpY, and ClpQ in elicitation of Ces phenotype was studied. The genetic analyses reported herein revealed that these functions do not play any role in the elicitation of Ces phenotype. The peculiar observation was that the introduction of clpA::kan insertion increased the β-galactosidase levels from cps::lac fusion to some extent even in the parental strains SG20780 and SG20781, which gives a clue that ClpA might play a role either in the degradation of RcsA or Cps::Lac fusion protein. However, this view should be treated conjectural till proven by biochemical means.
As β subunit being the very crucial subunit of the essential transcription machinery RNAP, one can imagine that the low-level expression of cps::lac might result from global transcription defect also. However, the expression levels of other candidate genes clearly indicate that perhaps it is not due to global transcription defect but could perhaps be specific to cps::lac. Expression levels of rcsA in these rpoB mutants indicate that the extent of transcription of rcsA indeed goes down in both mutants. Taken together, it is tenable to propose that these mutant RNAPs might be defective at either rcsA or cps promoters. In order to verify this view, the repressor of rcsA viz. HNS was knocked-off and the expression levels of cps::lac in these rpoB hns::kan strains were checked. However, the results reported herein indirectly imply that the RNAPs are not defective per se at rcsA promoter. Moreover, even after the introduction of rcsA+ allele in multiple copies, the cps::lac expression levels in both rpoB12 and rpoB77 mutants were not equivalent to that of the parental lon mutant, suggesting the involvement of hitherto unidentified players/mechanism in the elicitation of Ces phenotype besides rcsA. Studies on HNS and other nucleoid-associated proteins are gaining momentum these days (Atlung and Ingmer 1997; Browning et al. 2010; Rimsky and Travers 2011). It has been reported that HNS preferably binds to a curved region or sometimes binding of HNS itself bends the region, which is considered to be the mode of regulation of certain genes by HNS (Atlung and Ingmer 1997; Shin et al. 2005; Kahramanoglou et al. 2011).
In the case of rcsA, the exact binding sequence for HNS has not been reported so far. From this study, it could be suggested that the regulation of rcsA by HNS should also involve the same bending mechanism. Landick et al. (1990) have reported earlier that when a fast moving RNAP encounters competitors during initiation, it undergoes successive abortive transcription. It could be hypothesized that when a fast moving RNAP with rpoB12 mutation encounters a DNA bending molecules such as HNS, it might undergo abortive transcription which could be the possible explanation for the lower level expression of rcsA. But, results reported herein clearly indicate that both fast and slow moving RNAPs could elicit Ces phenotype. Therefore, bioinformatics analyses were carried out to check the structural change(s) in binding affinity to DNA template in these mutant RpoB subunits with the help of PYMOL software by taking the already available and reported structures as templates (PDB ID 4IGC and 2O5J) (Vassylyev et al. 2007; Murakami 2013). The Figure7A and B compares the structure of the region of wild type and mutant E. coli RpoB subunits corresponding to amino acid residues 507–536. It can be observed that change in amino acid at position 526 from Histidine to Tyrosine in the case of rpoB12 mutant results in change in the polar interaction with the nearby residues compared to the wild-type structure. Similarly, at the position 512, the amino acid Serine got mutated to Tyrosine in the rpoB77 mutant and in this case also there is change in the polar interaction between the nearby residues compared to the wild type. The Figure7C and D show the interaction of the mutant RpoB subunit region of Thermus thermophilus (amino acid range 385–425) with the template DNA–RNA hybrid. It can be clearly seen that, when His526 is present, the atom exposed toward the template DNA–RNA hybrid is Nitrogen that exhibit positive charge which probably has higher affinity toward the DNA–RNA hybrid, but when His526 is changed to Tyr526, the exposed atom is OH which is already negatively charged that might have lesser affinity toward the DNA–RNA hybrid. In the case of rpoB77, the negatively charged cloud is higher compared to the wild type which might result in the lesser affinity of the RNAP with the template DNA. Therefore, we believe that these mutant RNAPs might have lesser affinity to template DNA–RNA hybrid during transcription compared to wild type and thus we propose that in this situation when these mutant RNAPs encounters bending agents like HNS, probably it tends to undergo successive abortive transcription by slipping off from the template.
Figure 7.
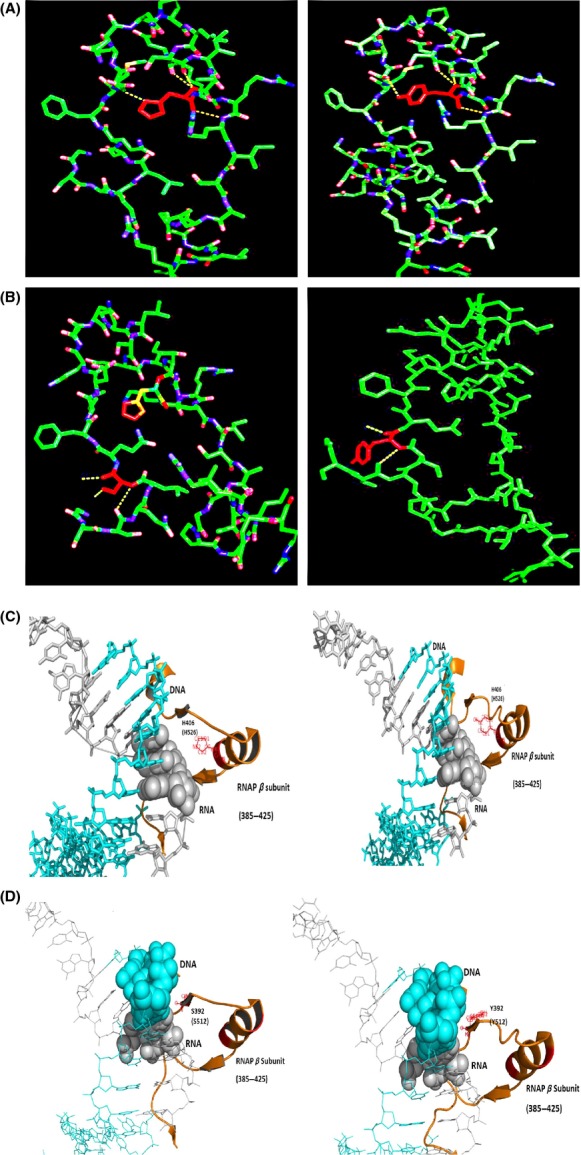
The structural analyses of mutant RpoB region of the rpoB mutants. Comparison of RpoB region (residues 507–563) from the mutant MMR6 (His526 to Tyr526) with that of wild type. (A) Comparison of RpoB region (residues 507–563) from the mutant MMR23 (Ser512 to Tyr512) with that of wild type. (B) Interaction of RpoB region from the MMR6 mutant (residues 385–425 in Thermus thermophilus) with the template DNA–RNA hybrid. (C) Interaction of RpoB region from the MMR23 mutant (residues 385–425 in T. thermophilus) with the template DNA–RNA hybrid. The structural prediction was made with PYMOL software. By taking the available structures in PDB (ID 2O5J and 4IGC) as template, we modeled and predicted the possible differences in the interaction with the DNA–RNA hybrid compared to the wild type. In the case of (A and B), the relevant residues are shown in red color and the yellow dotted line indicates the polar interactions with nearby residues. In the case of (C and D), the orange color represents the β-subunit (residues 385–425 of T. thermophilus, the position in Escherichia coli for the relevant residue is given in brackets). Cyan represents DNA, whereas gray represents RNA (refer text for more details).
To the best of our knowledge, this is perhaps a maiden report in which rif (rpoB) mutations are shown directly to affect expression of cps genes. In this study, although it has been shown that transcription level of rcsA is getting reduced in the case of rpoB12 and rpoB77 mutants, the levels of reduction in rcsA alone could not account for the 50–70% reduction in level of cpsB expression. Therefore, it is believed that in addition to the reduction in the level of rcsA there may be some other unidentified factors/mechanisms which might lead to the low-level synthesis of capsular polysaccharides in these rpoB mutants. Detailed study currently underway in the laboratory might probably help us to understand the actual mechanism by which these rpoB mutations elicit the suppression of capsule expression reported herein.
Acknowledgments
We thank R. Jayaraman for his continued encouragement, concern, and valuable advises, S. Gottesman NCI, NIH, USA for her continued support and concern in our laboratory progress and also for certain strains used in this study, S. Mahadevan, Indian Institute of Science, Bangalore for providing the HNS mutant, R. Harinarayanan, Centre for DNA Finger printing and Diagnostics, Hyderabad, for providing the rpoBC+ clone and rpoB8 mutant HR318, A. Rachid Rahmouni, CNRS, France for providing pλTR1 vector, M. K. Berlyn, CGSC, USA for providing E. coli strains. We thank the past and present Coordinators of School of Biological Sciences especially P. Gunasekaran, G. Marimuthu and G. S. Selvam and Sripati Kandula for their kind support and also for permitting to use the common facilities of SBS. We thank J. Kumaresan, Technical Officer, Department of Molecular Biology for his valuable help. Our special appreciation and thanks go to N. Arul Muthu Kumaran for his help in taking the pictures of plate as well as for proof reading the manuscript and Chandrasekaran for his enormous help in Bioinformatic analyses. We thank B. Singaravelan, T. Ponmani (Sangmyung University, Korea), Shanmugapriya Vinod, S. Vinodha, M. Karthik, S. Ashwin Sri Bala, I. Madhumathi, T. Nagaraj, J. C. Walter Devaa, Vel Murugan, G. Sutharsan (Hebrew University, Isreal), Vivek Raj (U.K.), Rajesh (Australia), and Aathmaja (Germany) for their help and support, S. Poovalingam and P. Jagadeesh for Laboratory errands. We thank the common programs given to School of Biological Sciences by UGC, Govt. of India namely, CEGS, CAS and NRCBS, the IPLS program funded by DBT, Govt. of India and the PURSE program given to MKU funded by DST, Govt. of India for the financial support. Shanmugaraja Meenakshi thanks Council for Scientific and Industrial Research, India for providing Junior and Senior Research Fellowships. We thank the authorities of the University for generously providing us part of the publication charge from the Unassigned Grant of the University.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Physiological characterization of the relevant strains at appropriate conditions. (A) Sequential spotting analyses of the relevant strains showing the growth pattern at 30°C and 42°C on LB agar plates containing appropriate antibiotics. (B) Sequential spotting analyses of the relevant strains showing the growth pattern at 30°C and 42°C on LB agar plates devoid of salt. (C) Sequential spotting analyses of the relevant strains showing the dominant/recessive phenotype on LB agar plates with and without Rifampicin. These experiments were performed more than twice and the pictures given are the representative for each of them (refer text for details).
Table S1. Distribution of Cps::Lac−/MMSR phenotype among spontaneous RifR mutants isolated from SG20780 (Δlon cps::lac).
Table S2. Percentage distribution of Cps::Lac+/− – RifR/S phenotype among KanR transductants obtained in the P1 transductional crosses involving P1 made of CAG18618 bearing KanR (thiC3178::Tn10kan) as donor and RifR mutants of SG20780 as recipients.
References
- Atlung T. Ingmer H. HNS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- Berlyn MK. Linkage map of Escherichia coli K-12, edition 10: the traditional map. Microbiol. Mol. Biol. Rev. 1998;62:814–984. doi: 10.1128/mmbr.62.3.814-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill JA, Quinlan-Walshe C. Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 1988;170:2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning DF, Grainger DC. Busby SJW. Effects of nucleoid-associated proteins on bacterial chromosome structure and gene expression. Curr. Opin. Microbiol. 2010;13:773–780. doi: 10.1016/j.mib.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Chen WP. Kuo TT. A simple and rapid method for the preparation of Gram negative bacterial genomic DNA. Nucleic Acids Res. 1993;21:2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DJ. The Rcs phosphorelay: more than just a two-component pathway. Future Microbiol. 2010;5:1173–1184. doi: 10.2217/fmb.10.83. [DOI] [PubMed] [Google Scholar]
- Demerec M, Adelberg EA, Clark AJ. Hartman PE. A proposal for uniform nomenclature in bacterial genetics. Genetics. 1966;54:61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel W. Trempy JE. Escherichia coli RcsA, a positive activator of colanic acid capsular polysaccharide synthesis, functions to activate its own expression. J. Bacteriol. 1999;181:577–584. doi: 10.1128/jb.181.2.577-584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Genetics of proteolysis in Escherichia coli. Annu. Rev. Genet. 1989;23:163–198. doi: 10.1146/annurev.ge.23.120189.001115. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Maurizi MR. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol. Rev. 1992;56:592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Trisler P. Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J. Bacteriol. 1985;162:1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Roche E, Zhou Y. Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin M, Robichon N, Rahmouni AR. Geiselmann J. A simple polypyrimidine repeat acts as an artificial Rho-dependent terminator in vivo and in vitro. Nucleic Acids Res. 1998;26:4895–4900. doi: 10.1093/nar/26.21.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwani D, Zangoui P. Mahadevan S. The β Glucoside (bgl) operon of Escherichia coli is involved in the regulation of oppA, encoding an oligopeptide transporter. J. Bacteriol. 2012;194:90–99. doi: 10.1128/JB.05837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DJ. Gross CA. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 1988;202:45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Jin DJ. Gross CA. Characterization of the pleiotropic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J. Bacteriol. 1989;171:5229–5231. doi: 10.1128/jb.171.9.5229-5231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DJ, Walter WA. Gross CA. Characterization of the termination phenotypes of rifampicin-resistant mutants. J. Mol. Biol. 1988;202:245–253. doi: 10.1016/0022-2836(88)90455-x. [DOI] [PubMed] [Google Scholar]
- Jin DJ, Burgess RR, Richardson JP. Gross CA. Termination efficiency at rho-dependent terminators depends on kinetic coupling between RNA polymerase and rho. Proc. Natl. Acad. Sci. USA. 1992;89:1453–1457. doi: 10.1073/pnas.89.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahramanoglou C, Seshasayee ASN, Prieto AI, Ibberson D, Schmidt S, Zimmermann J, et al. Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res. 2011;39:2073–2091. doi: 10.1093/nar/gkq934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby JE, Trempy JE. Gottesman S. Excision of a P4-like cryptic prophage leads to Alp protease expression in Escherichia coli. J. Bacteriol. 1994;176:2068–2081. doi: 10.1128/jb.176.7.2068-2081.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MS, Chen KP. Wu WF. Regulation of RcsA by the ClpYQ (HslUV) protease in Escherichia coli. Microbiology. 2004;150:437–446. doi: 10.1099/mic.0.26446-0. [DOI] [PubMed] [Google Scholar]
- Landick R, Stewart J. Lee DN. Amino acid changes in conserved regions of the β-subunit of Escherichia coli RNA polymerase alter transcription pausing and termination. Genes Dev. 1990;4:1623–1636. doi: 10.1101/gad.4.9.1623. [DOI] [PubMed] [Google Scholar]
- Majdalani N. Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 2006;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- Miller JH. A short course in bacterial genetics: a laboratory manual and hand book for Escherichia coli and related bacteria. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Munavar H, Zhou Y. Gottesman S. Analysis of the Escherichia coli Alp phenotype: heat shock induction in ssrA mutants. J. Bacteriol. 2005;187:4739–4751. doi: 10.1128/JB.187.14.4739-4751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami KS. X-ray crystal structure of Escherichia coli RNA polymerase sigma 70 holoenzyme. J. Biol. Chem. 2013;288:9126–9134. doi: 10.1074/jbc.M112.430900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimsky S. Travers A. Pervasive regulation of nucleoid structure and function by nucleoid associated proteins. Current Opinion in Microbiology. 2011;14:136–141. doi: 10.1016/j.mib.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Sambrook J. Russel DW. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sarkar P, Sardesai AA, Murakami KS. Chatterji D. Inactivation of the bacterial RNA polymerase due to acquisition of secondary structure by the omega subunit. J. Biol. Chem. 2013;288:25076–25087. doi: 10.1074/jbc.M113.468520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugapriya V. 2013. Analyses of unorthodox mode of mitomycin C resistance elicited by rif-nal mutations in SOS defective strains of Escherichia coli: allele specificity and key players involved. Ph.D. thesis.
- Shanmughapriya V. Munavar MH. Evidence for involvement of UvrB in elicitation of “SIR”phenotype by rpoB87gyrA87 mutations in lexA3 mutant of Escherichia coli. DNA Repair. 2012;11:915–925. doi: 10.1016/j.dnarep.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Shin M, Song M, Rhee JH, Hong Y, Kim YJ, Seok YJ, et al. DNA looping-mediated repression by histone-like protein H-NS: specific requirement of sigma70 as a cofactor for looping. Genes Dev. 2005;19:2388–2398. doi: 10.1101/gad.1316305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski D. Gottesman S. A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1995;92:2003–2007. doi: 10.1073/pnas.92.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V, Torres-Cabassa A, Maurizi MR, Gutnick D. Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol. 1991;173:1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Cabassa AS. Gottesman S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J. Bacteriol. 1987;169:981–989. doi: 10.1128/jb.169.3.981-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempy JE. Gottesman S. Alp, a suppressor of lon protease mutants in Escherichia coli. J. Bacteriol. 1989;171:3348–3353. doi: 10.1128/jb.171.6.3348-3353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempy JE, Kirby JE. Gottesman S. Alp suppression of lon: dependence on the slpA gene. J. Bacteriol. 1994;176:2061–2067. doi: 10.1128/jb.176.7.2061-2067.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisler P. Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K12. J Bacteriol. 1984;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassylyev DG, Vassylyeva MN, Zhang J, Palangat M, Artsimovitch I. Landick R. Structural basis for substrate loading in bacterial RNA polymerase. Nature. 2007;448:163–168. doi: 10.1038/nature05931. [DOI] [PubMed] [Google Scholar]
- Wickner S, Gottesman S, Skowyra D, Hoskins J, McKenney K. Maurizi MR. A molecular chaperone, ClpA, functions like DnaK and DnaJ. Proc. Natl. Acad. Sci. USA. 1994;91:12218–12222. doi: 10.1073/pnas.91.25.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WF, Zhou Y. Gottesman S. Redundant in vivo proteolytic activities of Escherichia coli Lon and the ClpYQ (HslUV) protease. J. Bacteriol. 1999;181:3681–3687. doi: 10.1128/jb.181.12.3681-3687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Horn V. Rifampin resistance mutations that alter the efficiency of transcription termination at the tryptophan operon attenuator. J. Bacteriol. 1981;145:1334–1341. doi: 10.1128/jb.145.3.1334-1341.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YN. Jin DJ. The rpoB mutants destabilizing initiation complexes at stringently controlled promoters behave like “stringent” RNA polymerases in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1998;95:2908–2913. doi: 10.1073/pnas.95.6.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YN, Lubkowska L, Hui M, Court C, Chen S. Court DL. Isolation and characterization of RNA polymerase rpoB mutations that alter transcription slippage during elongation in Escherichia coli. J. Biol. Chem. 2013;288:2700–2710. doi: 10.1074/jbc.M112.429464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Physiological characterization of the relevant strains at appropriate conditions. (A) Sequential spotting analyses of the relevant strains showing the growth pattern at 30°C and 42°C on LB agar plates containing appropriate antibiotics. (B) Sequential spotting analyses of the relevant strains showing the growth pattern at 30°C and 42°C on LB agar plates devoid of salt. (C) Sequential spotting analyses of the relevant strains showing the dominant/recessive phenotype on LB agar plates with and without Rifampicin. These experiments were performed more than twice and the pictures given are the representative for each of them (refer text for details).
Table S1. Distribution of Cps::Lac−/MMSR phenotype among spontaneous RifR mutants isolated from SG20780 (Δlon cps::lac).
Table S2. Percentage distribution of Cps::Lac+/− – RifR/S phenotype among KanR transductants obtained in the P1 transductional crosses involving P1 made of CAG18618 bearing KanR (thiC3178::Tn10kan) as donor and RifR mutants of SG20780 as recipients.