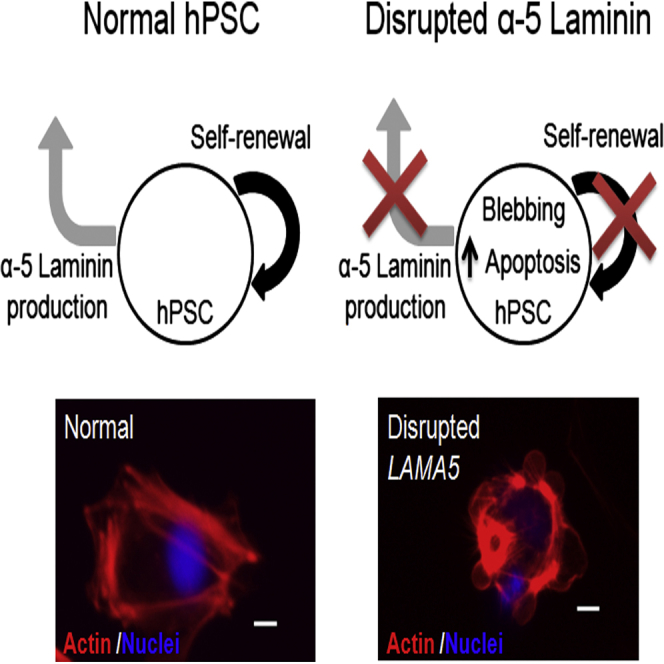
Summary
Substrate composition significantly impacts human pluripotent stem cell (hPSC) self-renewal and differentiation, but relatively little is known about the role of endogenously produced extracellular matrix (ECM) components in regulating hPSC fates. Here we identify α-5 laminin as a signature ECM component endogenously synthesized by undifferentiated hPSCs cultured on defined substrates. Inducible shRNA knockdown and Cas9-mediated disruption of the LAMA5 gene dramatically reduced hPSC self-renewal and increased apoptosis without affecting the expression of pluripotency markers. Increased self-renewal and survival was restored to wild-type levels by culturing the LAMA5-deficient cells on exogenous laminin-521. Furthermore, treatment of LAMA5-deficient cells with blebbistatin or a ROCK inhibitor partially restored self-renewal and diminished apoptosis. These results demonstrate that endogenous α-5 laminin promotes hPSC self-renewal in an autocrine and paracrine manner. This finding has implications for understanding how stem cells dynamically regulate their microenvironment to promote self-renewal and provides guidance for efforts to design substrates for stem cell bioprocessing.
Graphical Abstract
Highlights
-
•
α-5 laminin is produced by undifferentiated hPSCs under defined culture conditions
-
•
Disruption of endogenous α-5 laminin production causes hPSC apoptosis
-
•
Exogenous α-5 laminin rescues cell survival
-
•
α-5 laminin acts as a critical autocrine and paracrine factor for hPSC self-renewal
In this article, Masters and colleagues identify α-5 laminin as a critical component of the undifferentiated hPSC extracellular matrix that is necessary for hPSC survival and expansion. These findings demonstrate that endogenous α-5 laminin promotes hPSC self-renewal in both autocrine and paracrine manners and suggest a mechanism by which existing synthetic substrates are able to support undifferentiated hPSC culture.
Introduction
The extracellular matrix (ECM) is known to play an integral role in human pluripotent stem cell (hPSC) culture and maintenance. In the absence of appropriate substrate cues, hPSCs spontaneously differentiate, which has led to the development of numerous culture approaches and substrates intended to maintain pluripotency in hPSC cultures (Domogatskaya et al., 2008; Evseenko et al., 2009). Efforts in recent years (Chen et al., 2011; Mei et al., 2010; Melkoumian et al., 2010; Rodin et al., 2014b; Saha et al., 2011; Villa-Diaz et al., 2013) have specifically focused on the development of fully defined, xeno-free culture substrates as alternatives to the use of mouse embryonic fibroblast (MEF) feeder cells (Thomson et al., 1998) or Matrigel (Ludwig et al., 2006). Use of a defined, xeno-free culture environment is important for the performance of controlled in vitro investigations and for the clinical or commercial application of hPSCs (Azarin and Palecek, 2010; Chen et al., 2011).
Both natural and synthetic approaches have been used to generate fully defined hPSC culture substrates. ECM proteins such as vitronectin and laminins 511 and 521 have been shown to maintain hESC pluripotency and self-renewal (Braam et al., 2008; Domogatskaya et al., 2012; Miyazaki et al., 2012; Rodin et al., 2010, 2014a, 2014b; Yurchenco, 2011). However, not all ECM proteins are suitable for hPSC maintenance because collagens and fibronectin are not able to support an undifferentiated hPSC population (Azarin and Palecek, 2010; Braam et al., 2008; Evseenko et al., 2009; Miyazaki et al., 2012; Rodin et al., 2010; Villa-Diaz et al., 2013). Other fully defined hPSC culture environments have been produced by modification of substrates with recombinant E-cadherin (marketed as StemAdhere) (Nagaoka et al., 2010) or engineered peptide coatings (marketed as Synthemax) (Melkoumian et al., 2010).
Although many studies have applied exogenous ECM components to create hPSC culture substrates, relatively little attention has been given to the role of the endogenously produced ECM in hPSC self-renewal. Specifically, it is unknown whether there exists a common ECM “signature” produced by hPSCs cultured on substrates known to support the maintenance of undifferentiated hPSC culture. The identification of such an ECM signature—i.e., specific ECM components that are commonly produced across undifferentiated hPSCs maintained in different culture environments—could lead to a better understanding of how hPSCs regulate self-renewal.
In this study, we first identified α-5 laminin as a predominant ECM component produced endogenously by undifferentiated hPSCs—both human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs)—cultured on various defined substrates and then employed two different genetic manipulation strategies to disrupt α-5 laminin production to investigate the role of α-5 laminin in hPSC self-renewal and pluripotency. Our findings implicate α-5 laminin as a critical autocrine and paracrine factor that regulates hPSC survival and self-renewal.
Results
α-5 Laminin Is Produced by hPSCs under Defined Culture Conditions
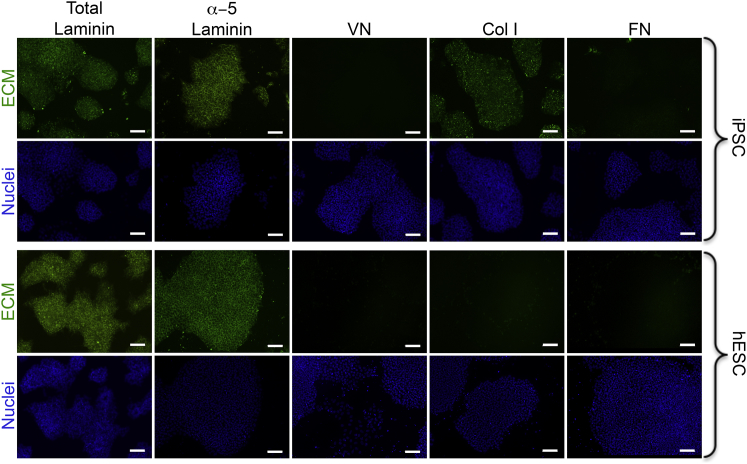
The ECM deposition profile of undifferentiated hPSCs cultured under defined conditions was evaluated by culturing H9 hESCs and 19-9-11 iPSCs on Synthemax, E-cadherin (StemAdhere), or recombinant human vitronectin in E8 medium for 5 days, followed by immunofluorescence detection of laminin, collagen I, fibronectin, and vitronectin (Figure 1; Figures S1A and S1B). Of these ECM proteins, only the production of laminin and, specifically, α-5 laminin was common across all substrates and both hPSC lines (Figure 1).
Figure 1.
Immunofluorescent Staining of ECM Proteins Deposited by hPSCs Cultured under Fully Defined Conditions
H9 hESCs and 19-9-11 iPSCs were cultured on Synthemax for 5 days in E8 medium and stained for the indicated ECM proteins. Production of laminin (and, in particular, α-5 laminin) was the only common ECM feature across all substrates and both cell lines. Scale bars, 100 μm. See also Figures S1 and S6. VN, vitronectin; Col I, collagen type I; FN, fibronectin.
Generation and Characterization of Inducible shRNA and Cas9 α-5 Laminin Knockout Lines
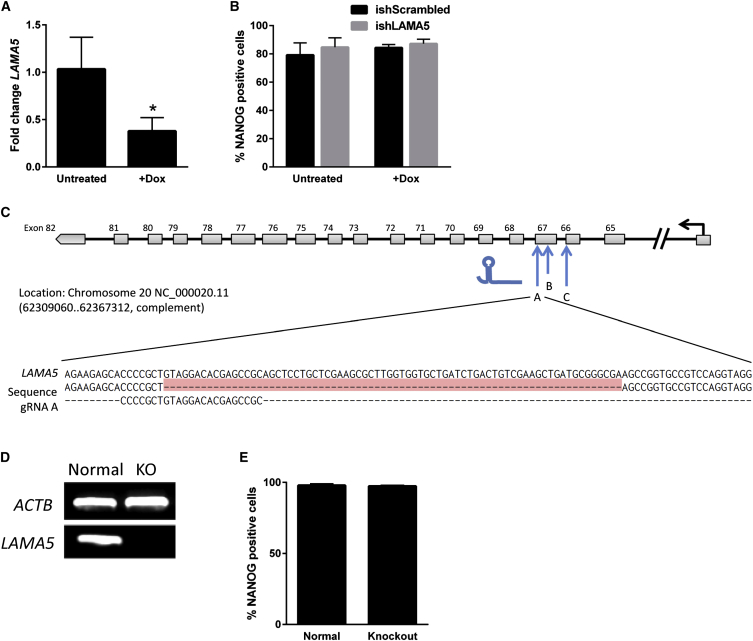
Two different genetic manipulation strategies were implemented to investigate the role of α-5 laminin in hPSC maintenance. In one approach, H9 and 19-9-11 cell lines were transduced with an inducible short hairpin RNA (shRNA) sequence (Table S1) targeting α-5 laminin (ishLAMA5) to achieve doxycycline (Dox)-induced knockdown of α-5 laminin production (Figure 2A; Figures S2A–S2D; Lian et al., 2013). Dox treatment of ishLAMA5 cell lines resulted in a 60% reduction in α-5 laminin expression (Figure 2A), and the karyotype of the 19-9-11 ishLAMA5 cell line was found to be normal (Figure S2E). Pluripotency characteristics were maintained during LAMA5 knockdown, as indicated by no significant change in the expression of the pluripotency marker Nanog (Figure 2B; Figures S2F and S2G).
Figure 2.
Generation of hPSC Knockdown and Knockout Lines
(A) Doxycycline treatment of the Dox-inducible LAMA5 19-9-11 shRNA cell line resulted in decreased LAMA5 expression relative to untreated ishLAMA5 cells, as measured by qRT-PCR after 3 days of culture on Synthemax (n = 3 independent samples/condition).
(B) Expression of the pluripotency marker Nanog was detected via flow cytometry and found to be maintained in ishLAMA5 19-9-11 cells and scrambled shRNA controls cultured on Synthemax for 5 days (n = 3 independent samples/condition).
(C) Schematic of the human LAMA5 locus showing gRNA targeting locations for Cas9-mediated gene disruption. Sanger sequencing results for the 19-9-11 KO line are shown below, with the red section indicating a homozygous deletion of the LAMA5 DNA sequence.
(D) Detection of LAMA5 in normal and homozygous knockout 19-9-11 iPSCs via RT-PCR using a primer within the deleted region of the knockout line. Lack of amplification in the KO line verifies homozygous deletion of the gene as detected by Sanger sequencing.
(E) Nanog expression by normal and KO 19-9-11 iPSCs as measured by flow cytometry after 5 days of culture on Synthemax (n = 3 independent samples/condition).
∗p < 0.01. Data are presented as mean ± SD. See also Figures S2 and S3.
In a separate approach, CRISPR-Cas9 gene editing was used in both H9 and 19-9-11 cell lines to create genetic mutations that resulted in loss of function of the LAMA5 gene (Ran et al., 2013b). A set of guide RNAs (gRNA) targeting LAMA5 (Figure 2C; Table S2) was transfected into the hPSCs concurrently with a Cas9-2A-GFP plasmid that encodes both Cas9 and GFP. Single GFP-positive cells were expanded into colonies, and the gRNA target sites amplified from the genomic DNA of each colony were Sanger-sequenced to confirm LAMA5 gene disruption (Figure 2C). From this screening process, we identified an H9 line containing a heterozygous mutation (Figures S3A and S3B) and a 19-9-11 line containing a homozygous deletion/frameshift mutation (Figure 2C). All mutated lines maintained expression of the pluripotency marker Nanog while exhibiting significantly reduced expression of α-5 laminin (Figures 2D and 2E; Figures S3C–S3E). The homozygous 19-9-11 knockout cell line was found to exhibit a normal human karyotype (Figure S3F).
Disrupted α-5 Laminin Production Increases Apoptosis, Causing Impaired Self-Renewal
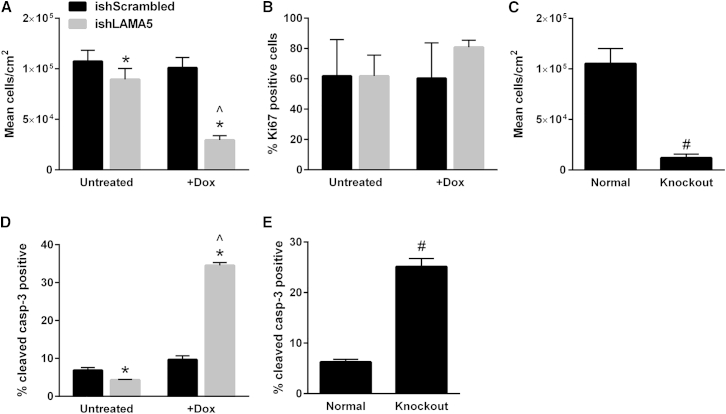
Under defined culture conditions on the Synthemax substrate, treatment of ishLAMA5 hPSC lines with Dox resulted in an immediately apparent reduction in cell number relative to both untreated and ishRNA sequence-scrambled controls (Figure 3A; Figure S4A). Several other factors related to cell self-renewal were then examined to identify the cause of this disparity in cell number. Production of Ki-67, a marker present during active progression through the cell cycle (Gerdes et al., 1984), was not significantly altered by α-5 laminin knockdown in either H9 (Figure S4B) or 19-9-11 (Figure 3B) cells. Cell-cycle distribution in α-5 laminin knockdown and untreated cells was also quantified and found not to be significantly affected by the disruption in α-5 laminin production (Figures S4C and S4D). H9 and 19-9-11 cells subjected to Cas9-mediated knockout of α-5 laminin function behaved similarly as the shRNA knockdown cell lines, exhibiting dramatically reduced cell numbers relative to control hPSCs cultured on Synthemax (Figure 3C; Figure S4E).
Figure 3.
Disruption of α-5 Laminin Production Impacts 19-9-11 Cell Number and Apoptosis
(A) Dox treatment of ishLAMA5 19-9-11 cells cultured on Synthemax for 5 days resulted in decreased cell density compared with untreated or scrambled ishRNA controls. Cells were initially seeded at 6,600 cells/cm2.
(B) Expression of the proliferation marker Ki67 as measured via flow cytometry after 5 days of culture on Synthemax.
(C) Cas9-mediated knockout of LAMA5 in 19-9-11 cells also resulted in decreased cell density.
(D) Dox treatment significantly increased apoptosis in 19-9-11 ishLAMA5 cells cultured for 5 days on Synthemax, as indicated by measurement of caspase-3 via flow cytometry.
(E) Cas9-mediated knockout of LAMA5 in 19-9-11 cells increased apoptosis relative to wild-type (normal) 19-9-11 iPSCs cultured on Synthemax for 5 days.
∗p < 0.001 versus untreated cells, ˆp < 0.001 versus Dox-treated scrambled control cells, #p < 0.001 versus wild-type 19-9-11 iPSCs (n = 3 independent samples/condition). Data are presented as mean ± SD. See also Figures S4 and S5.
Apoptosis in H9 and 19-9-11 cultures with disrupted α-5 laminin production was evaluated to further investigate the cause of the observed decline in cell number in α-5 laminin knockdown and knockout hPSC lines. Increased production of both cleaved caspase-3 (Li et al., 2014) and annexin V (van Engeland et al., 1998; Zhang et al., 1997) was found in both H9 and 19-9-11 inducible knockdown cell lines relative to scrambled controls (Figure 3D; Figure S4F). H9 and 19-9-11 α-5 laminin knockout cell lines also exhibited significantly increased expression of cleaved caspase-3 and annexin V relative to unmodified hPSCs (Figure 3E; Figures S5A and S5B).
Rescue of hPSC Self-Renewal via Culture on Laminin-521
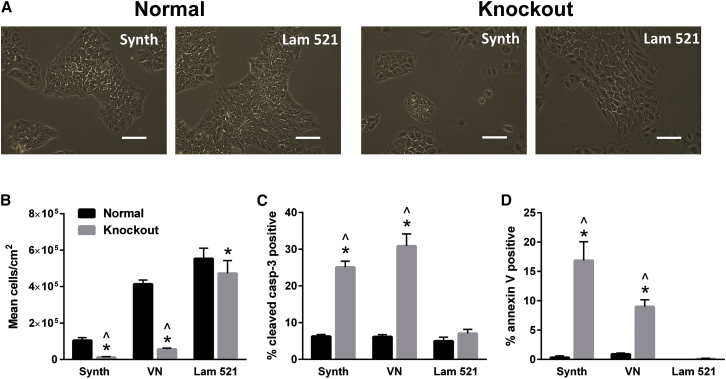
hPSCs possessing the Cas9-mediated disruption to LAMA5 were cultured on exogenously supplied laminin-521 to determine whether the noted decrease in cell number and increase in apoptosis could be reversed by the presentation of α-5 laminin to the cells. Indeed, culture of knockout H9 and 19-9-11 cells on exogenous laminin-521 restored cell expansion to levels near those found in normal hPSC controls (Figures 4A and 4B; Figures S5C–S5E). The extent of rescue was dependent on the laminin-521 coating density (Figure S5F). Although the expansion of normal hPSCs was improved by culture on exogenous vitronectin relative to Synthemax, use of vitronectin as a culture substrate was unable to fully rescue the expansion deficit resulting from LAMA5 knockout. This finding indicates that rescue of the knockout cells was likely specific to α-5 laminin and not simply due to the presence of an exogenous ECM component that supports undifferentiated hPSC culture. Laminin-111 was not used as a comparison condition because pure human laminin-111 does not support the attachment of normal hPSCs (Figure S6A).
Figure 4.
Culture of 19-9-11 LAMA5 Knockout Cells on Laminin-521, but Not Vitronectin, Results in Rescue of Cell Number
(A) Bright-field images of wild-type and LAMA5 KO 19-9-11 iPSCs cultured on laminin-521 (Lam 521) or Synthemax (Synth) for 3 days, illustrating differences in cell density.
(B) Culture of Cas9-mediated LAMA5 KO 19-9-11 iPSCs on laminin-521 rescued cell number, as indicated by analysis of mean cell density after 5 days of culture on Synthemax, vitronectin, or laminin-521. Cells were initially seeded at 6,600 cells/cm2.
(C and D) Apoptosis of 19-9-11 LAMA5 KO cells was also restored to wild-type levels by culture on laminin-521, as indicated by measurement of caspase-3 (C) and annexin V (D) via flow cytometry after 5 days of culture.
Scale bars, 100 μm. ∗p < 0.001 versus wild-type 19-9-11 iPSCs cultured on the same substrate, ˆp < 0.001 versus knockout cells cultured on laminin-521 (n = 3 independent samples/condition). Data are presented as mean ± SD. See also Figure S5.
Culture on exogenously supplied laminin-521 was also able to inhibit apoptosis in LAMA5 knockout H9 (Figures S6A and S6B) and 19-9-11 (Figures 4C and 4D) cells. Specifically, culture of knockout cells on laminin-521 resulted in a full reduction of the apoptosis markers cleaved caspase-3 and annexin V to levels observed in wild-type H9 and 19-9-11 cells. Culture on vitronectin was not able to reduce apoptosis markers in the knockout lines.
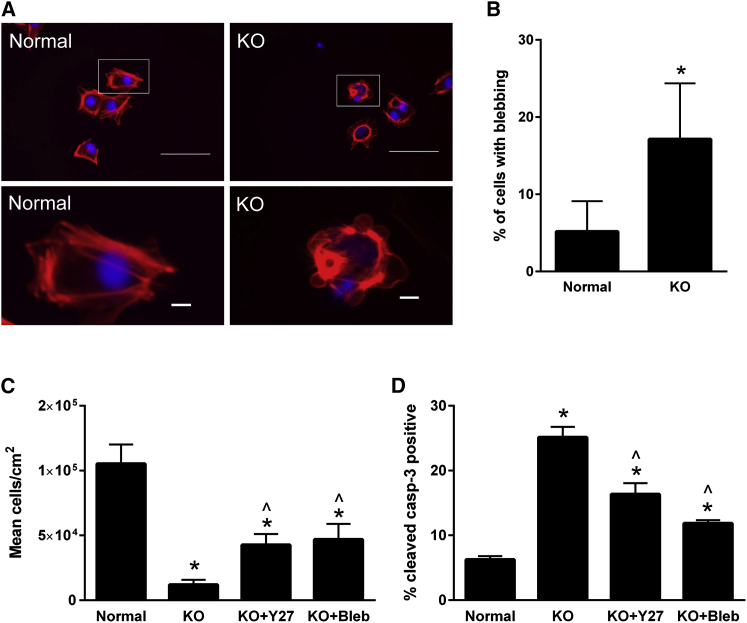
Apoptotic Response Arises from Cell Blebbing and Is Partly Reversed by Contractile Inhibition
The results reported in Figures 3 and 4 suggest that increased apoptosis is at least partly responsible for the decreases in cell expansion observed upon disruption of α-5 laminin production. Additionally, a significant increase in cell blebbing was observed in knockout cells cultured on Synthemax (Figures 5A and 5B; Figure S6B), indicating a role for cytoskeletal contractility in regulating apoptosis under these conditions. Cell survival was not improved by the addition of soluble laminin-521 to suspension cultures of LAMA5 knockout 19-9-11 hPSCs (Figure S6C), suggesting that binding of α-5 laminin to cell surface receptors alone is not sufficient for cell rescue but that the presentation of α-5 laminin on the substrate surface may be needed to provide appropriate ECM contacts for regulating cytoskeletal tension. Therefore, we next investigated mechanisms linking α-5 laminin to this apoptotic response by applying to H9 and 19-9-11 knockout lines a panel of small molecule inhibitors targeting a range of apoptotic pathway components as well as elements of the cytoskeleton (Table S3), followed by evaluation of cell expansion and apoptosis. In both the H9 and 19-9-11 cell lines with the LAMA5 knockout, treatment with either a Rho kinase (ROCK) inhibitor (Y27632) or the myosin inhibitor blebbistatin was able to increase cell expansion, resulting in partial rescue. These levels of expansion remained lower than those observed in normal hPSCs cultured on Synthemax (Figure 5C; Figure S6D). This partial rescue of cell expansion was accompanied by a decrease in the expression of apoptosis markers relative to the untreated knockout population (Figure 5D; Figure S6E). These results suggest that relieving cell tension can at least partly restore hPSC expansion by inhibiting apoptosis in the absence of α-5 laminin.
Figure 5.
Partial Rescue of Cell Self-Renewal by Treatment with Inhibitors of Cell Contractility
(A) F-actin staining of normal and KO 19-9-11 cultures after 48 hr on Synthemax. White boxes indicate regions examined at a higher magnification. Scale bars, 100 μm (top) and 10 μm (bottom).
(B) Percentage of observed blebbing cells in normal and KO 19-9-11 cultures, calculated as the number of cells with observed blebs over the total number of cells per image field.
(C) Treatment with inhibitors of cell contractility (blebbistatin [Bleb] and Y27632 [Y27]) was able to partially rescue cell number for LAMA5 KO 19-9-11 iPSCs cultured on Synthemax for 5 days. Cells were initially seeded at 6,600 cells/cm2.
(D) Treatment with blebbistatin or Y27632 was also able to decrease apoptosis relative to untreated LAMA5 KO 19-9-11 iPSCs, as measured by flow cytometric analysis of cleaved caspase-3 after 5 days of culture on Synthemax.
∗p < 0.001 versus wild-type 19-9-11 iPSCs, ˆp < 0.001 versus LAMA5 knockout iPSCs. n = 3 independent samples/condition. Data are presented as mean ± SD. See also Figure S6.
Discussion
In this study, we first identified laminin as a prevalent signature component of the ECM produced endogenously by undifferentiated H9 hESCs and 19-9-11 iPSCs cultured on three different defined substrates and subsequently examined the role of endogenously produced α-5 laminin in regulating the self-renewal and pluripotency marker expression of these stem cells. We targeted the α-5 chain specifically because the majority of integrin binding activity in the laminin molecule is thought to be conferred by the C-terminal globular domains encoded on the α strand (Beck et al., 1990; Deutzmann et al., 1990; Ido et al., 2006; Li et al., 2002; Miner and Yurchenco, 2004). Furthermore, hPSCs predominantly express the α6β1 integrin, which interacts strongly with these laminin globular domains (Evseenko et al., 2009; Li et al., 2002; Meng et al., 2010; Miyazaki et al., 2012; Rodin et al., 2010). Using two different techniques to disrupt α-5 laminin production in both hESC and iPSC lines, we found that hPSC expression of the pluripotency marker Nanog was not strongly dependent on α-5 laminin production but that hPSC self-renewal diminished as a result of apoptosis in the absence of this specific ECM component. Importantly, this apoptosis could be reduced by supplying the knockout cells with an exogenous source of α-5 laminin, such as culturing them on a laminin-521 substrate (Rodin et al., 2014b).
To link this apoptotic response to an ECM-dependent mechanism, we tested heterozygous and knockout LAMA5 lines against several small-molecule inhibitors and identified blebbistatin and the ROCK inhibitor Y27632 as being able to reduce the apoptotic response of the knockout lines cultured under defined conditions. Blebbistatin and Y27632 are known to act in pluripotent stem cells by preventing membrane blebbing, a phenomenon that was observed during culture of α-5 laminin knockout cells under defined conditions. These agents prevent blebbing via inhibition of contractility (Chen et al., 2010, 2014; Ohgushi et al., 2010; Ohgushi and Sasai, 2011; Watanabe et al., 2007), which may reduce anoikis, a type of apoptotic cell death that occurs because of a lack of cell-cell and cell-ECM contacts (Chen et al., 2010; Kovács et al., 2004; Ohgushi et al., 2010; Ohgushi and Sasai, 2011; Watanabe et al., 2007). Although contractility inhibition did reduce apoptosis in α-5 laminin-deficient cells, the restoration of hPSC self-renewal was only partial, which does suggest the involvement of additional signaling mechanisms in the hPSC response to α-5 laminin.
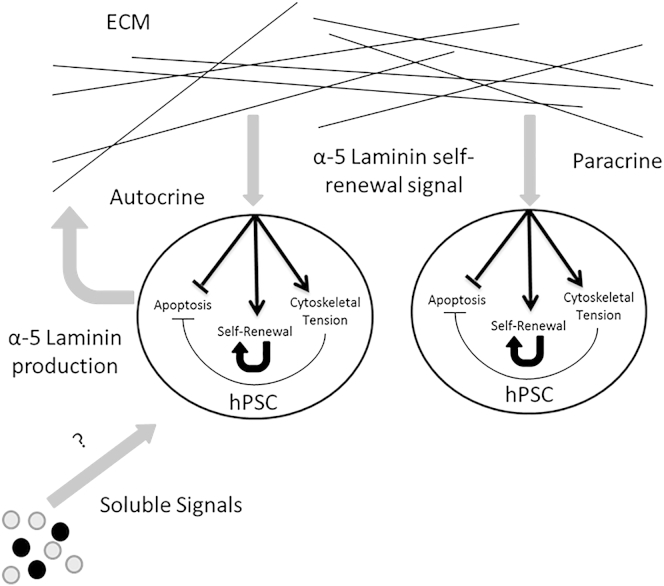
The specificity of the hPSC apoptotic response to a lack of proper ECM contacts highlights the importance of substrate interactions in regulating hPSC survival. Work by our own group suggests that differentiating pluripotent stem cells produce an ECM that is synergistic with the differentiation cues given to the cells (Laperle et al., 2015). Meanwhile, recent work by Meng et al. (2012) suggests that matrices, media, and the soluble growth factors to which hPSCs are exposed during culture have a synergistic effect on the growth and attachment of hPSCs under defined culture conditions. For instance, in mouse mesangial and early endoderm progenitor cells, transforming growth factor β (TGF-β) signaling has been shown to drive laminin production and increase the production of other ECM components (Jiang et al., 2005; Sugiyama et al., 2013; Tonary and Carnegie, 1996; Wells and Discher, 2008). It is possible that the α-5 laminin self-renewal signal, combined with other soluble components, drives hPSCs to produce a matrix that synergizes with these self-renewal signals. The hPSCs subsequently produce a matrix rich in α-5 laminin and receive supportive self-renewal signals from that matrix in an autocrine and paracrine signaling loop (Figure 6). Although this endogenous ECM may be necessary for efficient self-renewal, it is unlikely to be sufficient to fully support this process on its own.
Figure 6.
Proposed Autocrine and Paracrine Positive Feedback Loops in α-5 Laminin Self-Renewal Signaling
Undifferentiated hPSCs attach to a surface and receive soluble self-renewal cues such as TGF-β. As part of the cellular response to these maintenance cues, the hPSCs begin to produce an ECM enriched in α-5 laminin. Signaling from this ECM microenvironment, in turn, synergizes with the existing soluble cues and enables efficient self-renewal of cells in contact with the endogenous ECM.
In this work, we developed multiple tools to interrogate this autocrine and paracrine signaling loop involving α-5 laminin production by hPSCs. The use of the inducible short hairpin lines allowed us to control the dynamics of α-5 laminin production in a reversible manner. However, the interpretation of results is complicated by the action of the inducer, doxycycline, itself on cells; it is known to inhibit matrix remodeling proteinases (Gajbhiye et al., 2014; Nip et al., 1993). In addition, knockdown was partial and variable among hairpins from cell line to cell line, with our best hairpin providing only ∼60% knockdown efficiency. The Cas9-mediated knockout lines were developed to address these drawbacks with the hairpin system and provide a complete removal of the globular domains of α-5 laminin in hPSCs. The Cas9 system can easily be used, by simple modification of the guide RNA, to remove other domains of α-5 laminin and, in general, provides unprecedented precision in disrupting molecular signaling domains in large, complex proteins like ECM components. Application of the Cas9 tool in hPSCs offers a powerful way to study stem cell signaling, particularly in multicellular autocrine and paracrine contexts that involve reprogramming and differentiation.
Together, the findings reported here have implications with respect to not only understanding how existing substrates maintain hPSC self-renewal but also for designing new, defined substrates for hPSC culture. α-5 laminin is known to be present in numerous widely used but undefined hPSC culture systems. For instance, the functionality of MEF feeder cells in maintaining hPSCs is based on MEF production of laminin 511 (Hongisto et al., 2012). Additionally, α-5 laminin has been detected in Matrigel, perhaps the most widely used substrate for hPSC propagation (Hughes et al., 2010). Based on the results reported in this work, we put forth the hypothesis that the ability of many fully defined substrates to support hPSC self-renewal (Amit et al., 2011; Azarin and Palecek, 2010; Braam et al., 2008; Celiz et al., 2014; Chen et al., 2011; Evseenko et al., 2009; Klim et al., 2010; Mei et al., 2010; Melkoumian et al., 2010; Miyazaki et al., 2012; Rodin et al., 2010, 2014b; Saha et al., 2011; Villa-Diaz et al., 2010, 2013; Weber et al., 2010; Zweigerdt et al., 2011) is likely due, in part, to their support of α-5 laminin deposition by the cultured cells. An illustrative example of such a substrate is a peptide coating based on repeats of a heparin binding motif that is designed to promote stem cell attachment via binding of extracellular glycosaminoglycan (GAG) domains on the stem cell surface (Klim et al., 2010). However, because of the high-affinity interactions that occur between heparin and the globular domains of α-5 laminin (Hozumi et al., 2009; Katagiri et al., 2014), it is also likely that these GAG-binding domains are able to bind secreted ECM proteins, specifically laminins. In another example, polymer substrate screening studies have identified specific chemical functionalities, namely carboxylic acid and ester residues, as critical polymer features for the support of hPSC growth (Mei et al., 2010; Saha et al., 2011). These same residues, along with substrate surface potential, are thought to control the adsorption and conformation of laminin molecules on a surface (Lin et al., 2014; Rodríguez Hernández et al., 2007), suggesting that the ability of these materials to support hPSC culture may be at least partly related to laminin deposition. Synthetic polymer substrates such as poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH) (Qian et al., 2014; Villa-Diaz et al., 2010) may also support hPSC expansion via a similar mechanism because we have observed the production of α-5 laminin by hESCs cultured on this substrate (Figure S6F).
We conclude that α-5 laminin endogenously produced by hPSCs is a critical component of the undifferentiated pluripotent stem cell ECM and that it is necessary for the survival and expansion of these cells. This work describes an autocrine and paracrine mechanism of self-renewal signaling through the endogenous ECM of hPSCs and is important in understanding the signals regulating self-renewal and hPSC/substrate interactions. Our findings have implications for the design and optimization of hPSC culture substrates and scaffolds, whose performance in supporting undifferentiated hPSCs may be related to the ability of the environment to support the deposition and production of α-5 laminin.
Experimental Procedures
hPSC Culture
H9 hESCs, 19-9-11 iPSCs, and all genetically modified lines derived from these parental lines (WiCell) were maintained in E8 medium on tissue culture polystyrene (TCPS) plates (BD Falcon, Corning) coated with Matrigel (WiCell) as described previously (Chen et al., 2011). Briefly, hESCs were cultured in 6-well plates (BD Falcon) coated with Matrigel (83 μg/well) in 2.5 ml of E8 medium/well. Cells were split at a ratio of 1:12 every 5 days with Versene solution (Life Technologies). For matrix production studies, cells were singularized with Accutase (Innovative Cell Technologies) and seeded in E8 medium, with 5 μM Y27632 (Stemgent) present for the first 24 hr. In subsequent experiments involving genetically modified cell lines, cells were singularized with Accutase and seeded using mTeSR1 medium (WiCell), with 5 μM Y27632 present for the first 24 hr. H1 and CHB10 hESCs were cultured on PMEDSAH as described by Villa-Diaz et al. (2010).
ECM Coating Preparation
Six- and 12-well TCPS plates were coated with a range of defined substrates according to the manufacturer’s recommendations. Vitronectin (provided by the lab of Dr. Jamie Thomson, University of Wisconsin-Madison) or laminin-521 (BioLamina) were diluted in PBS and coated onto TCPS plates at a density of 0.61 μg/cm2 overnight at 37°C. Synthemax (Corning) was coated onto TCPS at a density of 3.3 μg/cm2 in PBS and incubated overnight at 37°C. StemAdhere coatings were achieved by diluting 240 μl StemAdhere (STEMCELL Technologies) in 6 ml of StemAdhere dilution buffer, with 1 ml of this solution (1.02 μg/cm2) placed into each well of a 6-well non-tissue culture polystyrene plate (BD Falcon) and incubated overnight at 37°C. Prior to cell seeding, all plates were washed with sterile PBS to remove any non-adsorbed coating.
Generation and Validation of Inducible shRNA hPSC Lines
An inducible knockdown vector targeting the α-5 chain of laminin (LAMA5) was constructed by combining a small interfering RNA (siRNA) sequence (LAMA5, catalog no. s8066, Life Technologies) with an existing Tet-pLKO-puro backbone (Addgene, plasmid 21915). These vectors were co-transfected with the helper plasmids psPAX2 and pMD2.G (Addgene, plasmids 12260 and 12259) into HEK293TN cells (System Biosciences) for lentivirus particle production. Virus-containing media were collected 48, 72, 96, and 120 hr after transfection. The supernatant was first concentrated using Lenti-XTM Concentrator (Clontech Laboratories) according to the manufacturer’s instructions for storage at −80°C, and then used for hPSC infection in the presence of 6 μg/ml polybrene (Sigma). Lentiviral transduction was performed on hPSCs by plating 100,000 cells/well in a 6-well plate with 2 ml mTeSR and incubating with 25 μl viral supernatant overnight. Transduced cells were cultured in mTeSR1 medium on Matrigel for at least 3 days and then selected and clonally isolated in mTeSR1 based on resistance to 1 μg/ml puromycin (Lian et al., 2013).
Screening for knockdown efficiency was conducted via RT-PCR and qRT-PCR against the human LAMA5 gene. For RT-PCR, total RNA was extracted using DNA/RNA Shield with Quick-RNA MiniPrep (Zymo Research) according to the manufacturer’s instructions. cDNA was generated from 1 μg of RNA using Omniscript reverse transcriptase (QIAGEN) and Oligo-dT(20) primers (Life Technologies). RT-PCR was performed using GoTaq Green Master Mix (Promega) using primers (Table S4) from Integrated DNA Technologies, and 2% agarose gel electrophoresis was performed to visualize the PCR products. GAPDH and β-actin (ACTB) were used as endogenous controls. qRT-PCR for LAMA5 was performed as described in a subsequent section. This approach was used to produce the H9 ishLAMA5 (WiCell line WIP01e-H9ishLAMA5), H9 ishSCR (WiCell line WIP02e-H9ishSCR), 19-9-11 ishLAMA5 (WiCell line WIP03i-IPSishLAMA5), and 19-9-11 ishSCR (WiCell line WIP04i-IPSishSCR) cell lines. The 19-9-11 ishLAMA5 cell line was submitted to WiCell for karyotyping.
Generation and Validation of Cas9 Gene-Disrupted Lines
Cas9-mediated knockout (KO) of LAMA5 in hESCs and iPSCs was performed according to the protocol published by Ran et al. (2013b). H9 and 19-9-11 cells were first singularized with Accutase (Innovative Cell Technologies) and electroporated using a Bio-Rad Gene Pulser XL with 2 μg of each guide RNA (Table S1), 2 μg Cas9 plasmid (Addgene, plasmid 44719), and 1 μg of GFP plasmid per one million cells. Three million cells were used in each electroporation. Electroporated cells were plated (100,000 cells/cm2) onto Matrigel-coated plates in mTESR1 medium containing 5 μM Y27632 (Stemgent) and cultured for 48 hr. After 48 hr, cells were again singularized with Accutase and sorted for GFP expression via fluorescence-activated cell sorting (FACS) (BD FACSAria). Sorted cells were again re-plated on Matrigel at a low density of 1000 cells/cm2 and allowed to expand for 7—10 days in mTeSR medium. Individual colonies were isolated using an AMG EVOS XL core cloning scope and expanded on Matrigel (H9) or laminin-521 (19-9-11) for another 7-14 days, after which genomic DNA was isolated from each clonal sample for an assay of LAMA5 disruption.
To assay target gene disruption, 500 ng of genomic DNA was isolated from cells using the QIAGEN DNeasy blood and tissue kit and combined with genomic DNA primers (final concentration, 0.2 μM) from IDT (Table S5) and AccuPrime high-fidelity Taq polymerase (Life Technologies) at the recommended annealing temperatures for 35 cycles on an Eppendorf Mastercycler Nexus GSX1. PCR products were purified using a QIAGEN QIAquick PCR purification kit. Genomic sequencing was performed by mixing 500 ng of purified genomic PCR product with 1 μl of the genomic primer used to amplify that segment, and purified water was added to bring the total volume to 25 μl. Samples were submitted to the University of Wisconsin Biotechnology Center DNA Sequence Facility for Sanger sequencing. Sequencing results were visualized using Benchling software.
Heterozygous mutation of LAMA5 and gene disruption efficiency were assessed using a T7 endonuclease I assay as described previously (Cho et al., 2013; Ran et al., 2013a, 2013b; Yang et al., 2013). Briefly, 1 μg of purified genomic DNA was hybridized in NEB buffer II (New England Biolabs), followed by addition of 1 μl of T7 endonuclease to each T7 digestion and incubation at 37°C for 15 min prior to addition of 2.1 μl stop solution. Purified genomic DNA, hybridization product, and T7 digest product were visualized on a 2% agarose gel and imaged using a Bio-Rad Gel Doc XR+. This approach was used to generate the H9 Cas9 Het (WiCell line WIP07e-H9Cas9Het) and 19-9-11 Cas9 KO (WiCell line WIP05i-IPSCas9KO) cell lines, and the latter was submitted for karyotype analysis (WiCell).
Immunocytochemical Detection of ECM
The deposition of endogenous ECM proteins onto uncoated and ECM-coated TCPS plates by H9 hESCs and 19-9-11 iPSCs was assessed via immunocytochemical staining. Samples were fixed in 10% formalin (Sigma) and permeabilized using 1% Triton X-100 (Sigma) in PBS. Primary antibodies against human fibronectin (Santa Cruz Biotechnology, catalog no. SC80559), laminin (Abcam, catalog no. ab30320), α-5 laminin (Millipore, catalog no. MAB1924), vitronectin (Santa Cruz Biotechnology, catalog no. SC74485), collagen type IV (Santa Cruz Biotechnology, catalog no. SC52317), and collagen type 1 (Sigma, catalog no. C2456) were diluted 1:500 in 3% BSA in PBS and allowed to incubate overnight at 4°C, followed by rinsing in PBS and incubation with Alexa Fluor 488- and 568-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Life Technologies, catalog nos. A10667 and A11031, respectively) in 3% BSA at room temperature for 1 hr. Cell nuclei were counterstained with 1 μg/ml DAPI (4′,6-diamidino-2-phenylindole dihydrochloride, Life Technologies, catalog no. D1306), and fluorescent images were captured using a Nikon IX51 epifluorescence microscope.
Apoptosis, Cell Cycle, and Pluripotency Analysis via Flow Cytometry
hPSCs cultured on the aforementioned coatings were singularized with Accutase and fixed in cold 90% methanol. Primary antibodies against annexin V (1:500, Santa Cruz Biotechnology, catalog no. SC74458), Nanog (1:500, Cell Signaling Technology, catalog no. 4893), cleaved caspase-3 (1:250, Cell Signaling Technology, catalog no. 9664), Ki67 (1:500, Abcam, 15580), and an isotype control (1:500 and 1:250, mouse immunoglobulin G1 [IgG1], BD Biosciences, catalog no. 550878) were prepared in 0.3% BSA in PBS and incubated with samples overnight at 4°C. After washing, samples were incubated for 1 hr at room temperature with Alexa Fluor 488- or 568-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Life Technologies, catalog nos. A10667 and A11031, respectively) diluted 1:500 in 0.3% BSA. Samples were analyzed on a BDCanto flow cytometer using BD FACSdiva software, and negative control gating was performed on samples treated with an isotype control primary antibody and a secondary antibody identical to that used for samples. For cell-cycle analysis, samples were stained with propidium iodide (Life Technologies), and cell-cycle distribution was determined using the ModFit LT version 4 software package from Verity Software House.
qRT-PCR
mRNA was isolated from cultured cells using the QIAGEN RNeasy Plus mini kit. cDNA was produced using the Applied Biosystems high-capacity cDNA reverse transcription kit. Gene expression was assessed via qRT-PCR using the NANOG, and LAMA5 human TaqMan gene expression assays from Applied Biosystems. Samples were measured using a StepOne real-time PCR system (Applied Biosystems), and data analysis was performed using the ΔΔCt method relative to GAPDH.
Statistical Analysis
Statistical analysis was performed via one-way ANOVA with a Tukey honest significant difference (HSD) post hoc test using the KaleidaGraph software package. p Values less than or equal to 0.05 were considered statistically significant. Data are presented as mean ± SD. All experiments were repeated a minimum of two times with three independent samples per condition each time.
Acknowledgments
This work was supported by NIH grants R01 EB007534 and R01 HL093281. We would like to thank Nicholas Propson and Dr. James Thomson from the Regenerative Biology Group at the Morgridge Institute for Research for providing recombinant human vitronectin and for the use of their flow cytometry and FACS facilities. We also thank Jared Carlson-Stevermer for assistance with guide RNA design and protocols. We acknowledge generous financial support from the Wisconsin Institute for Discovery (to K.S.), NSF (CBET-1350178, to K.S.), and the Society in Science Foundation (to K.S.).
Published: July 30, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes six figures and five tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.06.009.
Supplemental Information
References
- Amit M., Laevsky I., Miropolsky Y., Shariki K., Peri M., Itskovitz-Eldor J. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat. Protoc. 2011;6:572–579. doi: 10.1038/nprot.2011.325. [DOI] [PubMed] [Google Scholar]
- Azarin S.M., Palecek S.P. Matrix revolutions: a trinity of defined substrates for long-term expansion of human ESCs. Cell Stem Cell. 2010;7:7–8. doi: 10.1016/j.stem.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Beck K., Hunter I., Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Braam S.R., Zeinstra L., Litjens S., Ward-van Oostwaard D., van den Brink S., van Laake L., Lebrin F., Kats P., Hochstenbach R., Passier R. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- Celiz A.D., Smith J.G.W., Langer R., Anderson D.G., Winkler D.A., Barrett D.A., Davies M.C., Young L.E., Denning C., Alexander M.R. Materials for stem cell factories of the future. Nat. Mater. 2014;13:570–579. doi: 10.1038/nmat3972. [DOI] [PubMed] [Google Scholar]
- Chen G., Hou Z., Gulbranson D.R., Thomson J.A. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., Smuga-Otto K., Howden S.E., Diol N.R., Propson N.E. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.K., Chen X., Lim Y.M., Reuveny S., Oh S.K. Inhibition of ROCK-myosin II signaling pathway enables culturing of human pluripotent stem cells on microcarriers without extracellular matrix coating. Tissue Eng. Part C Methods. 2014;20:227–238. doi: 10.1089/ten.TEC.2013.0191. [DOI] [PubMed] [Google Scholar]
- Cho S.W., Kim S., Kim J.M., Kim J.-S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- Deutzmann R., Aumailley M., Wiedemann H., Pysny W., Timpl R., Edgar D. Cell adhesion, spreading and neurite stimulation by laminin fragment E8 depends on maintenance of secondary and tertiary structure in its rod and globular domain. Eur. J. Biochem. 1990;191:513–522. doi: 10.1111/j.1432-1033.1990.tb19151.x. [DOI] [PubMed] [Google Scholar]
- Domogatskaya A., Rodin S., Boutaud A., Tryggvason K. Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26:2800–2809. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- Domogatskaya A., Rodin S., Tryggvason K. Functional diversity of laminins. Annu. Rev. Cell Dev. Biol. 2012;28:523–553. doi: 10.1146/annurev-cellbio-101011-155750. [DOI] [PubMed] [Google Scholar]
- Evseenko D., Schenke-Layland K., Dravid G., Zhu Y., Hao Q.L., Scholes J., Wang X.C., Maclellan W.R., Crooks G.M. Identification of the critical extracellular matrix proteins that promote human embryonic stem cell assembly. Stem Cells Dev. 2009;18:919–928. doi: 10.1089/scd.2008.0293. [DOI] [PubMed] [Google Scholar]
- Gajbhiye V., Escalante L., Chen G., Laperle A., Zheng Q., Steyer B., Gong S., Saha K. Drug-loaded nanoparticles induce gene expression in human pluripotent stem cell derivatives. Nanoscale. 2014;6:521–531. doi: 10.1039/c3nr04794f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H.H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Hongisto H., Vuoristo S., Mikhailova A., Suuronen R., Virtanen I., Otonkoski T., Skottman H. Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture. Stem Cell Res. (Amst.) 2012;8:97–108. doi: 10.1016/j.scr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Hozumi K., Suzuki N., Uchiyama Y., Katagiri F., Kikkawa Y., Nomizu M. Chain-specific heparin-binding sequences in the laminin alpha chain LG45 modules. Biochemistry. 2009;48:5375–5381. doi: 10.1021/bi900542u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.S., Postovit L.M., Lajoie G.A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- Ido H., Harada K., Yagi Y., Sekiguchi K. Probing the integrin-binding site within the globular domain of laminin-511 with the function-blocking monoclonal antibody 4C7. Matrix Biol. 2006;25:112–117. doi: 10.1016/j.matbio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Cheng D.W., Crook E.D., Singh L.P. Transforming growth factor-beta1 regulation of laminin gamma1 and fibronectin expression and survival of mouse mesangial cells. Mol. Cell. Biochem. 2005;278:165–175. doi: 10.1007/s11010-005-7327-z. [DOI] [PubMed] [Google Scholar]
- Katagiri F., Hara T., Yamada Y., Urushibata S., Hozumi K., Kikkawa Y., Nomizu M. Biological activities of the homologous loop regions in the laminin α chain LG modules. Biochemistry. 2014;53:3699–3708. doi: 10.1021/bi5003822. [DOI] [PubMed] [Google Scholar]
- Klim J.R., Li L., Wrighton P.J., Piekarczyk M.S., Kiessling L.L. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat. Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács M., Tóth J., Hetényi C., Málnási-Csizmadia A., Sellers J.R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Laperle A., Masters K.S., Palecek S.P. Influence of substrate composition on human embryonic stem cell differentiation and extracellular matrix production in embryoid bodies. Biotechnol. Prog. 2015;31:212–219. doi: 10.1002/btpr.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Harrison D., Carbonetto S., Fassler R., Smyth N., Edgar D., Yurchenco P.D. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 2002;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wu D., Chen X., Zhang T., Zhang L., Yi Y., Miao Z., Jin N., Bi X., Wang H. Current and emerging biomarkers of cell death in human disease. BioMed Res. Int. 2014;2014:690103. doi: 10.1155/2014/690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.H., Chang H.Y., Kao W.L., Lin K.Y., Liao H.Y., You Y.W., Kuo Y.T., Kuo D.Y., Chu K.J., Chu Y.H., Shyue J.J. Effect of surface potential on extracellular matrix protein adsorption. Langmuir. 2014;30:10328–10335. doi: 10.1021/la5020362. [DOI] [PubMed] [Google Scholar]
- Ludwig T.E., Bergendahl V., Levenstein M.E., Yu J., Probasco M.D., Thomson J.A. Feeder-independent culture of human embryonic stem cells. Nat. Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Mei Y., Saha K., Bogatyrev S.R., Yang J., Hook A.L., Kalcioglu Z.I., Cho S.-W., Mitalipova M., Pyzocha N., Rojas F. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkoumian Z., Weber J.L., Weber D.M., Fadeev A.G., Zhou Y., Dolley-Sonneville P., Yang J., Qiu L., Priest C.A., Shogbon C. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- Meng Y., Eshghi S., Li Y.J., Schmidt R., Schaffer D.V., Healy K.E. Characterization of integrin engagement during defined human embryonic stem cell culture. FASEB J. 2010;24:1056–1065. doi: 10.1096/fj.08-126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G., Liu S., Rancourt D.E. Synergistic effect of medium, matrix, and exogenous factors on the adhesion and growth of human pluripotent stem cells under defined, xeno-free conditions. Stem Cells Dev. 2012;21:2036–2048. doi: 10.1089/scd.2011.0489. [DOI] [PubMed] [Google Scholar]
- Miner J.H., Yurchenco P.D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Futaki S., Suemori H., Taniguchi Y., Yamada M., Kawasaki M., Hayashi M., Kumagai H., Nakatsuji N., Sekiguchi K., Kawase E. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka M., Si-Tayeb K., Akaike T., Duncan S.A. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev. Biol. 2010;10:60. doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nip L.H., Uitto V.J., Golub L.M. Inhibition of epithelial cell matrix metalloproteinases by tetracyclines. J. Periodontal Res. 1993;28:379–385. doi: 10.1111/j.1600-0765.1993.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Ohgushi M., Sasai Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends Cell Biol. 2011;21:274–282. doi: 10.1016/j.tcb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Ohgushi M., Matsumura M., Eiraku M., Murakami K., Aramaki T., Nishiyama A., Muguruma K., Nakano T., Suga H., Ueno M. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Qian X., Villa-Diaz L.G., Kumar R., Lahann J., Krebsbach P.H. Enhancement of the propagation of human embryonic stem cells by modifications in the gel architecture of PMEDSAH polymer coatings. Biomaterials. 2014;35:9581–9590. doi: 10.1016/j.biomaterials.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F.A., Hsu P.D., Lin C.Y., Gootenberg J.S., Konermann S., Trevino A.E., Scott D.A., Inoue A., Matoba S., Zhang Y., Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin S., Domogatskaya A., Ström S., Hansson E.M., Chien K.R., Inzunza J., Hovatta O., Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- Rodin S., Antonsson L., Hovatta O., Tryggvason K. Monolayer culturing and cloning of human pluripotent stem cells on laminin-521-based matrices under xeno-free and chemically defined conditions. Nat. Protoc. 2014;9:2354–2368. doi: 10.1038/nprot.2014.159. [DOI] [PubMed] [Google Scholar]
- Rodin S., Antonsson L., Niaudet C., Simonson O.E., Salmela E., Hansson E.M., Domogatskaya A., Xiao Z., Damdimopoulou P., Sheikhi M. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat. Commun. 2014;5:3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- Rodríguez Hernández J.C., Salmerón Sánchez M., Soria J.M., Gómez Ribelles J.L., Monleón Pradas M. Substrate chemistry-dependent conformations of single laminin molecules on polymer surfaces are revealed by the phase signal of atomic force microscopy. Biophys. J. 2007;93:202–207. doi: 10.1529/biophysj.106.102491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K., Mei Y., Reisterer C.M., Pyzocha N.K., Yang J., Muffat J., Davies M.C., Alexander M.R., Langer R., Anderson D.G., Jaenisch R. Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions. Proc. Natl. Acad. Sci. USA. 2011;108:18714–18719. doi: 10.1073/pnas.1114854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D., Kulkeaw K., Mizuochi C. TGF-beta-1 up-regulates extra-cellular matrix production in mouse hepatoblasts. Mech. Dev. 2013;130:195–206. doi: 10.1016/j.mod.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tonary A.M., Carnegie J.A. TGF-beta 1 regulation of laminin secretion by F9-derived primitive endoderm cells: a potential role in cell migration within the mouse blastocyst. Can. J. Physiol. Pharmacol. 1996;74:940–948. [PubMed] [Google Scholar]
- van Engeland M., Nieland L.J., Ramaekers F.C., Schutte B., Reutelingsperger C.P. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Villa-Diaz L.G., Nandivada H., Ding J., Nogueira-de-Souza N.C., Krebsbach P.H., O’Shea K.S., Lahann J., Smith G.D. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Diaz L.G., Ross A.M., Lahann J., Krebsbach P.H. Concise review: The evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings. Stem Cells. 2013;31:1–7. doi: 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Nishikawa S., Muguruma K., Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Weber J.L., Dolley-Sonneville P., Weber D.M., Fadeev A.G., Zhou Y., Yang J., Priest C.A., Brandenberger R., Melkoumian Z. Corning[reg] Synthemax[trade] Surface: a tool for feeder-free, xeno-free culture of human embryonic stem cells. Nat. Methods. 2010:7. [Google Scholar]
- Wells R.G., Discher D.E. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci. Signal. 2008;1:pe13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Guell M., Byrne S., Yang J.L., De Los Angeles A., Mali P., Aach J., Kim-Kiselak C., Briggs A.W., Rios X. Optimization of scarless human stem cell genome editing. Nucleic Acids Res. 2013;41:9049–9061. doi: 10.1093/nar/gkt555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P.D. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011;3:3. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Gurtu V., Kain S.R., Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques. 1997;23:525–531. doi: 10.2144/97233pf01. [DOI] [PubMed] [Google Scholar]
- Zweigerdt R., Olmer R., Singh H., Haverich A., Martin U. Scalable expansion of human pluripotent stem cells in suspension culture. Nat. Protoc. 2011;6:689–700. doi: 10.1038/nprot.2011.318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.