Summary
Identification of human satellite cells that fulfill muscle stem cell criteria is an unmet need in regenerative medicine. This hurdle limits understanding how closely muscle stem cell properties are conserved among mice and humans and hampers translational efforts in muscle regeneration. Here, we report that PAX7 satellite cells exist at a consistent frequency of 2–4 cells/mm of fiber in muscles of the human trunk, limbs, and head. Xenotransplantation into mice of 50–70 fiber-associated, or 1,000–5,000 FACS-enriched CD56+/CD29+ human satellite cells led to stable engraftment and formation of human-derived myofibers. Human cells with characteristic PAX7, CD56, and CD29 expression patterns populated the satellite cell niche beneath the basal lamina on the periphery of regenerated fibers. After additional injury, transplanted satellite cells robustly regenerated to form hundreds of human-derived fibers. Together, these findings conclusively delineate a source of bona-fide endogenous human muscle stem cells that will aid development of clinical applications.
Highlights
-
•
CD56 and CD29 mark PAX7 satellite cells in diverse human muscles
-
•
Transplanted human satellite cells engraft, self-renew, and regenerate
-
•
Human adult muscle stem cells reside within the CD56+/CD29+ satellite cell pool
In this article Pomerantz and colleagues demonstrate that human skeletal muscle stem cells reside within the satellite cell pool at similar frequencies in diverse adult muscles and engraft, populate the stem cell niche, and regenerate after transplantation.
Introduction
The ability to characterize, isolate, and transplant human muscle stem cells will lay the foundation for translational efforts to regenerate or engineer human muscles. However, the absence of approaches to expand limited amounts of available tissue ex vivo with retention of stem cell properties (Montarras et al., 2005), and difficulty developing xenograft model systems to test in vivo function (Boldrin et al., 2010; Silva-Barbosa et al., 2005; Zhang et al., 2014) make it difficult to study human muscle regeneration. Consequently, endogenous human muscle stem cells have not been characterized definitively, precluding the development of clinical applications.
Muscle regeneration in mice is mediated by satellite cells that are anatomically defined based on their position between the fiber plasma membrane and the basal lamina. A subset of mouse satellite cells fulfill criteria of adult stem cells in that they engraft, proliferate, respond to injury by regenerating mature muscle, reoccupy the muscle satellite cell niche, and self-renew (Collins et al., 2005; Kuang et al., 2007; Montarras et al., 2005; Sacco et al., 2008; Sherwood et al., 2004). In contrast, satellite cell progeny can be propagated in vitro and show some capacity for differentiation, but after even brief culture cannot engraft efficiently when isolated from mouse (Montarras et al., 2005) or human (Brimah et al., 2004; Cooper et al., 2001).
To date, most attempts to transplant adult human muscle cells have used cultured derivatives of endogenous or induced muscle cells, and few used freshly isolated or prospectively identified cells. Culture-expanded human myoblasts (Skuk et al., 2010) and CD133+ cells (Meng et al., 2014) can engraft and generate functional satellite cells after xenotransplantation of large numbers of cells, suggesting the potential for regenerative applications. Despite advances, the results of clinical trials (Miller et al., 1997; Partridge, 2002) and xenotransplantation experiments (Bareja et al., 2014; Castiglioni et al., 2014; Darabi et al., 2012; Ehrhardt et al., 2007; Miller et al., 1997; Partridge, 2002; Pisani et al., 2010; Silva-Barbosa et al., 2008) collectively show low transplantation efficiency, and satellite stem cell functions of self-renewal or expansion in vivo after injury have not been demonstrated from endogenous satellite cells. Thus, there is currently no established approach for directly isolating and transplanting endogenous bona-fide skeletal muscle stem cells from adult humans.
Mouse satellite cells have been well characterized (reviewed in Yin et al., 2013), providing a strong foundation for human translation. Although available evidence suggests that human and mouse satellite cells have similar morphological characteristics and surface marker expression (Boldrin et al., 2010; Kadi et al., 2004; Mackey et al., 2009), significant differences have been identified. For example, it has been suggested that the canonical satellite cell transcription factor Pax-7 (Seale et al., 2000) is not absolutely restricted to or expressed in all human satellite cells (Reimann et al., 2004). Moreover, surface marker expression is not identical between mouse and human satellite cells (Boldrin and Morgan, 2012) and there is no accepted set of surface markers upon which to base human satellite cell isolation. Whereas satellite cell frequency and function is heterogeneous in murine muscles (Collins et al., 2005; Kuang et al., 2007; Ono et al., 2010; Pavlath et al., 1998; Zammit, 2008), little is known about heterogeneity in human muscles. Human satellite cell heterogeneity could in theory constrain transplantation of particular recipient muscles. Finally, while direct transplantation of individual muscle fibers from mice preserves very robust satellite cell function (Collins et al., 2005; Hall et al., 2010) and bypasses flow cytometry, human fiber experimentation has not kept pace. Human fibers have been successfully cultured (Bonavaud et al., 2002) but are significantly longer than in the mouse, making them difficult to handle and deterring experimentation with them (Boldrin and Morgan, 2012). Recently, the feasibility of transplanting cultured human fiber fragments has been demonstrated (Marg et al., 2014).
Here, we report the successful transplantation of adult human muscle stem cells from diverse muscles by two distinct approaches, direct transplantation of niche-protected satellite cells on fibers and transplantation of flow-cytometry-enriched satellite cells. We analyze the frequency of satellite cells in diverse human muscles, corroborating CD29 and CD56 surface marker expression with satellite cell morphological characteristics and PAX-7 expression. Using irradiation and limited injury, we achieved efficient engraftment by fiber-derived or sorted satellite cells, and this enabled analysis of differentiation, response to injury, and self-renewal. Our results demonstrate stem cell phenotypes and establish approaches for transplantation of endogenous human satellite stem cells.
Results
Human Satellite Cells Co-express PAX7, CD56, and CD29 and Exist at Predictable Frequencies in Diverse Skeletal Muscles of Adults
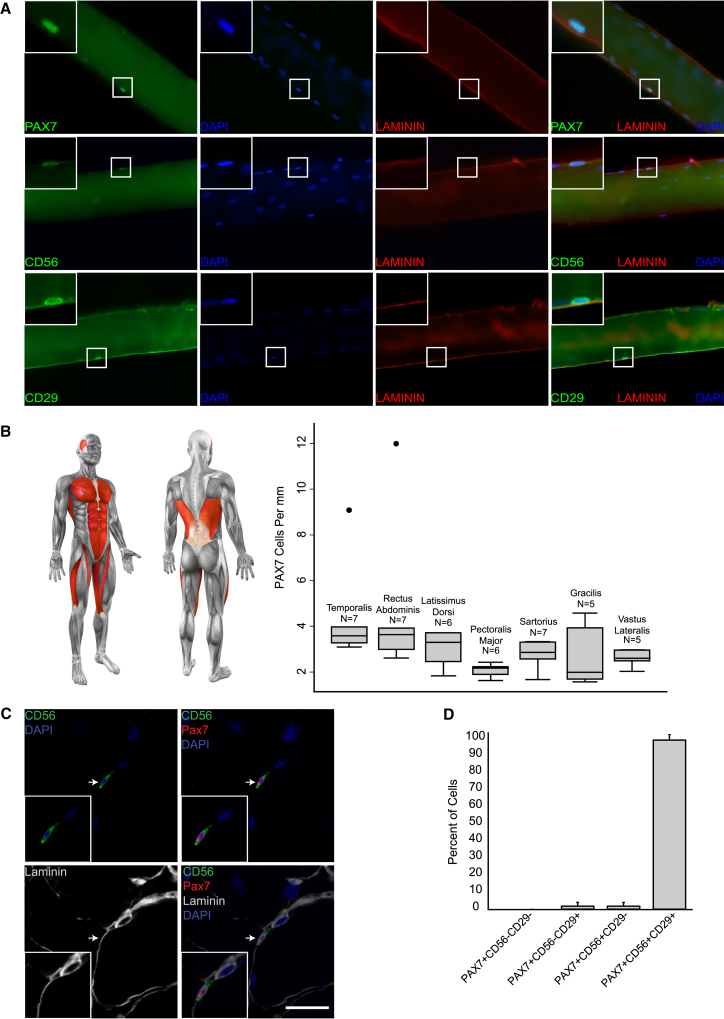
To investigate human satellite cell heterogeneity, we analyzed satellite cells in different human muscles. Muscle biopsies from males and females were sharply dissected during reconstructive surgery. None had systemic muscle disease and all biopsies were from healthy muscle not previously operated on. Mononucleated cells in the sublaminar position on the periphery of fixed individual fibers (satellite cells) were analyzed for expression of PAX7, CD29 (B-1 INTEGRIN), and CD56 (NCAM), which have been shown to be expressed by human satellite cells (Boldrin and Morgan, 2012; Schubert et al., 1989). Nuclear PAX7 (Figure 1A, top panels), and surface CD56 and CD29 (Figure 1A, middle and bottom panels) staining was readily detected on human satellite cells within the laminin-marked basement membrane in all biopsies (Figure 1). To determine whether satellite cell frequency is heterogeneous, we analyzed 7 different adult human skeletal muscle types in 43 patients—representing the trunk, lower limbs, and head (Figure 1B schematic; Table S1). In all but two outlier samples (5% of the total), PAX7-positive satellite cells existed at a relatively homogeneous frequency of 2–4 cells per millimeter of fiber length (Figure 1B, boxplot), with little variation within groups for one muscle type or among different muscles. We also estimated the total number of satellite cells in a given volume of muscle (Figure S1). Whereas the six analyzed body and limb muscles had similar estimated satellite cell contents, the number was significantly higher for the temporalis, a result of a greater number of fibers per unit area in that muscle. Collectively, our results indicate that under normal homeostatic conditions, most adult human skeletal muscle contains a relatively homogeneous frequency of PAX7 satellite cells per fiber and that variation in satellite cell content may be predicted based on differences in fiber area and density.
Figure 1.
Characterization of Satellite Cells of Diverse Human Skeletal Muscles
(A) Representative images showing immunostaining of satellite cells on isolated fixed human fibers for PAX7 (top row), CD56 (middle row), or CD29 (Serotec) (bottom row), each with LAMININ and with DAPI. Insets: enlargement of the indicated satellite cells.
(B) Left: schematic of analyzed human muscles highlighted in red. Right: boxplot representation of PAX7 cell frequency per millimeter myofiber in different individuals. Boxes are grouped by muscle type. n = 5–7 individuals per group. The middle line of each boxplot indicates the median. The inner lower and upper lines represent the 25th and 75th percentile, respectively. The outer lower and upper horizontal bars represent the 25th percentile − 1.5 interquartile range and 75th percentile + 1.5 interquartile range, respectively. Black dots indicate outlier samples.
(C) Immunostaining for PAX7, CD56, and LAMININ on cross section of the vastus lateralis muscle of a 54-year-old male. Arrows indicate satellite cell expressing all three markers. Scale bar, 20 μm.
(D) Cells expressing combinations of PAX7, CD56, and CD29 in three muscle types from five individuals. The aggregate number of cells expressing each combination is presented as a percentage of total PAX7-positive cells. Bars represent the average percentage of cells for each expression pattern: PAX7+CD56+CD29+, 96.89% ± 3.32%; PAX7+CD56+CD29−, 1.57% ± 2.15%; PAX7+CD56−CD29+, 1.54% ± 2.11%; PAX7+CD56−CD29−, 0% ± 0%. Error bars, SEM.
See also Figures S1–S3 and Tables S1 and S2.
Potential heterogeneity within the satellite cell pool prompted us to analyze surface marker co-expression with PAX7. We focused on CD29 and CD56 to lay the foundation for isolation by flow cytometry. In fiber preparations, CD56, CD29, and PAX7 expression correlated closely in terms of frequency per millimeter (Table S2). We also analyzed cross sections to more definitively evaluate position and co-expression of all three markers. This showed a nearly uniform correlation of CD29 and CD56 expression in PAX7-positive cells (Figures 1C, 1D, and S2). PAX7 satellite cells had a characteristic pattern of CD56 expression concentrated on the apical surface opposite the basal lamina and adjacent to the fiber plasma membrane, whereas the CD29 pattern marked the fiber plasma membrane as well as the entire perimeter of satellite cells (Figures 1C and S2). Frequent CD29 cells were observed that did not express CD56, and these cells were also negative for PAX7 (Figure S2). Quantification of co-expression patterns showed that most PAX7 cells expressed both CD56 and CD29. Very rare PAX7 cells had one, but not both, surface markers detectable (Figure 1D). Finally, since vessel-associated cell populations within skeletal muscle have previously been shown to exhibit engraftment capacity (reviewed in Chen et al., 2012), we analyzed sections for pericyte markers to confirm that our analysis of satellite cells did not include non-satellite, vessel-associated cells. We found that CD56+/PAX7+ satellite cells do not express pericyte markers, confirming that satellite and pericyte muscle cells are distinct populations (Figure S3). We conclude that CD56 and CD29 are robust markers of human PAX7 satellite cells, which are a relatively homogeneous population with respect to these three proteins in adult healthy muscle.
Evaluation of Muscle Stem Cell Function by Transplantation of Individual Human Myofibers
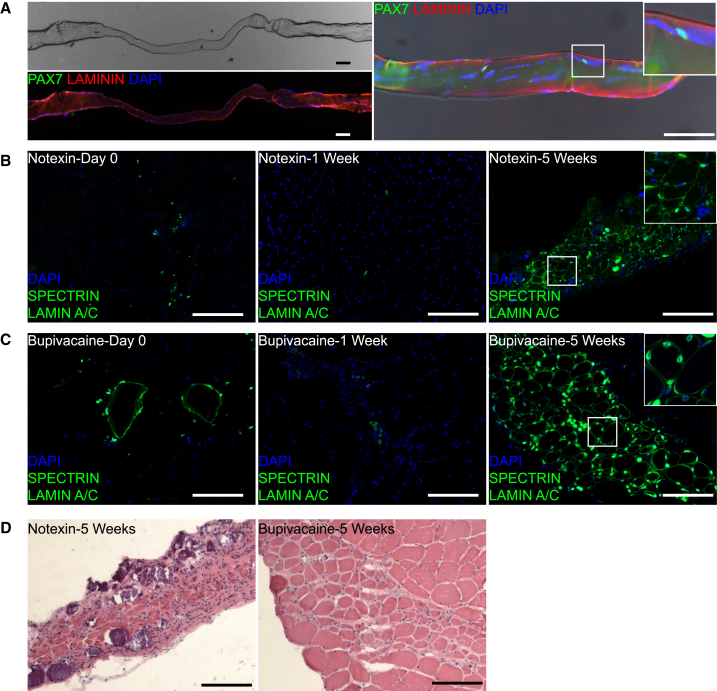
To evaluate human satellite cell function, we first investigated transplantation of individual myofibers, an unbiased approach independent of surface marker phenotype. We modified established techniques for fiber transplantation to work with human fibers (Bonavaud et al., 2002; Collins et al., 2005; Rosenblatt et al., 1995). Freshly prepared single live fibers yielded myoblasts in culture (Figure S4) and when fixed just after dissection satellite cell frequency (Figure 2A; Table 1; Table S3) was in accord with observations on directly fixed muscle samples (Figure 1).
Figure 2.
Satellite Cells from Isolated Live Human Muscle Fibers Engraft and Differentiate after Transplantation
(A) Myofiber isolated as in Figure S4, but fixed. Upper left: phase image of single fiber. Lower left: immunostaining of same fiber for LAMININ, PAX7, and DAPI. Right: enlarged image of the fiber with Inset showing PAX7 satellite cells, which are not visible on the lower left image.
(B) Human myofiber (vastus lateralis, 54-year-old male) transplantation into irradiated TA treated with notexin. Analysis of cross sections immunostained for human SPECTRIN and LAMIN A/C immediately after transplantation (left), at 1 week (middle) and 5 weeks (right). Inset: enlargement of the indicated area showing location of nuclei relative to fiber. Right: 46% of the human nuclei are located within fibers bound by human SPECTRIN. 70.4% (SD 9.2%, n = 3 TA muscles) of SPECTRIN-positive fibers contained a LAMIN A/C positive nucleus within the same section. On serial sections, LAMIN A/C positive nuclei could be identified within each fiber.
(C) Human myofiber (rectus abdominis, 64-year-old female) transplantation into irradiated TA treated with bupivacaine. Analysis as in (B). Right: 83.5% (SD 5.9%, n = 4 TA muscles) of SPECTRIN-positive fibers contained a LAMIN A/C positive nucleus within the same section. On serial sections, LAMIN A/C positive nuclei could be identified within each fiber.
(D) H&E of consecutive sections from 5-week samples shown in (B) and (C).
Scale bars, 100 μm. Sections were co-stained with DAPI. See also Figure S4 and Table S3.
Table 1.
Human Myofiber Transplants
| Animal | Human Muscle | Myotoxin | Length of Human Fiber Transplanted (mm) | Estimated Satellite Cells Transplanted | Human SPECTRIN Fibers |
|---|---|---|---|---|---|
| 1 | VL, 52M | Ntx | 20 | 52 | 165 |
| 2 | VL, 52M | Ntx | 20 | 52 | 99 |
| 3 | VL, 52M | Ntx | 20 | 52 | 11 |
| 4 | RA, 64F | Bup | 15 | 70 | 119 |
| 5 | RA, 64F | Bup | 15 | 80 | 43 |
| 6 | RA, 64F | Bup | 15 | 80 | 38 |
| 7 | RA, 64F | Bup | 15 | 80 | 6 |
| 8 | RA, 60F | Ntx | − | − | 3 |
| 9 | RA, 60F | Ntx | − | − | 55 |
| 10 | G, 17M | Ntx | − | − | 68 |
| 11 | G, 17M | none | − | − | 20 |
| 12 | G, 17M | none | − | − | 5 |
| 13 | RA, 46F | none | − | − | 55 |
| 14 | RA, 46F | Ntx | − | − | 89 |
| 15 | RA, 46F | Ntx | − | − | 107 |
| 16 | RA, 46F | none | − | − | 8 |
| 17 | S, 60M | Ntx | − | − | 24 |
| 18 | S, 60M | Ntx | − | − | 35 |
| 19 | S, 60M | Bup | − | − | 5 |
| 20 | S, 60M | Bup | − | − | 53 |
For transplants in which fiber length was measured (#1–7), the length was used to estimate the number of satellite cells using the average satellite cell frequency for that particular donor muscle type (Figure 1). Engraftment was analyzed at 5 weeks. − indicates not determined. All transplants were engrafted from the five muscle types in six muscles from six individuals. Ntx, notexin; Bup, bupivacaine; RA, rectus abdominis; VL, vastus lateralis; G, gastrocnemius; S, soleus; M, male; F, female.
Human fiber fragments (15–20 mm) were transplanted into tibialis anterior (TA) muscles of NOD SCID Gamma (NSG) mice after hindlimb irradiation (18 Gy) to incapacitate endogenous satellite cells, and notexin treatment at the time of transplantation to induce myofiber injury. Human-derived myofibers and mononucleated cells were identified by detecting human SPECTRIN and LAMIN A/C, a combination of markers that simultaneously identify human muscle fibers and human nuclei (Brimah et al., 2004). Mice were sacrificed immediately after transplantation or after 1 or 5 weeks. Immediately after transplantation, fragmented SPECTRIN staining and intact LAMIN A/C positive nuclei were identified within the TA (Figures 2B and S4B). By 1 week, human fibers had degenerated as evidenced by absence of SPECTRIN staining, and rare mononucleated LAMIN A/C positive human nuclei persisted in areas of injury surrounded by degenerating mouse fibers and mononucleated cells (Figure 2B, middle). At 5 weeks, most mouse fibers had degenerated and large clusters of human-derived fibers expressing LAMIN A/C and SPECTRIN appeared (Figure 2B, right) and were identified on sections along most of the length of the TA. Human fibers were of varying diameter with peripheral and central human nuclei and some mononucleated human cells in the interstitial space. Most SPECTRIN-positive fibers contained at least one LAMIN A/C positive nucleus within the section analyzed (see Figure 2 legend), and on serial sections LAMIN A/C nuclei could be found in virtually all SPECTRIN fibers, fulfilling human origin criteria as described in Meng et al. (2014). Fiber transplantation experiments with various donor muscles (Table 1) show that transplantation results in robust formation of human-derived fibers and that satellite cells survive, engraft, and contribute to human-derived fiber generation in excess of the original transplanted fiber over a period of weeks.
The recipient muscle was severely damaged by the radiation and notexin protocol (Figure 2D, left). To induce engraftment but minimize damage, we investigated other concomitant injury methods. Bupivacaine, a clinically used local anesthetic, induces muscle injury and regeneration upon intramuscular injection, but is less destructive than notexin (Plant et al., 2006). Transplantation of human fibers with bupivacaine into irradiated muscle resulted in successful engraftment (Figure 2C; Table 1) and much better preservation of recipient muscle architecture (Figure 2D, right). Human fiber structure was well preserved immediately after transplantation, followed by fiber degradation and retention of mononucleated LAMIN A/C cells at 1 week (Figure 2C), as with notexin. At 5 weeks, formation of human-derived muscle had occurred as clusters of SPECTRIN and LAMIN A/C fibers within intact host muscle. Transplantation of human myofibers into irradiated muscle with either notexin or bupivacaine injury demonstrates that significant fiber formation can be generated from fewer than 100 transplanted niche-protected satellite cells.
Human Satellite Cells Populate the Satellite Niche after Transplantation
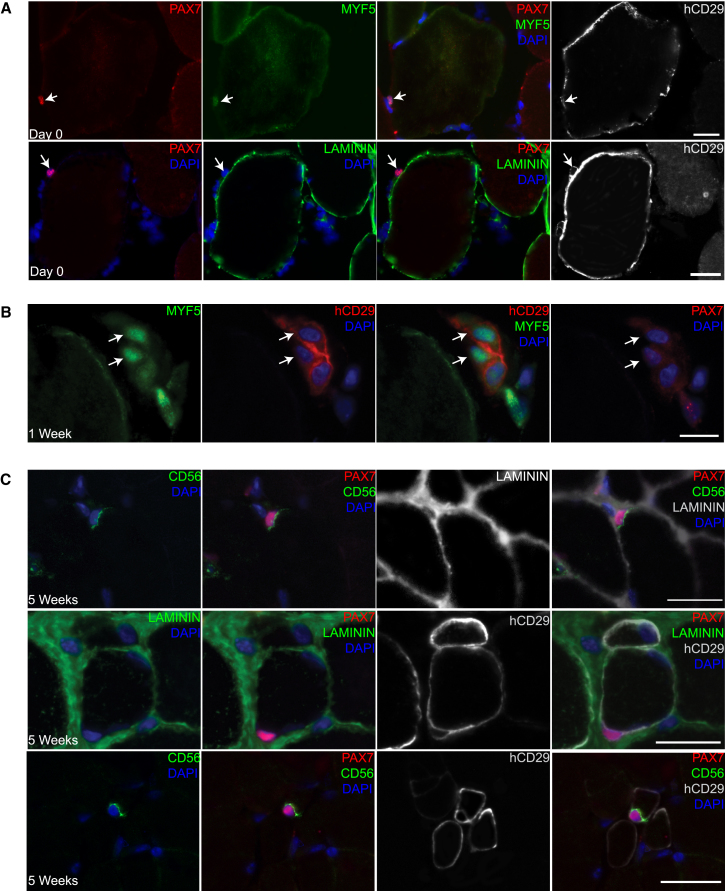
To investigate human satellite cell activation and progeny during engraftment, we analyzed the expression of PAX7, MYF5, LAMININ, and human CD29 (hCD29) after fiber transplantation. Immediately after transplantation, human PAX7 cells were associated with the periphery of transplanted fibers and expressed MYF5 (Figure 3A, top row). Like satellite cells in uninjured muscle, human PAX7 cells were hCD29 positive. However, the hCD29 pattern after transplantation suggested the position on the fiber altered from tightly juxtaposed (as in Figure S2B) to a more dissociated location further from the periphery (Figure 3A, right panels). This was confirmed using LAMININ staining, which demonstrated that after fiber transplantation human PAX7 cells assumed extralaminar positions adjacent to human fibers (Figure 3A, bottom row). hCD29 expression confirmed human origin of the PAX7 cells (Figure 3A, bottom right). The expression phenotype of PAX7+/MYF5+ and extralaminar position may indicate activated satellite cells or committed progenitors similar to what has been described in mice (Kuang et al., 2007). 1 week after transplantation, PAX7−/MYF5+ was the predominant transcription factor phenotype, likely reflecting a population of committed progenitors (Figure 3B).
Figure 3.
Analysis of Human Mononucleated Muscle Cells after fiber Transplantation
(A) Immediately after fiber injection. PAX7 cells survive and localize outside of the human fiber basement membrane. Top: immunostaining for PAX7, LAMININ, and CD29. Human cells are marked by anti-CD29 antibody (BioLegend TS2/16) (hCD29) that detects human, but not mouse, CD29 by immunostaining (see Figure S2 for antibody controls). Bottom: PAX7, MYF5, and CD29. Human PAX7 cells express MYF5 after transplantation. Arrows indicate PAX7-positive nuclei.
(B) 1 week after fiber injection. Human fibers have degenerated. Immunostaining for MYF5, hCD29, and PAX7. Surviving human cells (marked by hCD29 cy5, red pseudocolor, arrows) express MYF5. PAX7 (cy3, red pseudocolor) is not detected in these cells (right panel).
(C) 5 weeks after fiber injection. Human PAX7 cells occupy the satellite cell niche on new human fibers. Top row: immunostaining for PAX7, CD56, and LAMININ. Sublaminar PAX7 cell expresses apical hCD56. Middle row: PAX7, hCD29, and LAMININ. Note sublaminar PAX7 cell and marking of the satellite cell and the fiber membrane by hCD29. Bottom row: PAX7, CD56, and CD29. Human satellite cell expressing high PAX7, apical CD56, and circumferential CD29.
Sections were co-stained with DAPI. Scale bars, 20 μm. See also Figure S2.
5 weeks after transplantation, human cells were common (Figure 2C, right) and human PAX7 cells were readily identified in the satellite cell position (Figure 3C). Sublaminar cells with high levels of PAX7 were located on the periphery of human fibers and expressed apical CD56 similar to endogenous human satellite cells (Figure 3C, top and bottom rows). Moreover, human PAX7 cells were enveloped by CD29 (Figure 3C, middle and bottom rows) in a pattern like that seen in satellite cells in human muscle biopsies (Figure S2). On sections taken at 5 weeks, 20.2% (±6.5%) of human-derived fibers had sublaminar PAX7 cells, compared to 13.8% (±3.8%) on a native human control sample. The morphology and expression phenotype of human sublaminar mononucleated cells 5 weeks after transplantation indicates that a portion of transplanted cells repopulate the satellite cell niche.
Isolation of CD56/CD29 Satellite Cells from Healthy Adult Muscle by Flow Cytometry
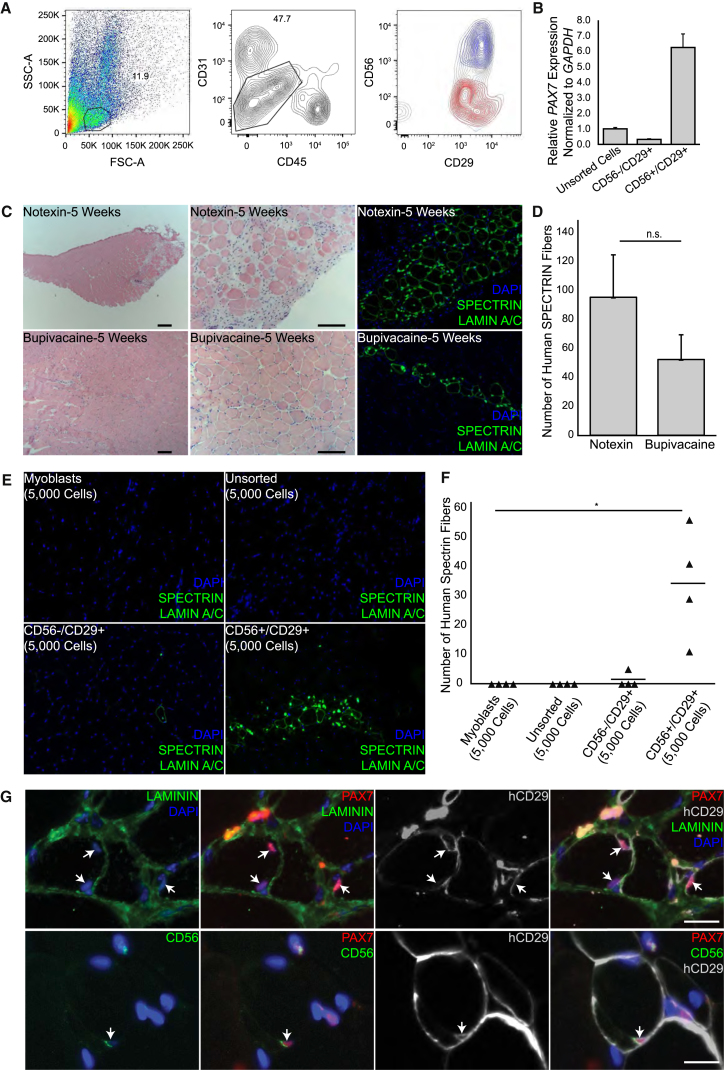
To evaluate satellite cell function in a defined population, we used flow cytometry to isolate satellite cells from human muscle biopsies. Expression patterns of CD56 and CD29 (Figure 1) supported a strategy based on these markers. After muscle digestion, live cells were depleted for CD31 and CD45 and cells that were singly or doubly positive for CD56 and CD29 were selected (Figures 4A and S5). Smaller populations of CD56+/CD29− cells were identified in some muscle samples in contrast to consistent larger populations of CD56−/CD29+ and CD56+/CD29+ cells, in agreement with our findings on muscle sections. The CD31−/CD45−/CD56+/CD29+ population comprised about 1% of the total events and based on our immunostaining data should represent the satellite cell population. This was confirmed by amplifying PAX7 transcripts from different human muscle cell populations (Figure 4B) and by staining sorted cells for PAX7 (Figure S5). When compared to unsorted muscle preparations, the CD56+/CD29+ population, but not the CD56−/CD29+ population, is highly enriched for PAX7 transcripts, and the vast majority of CD56+/CD29+ cells express detectable levels of PAX7 protein, supporting use of the combination of CD56 and CD29 to isolate human satellite cells (Figure 4B).
Figure 4.
Human CD56+/CD29+ Cells Engraft and Generate Human Muscle Fibers in Recipient Mice
(A) Representative FACS plot showing isolation of human CD31−/CD45−/CD56+/CD29+ cells prepared from the vastus lateralis muscle of an 81-year-old male. Left: forward scatterplot (FSC-A) and side scatterplot (SSC-A); gated area shown by solid line represents 11.9% of the total events. Middle: depletion of live CD45 and CD31 cells; gated area shown by solid line represents 47.7% of the cells. Right: live cells after depletion. Blue indicates CD56+/CD29+ selected cells; red indicates CD56−/CD29+ selected cells; and the backdrop indicates the total population on the CD56/CD29 plot. Cells that were CD56+/CD29+ or CD56−/CD29+ were separated for further experimentation. See also Figure S6 for isotype controls.
(B) qRT-PCR for PAX7 expression normalized to GAPDH in indicated cell populations. Error bars, SEM of three replicates. Representative experiment is shown from three biological replicates.
(C) Human CD56+/CD29+ transplantation. Human muscle: rectus abdominis, 40-year-old female. Cross sections, 5 weeks after transplantation of 5,000 cells. Left panels: H&E. Middle panels: enlarged. Right panels: consecutive sections immunostained for human SPECTRIN and human LAMIN A/C. NSG TAs were treated with notexin (top row; n = 3 mice) and with bupivacaine (bottom row; n = 3 mice). Sections were co-stained with DAPI. Scale bars, 100 μm.
(D) Analysis of the number of human SPECTRIN fibers identified in each TA 5 weeks after transplantation with 5,000 CD56+/CD29+ cells. n = 3 animals for each group. Bar represents the average number of human SPECTRIN fibers for each group (bupivacaine, 52 ± 17; notexin, 95 ± 29). Statistical analysis was determined using paired Student’s t tests. Error bar, SEM; n.s., not significant. 73.9% (SD 6.3%) of SPECTRIN-positive fibers in the bupivacaine samples and 78.3% (SD 3.9%) in the notexin samples contained a LAMIN A/C positive nucleus within the same section.
(E) Comparison of engraftment of different human muscle cell populations. Representative cross sections 5 weeks after transplantation of 5,000 human cells and stained for human SPECTRIN and LAMIN A/C. Sections co-stained with DAPI. Top left: cultured primary myoblasts. Top right: unsorted cells. Bottom left: CD56−/CD29+ cells. Bottom right: CD56+/CD29+ cells. All except myoblasts are from the same latissimus dorsi, 60-year-old male. n = 4 mice for each group. In the CD56+/CD29+ sample, 73.4% (SD 22.5%) of the SPECTRIN-positive fibers contained a LAMIN A/C positive nucleus within the same section.
(F) Dot plot showing the number of human SPECTRIN fibers (triangles) identified in each TA 5 weeks after transplantation with the indicated populations of human muscle cells. n = 4 recipient mice for each group. Myoblasts, 0 ± 0; unsorted human muscle cells, 0 ± 0; human CD56−/CD29+ cells, 1 ± 3; human CD56+/CD29+ cells, 34 ± 19. Bars represents the average number of human SPECTRIN fibers for each transplanted population. Statistical analysis was determined using paired Student’s t tests. p values are ∗ < 0.05.
(G) CD56+/CD29+ transplantation—analysis for human satellite cells. Cross sections from same experiment as (C) are shown 5 weeks after transplantation of 5,000 cells. Top row: human satellite cells identified by PAX7 expression and sublaminar location (LAMININ). Human origin identified by hCD29. Bottom row: human PAX7 cells retain CD29 and apical CD56 expression. Arrows indicate human satellite cells. Sections were co-stained with DAPI. Scale bar, 20 μm.
Engraftment of Human CD56/CD29 Cells after Transplantation
To test human satellite cell function, we transplanted 1,000 to 5,000 CD56+/CD29+ cells into irradiated TA muscles. In all transplanted mice analyzed at 5 weeks (n = 24) (Table 2), recipient muscle contained clusters of human-derived myofibers with peripherally and centrally located human nuclei along the length of the muscle (Figure 4C) at levels slightly, but not significantly, higher with notexin than bupivacaine (Figure 4D).
Table 2.
Human CD56+/CD29+ Satellite Cell Transplants
| Animal | Human Muscle | Myotoxin | Satellite Cells Transplanted | Human SPECTRIN Fibers |
|---|---|---|---|---|
| 1 | TA, 45F | Ntx | 3,500 | 5 |
| 2 | TA, 53F | Bup | 4,000 | 82 |
| 3 | RA, 56F | Ntx | 4,000 | 104 |
| 4 | VL, 81M | Ntx | 1,300 | 20 |
| 5 | VL, 81M | Ntx | 1,300 | 131 |
| 6 | RA, 54F | Ntx | 5,000 | 97 |
| 7 | RA, 54F | Ntx | 5,000 | 99 |
| 8 | RA, 54F | Bup | 5,000 | 2 |
| 9 | RA, 54F | Bup | 5,000 | 10 |
| 10 | LD, 60M | Bup | 5,000 | 56 |
| 11 | LD, 60M | Bup | 5,000 | 11 |
| 12 | LD, 60M | Bup | 5,000 | 29 |
| 13 | LD, 60M | Bup | 5,000 | 41 |
| 14 | LD, 54M | Bup | 5,000 | 108 |
| 15 | LD, 54M | Bup | 5,000 | 73 |
| 16 | LD, 54M | Bup | 5,000 | 112 |
| 17 | LD, 54M | Bup | 5,000 | 70 |
| 18 | RA, 40F | Ntx | 5,000 | 65 |
| 19 | RA, 40F | Ntx | 5,000 | 99 |
| 20 | RA, 40F | Ntx | 5,000 | 122 |
| 21 | RA, 40F | Bup | 5,000 | 35 |
| 22 | RA, 40F | Bup | 5,000 | 54 |
| 23 | RA, 40F | Bup | 5,000 | 68 |
| 24 | RA, 64M | Bup | 1,000 | 20 |
Engraftment was analyzed at 5 weeks. All transplants were engrafted from four muscle types from nine muscles of nine individuals. Ntx, notexin; Bup, bupivacaine; TA, tibialis anterior; RA, rectus abdominis; VL, vastus lateralis; LD, latissimus dorsi; M, male; F, female.
When 5,000 cells of different populations were transplanted from the same muscle sample, only the CD56+/CD29+ fraction engrafted (Figures 4E and 4F). In CD56−/CD29+ transplants, human cells were identified in 2 out of 7 mice but existed as mononucleated cells. Rare fibers were detected in 1 out of 7 mice, probably reflecting contamination with CD56+/CD29+ cells or very limited myogenic potential of CD56−/CD29+ cells. Transplantation of 5,000 unsorted muscle cells or passaged primary human myoblasts did not yield detectable human cells. Together, these results indicate the presence of comparable populations of satellite cells in different muscles of different individuals and show that most transplantable human satellite cells exist within the CD56+/CD29+ population.
As in fiber transplantation, transplanted human CD56+/CD29+ cells or their progeny populated the satellite cell niche and retained morphological and expression phenotypes of the parent population (Figure 4G). Sublaminar PAX7 cells were readily detected on the periphery of human fibers. Human CD29 expression confirmed human origin and apical CD56 staining was similar to that of satellite cells in human muscle (Figures 1 and S2). This replenishment of the satellite niche implies that regenerated fibers also acquired human-derived stem cell reserve capable of fiber maintenance and repair.
Transplanted Human Satellite Cells Respond to Injury by Expansion and Differentiation into Mature Human-Derived Skeletal Muscle
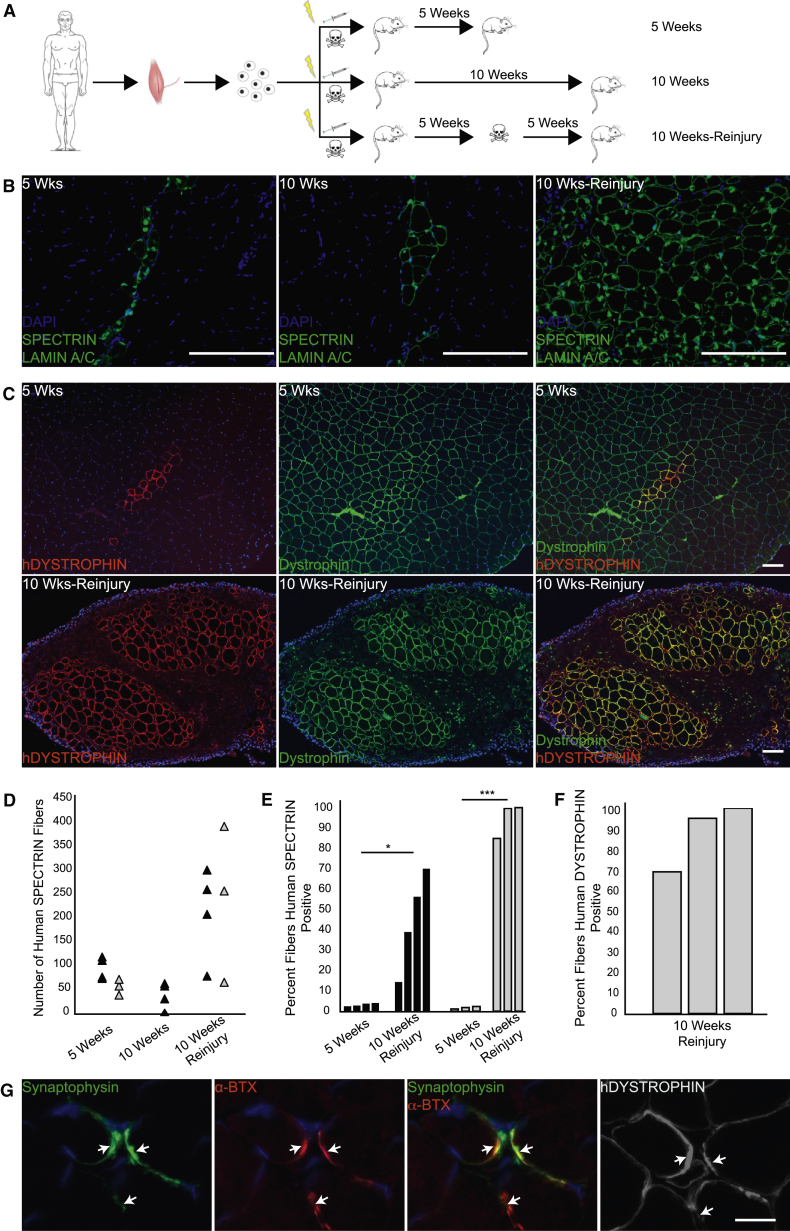
To evaluate whether engrafted human satellite cells can regenerate in response to injury, we performed reinjury experiments. Reinjured muscles were compared to parallel non-reinjured muscles, according to the schematic (Figure 5A). After transplantation of 5,000 CD56+/CD29+ cells with bupivacaine, expected engraftment occurred at 5 weeks (Figures 5B and 5C, left, and 5D, black triangles; Table 2). Since it has been reported that anti-human SPECTRIN can cross react with mouse fibers (Rozkalne et al., 2014), we confirmed that the SPECTRIN and LAMIN A/C staining specifically identifies human fibers by staining adjacent sections with human specific anti-DYSTROPHIN antibody, and the staining patterns correlated well (Figure S6). In mice analyzed at 10 weeks without reinjury, engrafted human cells and human fibers persisted in clusters of similar numbers of human-derived fibers comparable to that at 5 weeks (Figures 5B, middle, and 5D), but human fiber diameter increased suggesting maturation (Figure S7). Human fibers accounted for less than 5% of the overall muscle fibers at 5 weeks, and at 10 weeks without reinjury, the composition was similar (Figures 5B, middle, and 5E). Human satellite cells were identified 5 weeks after transplantation by co-staining with PAX7, LAMININ, and LAMIN A/C after (Meng et al., 2014; Skuk et al., 2010) (Figure S7F). Reinjury with notexin resulted in abundant generation of new human fibers in large groups indicating a robust proliferative response (Figures 5B, right, and 5D). After reinjury, human fiber diameter varied more than in 10-week controls (Figure S7E), and many fibers contained centrally located human nuclei. To better characterize the degree of human muscle cell regeneration, we examined longitudinal sections of reinjured TA muscles for expression of human DYSTROPHIN, LAMIN A/C, and satellite cell markers (Figure S7). The vast majority of the myonuclei in human-derived fibers were of human origin (Figures S7A and S7B), and in contrast to a patchy, domain-limited human protein expression pattern that would be expected in highly chimeric fibers (Brimah et al., 2004), human DYSTROPHIN staining was uniformly present in fibers containing LAMIN A/C positive myonuclei (Figures S7A and S7B). 10,000–20,000 human myonuclei were distributed over the length of reinjured muscles, and like in cross sections, human-derived satellite cells were identified in longitudinal sections (Figures S7C and S7D).
Figure 5.
Engrafted CD56+/CD29+ Cells Respond to Additional Injury by Proliferating and Generating New Human Fibers
(A) Schematic of reinjury experiments. 5,000 human CD56+/CD29+ cells were transplanted with bupivacaine. One cohort was sacrificed at 5 weeks; one cohort was reinjured with notexin at 5 weeks and sacrificed at 10 weeks; and one cohort was sacrificed at 10 weeks without reinjury. Lightning bolt indicates 18 Gy irradiation; syringe indicates cell transplantation; and skull and crossbones indicates toxin.
(B) Immunostaining for human SPECTRIN and LAMIN A/C on transverse sections at 5 weeks (left), 10 weeks without reinjury (middle), and 10 weeks with notexin reinjury (right). Human muscle: latissimus dorsi, 54-year-old male. Representative sections are shown; n = 4 mice for each group. All sections were co-stained with DAPI. Scale bar, 100 μm.
(C) Immunostaining for pan DYSTROPHIN (DYSTROPHIN) and human DYSTROPHIN (hDYSTROPHIN) at 5 weeks (top row) and at 10 weeks after reinjury with notexin (bottom row). Human muscle: rectus abdominis, 40-year-old female. Representative sections are shown; n = 3 mice for each group. Sections were co-stained with DAPI. Scale bar, 100 μm.
(D) Dot plot showing the number of human SPECTRIN fibers (triangles) identified in individual recipient mice. Results are presented from separate experiments using two different donor muscles: black triangles indicate latissimus dorsi, 54-year-old male; gray triangles indicate rectus abdominis, 40-year-old female. n = 3–4 mice for each group. In the 10 week reinjury animals, 89.8% (SD 5.1%) of the SPECTRIN-positive fibers contained a LAMIN A/C positive nucleus within the same section analyzed for SPECTRIN.
(E) Percentage of total myofibers that express human SPECTRIN after transplantation with human CD56+/CD29+ cells. Each bar represents the percentage per mouse. Donor cells: black bars represent latissimus dorsi, 54-year-old male; gray bars represent rectus abdominis, 40-year-old female, as in (D). n = 3–4 mice for each group. Statistical analysis was determined using paired Student’s t tests; p values are ∗ < 0.05, ∗∗∗ < 0.001. See also Figure S4.
(F) Percentage of DYSTROPHIN-expressing fibers that express human DYSTROPHIN after reinjury. See also Figure S5.
(G) Analysis of neuromuscular junctions on human myofibers after reinjury. Neuromuscular junctions identified by SYNAPTOPHYSIN localization with α-BUNGAROTOXIN (α-BTX). Human origin of myofibers is indicated by human DYSTROPHIN. Arrows point to neuromuscular junctions. Sections were co-stained for DAPI. Scale bar, 20 μm.
See also Figures S6 and S7.
A significant growth advantage of engrafted human satellite cells is shown by the global replacement of the muscle with human-derived cells after reinjury (Figure 5C). This is most likely a result of the use of irradiation of the host muscle to incapacitate endogenous mouse satellite cells, as evidenced by the abundance of human-derived cells and absence of host PAX7 cells (Figure S7). At 5 weeks, small clusters of muscle express human DYSTROPHIN in contrast to a majority of mouse DYSTROPHIN-expressing fibers (Figure 5C, top row). After reinjury, most muscle fibers were of human origin, accounting for up to 100% of the muscle (Figure 5E), and they matured to the point that they expressed human DYSTROPHIN (Figures 5C, right, and 5E). Human DYSTROPHIN expression correlated with SPECTRIN and LAMIN A/C staining of adjacent sections (Figure S7G). To further evaluate maturation, we analyzed samples for neuromuscular junctions on human-derived fibers (Figure 5G). Co-staining with antibodies to SYNAPTOPHYSIN, α-BUNGAROTOXIN, and human DYSTROPHIN identified mature neuromuscular junctions by adjacent layers of SYNAPTOPHYSIN (presynaptic) and α-BUNGAROTOXIN (postsynaptic) staining on the fiber periphery. Junctions were observed on approximately 2% of human DYSTROPHIN-expressing fibers on sections after reinjury. Together, these reinjury experiments demonstrate a robust capacity of engrafted human satellite cells to expand and to generate quantities of mature muscle in excess of the original number of transplanted cells, a standard criterion of a tissue stem cell.
Discussion
We demonstrate that a population of adult human satellite cells fulfills conventional criteria of tissue-specific stem cells. Many characteristics of mouse satellite cells are found to be conserved in adult humans, substantiating translational approaches that extend investigations of mouse muscle regeneration. This study identifies a distinct stem cell population that can be investigated, targeted, or transplanted to treat muscle disorders.
Our analysis of satellite cell frequency in muscles of the head, trunk, and limbs suggests considerable homogeneity among different healthy human muscle types. However, when considering muscle volume, higher satellite cell content of the temporalis may relate to physiological differences of that muscle and also suggests satellite cell content may be heterogeneous in other muscles yet to be examined. Moreover, potential functional heterogeneity within the human PAX7-positive satellite cell compartment remains to be examined and important variations in satellite stem cells may yet be identified. In the two outliers we found, recent physical activity or injury may have influenced satellite cell activation and frequency as has been shown to occur in humans (Crameri et al., 2004; Mackey et al., 2009). It will be important to determine whether activated human satellite cells retain satellite stem cell properties. Our analyses demonstrate that human skeletal muscles derived from either somitic or cranial mesoderm retain an abundance of satellite cells in adulthood. It appears feasible to obtain enriched populations of relatively large numbers of satellite cells from diverse human muscles (Table S4). Our data suggest that when optimized, the yield from individual human donor muscle harvests could be expected to be adequate for regeneration of smaller recipient muscles without ex vivo expansion. The consistent engraftment that occurred after transplantation of similar numbers of fiber-associated or sorted satellite cells from six different muscle types from males and females aged 17–81 years suggests substantial diversity of potential satellite cell donor muscles. An implication of our findings is that choice of donor muscle(s) in future applications including clinical transplantation can be based on previously established criteria for human muscle expendability and donor morbidity (Mathes and Nahai, 1997) and on ease of muscle sample preparation, rather than on a muscle-specific preference based on properties of the satellite cells within it.
The different approaches to transplant satellite cells each have advantages and disadvantages from the standpoint of potential clinical application. Like in mouse experiments in which transplantation of single fibers carrying <10–20 satellite cells yield up to 100 fibers without additional injury (Collins et al., 2005; Hall et al., 2010), we found that human muscle fibers retain similarly high regenerative capacity derived from tens of satellite cells (Figure 2; Table 1) that are presumably better protected within their endogenous niche and have better preservation of surface proteins because of less enzyme exposure. Fiber transplantation also is not limited by selection of arbitrary surface markers, which could in theory exclude some stem cells. Not unlike currently used techniques of grafting adipose tissue or skin, both of which carry tissue stem cells that support graft homeostasis throughout the life of the organism, transplantation of muscle grafts (Zhang et al., 2014) is a theoretically feasible approach to regenerate small muscles that have depleted regenerative capacity. However, inherent technical difficulty complicates muscle fiber grafting.
Prospective isolation helped to better define and characterize adult human satellite cells. Our analysis of PAX7, CD56, and CD29 in adult human muscle biopsies demonstrated that the co-expression and staining pattern of CD56 and CD29, as opposed to either alone, faithfully marks PAX7-expressing satellite cells. We observed a distinct staining pattern of apical CD56 expression that was particular to sublaminar satellite cells, similar to that previously shown for M-CADHERIN (Reimann et al., 2004), which may be specific to quiescent satellite cells. It has been described previously (Lindström and Thornell, 2009) that CD56 expression can occur on PAX7-negative human mononuclear muscle cells that could represent activated satellite cells or myoblasts. The relative scarcity of this population in our study could reflect the fact that most human muscle is quiescent with little proliferation in the absence of injury. We observed a population of CD56+/CD29+ cells that did not stain for PAX7 on sections. It is unclear what the lineage of these cells is, but during flow cytometry, a population of CD56+/CD29+ cells was depleted because of co-expression of CD31. In addition to satellite cells, the myofiber and some non-muscle cells within muscle tissue also express CD29. This makes CD29, if used alone, inadequate for identification of human satellite cells. Therefore, when using CD29 to evaluate human satellite cells, CD29 must be used in conjunction with other markers such as PAX7 and LAMININ. Additionally, it is important to confirm that sublaminar CD29 staining on satellite cells is distinguished from staining of the fiber plasma membrane. Our analysis of biopsies and transplanted populations provides strong evidence that most adult muscle stem cell activity resides within the CD56+/CD29+ population. This study does not exclude the possibility that extralaminar PAX7 cells or rare PAX7 satellite cells that do not exhibit CD56 and CD29 co-expression have myogenic properties. Moreover, the possibility that a subpopulation of PAX7+/CD56+/CD29+ cells can be further enriched for muscle stem cell functions merits investigation.
Engraftment, repopulation of the satellite niche, and response to additional injury clearly demonstrate that the human CD56+/CD29+ satellite cell population contains muscle stem cells. With respect to engraftment, our experiments resulted in a greater efficiency of human-derived fiber generation (Figures 4 and 5; Table 2) compared with other reports in which up to 100-fold greater (105) (Castiglioni et al., 2014) or similar (103) (Bareja et al., 2014) numbers of transplanted cells yielded inconsistent engraftment and lower fiber number. This discrepancy in engraftment among reports and the absence of evidence of repopulation of the niche or self-renewal in prior reports may reflect differences in cell populations studied or in the transplantation protocols such as the use of irradiation in our study, or differences in recipient mouse strains. Future improvements in isolation efficiency of CD56+/CD29+ satellite cells and engraftment protocols should maximize stem cell yield and function. Since the anesthetic bupivacaine is also an established agent to induce muscle injury in clinical settings (Scott et al., 2009) and may aid engraftment (this study), it is attractive for potential use in future muscle transplantation applications. Our study did not assess the potency of individual human muscle stem cells on a cell per cell basis, and definitive conclusions cannot be made about the full potential of adult human satellite cells. Such analysis is also complicated in xenotransplantation, where the severely immunocompromised host state would possibly affect muscle regeneration and therefore engraftment (Cooper et al., 2001; Ehrhardt et al., 2007). However, the robust engraftment after transplantation of tens of fiber-associated satellite cells or 1,000–5,000 sorted cells and the generation of tens of thousands of human myonuclei after reinjury suggest that the potency of human satellite cells is on par with their mouse counterparts.
With the approaches described in this paper, xenotransplantation of human muscle stem cells will be useful for evaluating differences in human muscle stem cell function in vivo in aged or diseased muscle and in response to pharmacological treatments or genetic modification. We also expect this system to be useful for comparing the regenerative capability of human-embryonic-stem-cell-derived or induced-pluripotent-stem-cell-derived muscle cells against naturally existing bona fide muscle stem cells.
In summary, we have characterized, isolated, and transplanted endogenous human satellite stem cells that can engraft, differentiate, replenish the niche, self-renew, and respond to injury. We show direct experimental evidence that in humans, muscle stem cell function resides within the satellite cell compartment and can be transferred directly within endogenous niches by fiber grafting or after isolation of satellite cells based on surface marker expression phenotype. Resident muscle stem cells in adult humans are a targetable cell population for clinical applications.
Experimental Procedures
Human Muscle Procurement
This study was conducted under the approval of Committee on Human Research at the University of California, San Francisco (UCSF). Biopsies were obtained from individuals undergoing surgery at UCSF. Written consent was obtained from all subjects.
Animal Care and Transplantation Studies
Mice were housed in a pathogen-free facility at the UCSF. All procedures were approved and performed in accordance with the UCSF Institutional Animal Care and Use Committee. All experiments were performed using (NSG) mice (The Jackson Laboratory). Irradiation was done on the day of transplantation. Human cells or myofibers were injected along with toxin or media in 50 μl into the NSG TA after radiation.
Analysis of Human Muscle Satellite Cells
Human samples were split and fixed fibers were prepared as previously described (Boldrin and Morgan, 2012) and immunostained. The remaining portion of the sample was frozen in isopentane chilled in liquid nitrogen. Transverse 6 μm (for analysis of satellite cell markers on human muscle sections) or 10 μm (for H&E staining) sections were prepared.
Live Human Muscle Fiber Isolation and Analysis
Human muscle samples were incubated for 1 hr in 0.2% type I collagenase (Sigma-Aldrich). Individual fibers were separated using a dissecting microscope, measured for length, and then taken up in a syringe for transplantation, culture, or immunostaining.
CD56+/CD29+ Cell Sorting
Freshly harvested human muscle was digested either immediately or after overnight storage in 30% fetal bovine serum (FBS) at 4°C. Viable singlets were depleted for CD45 and CD31 expressing cells and remaining cells were sorted for CD56+/CD29− and CD56+/CD29+ and collected. Cells to be transplanted were taken up in 50 μl of 20%FBS in DMEM supplemented with 10 μM Rho-associated protein kinase inhibitor in order to improve viability of dissociated cells (Goudenege et al., 2012).
qRT-PCR
Relative gene expression was determined from extracted RNA with primers for PAX7 and housekeeping gene GAPDH. The cycle threshold (Ct) value for detection of gene of interest was normalized against the Ct value of GAPDH, and relative changes were calculated according the ΔΔCt method.
NSG TA Analysis
Harvested muscles were frozen in isopentane chilled in liquid nitrogen. Serial 10-μm transverse sections of the whole muscle were analyzed by immunostaining.
Statistical Analysis
Means between or across groups were compared using t tests for experiments involving two groups, or ANOVA was used when comparisons were made across more than two groups. Because the experiments were exploratory, multiple comparisons adjustments were not made. The p values are indicated by asterisks (∗p < 0.05, ∗∗p < 0.001, and ∗∗∗p < 0.0001) and NS (not significant).
See the Supplemental Experimental Procedures for details regarding antibodies, primers, fluorescence-activated cell sorting (FACS) controls, and staining procedures.
Author Contributions
X.X. designed experiments, performed the majority of the research, analyzed data, and wrote the manuscript. K.J.W., G.K., R.H., C.G., and H.T. designed and performed experiments and helped analyze data. H.S., S.H., R.S., P.D.K., and W.Y.H. acquired human muscle samples and commented on the manuscript. J.H.P. designed the research, interpreted data, oversaw the research, and wrote the manuscript.
Acknowledgments
This work was funded by CIRM New Faculty Physician Scientist Award RN3-06504 and the University of California, San Francisco Program in Breakthrough Biomedical Research Opportunity Award to J.H.P. X.X. is supported by NIH training grant T32DK007573-23. We thank Nancy Hills for statistical analysis, Pamela Derish for editorial comments, Joanna Dreux for comments and figure preparation, and Stanley Tamaki for the conjugated PAX7 antibody. We gratefully acknowledge the constructive suggestions of Ophir Klein, Sarah Knox, and Jeffrey Bush and the sharing of unpublished data by Stephane Corbel and Helen M. Blau early on in the project.
Published: September 8, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.07.016.
Supplemental Information
References
- Bareja A., Holt J.A., Luo G., Chang C., Lin J., Hinken A.C., Freudenberg J.M., Kraus W.E., Evans W.J., Billin A.N. Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis. PLoS ONE. 2014;9:e90398. doi: 10.1371/journal.pone.0090398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L., Morgan J.E. Human satellite cells: identification on human muscle fibres. PLoS Curr. 2012;3:RRN1294. doi: 10.1371/currents.RRN1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L., Muntoni F., Morgan J.E. Are human and mouse satellite cells really the same? J. Histochem. Cytochem. 2010;58:941–955. doi: 10.1369/jhc.2010.956201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavaud S., Agbulut O., D’Honneur G., Nizard R., Mouly V., Butler-Browne G. Preparation of isolated human muscle fibers: a technical report. In Vitro Cell. Dev. Biol. Anim. 2002;38:66–72. doi: 10.1290/1071-2690(2002)038<0066:POIHMF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Brimah K., Ehrhardt J., Mouly V., Butler-Browne G.S., Partridge T.A., Morgan J.E. Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum. Gene Ther. 2004;15:1109–1124. doi: 10.1089/hum.2004.15.1109. [DOI] [PubMed] [Google Scholar]
- Castiglioni A., Hettmer S., Lynes M.D., Rao T.N., Tchessalova D., Sinha I., Lee B.T., Tseng Y.H., Wagers A.J. Isolation of progenitors that exhibit myogenic/osteogenic bipotency in vitro by fluorescence-activated cell sorting from human fetal muscle. Stem Cell Reports. 2014;2:92–106. doi: 10.1016/j.stemcr.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.W., Corselli M., Péault B., Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J. Biomed. Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.A., Olsen I., Zammit P.S., Heslop L., Petrie A., Partridge T.A., Morgan J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cooper R.N., Irintchev A., Di Santo J.P., Zweyer M., Morgan J.E., Partridge T.A., Butler-Browne G.S., Mouly V., Wernig A. A new immunodeficient mouse model for human myoblast transplantation. Hum. Gene Ther. 2001;12:823–831. doi: 10.1089/104303401750148784. [DOI] [PubMed] [Google Scholar]
- Crameri R.M., Langberg H., Magnusson P., Jensen C.H., Schrøder H.D., Olesen J.L., Suetta C., Teisner B., Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J. Physiol. 2004;558:333–340. doi: 10.1113/jphysiol.2004.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R., Arpke R.W., Irion S., Dimos J.T., Grskovic M., Kyba M., Perlingeiro R.C. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt J., Brimah K., Adkin C., Partridge T., Morgan J. Human muscle precursor cells give rise to functional satellite cells in vivo. Neuromuscul. Disord. 2007;17:631–638. doi: 10.1016/j.nmd.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Goudenege S., Lebel C., Huot N.B., Dufour C., Fujii I., Gekas J., Rousseau J., Tremblay J.P. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol. Ther. 2012;20:2153–2167. doi: 10.1038/mt.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.K., Banks G.B., Chamberlain J.S., Olwin B.B. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci. Transl. Med. 2010;2:57ra83. doi: 10.1126/scitranslmed.3001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F., Charifi N., Denis C., Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004;29:120–127. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström M., Thornell L.E. New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-lifters and sedentary men. Histochem. Cell Biol. 2009;132:141–157. doi: 10.1007/s00418-009-0606-0. [DOI] [PubMed] [Google Scholar]
- Mackey A.L., Kjaer M., Charifi N., Henriksson J., Bojsen-Moller J., Holm L., Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve. 2009;40:455–465. doi: 10.1002/mus.21369. [DOI] [PubMed] [Google Scholar]
- Marg A., Escobar H., Gloy S., Kufeld M., Zacher J., Spuler A., Birchmeier C., Izsvák Z., Spuler S. Human satellite cells have regenerative capacity and are genetically manipulable. J. Clin. Invest. 2014;124:4257–4265. doi: 10.1172/JCI63992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes S.J., Nahai F. Quality Medical Publishing; 1997. Reconstructive Surgery: Principles, Anatomy and Technique. [Google Scholar]
- Meng J., Chun S., Asfahani R., Lochmuller H., Muntoni F., Morgan J. Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol. Ther. 2014;22:1008–1017. doi: 10.1038/mt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.G., Sharma K.R., Pavlath G.K., Gussoni E., Mynhier M., Lanctot A.M., Greco C.M., Steinman L., Blau H.M. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Montarras D., Morgan J., Collins C., Relaix F., Zaffran S., Cumano A., Partridge T., Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Ono Y., Boldrin L., Knopp P., Morgan J.E., Zammit P.S. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev. Biol. 2010;337:29–41. doi: 10.1016/j.ydbio.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. Myoblast transplantation. Neuromuscul. Disord. 2002;12(Suppl 1):S3–S6. doi: 10.1016/s0960-8966(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Pavlath G.K., Thaloor D., Rando T.A., Cheong M., English A.W., Zheng B. Heterogeneity among muscle precursor cells in adult skeletal muscles with differing regenerative capacities. Dev. Dyn. 1998;212:495–508. doi: 10.1002/(SICI)1097-0177(199808)212:4<495::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Pisani D.F., Dechesne C.A., Sacconi S., Delplace S., Belmonte N., Cochet O., Clement N., Wdziekonski B., Villageois A.P., Butori C. Isolation of a highly myogenic CD34-negative subset of human skeletal muscle cells free of adipogenic potential. Stem Cells. 2010;28:753–764. doi: 10.1002/stem.317. [DOI] [PubMed] [Google Scholar]
- Plant D.R., Colarossi F.E., Lynch G.S. Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve. 2006;34:577–585. doi: 10.1002/mus.20616. [DOI] [PubMed] [Google Scholar]
- Reimann J., Brimah K., Schröder R., Wernig A., Beauchamp J.R., Partridge T.A. Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res. 2004;315:233–242. doi: 10.1007/s00441-003-0833-y. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J.D., Lunt A.I., Parry D.J., Partridge T.A. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell. Dev. Biol. Anim. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- Rozkalne A., Adkin C., Meng J., Lapan A., Morgan J.E., Gussoni E. Mouse regenerating myofibers detected as false-positive donor myofibers with anti-human spectrin. Hum. Gene Ther. 2014;25:73–81. doi: 10.1089/hum.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A., Doyonnas R., Kraft P., Vitorovic S., Blau H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W., Zimmermann K., Cramer M., Starzinski-Powitz A. Lymphocyte antigen Leu-19 as a molecular marker of regeneration in human skeletal muscle. Proc. Natl. Acad. Sci. USA. 1989;86:307–311. doi: 10.1073/pnas.86.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A.B., Miller J.M., Shieh K.R. Treating strabismus by injecting the agonist muscle with bupivacaine and the antagonist with botulinum toxin. Trans. Am. Ophthalmol. Soc. 2009;107:104–109. [PMC free article] [PubMed] [Google Scholar]
- Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Sherwood R.I., Christensen J.L., Conboy I.M., Conboy M.J., Rando T.A., Weissman I.L., Wagers A.J. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Silva-Barbosa S.D., Butler-Browne G.S., Di Santo J.P., Mouly V. Comparative analysis of genetically engineered immunodeficient mouse strains as recipients for human myoblast transplantation. Cell Transplant. 2005;14:457–467. doi: 10.3727/000000005783982837. [DOI] [PubMed] [Google Scholar]
- Silva-Barbosa S.D., Butler-Browne G.S., de Mello W., Riederer I., Di Santo J.P., Savino W., Mouly V. Human myoblast engraftment is improved in laminin-enriched microenvironment. Transplantation. 2008;85:566–575. doi: 10.1097/TP.0b013e31815fee50. [DOI] [PubMed] [Google Scholar]
- Skuk D., Paradis M., Goulet M., Chapdelaine P., Rothstein D.M., Tremblay J.P. Intramuscular transplantation of human postnatal myoblasts generates functional donor-derived satellite cells. Mol. Ther. 2010;18:1689–1697. doi: 10.1038/mt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P.S. All muscle satellite cells are equal, but are some more equal than others? J. Cell Sci. 2008;121:2975–2982. doi: 10.1242/jcs.019661. [DOI] [PubMed] [Google Scholar]
- Zhang Y., King O.D., Rahimov F., Jones T.I., Ward C.W., Kerr J.P., Liu N., Emerson C.P., Jr., Kunkel L.M., Partridge T.A., Wagner K.R. Human skeletal muscle xenograft as a new preclinical model for muscle disorders. Hum. Mol. Genet. 2014;23:3180–3188. doi: 10.1093/hmg/ddu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.