Abstract
Plant-based diets rich in crucifers are effective in preventing cancer and other chronic diseases. Crucifers contain very high concentrations of glucosinolates (GS; β-thioglucoside-N- hydroxysulfates). Although not themselves protective, GS are converted by coexisting myrosinases to bitter isothiocyanates (ITC) which defend plants against predators. Coincidentally, ITC also induce mammalian genes that regulate defenses against oxidative stress, inflammation, and DNA-damaging electrophiles. Consequently, the efficiency of conversion of GS to ITC may be critical in controlling the health-promoting benefits of crucifers. If myrosinase is heat-inactivated by cooking, the gastrointestinal microflora converts GS to ITC, a process abolished by enteric antibiotics and bowel cleansing. When single oral doses of GS were administered as broccoli sprout extracts (BSE) to two dissimilar populations (rural Han Chinese and racially mixed Baltimoreans) patterns of excretions of urinary dithiocarbamates (DTC) were very similar. Individual conversions in both populations varied enormously, from about 1% to more than 40% of dose. In contrast, administration of ITC (largely sulforaphane)-containing BSE, resulted in uniformly high (70-90%) conversions to urinary DTC. Despite the remarkably large range of conversion efficiencies between individuals, repeated determinations within individuals were much more consistent. The rates of urinary excretion (slow or fast) were unrelated to the ultimate magnitudes (low or high) of these conversions. Although no demographic factors affecting conversion efficiency have been identified, there are clearly diurnal variations: conversion of GS to DTC was greater during the day, but conversion of ITC to DTC was more efficient at night.
Keywords: glucoraphanin, myrosinase, sulforaphane, dithiocarbamate
Introduction
It is widely accepted that human consumption of plant-rich diets, and consequently lower intake of other foods, reduces the risk of developing cancer and other age-related chronic diseases (1, 2). This landmark scientific consensus not only supports the view that many major chronic diseases can be prevented, but also suggests an explicit dietary strategy for prevention. In a recent illustration, British vegetarians had relative risks of stomach, ovarian, bladder, lymphatic/ hematopoetic, and “all” cancers, that were 0.36, 0.69, 0.47, 0.55 and 0.88, respectively, compared to those of meat-eaters (3). A significant refinement of this concept is the finding that one specific family of vegetables, the Cruciferae, is more highly protective than many other dietary plants. Thus, the risk of bladder cancer was approximately halved in men who ate high amounts of cruciferous vegetables compared to low quantities, but not in those who ate fruits and other vegetables or their combinations (4). Consumption of both raw and total cruciferous vegetables (highest vs. lowest quintile) were associated with an over 40% lower risk of lung cancer among smokers (5). Consumption of five or more servings compared with less than two servings per week of crucifers was associated with a 33% lower risk of non-Hodgkins lymphoma in women (6). There was a decreasing (31-39%) association of prostate cancer with increased intake of crucifers in two separate studies (7,8), as well as substantial reductions in colorectal cancer (9). Cruciferous vegetables include two noteworthy edible genera: Brassica. (broccoli, cabbage, cauliflower), and Raphanus. (radish and daikon). Crucifers are unique in the Western diet, because they are rich in glucosinolates (GS), which are believed to be largely responsible for their protective properties (10-14). GS are β-thioglucoside N-hydroxysulfates that are converted to isothiocyanates (ITC; also known as mustard oils) by myrosinases [E.C. 3.2.1.147], abundant enzymes that co-exist, but are physically segregated in intact plant cells. Myrosinase does not occur in cells of mammals, but is found in their gastrointestinal microflora. Upon damage to plant cells, myrosinases gain access to their relatively inert GS substrates and convert them to highly reactive and bitter-tasting ITC that defend plants against injury by insects, bacteria, and fungi (see reviews 15-17). Conversion of GS to ITC is one of the most ingenious methods that plants, especially crucifers, have evolved for protection against predators (15).
By coincidence, ITC have been recognized for many years as powerful protectors against carcinogenesis in animals, and these effects have been attributed to the induction of cytoprotective enzymes (18-20). A widely-studied example of a potent anticarcinogenic ITC is sulforaphane [SF; 1-isothiocyanato-4R-(methylsulfinyl)butane] isolated from broccoli where it is formed by myrosinase action on its GS precursor (glucoraphanin; GR).
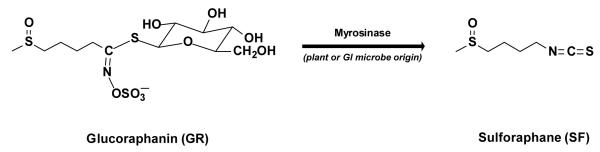
The myrosinase reaction therefore also plays a central role in the protection of animal cells against cancer and other chronic diseases. Consumption of lyophilized boiling water extracts of fresh broccoli sprouts (BSE) is an efficient method for administering standardized doses of GS to human volunteers. If these preparations are treated with myrosinase from daikon sprouts, the resultant extracts deliver ITC, principally SF, derived from its GS precursor glucoraphanin (GR) (19, 21). Earlier clinical studies with this system have established that: (i) administration of SF-rich BSE resulted in uniformly high urinary excretion of dithiocarbamate metabolites (DTC) that accounted for about 70-90% of the administered dose of SF in 24 h (22). (ii) GS to DTC and ITC to DTC conversions in individual subjects are strictly proportional to GS and ITC doses, respectively (23-24). (iii) When BSE in which myrosinase had been heat-inactivated are administered to humans, conversion of GS to DTC is promoted by the microflora of the gastrointestinal tract. Evidence for this mechanism was obtained by suppression of bowel microflora with enteric antibiotics and mechanical bowel cleansing, which almost completely abolished conversion of GS to DTC, followed by very rapid recovery (within days) (23). In contrast to administration of SF-rich BSE, however, the conversion of GS to DTC was highly variable among individuals, ranging from a low of about 1% to a high of more than 40% of the administered dose (22, 25).
It is well-established that isothiocyanates, not glucosinolates, are principally responsible for regulating genes coding for proteins that protect cells against oxidative stress, inflammation, and electrophile toxicity (10-12, 20). Since the majority of cruciferous vegetables are generally cooked prior to eating, thus inactivating plant myrosinase, the efficiency with which the gastrointestinal microbial flora of individuals converts dietary GS to ITC may either limit or augment the beneficial effects of high GS consumption. A differential effect of raw vs. cooked broccoli consumption is strongly supported by epidemiologic data (14). In the hope of decreasing risk of chronic disease, our ultimate goal is to devise interventions to enhance the efficiency of the conversion of GS to pharmacologically active ITC, especially in individuals who do not do so efficiently. Therefore, our current study examines the factors responsible for the bioavailability and metabolism of SF in humans consuming GR delivered in BSE. Since the principal GS in these preparations of BSE is GR, the precursor of SF, we refer from here on to the conversion process as occurring from GR to SF. Sulforaphane is conjugated with glutathione in animal tissues by glutathione S-transferase, to form glutathionyl-sulforaphane (a DTC) which then undergoes further stepwise enzymatic degradation via the mercapturic acid pathway to N-acetylcysteinyl-sulforaphane which is excreted in the urine. Sulforaphane and its DTC metabolites can be quantified collectively by a spectroscopic HPLC method that depends on a cyclocondensation reaction with 1,2-benzenedithiol developed in our laboratory (26, 27). Determination of urinary excretion of unchanged SF and its DTC metabolites provides a quantitative measure not only of the overall bioavailability of SF but also of its DTC metabolites, some of which are probably also active as regulators of cytoprotective enzymes (28-30). Most of the conversion of single doses of GR to SF and DTC is complete within 24 h (22, 24; and experiments reported below). We refer in this paper to the “efficiency of conversion” of single doses of GR administered in BSE, to urinary metabolites which include free SF and its DTC conjugates, as measured by the cyclocondensation reaction, and express these as a percentage of administered dose. We recognize that the overall formation of urinary DTC from orally administered GR involves multiple processes, including intestinal absorption, enzymatic conversion of GR to SF, enzymatic conjugations of SF via the mercapturic acid pathway, and renal excretion. The rate-limiting step in “conversion efficiency” reflects largely the formation of SF from GR, since the efficiency of conversion of administered SF to DTC in human volunteers is very high (70-90%). Conversion efficiency is therefore a reasonable index of bioavailability of the pharmacologically active chemical species.
Methods and Materials
Preparation of Broccoli Sprout Extract (BSE)
Preparation of extracts was essentially as described previously (22, 31-33). Briefly, broccoli sprouts were grown from selected seeds with adequate GR levels to yield 3-day-old fresh green sprouts with levels of at least 6 μmol of GR per gram. Seeds were surface-disinfected, and grown in a commercial green-sprouting facility that adheres to FDA-mandated sanitary regulations for sprout production. After 3 days of growth in which water and light were the only inputs, an aqueous extract was prepared in a steam-jacketed kettle at a food processing facility (Oregon Freeze Dry, Albany, OR). Sprouts were plunged into boiling deionized water and maintained at >95 °C for 30 min, and the sprout residues removed by filtration. The aqueous extract containing about 5 μmol of GR per ml, and essentially no SF, was frozen rapidly, and lyophilized in industrial freeze-driers. Total GR titer of the resulting powder was at least 200 μmol/g as assayed by HPLC (34), as well as by cyclocondensation after myrosinase hydrolysis (26), and by bioassays to determine induction potency for NQO1 (nicotinamide quinone oxidoreductase) activity (35). The bulk powders were tested for microbial contaminants, shipped to Baltimore, and stored in sealed bags in a locked, dedicated freezer until use, and confirmatory microbiological analyses (e.g., total aerobic plate count, yeast, mold, absence of specific pathogens) were performed by a commercial laboratory (Eurofins - Strasberger and Siegel, Hanover, MD) according to standard methods. Before clinical use, the preparations were re-analyzed for GR and SF content.
Clinical Study Protocols
All human studies were conducted on healthy volunteers who provided informed consent. The protocols were approved by the Johns Hopkins University Institutional Review Board, and informed consent was obtained from each study subject. Subjects were restricted from eating any cruciferous vegetables or condiments that might contain GS or ITC for 3 days before initiation of the study and through the conclusion of testing. They were required to have been free of antibiotic use for 14 days prior to screening since we showed that enteric antibiotic effects on conversion efficiency rebound within a few days (23). They were asked to maintain a food diary, to complete a medical history that included a list of medications (no antibiotic use within 2 weeks of study), and in a subset of volunteers, included information on stool frequency. A urine sample was obtained just before dosing and tested for DTC to motivate compliance with the restriction against crucifer consumption. At 8 a.m. on the day of the intervention, after overnight fasting, subjects consumed 200 μmol of GR contained in a GR-rich BSE powder (about 2 g) dissolved in 50 ml of deionized water just before consumption. Subjects were permitted to eat immediately thereafter. In most of the studies, the entire urine was collected from 8 a.m. to 4 p.m., and then from 4 p.m. until 8 a.m. on the following morning. For some studies, the urine collection intervals during the first 24-h period were further subdivided, and occasionally extended to 26 h.
Urine collection, measurement of sulforaphane metabolites, and compliance
Urine volumes were measured, and aliquots were taken for measurement of creatinine (Hagerstown Medical Laboratory, Hagerstown, MD), and for cyclocondensation, which was performed as described by Ye et al. (27). We discarded eight clearly incomplete urine collections, as judged by creatinine measurements, out of a total of 139 collections.
Analysis of sulforaphane metabolites
Sulforaphane metabolites were analyzed by isotope dilution mass spectrometry as described by Egner et al. (36), and were measured in the first overnight urines collected from participants randomized to receive a daily dose of 400 μmol GR in BSE for 14 days. Details of the study conducted in Qidong, China, have been reported (25).
Statistics
Descriptive statistics, as well as ANOVA, trend analyses (nptrend, ranksum), pairwise correlations (pwcorr), Students t-test comparisons, and random intercept linear mixed effect models to evaluate between-person covariate effects, were all made using Stata 10.0 (StataCorp, College Station, TX, USA).
Results and Discussion
Efficiency of conversion of glucosinolates to isothiocyanate
Reliable quantitative conversion efficiencies were obtained on 45 healthy volunteers living in Baltimore (27 women and 18 men, aged 21 to 87 years). Thirteen subjects were evaluated only once, whereas 32 subjects were tested from 2 to 7 times, to provide a total of 131 observations. At 8 a.m. each subject received a single oral dose of broccoli sprout extract (BSE) containing 200 μmol of glucosinolates of which 85% was GR, the precursor of sulforaphane (SF). Total 24-h urine was collected in two aliquots: for the first 8 h and the subsequent 16 h. (Completeness of urine collections was confirmed by creatinine determinations.) The sum of the urinary excretion of non-metabolized SF and its dithiocarbamate metabolites (DTC) was determined by the cyclocondensation reaction (27). These determinations permit comparisons with the conversions of GR to SF metabolites in two earlier studies: (i) a safety and tolerance study in which cohorts of three subjects each received similar BSE preparations containing either 25 or 100 μmol of GR, every 8 h for 7 days, and in which complete urine collections were obtained during each 8-h interval for 7 days (21 samples) (22). (ii) Our study in China in which 99 healthy volunteers received doses of BSE extract containing 400 μmol of GR nightly for 14 days, and overnight urines (approximately 12 h) were collected after each dose (25). Comparisons of conversion efficiencies between studies that used a wide range of oral doses of glucosinolates are valid, since at least over a dose range of 25 to 200 μmol glucosinolates, there is a strict linear relation between DTC excretion and dose, and the time-courses of excretion of DTC over this range of doses were also similar (22).
Magnitude of total conversions
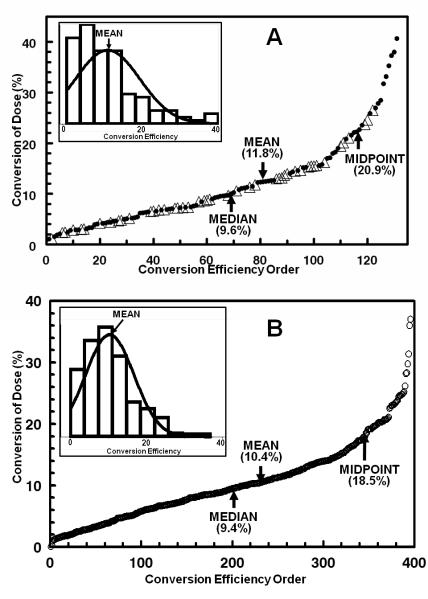
In the 131 observations obtained on 45 subjects, the range of total 24-h DTC excretions following a single 200 μmol glucosinolate dose varied enormously, from 2.2 to 81.3 μmol of DTC (i.e., 1.1 – 40.7% of dose) with a mean of 23.5 μmol (11.8% of dose). The distribution of magnitudes of conversions was skewed dramatically toward lower efficiencies. Thus, more than 80% (106/131) of the conversion determinations were below the mid-point value (20.9% of dose), and only 9 of the 45 subjects exceeded this level (Fig.1A). High conversion is therefore uncommon.
Figure 1.
Efficiencies of conversion of BSE GR to urinary SF and DTC in two populations. A, Baltimore: 131 conversion determinations on 45 volunteers who received a single oral dose of BSE containing 200 μmol of GR. The total 24-h excretion of SF and DTC, measured by cyclocondensation reaction, is expressed as a percentage of dose. The first observation made on each subject is identified separately (△) from subsequent determinations (●). The lowest conversion was 2.2 μmol (1.1% of dose) and the highest was 81.3 μmol (40.7%). B, Qidong, China. The subjects (n = 99) received BSE containing 400 μmol of GR each evening for 14 days. All overnight urine (approximately 12 h) was analyzed by cyclocondensation reaction. The 396 values shown represent the means for each subject determined on four days. Excretion values ranged from 4.1- to 180 μmol DTC (1.02 – 45% of administered dose). Normal distributions are superimposed on distribution histograms for each dataset, in insets to Figs. 1A and 1B. Because collection periods are not the same in the two studies, the results are not directly quantitatively comparable, but the patterns are remarkably similar. Thus the mean excretion was 10.4 −11.8% of dose, and only about 20% of the observations in both studies exceeded the midpoint (ca. 20%) of conversion of the administered dose.
By comparison, in our 2006 study (22) in which subjects were dosed every 8 h for 7 days, the mean excretion efficiencies for all 21 determinations in 3 individuals receiving doses of 25 μmol BSE GR were 17.5 % (range 5.87 – 41.2%), and in those receiving doses of 100 μmol GR were 18.5 % (range 4.14 – 38.3%). The finding of large between-subject differences led to the recognition that there appeared to be a much greater consistency of conversion efficiency over time within-subjects compared to between-subjects. This conclusion is further discussed below.
Although the doses of GR were different in our 2005 study in China (25), the results were quite similar to those obtained in the present study in Baltimore. In Qidong, China, the mean overnight DTC excretion from a 400-μmol daily dose of GR in 99 subjects, measured on 4 days of the 14-day administration period, was 42.1 μmol (10.5% of dose), but again ranged enormously from 4.1 to 180 μmol (1.02 – 45.1% of dose) (Fig. 1B). The distribution of conversion efficiencies was also markedly skewed toward low converters. Out of the 396 observations (4 days on 99 individuals), only 50 conversion determinations (12.6%; in 31 of 99 individuals) exceeded the mid-point of conversion efficiency (74 μmol or 18.5% of dose) (Fig. 1B).
It is therefore notable that in two highly dissimilar populations (urban Caucasian and African-American Baltimoreans and rural Han Chinese), who also have radically different dietary habits, there are similar and enormous differences in GR to DTC conversion efficiencies, but the mean conversions of single GR doses in the two populations were quite similar, about 11%. Only about one fifth of the determinations in both studies exceeded the midpoint of conversion of the administered dose (ca. 20%).
Kinetics of conversion patterns
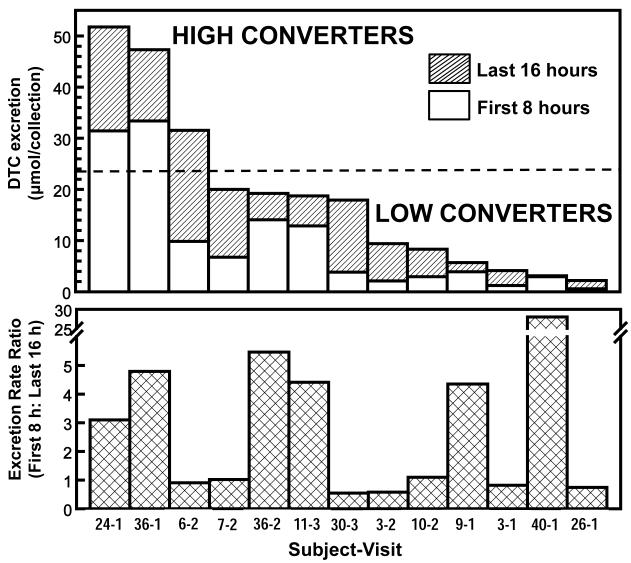
In a small number of subjects from whom additional urine samples were collected at both shorter (<8 h) and longer (>24 h) time intervals, we observed: (i) all of the DTC derived from a single dose of GR dose was excreted within 24 h. (ii) Median peak excretion in all of the tests occurred at about 8 h, but the excretion patterns during the 24-h period were dramatically different among individuals. Whereas in some subjects more than two-thirds of the total excretion of DTC occurred in the first 8 h after dosing, in others two-thirds of total DTC excretion occurred during the 8- to 24-h collection period. There is therefore a continuum of conversion velocities ranging from fast to slow. Furthermore the ratios of the rates of DTC excretion per hour during these collection periods varied greatly among subjects. We selected a random sample of 13 of the 131 conversion determinations, ranging from low to high conversion efficiency to examine the relation between rate and total quantity of conversion (Fig. 2). The ratio of DTC excretion rates (during the first 8 h to subsequent 16 h) ranged enormously and apparently continuously from 0.55 to 28. Interestingly, there was absolutely no relation between the rates (slow to fast) of converter phenotypes, and the total magnitude (low to high) of conversion during the 24-h time period (Fig. 2).
Figure 2.
Time-course of conversion of GR to DTC in a random selection of 13 observations representing both high and low converters. Each subject received a single dose of BSE containing 200 μmol of GR at 8 a.m. each morning. Urine was collected for the first 8 and the subsequent 16 h after dosing. The upper panel shows the total quantity of DTC excreted (range 2.2 - 52 μmol, or from 1 to 26% of dose). The dotted line represents the overall mean for 131 conversion observations (selected from Fig. 1A). The lower panel shows the ratio of the DTC excretions in the first 8 h to the last 16 h. Note that there are High/Fast, High/Slow, Low/Fast, and Low/Slow converter phenotypes (subjects 36-1, 6-2, 40-1, and 3-1, respectively). The horizontal axis designates the number of the subject and which test in a sequence is shown (i.e., 36-2 is the second test in Subject 36).
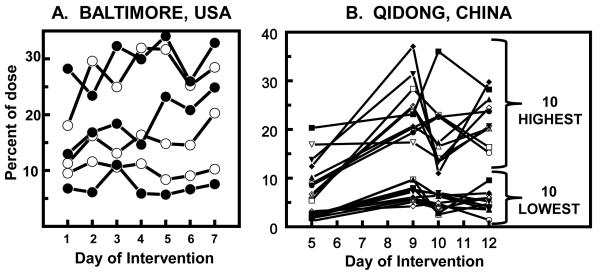
Variability in conversion efficiency between and within subjects over time
Figure 3 compares the large conversion efficiency differences between subjects as well as the greater consistency over time within subjects in two populations. In Baltimore (Fig. 3A) the conversion magnitudes on two cohorts who received 75 or 300 μmol of GR in three divided doses daily (3 subjects each) varied from about 5 to 30%, but the conversion within individuals over a 7-day period was much more consistent (22). A similar pattern was observed in China (25) where 99 subjects received 400 μmol of glucosinolates daily for 14 days. Figure 3B shows the excretion of DTC on 4 days for the 10 lowest and the 10 highest converters. Furthermore, there are very large quantitative differences between the highest and lowest converters, but individuals were much more consistent.
Figure 3.
Urinary DTC excretion after administration of a broccoli sprout extract rich in GR and expressed as percent of dose recovered. A, Baltimore (22). Each line represents a separate subject (means of three daily 8-h values) who received either 25 μmol (○), or 100 μmol of GR (●). Mean overall 24-h excretion is 18%. B, Qidong (25). Overnight urine (approximately 12-h) DTC on Days 5, 9, 10, and 12 from participants who received BSE containing 400 μmol of GR daily for 14 days. Timelines for the 10 individuals whose mean excretion levels of DTC across the 4 days evaluated were the highest and lowest, are shown.
In order to establish and then validate protocols to assess the effects of potential interventions on the efficiency of conversion of GS to ITC, we compared the variances of the conversion efficiencies of repeated observations within-subjects to those between-subjects including only the first observation in those who had multiple tests. The intervals between re-testing varied between a few days to 2.5 years. The first test for each subject is highlighted in Figure 1A. The 131 observations in a total of 45 subjects were distributed as follows: 13 subjects (single test), 12 subjects (1 re-test), 6 subjects (2 re-tests), 3 subjects (3 re-tests), 4 subjects (4 re-tests), 5 subjects (5 re-tests), and 2 subjects (6 re-tests). The 118 observations in 32 subjects with multiple observations had a mean coefficient of variation (CVbetween-subjects) of 41.1%. This variance is close to the CVbetween-subjects of 47.7% for the combined observations obtained on the 13 subjects who had only a single determination and the first observations of the remaining 32 subjects. Based on a one-way ANOVA, with subject as a fixed effect, differences between subjects were highly significant (p = 0.00009), and the ratio of between- to within-subject variance was 3.17.
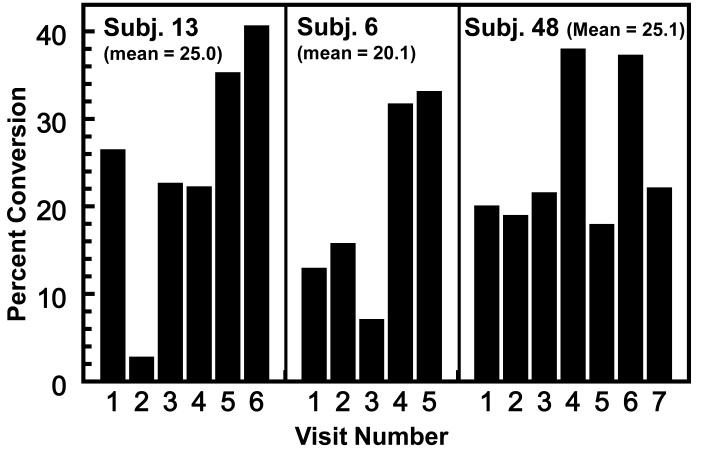
Although the within-subject variance in conversion efficiencies is smaller than that between subjects, there are unexplained variations over time in these observations. Figure 4 shows the repetitive (4-6 repeats) observations on the three highest converters studied (20.1-25.1% conversions of single 200 μmol doses of GS). Although there is consistency among repeat observations on each individual, there are occasional very low conversions and the reasons for these anomalies are unclear. Nevertheless, a total analysis of all repetitive observations on individual subjects showed clearly that the interval between repetitive studies (from a few days to 2.5 years) did not explain this variability and provides the basis for power calculations for intervention studies (results not shown).
Figure 4.
Consistency of conversion of GR to urinary ITC in 3 high-converter phenotypes who were tested on 5-7 occasions (selected from the 131 determinations shown in Fig. 1A). Each determination involved a single oral administration of 200 μmol of GR and measurement of 24-h DTC excretion. The results are expressed as percent of administered dose. The intervals between tests varied from a few days to 2.5 years.
Diurnal variation in conversion/conjugation efficiencies
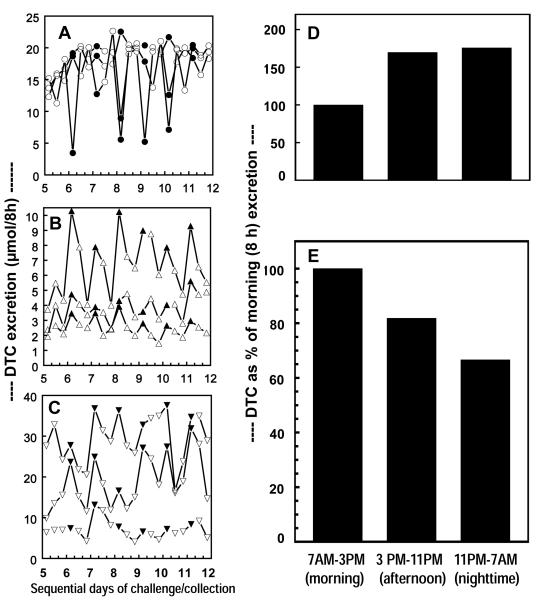
We have now analyzed in greater detail our previous observations on the time-course of DTC excretion rates in healthy hospitalized volunteers in which BSE containing 25 μmol of SF was given orally at 8-h intervals (at 7a.m., 3 p.m., and 11 p.m.) for 7 days, and compared them with rates observed when BSE delivering either 25- or 100-μmol doses of GR was administered on the same schedule (22). Urine was collected during the corresponding 8-h intervals. Three subjects were in each cohort. There is a marked and regular periodicity in the DTC excretion patterns during each day (Fig. 5A, B, C). Whereas for the SF treatment, the mean urinary DTC excretion for all subjects was lowest during the morning (7 a.m. to 3 p.m.) and rose during the subsequent two 8-h intervals, the excretion rate of GR metabolites showed the opposite pattern, falling progressively from day to night. These marked diurnal variations were nearly constant on each day of the 7 days studied, and are summarized graphically in Fig. 5D and 5E which compare the mean DTC excretions during the three daily time periods for the last 6 days for the two cohorts that received SF-rich and GR-rich BSE, respectively. The SF conversions are thus based on 18 measurements for each time period, and the GR conversions for each time period are based on 36 measurements. For ease of comparison, we have normalized the 7 a.m. to 3 p.m. (morning) DTC excretion values for all treatment groups to 100%. Compared to the morning values, the corresponding mean DTC excretion values for SF administration rose to 170% and 175% for the afternoon (3 p.m. to 11 p.m.) and nighttime (11 p.m. to 7 a.m.), respectively (Fig 5D). In contrast, following GR administration, the afternoon and the nighttime excretions were 81.8% and 66.6%, respectively. Differences in conversion by time of dosing were highly significant by one-way ANOVA (F=62.1, p<0.00009) (Fig. 5E).
Figure 5.
Diurnal fluctuation in conversion of oral GR and SF from broccoli sprout extracts to urinary DTC. There were 3 cohorts (A, B, and C) each comprising 3 male subjects. All subjects were dosed at 8-h intervals (7 AM, 3 PM, and 11 PM) and complete urines were collected during the ensuing 8-h periods for 7 days (20 doses and 21 urine collections). The doses (and ages) were as follows: Cohort A (28, 45 and 46 y old), 25 μmol of SF; Cohort B (45, 46, and 57 y old), 25 μmol of GR; Cohort C (45, 45, and 48 y old), 100 μmol of GR. Mean excretion per time period (morning, afternoon, and nighttime) for all 3 subjects who received SF-rich BSE are plotted in 5D, and mean excretion per time period for the 6 subjects who received GR-rich BSE are shown in 5E. Percent conversion of dose values are normalized to 100% for the morning time period for both 5D and 5E.
Since the formation of the DTC from GR presumably proceeds through SF as an obligatory intermediate, and the conversion of SF to DTC increases during the 24-h day, while the overall conversion of GR to DTC declines during this time period, it seems likely that the rate-limiting step in the latter decline occurs during the conversion of GR to SF. It also seems possible that the actual decrease in conversion efficiency of GR to SF during the night may be even larger.
Multiple processes are involved in the conversion of GR to DTC and their excretion in the urine. Most importantly is the participation of the gastrointestinal microbial flora which differs between individuals and within individuals over time. However, given the absence of any significant differences in the bacterial community structure of high- and low ITC excreters reported recently (37), and the fact that conversion efficiency rapidly recovers from antibiotic challenge (23), the formidable task of detailed mapping of these microbial communities may not provide adequate insight into how the conversion of GR to SF can be enhanced in low converters. Other processes include the absorption and hydrolysis of GR to SF in the gastrointestinal tract, the conjugation of SF with glutathione, and their subsequent sequential degradation and N-acetylation of cysteine as part of the mercapturic acid pathway (38). Diurnal variation of renal excretion rates of these metabolites with substantial participation of tubular secretion (27) also may play a role in the observed diurnal kinetics of the observed conversion/excretion patterns. Thus diurnal changes in gastrointestinal mobility and in renal clearance may be involved. Diurnal changes in the activities of glutathione transferases are less likely to be responsible since polymorphism in at least some of these enzymes did not appear to influence SF metabolite excretion patterns (25). In light of the recently described dramatic effects of circadian rhythm on UV induced skin carcinogenesis (39), the observations reported herein may have special relevance.
Relation of conversion efficiencies to demographic characteristics
Sample size was too small to make any but the most basic observations on the correlations between conversion efficiencies and demographic characteristics for the 131 observations on the 45 subjects studied, which were as follows: Gender (27 female, 18 male); Race: African-Americans 19, Caucasians 25, Asians 1; Age 21 - 87 years (mean 46.0); Body Mass Index (BMI): 20.7 - 46.0 (mean 29.2); Stool frequency per week: 2.3 - 14 (mean 8.5). A random intercept, linear mixed-effect model used to evaluate within-cluster and between-cluster covariate effects verified that there were no significant correlations of DTC excretion magnitudes with BMI, stool frequency, age, number of repeat visits, or race. Gender was only of marginal significance (t = 2.06, p = 0.046) with mean conversion by males (47 observations on 18 subjects) of 31.7 μmol (15.8% of dose), and mean conversions by females (84 observations on 27 subjects) of 18.9 μmol (9.5% of dose). In contrast, there was no relation of gender to efficiency of conversion of GS to total DTC in the 99 subjects studied in Qidong, China (25), nor was there an effect in our smaller China study (31). A more intensive examination of demographic factors influencing conversion/excretion efficiencies is therefore justified.
Patterns of sulforaphane metabolites
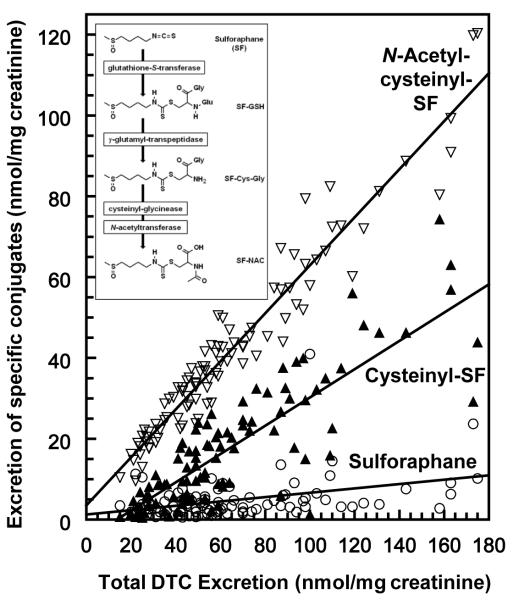
The cyclocondensation reaction provides generic quantification of ITC and their DTC metabolites (Fig. 6 insert), but does not distinguish their individual components. Availability of mass spectrometry for determining individual DTC metabolites provided a means for determining whether the quantitative differences in the excretion patterns observed among subjects were related to changes in patterns of metabolism (36). The two major metabolites of SF (N-acetylcysteinyl-SF, and cysteinyl-SF) and free SF, all tracked monotonically with increasing total DTC excretion in our study in China (25). This is shown clearly in Fig. 6 which leads to the conclusion that conversion phenotypes neither result from-nor create differences in the relative proportion of each of the major SF metabolites (Fig. 6). When evaluated by use of pairwise correlations, urinary excretion of free SF, and its major mercapturic acid conjugates cysteinyl-SF, and N-acetylcysteinyl-SF, and the minor metabolite cysteinylglycyl-SF were each highly correlated with total DTC excretion (p = 0.00009). The glutathionyl-SF conjugate (the SF metabolite found in lowest abundance in the urine), was not correlated with total DTC excretion (p = 0.147). There was no difference in the relative abundance of any of these compounds, or the less abundant cysteinylglycyl-SF and glutathione-SF (data not shown), irrespective of conversion phenotype. Furthermore, none of these metabolites were correlated with the absence of either glutathione S-transferase (GST) genotypes GSTT1, GSTM1, or the absence of both enzymes. Also, there was no interaction between GST genotypes and total DTC excretion and no disproportionate representation of GST genotype in either high- or low-efficiency converters.
Figure 6.
Urinary excretion of sulforaphane and its metabolites in the first overnight urine collections (~12 h) from 98 subjects in Qidong, China, who received 400 μmol of GR daily for 14 days (25, 36). Urinary excretion of free sulforaphane (SF) [○], and its major mercapturic acid conjugates: cysteinyl-SF [▲], and N-acetylcysteinyl-SF [=], as a function of total DTC excretion. Measurements of metabolites were for a single day, and are expressed as micromol of the specified metabolite, and normalized per mg creatinine.
Conclusions
A major contributor to the protective effects of cruciferous vegetable in reducing the risk of cancer and other chronic diseases is believed to be their high content of glucosinolates. Whereas glucosinolates themselves are not significant chemoprotectors, they are converted by coexisting plant myrosinases and the gastrointestinal microflora of humans to isothiocyanates, which are widely recognized as potent protective agents. The bioavailability of isothiocyanates from glucosinolates is relatively constant in individual subjects upon repeated determinations over time, but varies dramatically between individuals (ranging from 1 to 40% of administered dose). Surprisingly, similar patterns of bioavailability have been observed in two highly dissimilar (with respect to genetics, diet, and environment) populations: rural Han Chinese and a mixture of Caucasian and African-American residents of Baltimore. High conversion efficiencies above 20% of dose are rare. The reasons underlying these differences between individuals remain unexplained, yet the potential health benefits of interventions to increase the bioavailability of isothiocyanates from glucosinolates requires serious consideration. The only solid clue as to factors contributing to these differences is that conversion is considerably higher during the day than at night. This may need to be considered when addressing the timing of GS administration to humans in clinical trials and in diet-based prevention strategies. The complex interactions of these diurnal cycles of microbial metabolism in the gut lumen, with the daily cycling of mammalian enzymes that are directly associated with carcinogenesis (e.g. those enzymes involved in the repair of DNA damage; 39), suggests that circadian rhythmicity may have profound implications for cancer prevention. Although our observations suggest that the gut microbiome exerts primary control over GR processing, there are a large number of interacting external factors that can affect conversion and utilization of these phytochemicals.
We have therefore identified a potential limitation to the protective benefits of crucifers in reducing chronic disease risk: low efficiency of conversion of GS to ITC by the gastrointestinal microflora of most individuals globally.
Acknowledgments
We gratefully acknowledge technical assistance from Katherine K. Stephenson, Kristina L. Wade, Kelley E. Bryan, Ling Ye, Gayane Yenokyan, the NIH General Clinical Research Center staff at John Hopkins University, our study volunteers in both Baltimore and HeZou, China, and the laboratory and clinical staff at the Qidong Liver Cancer Institute.
Grant Support These studies were supported by NIH Grants R01 CA093780, P01 ES006052, Center Grant ES003819, the Prevent Cancer Foundation, and the Lewis B. and Dorothy Cullman Foundation.
Abbreviations and Definitions
- BSE
broccoli sprout extract
- DTC
dithiocarbamate
- GR glucoraphanin
the glucosinolate of sulforaphane
- GS
glucosinolate
- ITC
isothiocyanate
- SF
sulforaphane 1-isothiocyanato-4R-(methylsulfinyl)butane
Footnotes
Disclosure of Potential Conflicts of Interest The authors declare that they have no potential conflicts of interest.
References
- 1.Doll R, Peto R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. JNCI. 1981;66(6):1191–308. [PubMed] [Google Scholar]
- 2.World Cancer Research Fund / American Institute for Cancer Research (WCRF/AICR) Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 3.Key TJ, Appleby PN, Spencer EA, Travis RC, Allen NE, Thorogood M, et al. Cancer incidence in British vegetarians. Brit J Cancer. 2009;101:192–7. doi: 10.1038/sj.bjc.6605098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. JNCI. 1999;91(7):605–13. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 5.Tang L, Zirpoli GR, Jayaprakash V, Reid ME, McCann SE, Nwogu CE, et al. Cruciferous vegetable intake is inversely associated with lung cancer risk among smokers: a case-control study. BMC Cancer. 2010;10:162. doi: 10.1186/1471-2407-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang SM, Hunter DJ, Rosner BA, Giovannucci EL, Colditz GA, Speizer FE, et al. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin’s lymphoma among women. Cancer Epidemiol Biomarkers Prev. 2000;9:477–85. [PubMed] [Google Scholar]
- 7.Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: Findings from case-control studies in Canada. Nutr Cancer. 1999;34(2):173–84. doi: 10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- 8.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, et al. Vegetables, fruits, legumes, and prostate cancer: A multi-ethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 9.Hara M, Hanaoka T, Kobayashi M, Otani T, Adachi HY, Montani A, et al. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr Cancer. 2003;46(2):139–47. doi: 10.1207/S15327914NC4602_06. [DOI] [PubMed] [Google Scholar]
- 10.Mi L, Di Pasqua AJ, Chung F-L. Proteins as binding targets of isothiocyanates in cancer prevention. Carcinogenesis. 2011;32(10):1405–13. doi: 10.1093/carcin/bgr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KK, Hampton MB. Biological targets of isothiocyanates. Biochim Biophys Acta. 2011;1810:888–94. doi: 10.1016/j.bbagen.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28(9):1343–54. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrecher A, Nimptsch K, Husing A, Rohrmann S, Linseisen J. Dietary glucosinolate intake and risk of prostate cancer in the EPIC-Heidelberg cohort study. Int J Cancer. 2009;125:2179–86. doi: 10.1002/ijc.24555. [DOI] [PubMed] [Google Scholar]
- 14.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1806–11. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5–51. doi: 10.1016/s0031-9422(00)00316-2. [corrigendum: Phytochemistry 59:237.] [DOI] [PubMed] [Google Scholar]
- 16.Ratska A, Vogen H, Kliebenstein DJ, Mitchell-Olds T, Kroymann J. Disarming the mustard oil bomb. Proc Natl Acad Sci USA. 2002;99(17):11223–8. doi: 10.1073/pnas.172112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barto EK, Powell JR, Cipollini D. How novel are the chemical weapons of garlic mustard in North American forest understories? Biological Invasions. 2010;12:3465–71. [Google Scholar]
- 18.Wattenberg L. Inhibition of carcinogen-induced neoplasia by sodium cyanate, tert-butyl isocyanate, and benzyl isothiocyanate administered subsequent to carcinogen exposure. Cancer Res. 1981;41(8):2991–4. [PubMed] [Google Scholar]
- 19.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32(3&4):395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 21.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr Cancer. 2006;55(1):53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–100. [PubMed] [Google Scholar]
- 24.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Disposition of chemoprotective glucosinolates and isothiocyanates of broccoli sprouts. Cancer Epidemiol Biomarkers Prev. 2001;10:501–8. [PubMed] [Google Scholar]
- 25.Kensler TW, Chen J-G, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo Township, Qidong, PRC. Cancer Epidemiol Biomarkers Prev. 2005;14(11):2605–13. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal Biochem. 1996;239:160–7. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 27.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clinica Chimica Acta. 2002;316(1-2):43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y. Molecular mechanism of rapid accumulation of anticarcingenic isothiocyanates. Carcinogenesis. 2001;22(3):425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21(6):1175–82. [PubMed] [Google Scholar]
- 30.Ye L, Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxication enzymes. Carcinogenesis. 2001;22(12):1987–92. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 31.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, et al. Bioavailability of sulforaphane from two forms of broccoli sprout beverage: Results of a short term, cross-over clinical trial in Qidong, People’s Republic of China. Cancer Prev Res. 2011;4(3):384–95. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinkova-Kostova AT, Fahey JW, Benedict AL, Jenkins SN, Ye L, Wehage SL, et al. Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice. Photochem Photobiol Sci. 2010;9:597–600. doi: 10.1039/b9pp00130a. (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang L, Zhang Y, Jobson HE, Li J, Stephenson KK, Wade KL, et al. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Mol Cancer Ther. 2006;5(4):935–44. doi: 10.1158/1535-7163.MCT-05-0476. [DOI] [PubMed] [Google Scholar]
- 34.Wade KL, Garrard IJ, Fahey JW. Improved hydrophilic interaction chromatography method for the identification and quantification of glucosinolates. J Chrom A. 2007;1154:469–72. doi: 10.1016/j.chroma.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahey JW, Dinkova-Kostova AT, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. In: Sies H, Packer L, editors. Chapter 14 in Methods in Enzymology. Part B. Vol. 382. Elsevier Science; San Diego, CA: 2004. pp. 243–258. [DOI] [PubMed] [Google Scholar]
- 36.Egner PA, Kensler TW, Chen J-G, Gange SJ, Groopman JD, Friesen MD. Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem Res Toxicol. 2008;21(10):1991–6. doi: 10.1021/tx800210k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Hullar MAJ, Beresford SAA, Lampe JW. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Brit J Nutr. 2011 doi: 10.1017/S0007114511000274. doi:10.1017/S0007114511000274:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens JL, Jones DP. The mercapturic acid pathway: Biosynthesis, intermediary metabolism, and physiological disposition. In: Dolphin D, Poulson R, Avramovic O, editors. Glutathione: Chemical, Biochemical, and Medicinal Aspects. Part B. Wiley; New York: 1989. pp. 45–85. [Google Scholar]
- 39.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108(46):18790–5. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]