The connection between cerebrovascular health and cognition has been of empirical interest to scientists for over a century. In 1894, Swiss neurologist, Otto Binswanger, described an association between postmortem cerebrovascular changes, including atherosclerosis, and cognitive impairment preceding death in middle-aged and older adults.1 One-hundred and twenty years later, the field has evolved beyond Binswanger’s seminal work to include midlife systemic vascular health factors as potential mechanistic drivers in abnormal cognitive aging, including the most common form of dementia, Alzheimer's disease.2, 3
In this issue of Circulation, Yaffe and colleagues push the envelope further by reporting that longitudinal exposure to one or more vascular risk factors in early and midadulthood is associated with worse midlife cognitive performance.4 Participants from the Coronary Artery Risk Development in Young Adults (CARDIA) Study who were 18 to 30 years of age at baseline underwent vascular risk factor assessments every 2 to 5 years over a 25-year period. Cognitive assessment, conducted at the end of the follow-up period, included delayed episodic memory (Rey Auditory Verbal Learning Test), information processing speed (Digit Symbol Coding), and 1 key aspect of executive functioning (inhibition assessed by the Stroop Test).
In unadjusted models, cumulative exposure of each vascular risk factor (with the exception of total cholesterol) was individually associated with poorer performance on all 3 cognitive measures at midlife. However, in models adjusting for or excluding participants with incident cardiovascular events (eg, myocardial infarction, coronary revascularization, congestive heart failure), findings were less consistent, and the significant effects that remained were diminished. Normal levels of each risk factor (defined by American Heart Association guidelines)5 were unrelated to midlife cognitive performance in models adjusting for age, sex, race, and education. Yet, when models included the same key demographic covariates, elevated systolic blood pressure, diastolic blood pressure, and fasting blood glucose were each individually associated with poorer cognitive performance.
Collectively, the results suggest that longitudinal exposure to one or more vascular risk factors across early and middle adulthood may have modest effects on midlife cognitive performance. Recent literature indicates the pathogenesis of Alzheimer's disease unfolds decades before late-life clinical symptom manifestation. For example, postmortem evidence of abnormal phosphorylated tau (ie, a precursor to neurofibrillary tangles associated with Alzheimer's disease) can be found in children and young adults aged 4 to 29 years.6 Neuroimaging evidence suggests infant ε4 carriers of the apolipoprotein E genotype (APOEε4, a susceptibility gene for Alzheimer's disease) have structural brain differences in comparison with their APOEε4-negative counterparts.7 Thus, it is similarly plausible that the intersection of vascular risk exposure and initial changes in brain structure may occur much earlier than previously appreciated, although such effects may not clinically manifest until midlife or late adulthood. The current findings by Yaffe and colleagues highlight this possibility.
The authors speculate that a diverse number of causal mechanisms account for the reported associations, including ischemia (especially of a subcortical nature), alterations in amyloid production or clearance, inflammatory or oxidative stress–induced neuronal injury, or gene-environment interactions. The underlying mechanism(s) are probably complex and may or may not reflect a causal pathway.
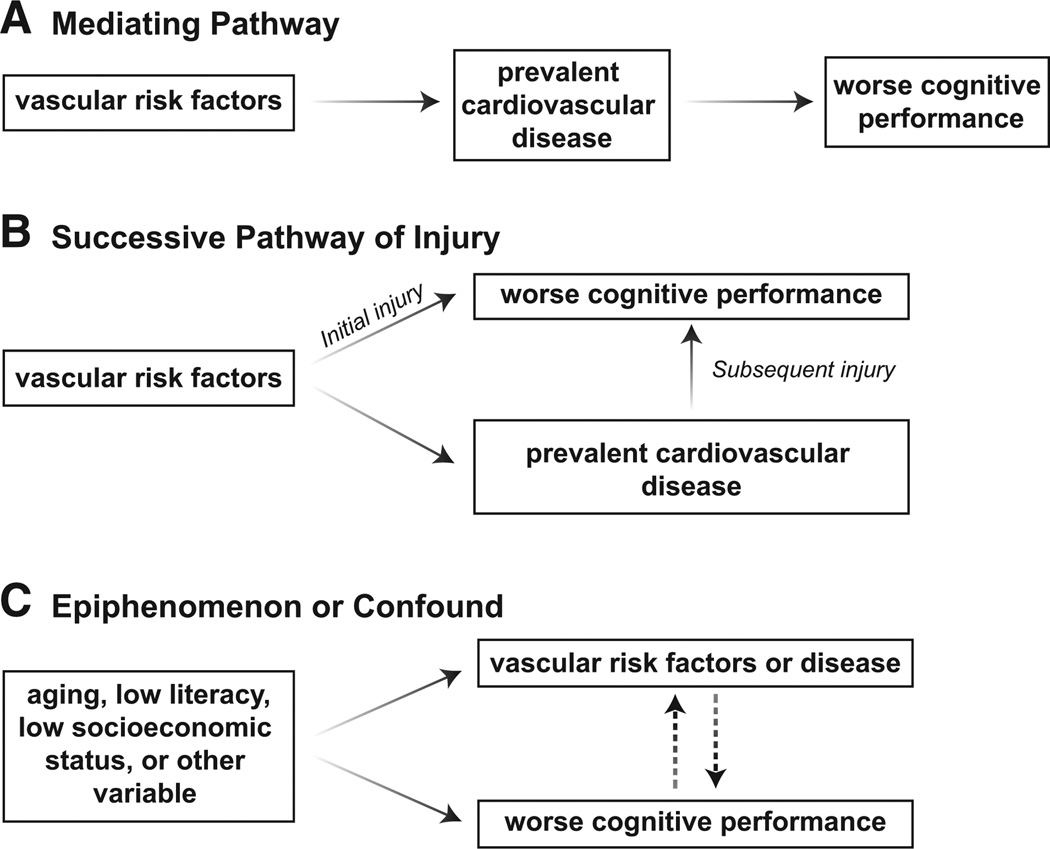
If a causal mechanism exists, there are at least 2 fundamental pathways through which vascular risk factors negatively impact cognition. First, prevalent cardiovascular disease (eg, myocardial infarction, congestive heart failure) may act as a mediating variable in the association between vascular risk factors and poor cognitive outcomes (Figure A). That is, the presence of prevalent cardiovascular disease, rather than any 1 risk factor, drives observations between vascular risk factors and cognition. Most results from Yaffe et al remained statistically significant after adjusting for or excluding incident cardiovascular events, although effect sizes were reduced. Thus, their observed pattern of results supports an alternative explanation—a successive pathway of injury (Figure B). In this latter account, vascular risk factors, such as hypertension8 and diabetes mellitus,9 contribute an initial pathway of injury to cognition by disrupting the brain’s capillary ultrastructure. These initial basement membrane morphological changes (eg, pericytic degeneration)10 result in compromised blood-brain barrier permeability and microcirculation, which manifest as subtle cognitive changes. Over time, vascular risk factor burden can contribute to prevalent cardiovascular disease. Such interim cardiovascular events create a second, subsequent pathway of injury to the brain by further exacerbating small-vessel changes (and perhaps affecting larger vessels, too) with corresponding cognitive decline.
Figure.
Hypothetical pathways that may account for the association between worse vascular health and abnormal cognitive changes with age. A, Mediating pathway. B, Successive pathway of injury. C, Epiphenomenon or confound.
Alternatively, it is plausible that no such causal pathway exists, and any reported connection between vascular risk factors and abnormal cognitive aging is epiphenomenological in nature. One confounding variable that could explain this phenomenon is age. Vascular risk factors and prevalent cardiovascular disease increase with age as does the prevalence of cognitive impairment. Shared age-related biological change(s) not adequately captured by chronological age could reasonably account for the results reported by Yaffe and colleagues (Figure C). Another possible epiphenomenon (or confound) is socioeconomic status or any one of its complex correlates like literacy, nutrition habits, or environmental enrichment versus impoverishment. Individuals from lower socioeconomic backgrounds11 or with lower literacy levels12 (in comparison peers with higher socioeconomic or literacy levels) may have lower baseline cognitive performance levels and engage in poorer health choices, thereby increasing vascular risk factor burden. Previous studies from the CARDIA cohort11 and others13 have shown that lower socioeconomic status is a major predictor of poorer cardiovascular health outcomes. However, the current study design does not take into account the potential impact of socioeconomic status or literacy level on results, and the absence of baseline cognitive testing precludes the evaluation of the potential confounding effects of interindividual differences in literacy or socioeconomic status within the cohort.
Much of the existing vascular risk factor and cognitive aging literature focuses on risk exposure at a single time point, likely inadequately capturing risk development and burden over the life course. In contrast, Yaffe and colleagues leverage longitudinal data from 8 examinations spanning 25 years across early adulthood and midlife. Although the reported effects are small and the clinical significance is unclear, a key strength of the study is its emphasis on cumulative temporal exposure to one or more risk factors. Unfortunately, cognitive assessment is restricted to a single evaluation at the end of the follow-up period, which precludes inferences about how the cumulative burden of each risk factor affects cognitive trajectory.
There are a few additional caveats to consider with respect to the results put forth by Yaffe and colleagues. First, the analytic models consider each risk factor individually without statistical consideration of the intercorrelated nature of these variables (eg, examining fasting blood glucose without adjusting for the potential confounding role of systolic blood pressure). Just as the authors advocate for capturing exposure duration and intensity, it is similarly important to capture the shared effects of vascular risk factors on brain health. Another consideration is that increasing evidence supports APOEε4 as an effect modifier in the association between midlife vascular risk factor exposure and midlife14 and late-life cognition.15 Unfortunately, analytic models in the current study did not consider possible APOE genotype effects, perhaps because such data are unavailable in the cohort. Finally, in light of the extensive number of models analyzed, the absence of a correction factor could have yielded spurious findings, resulting in a type I error. Replication of these observations is essential.
Despite these modest limitations, the current work by Yaffe and colleagues is compelling and suggests that better vascular health in early life benefits cognitive aging in midlife. With respect to next steps, most essential is the need to unequivocally establish whether a causal connection exists between vascular risk factor exposure and worse cognitive trajectory (or whether these observations are explained by an epiphenomenon). If there is a causal connection, then the efficacy of therapeutic or lifestyle interventions in young adulthood and midlife can be determined. Once these essential aspects of the field are better understood, we can begin evaluating whether early screening coupled with more aggressive management of vascular risk factors in young adulthood is warranted to reduce the public health burden and associated costs of abnormal cognitive aging.
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Binswanger O. Die abgrenzung der allgemeinen progressiven paralyse. Berl Klin Wochenschr. 1894;31:1103–1105. 1137–1139, 1180–1186. [Google Scholar]
- 2.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Pletcher M, Whitmer R, Coker L, Sidney S. Early adult to mid-life cardiovascular risk factors and cognitive function. Circulation. 2014;129:XX–XXX. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 7.Dean DC, 3rd, Jerskey BA, Chen K, Protas H, Thiyyagura P, Roontiva A, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Siniard AL, Turk MN, Hua X, Madsen SK, Thompson PM, Fleisher AS, Huentelman MJ, Deoni SC, Reiman EM. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 2014;71:11–22. doi: 10.1001/jamaneurol.2013.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagami M, Nara Y, Kubota A, Fujino H, Yamori Y. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke. 1990;21:1064–1071. doi: 10.1161/01.str.21.7.1064. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PC, Brendel K, Meezan E. Thickened cerebral cortical capillary basement membranes in diabetics. Arch Pathol Lab Med. 1982;106:214–217. [PubMed] [Google Scholar]
- 10.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlamangla AS, Singer BH, Williams DR, Schwartz JE, Matthews KA, Kiefe CI, Seeman TE. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: the CARDIA Study (USA) Soc Sci Med. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson AL, Massaro JM, Beiser AS, Seshadri S, Larson MG, Wolf PA, Au R, Benjamin EJ. Inflammatory markers and neuropsychological functioning: the Framingham Heart Study. Neuroepidemiology. 2011;37:21–30. doi: 10.1159/000328864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Community Health. 1998;52:399–405. doi: 10.1136/jech.52.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, Bondi MW, Seshadri S, Wolf PA, Au R. APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis. 2013;22:1361–1369. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology. 1999;52:1119–1124. doi: 10.1212/wnl.52.6.1119. [DOI] [PubMed] [Google Scholar]