Abstract
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide. The multityrosine kinase inhibitor sorafenib is used in the therapy of advanced disease. However, the effects of sorafenib are limited, and combination treatments aiming at improved survival are encouraged. The sphingosine analog FTY720 (Fingolimod), which is approved for treatment of multiple sclerosis, has shown tumor suppressive effects in cell lines and animal models of HCC. In the present study, we combined sorafenib with FTY720 in order to sensitize the HCC cell lines Huh7 and HepG2 to sorafenib treatment. Using the XTT assay we show that noncytotoxic doses of FTY720 synergistically enhanced the decrease in viability caused by treatment of both cell lines with increasing doses of sorafenib. Further studies in Huh7 revealed that combined treatment with FTY720 and sorafenib resulted in G1 arrest and enhanced cell death measured using flow cytometry analysis of cells labeled with propidium iodide (PI)/Annexin-V and PI and 4′,6-diamidino-2-phenylindole-staining of nuclei. In addition, signs of both caspase-dependent and – independent apoptosis were observed, as cotreatment with FTY720 and sorafenib caused cytochrome c release and poly-ADP ribose polymerase-cleavage as well as translocation of Apoptosis-inducing factor into the cytosol. We also detected features of autophagy blockage, as the protein levels of LC3-II and p62 were affected by combined treatment with FTY720 and sorafenib. Together, our results suggest that FTY720 sensitizes HCC cells to cytotoxic effects induced by treatment with sorafenib alone. These findings warrant further investigations of combined treatment with sorafenib and FTY720 in vivo in order to develop more effective treatment of HCC.
Keywords: Cytotoxicity, FTY720, hepatocellular carcinoma, sensitization, sorafenib
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and the third cause of cancer-related mortality worldwide (Jemal et al. 2011). HCC is generally diagnosed at a late stage, and the majority of HCC patients die within 1 year after diagnosis. The only curative treatment options are resection and transplantation, but recurrence rates are high (Forner et al. 2012). HCC is highly resistant to conventional chemotherapy, and targeted therapy with the multi-kinase inhibitor sorafenib was a breakthrough in the treatment of unresectable, advanced HCC (Llovet et al. 2007). Sorafenib blocks tumor cell proliferation and angiogenesis mainly by targeting Raf kinases and vascular endothelial growth factor (VEGF)/platelet-derived growth factor (PDGF) receptor tyrosine kinases (Wilhelm et al. 2004; Liu et al. 2006). Based on the results of a large randomized phase III study, sorafenib is presently the standard systemic therapy for HCC patients with advanced disease, whereas other targeted therapies for treatment of HCC are under development (Worns and Galle 2010). However, the improved median survival of HCC patients treated with sorafenib is limited to 3 months (Llovet et al. 2007), and therefore combination therapies with sorafenib were encouraged in the future clinical trial design for HCC (Llovet et al. 2008). Aiming at prolonged survival, both preclinical (Chen et al. 2014; Zhang et al. 2014) and clinical studies (Abdel-Rahman and Fouad 2014) of sorafenib-based combination treatments have been performed.
There is accumulating evidence for the involvement of sphingosine 1-phosphate (S1P) and sphingosine kinase 1 (SK1) in cancer (Pyne and Pyne 2010). SK1 converts tumor suppressive sphingosine to S1P, which promotes proliferation, migration, angiogenesis and metastasis. The sphingosine analog FTY720 (Fingolimod, Gilenya™ Stein, Switzerland) is an immunosuppressant that has recently been approved by the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of relapsing multiple sclerosis. In addition to its immunomodulatory effects, FTY720 acts as a competitive inhibitor to SK1 and targets the enzyme for degradation (Tonelli et al. 2010), thereby preventing the conversion of sphingosine to S1P. Sphingosine kinase 2 converts FTY720 to (S)-FTY720 phosphate, which inhibits the effects of S1P signaling by acting directly as a functional antagonist to the S1P1 receptor (Brinkmann et al. 2002). FTY720 also exerts additional S1P receptor-independent antitumor effects (Brinkmann et al. 2001; Pyne and Pyne 2010; Pitman et al. 2012). Combined treatment with sorafenib and a synthetic inhibitor to SK2 has earlier shown antitumor activity in HCC xenografts (Beljanski et al. 2011).
FTY720 has shown antitumor and antiangiogenic effects in animal models of breast cancer, prostate cancer, lung cancer and malignant melanoma (Azuma et al. 2002; Chua et al. 2005; Schmid et al. 2005; LaMontagne et al. 2006; Zhou et al. 2006; Pereira et al. 2013; Saddoughi et al. 2013). Moreover, FTY720 has been shown to selectively induce cytotoxicity in hepatoma cell lines, and to suppress tumor growth with no apparent side-effects in a mouse model of HCC (Lee et al. 2004). Whereas the cytostatic and antiangiogenic effects of sorafenib counteract tumor development, sorafenib has also been shown to promote tumor invasiveness and metastasis in a mouse model of HCC (Zhang et al. 2012). In contrast, treatment with FTY720 suppressed liver tumor metastasis after liver transplantation and resection in rat models of HCC (Ushitora et al. 2009; Li et al. 2012). FTY720 has also been shown to enhance the effects of chemotherapeutic treatment of cell lines and animal models representing breast cancer, lymphoma, neuroblastoma, and prostate cancer cells (Pchejetski et al. 2010; Alinari et al. 2011; Mousseau et al. 2012; Li et al. 2013; Marvaso et al. 2014). In view of these observations, we hypothesized that sorafenib and FTY720 could act synergistically to suppress survival and growth of HCC cells, and therefore investigated the cytotoxic effects of combined treatment of these two compounds on HCC cell lines.
Materials and Methods
Sorafenib tosylate (BAY 54-9085) was provided by Bayer HealthCare Pharmaceuticals (Montville, NJ), FTY720 (Fingolimod) was purchased from Cayman Chemical Ann Arbor, Michigan, USA. (MI). Stock solutions of 100 mmol/L sorafenib and of 20 mmol/L FTY720 dissolved in Dimethyl sulfoxide (DMSO) were aliquoted and deep-frozen. Primary antibodies against poly-ADP ribose polymerase (PARP) and light chain 3II (LC3II) were from Cell Signaling (Cell Signaling Technology Inc., Danvers, MA). p62 from Sigma-Aldrich LLC. (St. Louis, MO), Apoptosis-inducing factor (AIF) from Santa Cruz Biotechnology, Inc. (Texas, USA), cytochrome c oxidase (Cox IV) from Novus Biologicals, LLC, (Littleton, Colorado, USA), Cytochrome c (Cyt c) from BD Bioscience (San Jose, CA), α-Tubulin and β-Actin from Sigma-Aldrich Co. LLC. were used. Secondary fluorescent antibodies, goat anti-rabbit and goat anti-mouse, were purchased from LI-COR® (Nebraska, USA). Cell culture medium and supplements were purchased from Invitrogen (Eugene, OR).
Cell culture
The human hepatocellular-carcinoma cell line Huh7 was purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) and HepG2 cell line was purchased from ATCC. Huh7 cells were cultured in Dulbecco's modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum, penicillin 100 U/mL and streptomycin 100 μg/mL at 37°C in an atmosphere containing 5% CO2, whereas HepG2 cells were cultured in DMEM supplemented with 10% fetal bovine serum at 37°C in an atmosphere containing 5% CO2.
Viability assay
Viability/cytotoxicity caused by sorafenib and FTY720 was determined using the cell proliferation kit II ((2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetra-zolium-5-Carboxanilide) XTT) from Roche Diagnostics (Basel, Switzerland) according to the manufacturer's instructions. The cells were seeded at a density of 10 × 103, 6 × 103 or 5 × 103 cells per well for Huh7 cell line and 15 × 103, 10 × 103 or 8 × 103 cells per well for HepG2 cell line in 96-well plates depending on the treatment time and allowed to adhere overnight. Subsequently the cells were treated for 24, 48 and 72 h with different doses of sorafenib alone or in combination with FTY720 or vehicle (0.04% DMSO). In the final step they were incubated with the XTT solution for two hours. The optical density reflecting viable cells was obtained by measuring the absorbance at 470 nm and subtracting the background absorbance at 750 nm. Dose–response curves were constructed for treatment with FTY720. The least cytotoxic dose, which had a marginal effect on the viability of cells when used as a single agent, was determined from two individual experiments by comparing the top and bottom plateaus of the dose–response curve. In order to study sensitization of HCC cells to treatment with sorafenib, this dose was used in the subsequent experiments.
Cell death analysis by flow cytrometry
The Annexin-V-FLUOS Staining Kit, ROCHE Diagnostics GmbH (Mannheim, Germany) was used to distinguish between viable and dead cells following treatment with sorafenib in the presence or absence of FTY720. Huh7 cells were seeded at a density of 8.0 × 105 in 25 cm2 flasks and allowed to adhere overnight. Thereafter, the cells were treated for 6, 12, or 24 h with different doses of sorafenib/sorafenib and FTY720 or DMSO as indicated in the Results section. Cells were harvested and labeled with annexin-V and propidium iodide (PI) according to the manufacturer's instruction and analyzed by flow cytometry in BD FACS caliber (BD Bioscience).
Cell cycle analysis by flow cytrometry
The BD Cycletest Plus reagent kit, BD Biosciences was used to determine the cell cycle distribution and sub G1 fractions. Huh7 cells were seeded at the densities of 4.0 × 105, 4.8 × 105 or 8.0 × 105 cells in 25 cm2 flasks depending on the treatment time and allowed to adhere overnight. Subsequently the cells were treated for 24 and 48 h with different doses of sorafenib alone or in combination with FTY720 or vehicle (0.04% DMSO). The cells were harvested and stained with PI according to the manufacturer's instruction. Cell cycle distribution was analyzed using BD LSRIl (BD Bioscience) and the data were analyzed using the ModFit LT v3.3, (BD Bioscence).
Western blot analysis
Whole cell protein was extracted from the Huh7 cells, using NP-40 lysis buffer (Invitrogen) containing 50 mmol/L Tris, 250 mmol/L NaCl, 5 mmol/L Ethylenediaminetetraacetic acid (EDTA), 50 mmol/L NaF, 1 mmol/L Na3VO4, 1% NP-40 and 0.02% NaN3, to which protease inhibitor cocktail p8340 had been added. Protein concentration was measured using Biorad DC protein concentration determination (Bio-Rad Laboratories Inc., Berkeley, California, USA) and 100 μg of total protein was separated with Nu-PAGE (Invitrogen) using gels of different concentrations depending on the size of the protein of interest. Proteins were transferred to either PVDF (Immobilon®-P; Millipore, Billerica, MA) or to nitrocellulose membranes (Thermo Scientific, Waltham, Massachusetts, USA) at 33 mAmp for 1 h using a semidry blotter HoeferTM TE 70 (Amersham Biosciences Corp. New Jersey, USA). The membranes were blocked in 5% non-fat milk solution in Phosphate-buffered saline (PBS)-T (0, 1% Tween 20) at room temperature for 1 h before incubation with individual primary antibodies at 4°C over-night. The membranes were washed for 6 × 5 min with PBS-T followed by 1 h incubation with HRP/Fluorescence-conjugated secondary antibodies at room temperature. HRP-conjugated antibodies were detected using Supersignal west Femto Maximum Sensitivity Substrate (Thermo Scientific) whereas Fluorescence conjugated antibodies were imaged directly using LICOR imaging system (LI-COR Biosciences, Ltd., Nebraska, USA). Relative density from western blots was calculated using ImageJ 1.48v software, background was subtracted from individual bands.
Confocal microscopy
Huh7 cells were grown on sterile coverslips in six-well plates at the density of 3.0 × 105 cells overnight. Cells were then treated by different doses of sorafenib in the presence or absence of FTY720 for 24 h. Thereafter, the cells were fixed with ice-cold methanol, permeabilized with 3:1 ethanol: acetic acid solution. Cells were blocked with 5% BSA in PBS for 1 h at room temperature. Preparations were mounted on slides using mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc. Burlingame, CA) and examined with a confocal microscope (Nikon Confocal Microscope; Nikon Corporation, Tokyo, Japan).
Cytosolic and mitochondrial fraction
Harvested Huh7 cells (2 × 106) were washed in ice-cold PBS and incubated for 25 min on ice with 300 μL of ice cold buffer A (250 mmol/L Sucrose, 20 mmol/L (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) HEPES-KOH (pH 7.4), 10 mmol/L KCl, 1 mmol/L Na-EGTA, 1 mmol/L Na-EDTA, 1.5 mmol/L MgCl2, 1 mmol/L dithiothreitol (DTT), 0.1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and cocktail of protease inhibitors [Sigma-Aldrich Co. LLC.]). Cells were then lysed with 30 strokes using a syringe fitted with a needle (22 gauges and 30 mm) and centrifuged at 4°C (600 g). The supernatant was centrifuged at 21,000g for 10 min. The supernatant (cytosol) was saved at −20°C and the pellet (mitochondria) was incubated for 20 min on ice in 100 μL of buffer B (50 mmol/L HEPES (pH 7.4), 1% Nonidet P-40 (NP-40), 10% (v/v) glycerol, 1 mmol/L Na-EDTA, 2 mmol/L DTT, 0.1 mmol/L PMSF, and cocktail of protease inhibitors [Sigma-Aldrich Co. LLC.]). Samples were centrifuged at 21,000g for 15 min, the supernatant containing mitochondrial proteins was stored at −20°C. 40 μg protein of each fraction were electrophoresed in 4–10% precasted SDS-PAGE gel (Thermo Scientific) prior to immunoblotting (see previous section).
Statistical analyses
Paired T-test was performed to assess the difference in viability between cells treated with sorafenib alone and with sorafenib and 8 μmol/L or 2 μmol/L of FTY720. Differences in cell death and cell cycle distribution between groups were analyzed by analysis of variance, with the least significance difference (LSD) as post hoc. *P < 0.05, **P < 0.005, ***P < 0.0005.
Results
Increased cytotoxicity after combined treatment with sorafenib and FTY720
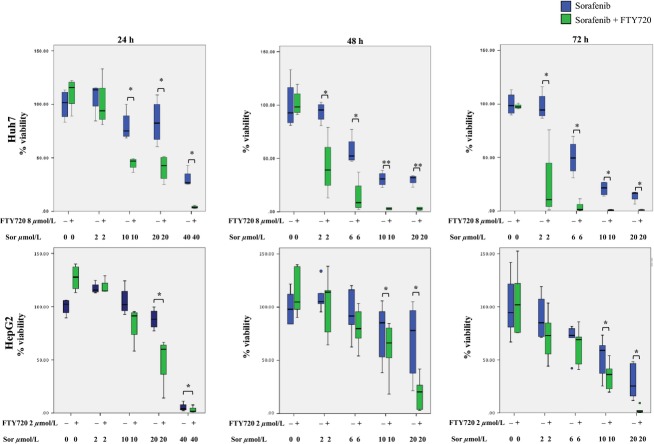
FTY720 has earlier shown cytotoxicity toward HCC cells (Lee et al. 2004; Ushitora et al. 2009; Li et al. 2012). In order to investigate whether FTY720 could sensitize HCC cells to treatment with sorafenib, we initially titrated the dose of FTY720 using the XTT assay as an estimation of viability. The least cytotoxic dose for FTY720 was determined to 8 and 2 μmol/L for Huh7 and HepG2, respectively (see Materials and Methods). The decreased viability induced by treatment of HCC cells with increasing doses of sorafenib for 24, 48 or 72 h was significantly enhanced after cotreatment of Huh7 and HepG2 with 8 μmol/L or 2 μmol/L of FTY720 respectively, Figure1. The effect increased over time and was more pronounced in Huh7 as compared to HepG2. These results indicate that FTY720 sensitizes HCC cells to cytotoxicity induced by sorafenib.
Figure 1.
Cell viability following combined treatment with sorafenib and FTY720. Huh7 and HepG2 cells were treated with increasing doses of sorafenib in the presence or absence of 8 or 2 μmol/L of FTY720, respectively for 24, 48 or 72 h, where after XTT assay was performed. The graphs are based on at least four individual experiments. The boxes represent the interquartile range (IQR) for each treatment, the bands represent the median, while the whiskers represent the highest and lowest values, respectively, that are not considered outliers. Outliers that are 1.5× the IQR or more are presented as circles. The paired T-test was performed to assess the difference in viability between cells treated with sorafenib alone and with sorafenib and 8 or 2 μmol/L of FTY720. *P < 0.05, **P < 0.005.
Effects on cell cycle distribution following combined treatment with sorafenib and FTY720
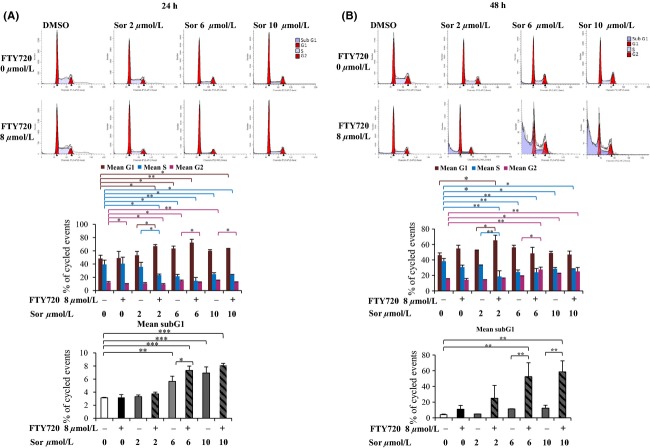
In order to examine the mechanisms behind the enhanced cytotoxicity following combined treatment, further studies were performed in the Huh7 cell line, which, similarly to a majority of primary HCC, and in contrast to HepG2, harbor mutated p53 (Bressac et al. 1990). To investigate whether the cell cycle distribution was affected by the combined treatment, Huh7 cells were treated with sorafenib in the presence or absence of FTY720 for 24 or 48 h. After both 24 and 48 h, combined treatment with 2 μmol/L of sorafenib and 8 μmol/L of FTY720 caused a significant accumulation of cells in the G1 phase and a concomitant decrease of the number of cells in the S phase compared to treatment with sorafenib alone, Figure2. In contrast, at higher doses of sorafenib, cotreatment with 8 μmol/L of FTY720 caused a significant decrease or increase of cells in the G2 phase after 24 or 48 h, respectively. At the latter time point, FTY720 also significantly increased the sub G1 fraction, representing dead cells, observed after treatment with 6 μmol/L or 10 μmol/L doses of sorafenib alone, Figure2.
Figure 2.
Cell cycle distribution following combined treatment with sorafenib and FTY720. Huh7 cells were treated with sorafenib in the absence or presence of 8 μmol/L FTY720 and stained with propidium iodide prior to analysis by flow cytometry for (A) 24 h or (B) 48 h. Cytograms show one representative experiment out of two (upper part). The columns in the histograms (lower part) show mean of two individual experiments, error bars represent standard deviation. Differences between groups were analyzed by analysis of variance, with the least significance difference as post hoc. *P < 0.05, **P < 0.005, ***P < 0.0005.
Together, the cell cycle analysis shows synergistic effects of combined treatment with FTY720 and sorafenib on both cell cycle progression and cell death.
Induction of apoptosis after combined treatment with sorafenib and FTY720
To further investigate the mechanisms behind the cytotoxic effect of combined treatment with sorafenib and FTY720, early signs of apoptosis were assessed by flow cytometry analysis of cells labeled with Annexin-V-Fluos and PI. A significant increase in presumably apoptotic, secondary necrotic and necrotic cells in the lower right, upper right and upper left quadrants, respectively, was observed after combined treatment with 10 μmol/L of sorafenib and 8 μmol/L of FTY720 as compared to treatment with sorafenib alone for 6 hours, Figure3. Condensation of nuclei was observed after treatment with 10 and 20 μmol/L of sorafenib for 24 h, and was further enhanced after cotreatment with 8 μmol/L of FTY, Figure4A. In order to investigate different apoptotic pathways, cleaved PARP and cytosolic AIF, markers for caspase-dependent and -independent apoptosis, respectively, were analyzed by western blotting. Treatment with a high dose of sorafenib (20 μmol/L) for 24 h caused cleavage of PARP in the absence of FTY720, whereas lower doses did not, Figure4B. The ratio of cleaved PARP increased when the cells were treated with 20 μmol/L of sorafenib together with 8 μmol/L of FTY720. Furthermore, a weak band representing cleaved PARP was observed when 10 μmol/L of sorafenib was combined with 8 μmol/L of FTY720. Treatment with either 2 or 10 μmol/L of sorafenib or 8 μmol/L of FTY720 alone for 24 h induced a slight release of AIF into the cytosol, which was further increased after combination treatment, Figure4C. The purity of the mitochondrial and cytosolic fractions was confirmed using the mitochondrial and cytoskeletal markers COX IV and β-actin, respectively. The expression of COX IV, declined after treatment with 10 μmol/L sorafenib ±8 μmol/L FTY720. The role of mitochondria-mediated apoptosis was supported by release of Cyt c into the cytosol. Taken together, these results indicate that the induction of cell death by combined treatment with sorafenib and FTY720 could partly be attributed to mitochondria-mediated apoptosis in both caspase-independent and, at higher doses of sorafenib, caspase-dependent manners.
Figure 3.
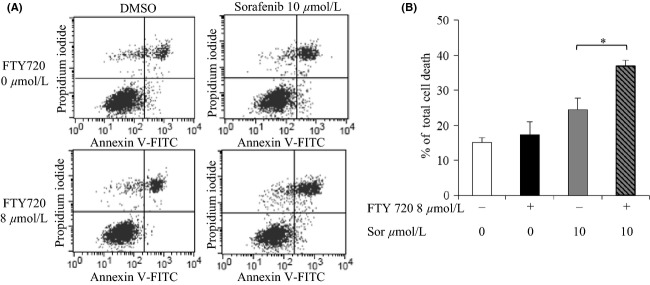
Induction of cell death following treatment with sorafenib and FTY720. Huh7 cells were exposed to 10 μmol/L of sorafenib and/or 8 μmol/L of FTY720 for 6 h and stained with FITC-Annexin V and propidium iodide (PI) prior to analysis by flow cytometry. (A) Cytograms show one representative experiment out of two, the percentages show unaffected cells which are negative for both stains (lower left quadrant), early apoptotic cells stained with Annexin V (lower right quadrant), late apoptotic cells stained with both Annexin V and PI (upper right quadrant) and necrotic cells stained with only PI (upper left quadrant). (B) Histogram showing total percentage of apoptotic and necrotic cells which were stained with Annexin V, PI or both, mean of two individual experiments. Error bars represent standard deviation. Differences between groups were analyzed by analysis of variance, with the least significance difference as post hoc. *P < 0.05.
Figure 4.
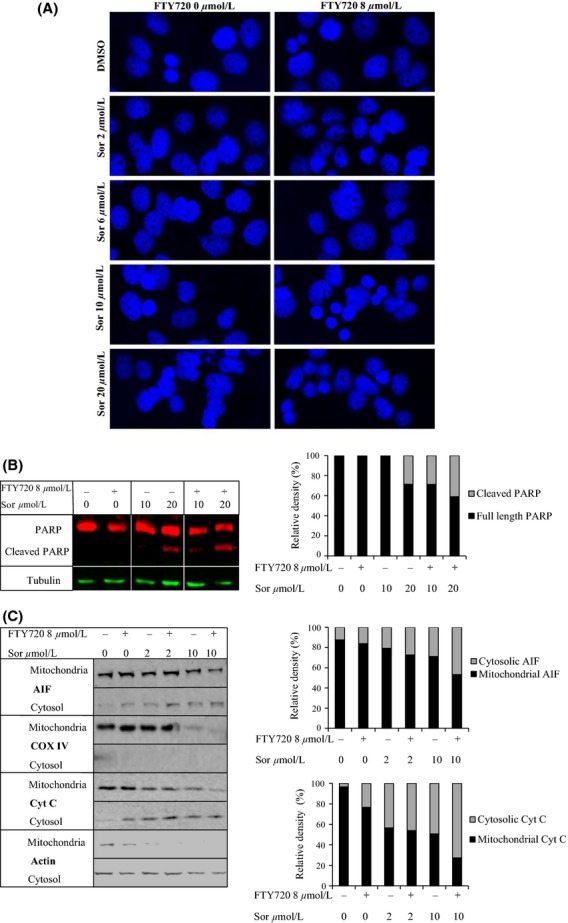
Apoptosis following combined treatment with sorafenib and FTY720. (A) Huh7 cells were treated with increasing doses of sorafenib in the presence or absence of 8 μmol/L FTY720 for 24 h, fixed with ice-cold methanol and stained with 4′,6-diamidino-2-phenylindole (DAPI) prior to visualization of nuclei (in blue) by confocal microscopy. (B) Western blot showing poly-ADP ribose polymerase (PARP) cleavage as an indicator of caspase-mediated apoptosis following treatment with 10 or 20 μmol/L of sorafenib in the presence or absence of 8 μmol/L FTY720. Tubulin was used as a loading control. In the histogram relative density was used to calculate the percentage of cleaved PARP compared to total PARP. (C) Western blots showing translocation of apoptosis-inducing factor (AIF) from mitochondria into the cytosol as an indicator of caspase-independent apoptosis. Huh7 cells were treated with 2 or 10 μmol/L of sorafenib in the presence or absence of 8 μmol/L FTY720 for 24 h, fractionated into mitochondrial and cytosolic compartments and analyzed for the protein levels of AIF and cytochrome c. In the histogram, relative density was used to calculate the percentages of cytosolic and mitochondrial fractions for both proteins. β-actin was used as cytoskeletal and COX IV as mitochondrial control. In all cases, one of two individual experiments is shown.
Effect of combined treatment with sorafenib and FTY720 on proteins involved in autophagy
The earlier observed opposing effects of sorafenib and FTY720 on autophagy directed us to investigate hallmarks of this cellular process following combined treatment of Huh7 cells with sorafenib and FTY720. Treatment with high doses of sorafenib alone caused increased levels of LC3II protein concurrently with decreased p62 protein levels, Figure5. In contrast, an increase in both LC3II and p62 protein levels was observed following treatment with FTY720 alone. Cotreatment with FTY720 further increased the induction of LC3II protein observed after treatment with sorafenib alone, and attenuated the sorafenib-induced decrease in p62 protein levels. These results indicate that sorafenib may induce autophagosome formation, whereas FTY720 may inhibit the fusion of autophagosomes with lysosomes.
Figure 5.
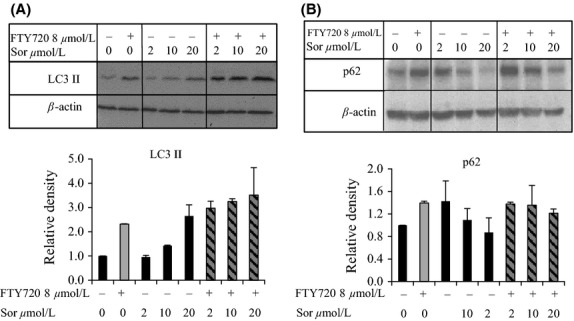
Western blots showing the levels of autophagic proteins following cotreatment with sorafenib and FTY720. Huh7 cells were treated with increasing doses of sorafenib with or without 8 μmol/L FTY720 for 24 h. Protein levels of (A) LC3-ll and (B) p62 are shown in the blot and in the histograms. Relative densities from two different experiments are presented in the histograms. Values are normalized against vehicle control as well as β-actin. Error bars represent standard deviation.
Discussion
At present, sorafenib is the standard systemic therapy for treatment of patients with advanced HCC. Combination of sorafenib with compounds that target alternate mechanisms of action may lead to new therapeutic options resulting in decreased risk for metastasis and increased survival. Among the combination therapies undergoing clinical trials (Villanueva and Llovet 2011), analogs of bioactive sphingolipids are currently not being tested in combination with sorafenib, despite their suggested potential as anticancer agents (Pyne et al. 2011; Delgado et al. 2012). In this study, we have investigated the effects of combined treatment of HCC cells with sorafenib and the sphingosine analog FTY720, which is approved for treatment of relapsing multiple sclerosis. We have presently shown that the sphingosine analog FTY720 sensitized HCC cells to treatment with sorafenib. At doses of FTY720 that itself showed no cytotoxicity, cotreatment with sorafenib significantly increased the effects on viability, cell cycle progression and cell death observed after treatment with sorafenib alone. Furthermore, whereas sorafenib alone has been shown to favor cancer cell survival by inducing autophagy (Shi et al. 2011; Shimizu et al. 2012), the autophagic process appeared to be blocked by cotreatment with FTY720.
Both FTY720 and sorafenib have earlier been shown to induce arrest in the G1 phase of the cell cycle in human cancer cells of various origins including HCC (Permpongkosol et al. 2002; Lee et al. 2004; Huether et al. 2007; Zheng et al. 2010; Marvaso et al. 2014). The relatively low dose of FTY720 used in this study had little effect on the cell cycle progression. However, it sensitized the Huh7 cells to treatment with sorafenib, as combined treatment with FTY720 and sorafenib caused significant accumulation of cells in the G1 phase at a dose of sorafenib that alone had no effect. At higher doses of sorafenib, combined treatment also caused a significant increase in the sub G1 fraction, indicating cell death. Earlier studies have shown induction of both apoptosis and necrosis in response to FTY720 treatment (Zhang et al. 2010; Pitman et al. 2012). We observed a significant synergistic increase in the fraction of apoptotic and necrotic cells after combined treatment with sorafenib and FTY720 for six hours. Similarly to earlier observations (Hung et al. 2008), no PARP-cleavage was observed following treatment with 8 μmol/L of FTY720 for 24 h. However, the ratio of cleaved PARP resulting from treatment with sorafenib was increased by cotreatment with FTY720. The relatively high doses of sorafenib required for PARP cleavage are in accordance with earlier observations of sorafenib-induced apoptosis (Yu et al. 2005). This result raised the possibility that caspase-independent apoptosis may have contributed to the cell death observed by detection of Annexin-V and PI. Our results support a modest induction of caspase-independent apoptosis, as a partial release of AIF into the cytosol was observed, whereas a fraction of AIF remained in the mitochondria after treatment of Huh7 cells with sorafenib and FTY720. The observed release of AIF into the cytosol might have triggered the extramitochondrial release of cyt c as demonstrated earlier (Loeffler et al. 2001; Liu et al. 2004).
Treatment with sorafenib has been shown to induce autophagy in HCC cells (Tai et al. 2013), and involvement of autophagy in acquired resistance to sorafenib in HCC cell lines has been demonstrated (Zhai et al. 2014). As autophagy counteracts the anti-proliferative effects of sorafenib, we investigated the effects of combined treatment with sorafenib and FTY720. Different studies have reported either induction (Zhang et al. 2010; Wallington-Beddoe et al. 2011; Liao et al. 2012) or blockage of autophagy by treatment of cancer cells with FTY720, the latter leading to enhanced effect of milatuzumab in mantle cell lymphoma (Alinari et al. 2012). During cytoprotective autophagy, the p62 protein levels decrease due to degradation in the autolysosome, whereas increased p62 levels may reflect a blockage in autophagy. Our results indicate that treatment with sorafenib alone induced autophagy in Huh7, as increasing doses of sorafenib caused a gradual slight increase in LC3II protein and concomitant decrease in p62 protein levels. In contrast, we observed higher protein levels of both LC3II and p62 following combined treatment with sorafenib and FTY720, indicating that FTY720 protects both proteins from degradation, possibly through blocking the conversion of autophagosomes to autolysosomes. Inhibition of autophagy with chloroquine has earlier been shown to potentiate sorafenib-induced apoptosis in vitro and antitumor effects in vivo in HCC cell lines and Huh7 xenografts, respectively (Shi et al. 2011; Shimizu et al. 2012). In line with these observations, inhibition of autophagy by FTY720 may underlie our observed synergistic effects on cell cycle arrest and cell death in response to combined treatment with sorafenib and FTY720.
In conclusion, we have shown that FTY720 enhanced the decrease in viability observed after treatment of Huh7 and HepG2 cells with sorafenib alone. Combined treatment of Huh7 cells with sorafenib and FTY720 generated synergistic effects on cell cycle arrest and apoptosis, possibly through blockage of autophagy. Our results suggest that FTY720 sensitizes HCC cells to sorafenib, and enhances its cytotoxic effects. This encourages further studies aiming at combinatorial use of FTY720 and sorafenib, both of which are in clinical use, in order to improve the outcome of HCC treatment.
Acknowledgments
We acknowledge Bayer HealthCare Pharmaceuticals for providing us with sorafenib.
Glossary
- HCC
hepatocellular carcinoma
- S1P
sphingosine 1-phosphate
- PARP
poly-ADP ribose polymerase
- LC3II
light chain 3II
- AIF
apoptosis-inducing factor
- Cox IV
cytochrome c oxidase
- PI
propidium iodide
- DAPI
4′,6-diamidino-2-phenylindole
- DTT
dithiothreitol
Author Contributions
J. Flygare, P.J. de Verdier and D. Ahmed designed the research study, D. Ahmed and O. Lunqe performed the experiments, P. Stål contributed with clinical expertise and experimental design, C. Ryk and D. Ahmed performed statistical data analysis, D. Ahmed and J. Flygare wrote the paper, and P. de Verdier, P. Stål, and C. Ryk critically revised the manuscript.
Disclosures
None to declare.
References
- Abdel-Rahman O, Fouad M. Sorafenib-based combination as a first line treatment for advanced hepatocellular carcinoma: a systematic review of the literature. Crit Rev Oncol Hematol. 2014;91:1–8. doi: 10.1016/j.critrevonc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893–6903. doi: 10.1182/blood-2011-06-363879. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alinari L, Baiocchi RA, Praetorius-Ibba M. FTY720-induced blockage of autophagy enhances anticancer efficacy of milatuzumab in mantle cell lymphoma: is FTY720 the next autophagy-blocking agent in lymphoma treatment? Autophagy. 2012;8:416–417. doi: 10.4161/auto.19050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y, Otsuki Y. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62:1410–1419. , et al. ( [PubMed] [Google Scholar]
- Beljanski V, Lewis CS, Smith CD. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol Ther. 2011;11:524–534. doi: 10.4161/cbt.11.5.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressac B, Galvin KM, Liang TJ, Isselbacher KJ, Wands JR, Ozturk M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:1973–1977. doi: 10.1073/pnas.87.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Wilt C, Kristofic C, Nikolova Z, Hof RP, Chen S. FTY720: dissection of membrane receptor-operated, stereospecific effects on cell migration from receptor-independent antiproliferative and apoptotic effects. Transpl Proc. 2001;33:3078–3080. doi: 10.1016/s0041-1345(01)02312-0. , et al. ( [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. , et al. ( [DOI] [PubMed] [Google Scholar]
- Chen CH, Chen MC, Wang JC, Tsai AC, Chen CS, Liou JP. Synergistic interaction between the HDAC inhibitor, MPT0E028, and sorafenib in liver cancer cells in vitro and in vivo. Clini Cancer Res. 2014;20:1274–1287. doi: 10.1158/1078-0432.CCR-12-3909. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CW, Lee DT, Ling MT, Zhou C, Man K, Ho J. FTY720, a fungus metabolite, inhibits in vivo growth of androgen-independent prostate cancer. Int J Cancer. 2005;117:1039–1048. doi: 10.1002/ijc.21243. , et al. ( [DOI] [PubMed] [Google Scholar]
- Delgado A, Fabrias G, Bedia C, Casas J, Abad JL. Sphingolipid modulation: a strategy for cancer therapy. Anti-Cancer Agents Med Chem. 2012;12:285–302. doi: 10.2174/187152012800228643. [DOI] [PubMed] [Google Scholar]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- Huether A, Hopfner M, Baradari V, Schuppan D, Scherubl H. Sorafenib alone or as combination therapy for growth control of cholangiocarcinoma. Biochem Pharmacol. 2007;73:1308–1317. doi: 10.1016/j.bcp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Hung JH, Lu YS, Wang YC, Ma YH, Wang DS, Kulp SK. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res. 2008;68:1204–1212. doi: 10.1158/0008-5472.CAN-07-2621. , et al. ( [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- LaMontagne K, Littlewood-Evans A, Schnell C, O'Reilly T, Wyder L, Sanchez T. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–231. doi: 10.1158/0008-5472.CAN-05-2001. , et al. ( [DOI] [PubMed] [Google Scholar]
- Lee TK, Man K, Ho JW, Sun CK, Ng KT, Wang XH. FTY720 induces apoptosis of human hepatoma cell lines through PI3-K-mediated Akt dephosphorylation. Carcinogenesis. 2004;25:2397–2405. doi: 10.1093/carcin/bgh250. , et al. ( [DOI] [PubMed] [Google Scholar]
- Li CX, Shao Y, Ng KT, Liu XB, Ling CC, Ma YY. FTY720 suppresses liver tumor metastasis by reducing the population of circulating endothelial progenitor cells. PLoS ONE. 2012;7:e32380. doi: 10.1371/journal.pone.0032380. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Hla T, Ferrer F. FTY720 inhibits tumor growth and enhances the tumor-suppressive effect of topotecan in neuroblastoma by interfering with the sphingolipid signaling pathway. Pediatr Blood Cancer. 2013;60:1418–1423. doi: 10.1002/pbc.24564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao A, Hu R, Zhao Q, Li J, Li Y, Yao K. Autophagy induced by FTY720 promotes apoptosis in U266 cells. Eur J Pharm Sci. 2012;45:600–605. doi: 10.1016/j.ejps.2011.12.014. , et al. ( [DOI] [PubMed] [Google Scholar]
- Liu T, Brouha B, Grossman D. Rapid induction of mitochondrial events and caspase-independent apoptosis in Survivin-targeted melanoma cells. Oncogene. 2004;23:39–48. doi: 10.1038/sj.onc.1206978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. , et al. ( [DOI] [PubMed] [Google Scholar]
- Llovet J, Mazzaferro V, Ricci S, Hilgard P, Raoul J, Zeuzem S. Sorafenib improves survival in a large multi-center, randomized, placebo-controlled phase III trial in patients with hepatocellular carcinoma. Ejc Suppl. 2007;5:261–261. , et al. ( [Google Scholar]
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. , et al. ( [DOI] [PubMed] [Google Scholar]
- Loeffler M, Daugas E, Susin SA, Zamzami N, Metivier D, Nieminen AL. Dominant cell death induction by extramitochondrially targeted apoptosis-inducing factor. FASEB J. 2001;15:758–767. doi: 10.1096/fj.00-0388com. , et al. ( [DOI] [PubMed] [Google Scholar]
- Marvaso G, Barone A, Amodio N, Raimondi L, Agosti V, Altomare E. Sphingosine analog fingolimod (FTY720) increases radiation sensitivity of human breast cancer cells in vitro. Cancer Biol Ther. 2014;15:797–805. doi: 10.4161/cbt.28556. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau Y, Mollard S, Faucher-Durand K, Richard L, Nizou A, Cook-Moreau J. Fingolimod potentiates the effects of sunitinib malate in a rat breast cancer model. Breast Cancer Res Treat. 2012;134:31–40. doi: 10.1007/s10549-011-1903-6. , et al. ( [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, Golzio M. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010;70:8651–8661. doi: 10.1158/0008-5472.CAN-10-1388. , et al. ( [DOI] [PubMed] [Google Scholar]
- Pereira FV, Arruda DC, Figueiredo CR, Massaoka MH, Matsuo AL, Bueno V. FTY720 induces apoptosis in B16F10-NEX2 murine melanoma cells, limits metastatic development in vivo, and modulates the immune system. Clinics. 2013;68:1018–1027. doi: 10.6061/clinics/2013(07)21. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permpongkosol S, Wang JD, Takahara S, Matsumiya K, Nonomura N, Nishimura K. Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int J Cancer. 2002;98:167–172. doi: 10.1002/ijc.10178. , et al. ( [DOI] [PubMed] [Google Scholar]
- Pitman MR, Woodcock JM, Lopez AF, Pitson SM. Molecular targets of FTY720 (fingolimod) Curr Mol Med. 2012;12:1207–1219. doi: 10.2174/156652412803833599. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Pyne S, Bittman R, Pyne NJ. Sphingosine kinase inhibitors and cancer: seeking the golden sword of Hercules. Cancer Res. 2011;71:6576–6582. doi: 10.1158/0008-5472.CAN-11-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid G, Guba M, Papyan A, Ischenko I, Bruckel M, Bruns CJ. FTY720 inhibits tumor growth and angiogenesis. Transpl Proc. 2005;37:110–111. doi: 10.1016/j.transproceed.2004.12.278. , et al. ( [DOI] [PubMed] [Google Scholar]
- Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–1172. doi: 10.4161/auto.7.10.16818. , et al. ( [DOI] [PubMed] [Google Scholar]
- Shimizu S, Takehara T, Hikita H, Kodama T, Tsunematsu H, Miyagi T. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer. 2012;131:548–557. doi: 10.1002/ijc.26374. , et al. ( [DOI] [PubMed] [Google Scholar]
- Tai WT, Shiau CW, Chen HL, Liu CY, Lin CS, Cheng AL. Mcl-1-dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC-59 in hepatocellular carcinoma cells. Cell Death Dis. 2013;4:e485. doi: 10.1038/cddis.2013.18. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushitora Y, Tashiro H, Ogawa T, Tanimoto Y, Kuroda S, Kobayashi T. Suppression of hepatocellular carcinoma recurrence after rat liver transplantation by FTY720, a sphingosine-1-phosphate analog. Transplantation. 2009;88:980–986. doi: 10.1097/TP.0b013e3181b9ca69. , et al. ( [DOI] [PubMed] [Google Scholar]
- Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallington-Beddoe CT, Hewson J, Bradstock KF, Bendall LJ. FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy. 2011;7:707–715. doi: 10.4161/auto.7.7.15154. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. , et al. ( [DOI] [PubMed] [Google Scholar]
- Worns MA, Galle PR. Novel inhibitors in development for hepatocellular carcinoma. Expert Opin Investig Drugs. 2010;19:615–629. doi: 10.1517/13543781003767418. [DOI] [PubMed] [Google Scholar]
- Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–6869. doi: 10.1038/sj.onc.1208841. , et al. ( [DOI] [PubMed] [Google Scholar]
- Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13(6):1589–98. doi: 10.1158/1535-7163.MCT-13-1043. , et al. ( [DOI] [PubMed] [Google Scholar]
- Zhang N, Qi Y, Wadham C, Wang L, Warren A, Di W. FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: a protective role of autophagy. Autophagy. 2010;6:1157–1167. doi: 10.4161/auto.6.8.13614. , et al. ( [DOI] [PubMed] [Google Scholar]
- Zhang W, Sun HC, Wang WQ, Zhang QB, Zhuang PY, Xiong YQ. Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology. 2012;143:1641–1649. doi: 10.1053/j.gastro.2012.08.032. , et al. ( e1645. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Z, Wang K. Combining sorafenib with celecoxib synergistically inhibits tumor growth of non-small cell lung cancer cells in vitro and in vivo. Oncol Rep. 2014;31:1954–1960. doi: 10.3892/or.2014.3026. [DOI] [PubMed] [Google Scholar]
- Zheng T, Meng X, Wang J, Chen X, Yin D, Liang Y. PTEN- and p53-mediated apoptosis and cell cycle arrest by FTY720 in gastric cancer cells and nude mice. J Cell Biochem. 2010;111:218–228. doi: 10.1002/jcb.22691. , et al. ( [DOI] [PubMed] [Google Scholar]
- Zhou C, Ling MT, Kin-Wah Lee T, Man K, Wang X, Wong YC. FTY720, a fungus metabolite, inhibits invasion ability of androgen-independent prostate cancer cells through inactivation of RhoA-GTPase. Cancer Lett. 2006;233:36–47. doi: 10.1016/j.canlet.2005.02.039. [DOI] [PubMed] [Google Scholar]