Abstract
Infections by Helicobacter pylori are very common, causing gastroduodenal inflammation including peptic ulcers, and increasing the risk of gastric neoplasia. The isothiocyanate (ITC) sulforaphane [SF; 1-isothiocyanato-4-(methylsulfinyl)butane] derived from edible crucifers such as broccoli is potently bactericidal against Helicobacter, including antibiotic-resistant strains, suggesting a possible dietary therapy. Gastric H. pylori infections express high urease activity which generates ammonia, neutralizes gastric acidity, and promotes inflammation. The finding that SF inhibits (inactivates) urease (jack bean and Helicobacter) raised the issue of whether these properties might be functionally related. The rates of inactivation of urease activity depend on enzyme and SF concentrations and show first order kinetics. Treatment with SF results in time-dependent increases in the ultraviolet absorption of partially purified Helicobacter urease in the 280–340 nm region. This provides direct spectroscopic evidence for the formation of dithiocarbamates between the ITC group of SF and cysteine thiols of urease. The potencies of inactivation of Helicobacter urease by isothiocyanates structurally related to SF were surprisingly variable. Natural isothiocyanates closely related to SF, previously shown to be bactericidal (berteroin, hirsutin, phenethyl isothiocyanate, alyssin, and erucin), did not inactivate urease activity. Furthermore, SF is bactericidal against both urease positive and negative H. pylori strains. In contrast, some isothiocyanates such as benzoyl-ITC, are very potent urease inactivators, but are not bactericidal. The bactericidal effects of SF and other ITC against Helicobacter are therefore not obligatorily linked to urease inactivation, but may reduce the inflammatory component of Helicobacter infections.
Keywords: jack bean urease, glucosinolate, dithiocarbamate
1. Introduction
Colonization of the gastric mucosa by Helicobacter pylori is probably the most prevalent infection afflicting more than one-half of all humans and significantly increasing their risk for developing peptic ulcer, gastric malignancy and lymphoma [1,2]. The ability of H. pylori to thrive in the unfavorable acidic environment of the stomach depends on the generation of large amounts (10–15% of total protein) of the enzyme urease (urea aminohydrolase; EC 3.5.1.5) [3]. Although widely distributed in nature, this enzyme does not occur in mammalian tissues. By producing ammonia from host urea, urease neutralizes gastric acidity and permits Helicobacter to proliferate. Urease-deficient mutant strains of Helicobacter have never been isolated from patients and are presumed not to be infectious [4,5]. Interestingly, urease promotes mucosal inflammation and also contributes to the pathogenicity of several other important human infections: Mycobacterium tuberculosis, Cryptococcus neoformans (associated with lung infections), and Proteus spp. (associated with urinary tract infections) [6].
Ten years ago, we made the totally unexpected observation that sulforaphane [SF; CH3S(O)(CH2)4NCS], an isothiocyanate derived from its cognate glucosinolate (glucoraphanin) that is abundant in broccoli and other edible crucifers, is very potently and quite specifically bactericidal for H. pylori [7]. Moreover, SF was very active against a large number of clinical isolates of H. pylori, many of which were resistant to conventional antibiotics, such as clarithromycin and metronidazole. This immediately posed the question whether dietary administration of SF might be a practical and economically accessible therapeutic strategy to combat H. pylori infections globally. Both clinical cases and murine infections of H. pylori have responded [8], and while not curative, SF has reduced colonization and inflammation significantly [8].
Although urease was crystallized from jack beans in 1926 [9], its molecular structure was only elucidated much more recently [3,10]. Ureases from plants and bacteria are very large (1.1 million Da) and highly homologous molecules, comprising 12 thiol-rich catalytic subunits (12 cysteine residues per subunit) with two nickel ions (Ni2+) present at each active site. The reactivity of these cysteine thiols and their modification by both reversible inhibitors and irreversible inactivators has been extensively studied [11–20] and recently reviewed [21]. Many of the cysteine residues are susceptible to inhibition by Michael reaction acceptors such as α,β-unsaturated ketones [22]. It is therefore not surprising that isothiocyanates such as SF are efficient inactivators of urease.
This paper analyzes the mechanisms of the inhibitory effects of SF and related isothiocyanates on the urease of H. pylori, and how this process relates to the bactericidal activity of SF. Since SF and other ITC are derived from widely-consumed cruciferous plants, and have been administered orally and are well tolerated in a number of clinical trials [23], it is hoped that they can be developed as a global strategy to ameliorate H. pylori infections.
2. Materials and methods
2.1. Materials
Jack bean (Canavalia ensiformis) urease and other reagents were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). All work with live Helicobacter cultures was done in a biosafety level 2 laboratory.
2.2. Cell cultures
Five H. pylori strains were used in this study. All strains except SS-1 (kindly provided by Dr. James Fox, MIT) were obtained from the American Type Culture Collection: J99 (ATCC 700824), 26695 (ATCC 700392), 60190 (ATCC 49503); urease-negative variant of 60190 (ATCC 51110), Sydney Strain (SS-1). All H. pylori cultures were maintained on tryptic soy agar (Difco) supplemented with 5% defibrinated sheep blood (Hemostat Laboratories, Dixon, CA) and Difco Brucella Broth with 5% fetal bovine serum (Gibco, Invitrogen, Carlsbad, CA). All H. pylori cultures were maintained at 37 °C under microaerophillic conditions in the BBL Campy Pack Plus Systems (Becton Dickinson, Franklin Lakes, NJ), using 3 oxygen scavenging sachets per box, replaced every 2–3 days, or in an incubator supplied with 10% CO2.
2.3. Assay of urease activity
Assay mixtures were prepared in a 96-well microtiter plate by combining 25 μl/well 100 mM potassium phosphate buffer, pH 6.8, containing between 1 and 4 I.U. of urease and 25 μL/well of the potential inhibitor, and incubated at 25 °C for specified periods. Following the inactivation period, enzyme assay mixtures were added containing 100 mM potassium phosphate buffer, pH 6.8, up to 150 mM urea, and 0.002% phenol red in a volume of 200 μL. Linear changes in absorbance at 570 nm were measured over a 3-min period and expressed as milli-absorbance units per minute (mAU/min). For specific activity determinations, these velocities were normalized for protein concentrations.
2.4. Isolation and purification of H. pylori urease
Crude Urease Extraction
One-hundred 10-cm Petri plates (see above), were inoculated with H. pylori, and incubated for 4 days; bacterial lawns were scraped directly into 20 mM potassium phosphate buffer, pH 7.0, and frozen at −80 °C for > 8 h. Cold distilled water (1 – 4 °C) was added to the frozen cell pellet, which was vortexed to thaw, and centrifuged (5,600 × g) for 10 min. The supernatant fraction was removed, pellets re-extracted with 5 mL of cold water, and pooled supernatants were filtered (0.22 μm). Filtrate (total of ~35 mL) was centrifugally concentrated (100,000 MWCO Centricon) to a volume of 11 mL, mixed with glycerol to give a final concentration of 20% (v/v) in a volume of 13.2 mL, and stored at −20 °C in 0.5-mL aliquots.
Purification of H. pylori Urease
A portion of the crude H. pylori urease preparation was further purified by FPLC by employing a sizing column, followed by anion exchange in a modification of published techniques [24]. Briefly, urease activity in fractions was measured using the phenol red assay, and protein content was measured using the bis-cinchoninic acid assay [25]. Fractions containing urease activity were pooled, desalted and concentrated by dialysis (10,000 MWCO Slide-A-Lyzer cassettes; Thermo Scientific). The final set of pooled fractions was concentrated in a 10,000 MWCO Amicon “Ultracell” (Millipore, Billerica, MA). Fractions were assessed for purity by electrophoresis on SDS PAGE 12% separation gels (BioRad, Hercules, CA) [24]. The first FPLC separation utilized a Sephacryl S-300 HR 26/60 column (60 × 2.6 cm, Pharmacia) equilibrated with gel-permeation buffer; flow rate was 1 mL/min, and 4-mL fractions were collected continuously after elution of Vo volume. Pooled urease-containing fractions were dialyzed against ion-exchange loading Bis-Tris propane buffer (120 mM 1,3-bis[tris(hydroxymethyl)-methylamino]propane) adjusted to pH 6.9 with HCl. Pooled urease fractions were concentrated in a 100K MWCO Amicon Centricon device to minimal volume. Final purification was performed on a Mono Q HR 5/5 anion exchange column (5 × 0.5 cm, Pharmacia) equilibrated with the same buffer (flow rate 1 mL/min, UV peaks collected using a linear 0 – 500 mM NaCl-gradient over 18 mL). Fractions containing urease were pooled, dialyzed against gel-permeation buffer, and concentrated to 500 μL.
2.5. Spectroscopic studies of urease
The time course of the spectral change induced by a single addition of sulforaphane to H. pylori urease was measured (0 – 120 min) essentially as described previously [26–28]. Briefly, partially purified H. pylori urease (3 mg protein/mL) was diluted into 10 mM potassium phosphate buffer, pH 7.2, and 0.74 mM sulforaphane (LKT Laboratories, St. Paul, MN); (9 μg in final volume of 300 μL). Spectral measurements were made with a Varian Cary 1E UV/Visible Spectrophotometer (Varian, Walnut Creek, CA) using a matched pair of quartz cuvettes (4 mm window).
2.6. Determination of minimum bactericidal concentrations (MBC)
H. pylori strains were grown as stock cultures on tryptic soy agar containing 5% defibrinated sheep’s blood and cultured at 37 °C under micro-aerophilic conditions (10% CO2) for 3–4 days. Determination of minimum bactericidal concentrations (MBC) were performed in 96-well microtiter plates with test compounds serially diluted in tryptic soy broth containing 5% (v/v) fetal bovine serum (100 μL per well final volume) [29]. Each test well was inoculated with 5 μL of a suspension of H. pylori adjusted to ~107 CFU/mL final concentration (A600 nm = 0.12). Assay plates were incubated for 3 days, then samples from each treatment well were plated on solid medium for an additional 3–4 days. The MBC was scored as the lowest concentration of test compound that inhibited H. pylori colony formation.
3. Results and discussion
3.1. Measurements of urease activity
Urease activity was determined in 96-well microtiter plates in weakly buffered (pH 6.8) assay systems containing phenol red. Ionization of the phenolic hydroxyl groups of phenol red (pKa 8.2) by the generated NH3 resulted in increases in absorption at 570 nm. These absorption increases during the initial 3-min reaction period were used as a measure of enzyme activity as originally described by Van Slyke & Archibald [30]. This assay was adapted for a microtiter plate format. Since linearity of absorption change at 570 nm with respect to NH3 generation and pH was not demonstrated previously [22,30], we established that reaction velocity determined in this way was reasonably linearly related to enzyme quantity over a sufficiently wide range (Fig. 1) to provide a simple quantitative assay that is adequate for these studies.
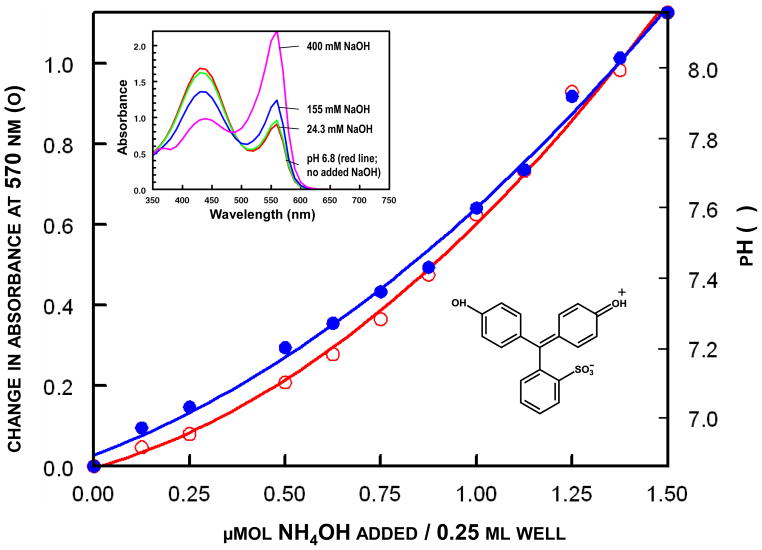
Figure 1.
Microtiter well calibration curve for measurement of ammonia by change in absorption of phenol red. Aliquots of NH4OH were added to 200 μL of 100 mM potassium phosphate, pH 6.8, containing 0.002% phenol red, and the changes in absorption at 570 nm (○) and the pH (●) determined. The insets show the absorption spectra of phenol red upon addition of increasing quantities of NaOH, and the structure of the chromophore.
By use of linear double reciprocal plots of velocity with respect to urea concentration, the Michaelis constants for urea were 20 mM for jack bean urease and 4.0 – 6.25 mM for several preparations of partially purified urease from H. pylori in 80 mM potassium phosphate buffer at pH 6.8 and 25 °C.
3.2. Kinetics of inactivation of Helicobacter urease by sulforaphane
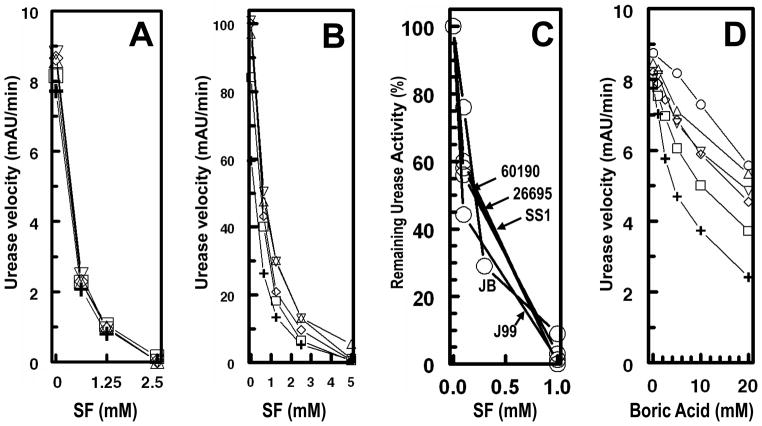
Direct addition of sulforaphane or of boric acid (a well-established inhibitor of urease) [31] to Helicobacter or jack bean urease in the standard assay system was without effect on the urease activity when measured in the presence of 20 – 120 mM urea. In contrast, incubation of these enzymes with inhibitors in the absence of urea resulted in loss of enzyme activity. This rate and extent of loss of urease activity depended on three factors: the concentrations of inhibitors, the duration of incubation with inhibitors, and the concentration of enzyme. With use of SF as inhibitor, the residual enzyme activity was completely unaffected by the concentration of urea (20 – 120 mM) used in the assay system, and once inactivation had occurred, the activity could not be restored by increasing the concentration of urea in the assay system (Fig. 2A and 2B). In this respect, urease from several strains of Helicobacter (Hp J99, Hp 26695, Hp SS-1, and Hp 60190) and from jack bean behaved similarly (Fig. 2C). In sharp contrast, prior incubation of urease with boric acid also reduced the enzymatic activity, in a time- and concentration-dependent manner, but increasing the urea concentration in the assay system at least partially reversed the inhibition (Fig. 2D). This behavior is consistent with an essentially irreversible inactivation of H. pylori urease by SF, suggesting a covalent reaction between enzyme and inhibitor, and the conclusion that boric acid is at least in part a reversible inhibitor.
Figure 2.
Inactivation of ureases by a series of concentrations of sulforaphane (A,B,C) and boric acid (D). The ureases from (A) H. pylori strain J99 and (B) jack bean were incubated for 1 h with inactivator prior to assay. The ureases from (C) 4 strains of H. pylori (60190, 26695, SS1, J99) and jack bean (JB), were incubated for 2 h with sulforaphane (SF) prior to assay. (D) Urease from H. pylori strain J99 was incubated with boric acid (BA) for 1 h prior to assay. Urease activities were measured in the presence of urea 120 (○), 100 (△), 80 (▽), 60 (◇), 40 (□), and 20 (+) mM urea.
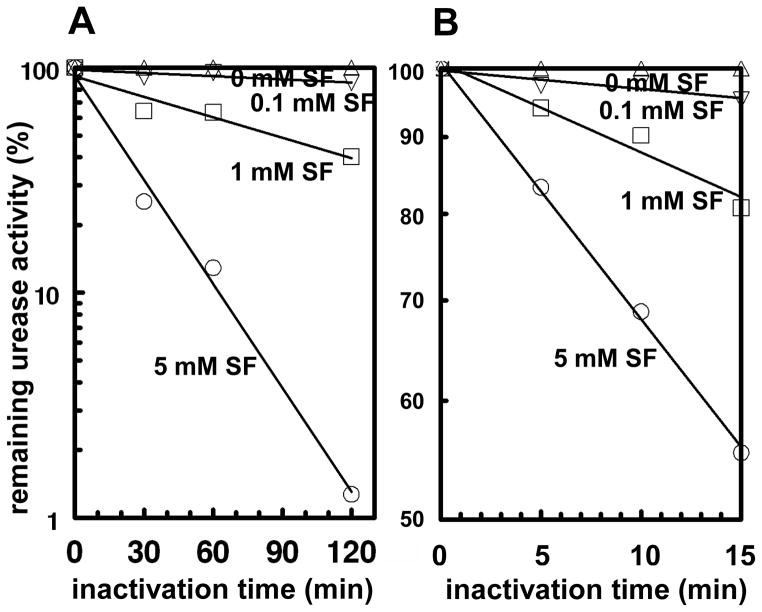
More detailed examination of the time-course of inactivation of H. pylori urease by a range of SF concentrations showed that the inactivation is a first order process whose rate depended both on the concentration of SF and of the enzyme (Figs. 3). Furthermore, double reciprocal plots of the pseudo-first-order rate constants of inactivation and the inactivator concentration were linear, consistent with the analysis of Kitz and Wilson [32] for another system.
Figure 3.
Time-course of inactivation of partially purified H. pylori urease by a series of concentrations of sulforaphane (SF). The urease concentrations were 26.7 μg/mL (A) and 80 μg/mL (B), and the remaining urease activities were measured in the presence of 20 mM urea. Semi-log plots of residual activity with respect to time of inactivation are linear, consistent with a first-order inactivation process.
3.3. Spectroscopic analysis of sulforaphane interaction with urease
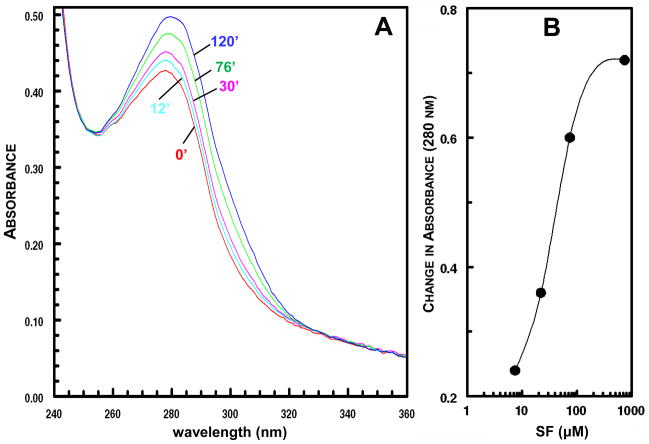
Consistent with the suggestion that SF inactivates urease by covalent interaction, incubation of purified H. pylori urease with SF resulted in a time-dependent increase in ultraviolet absorption in the 260–320 nm region (Fig. 4). The magnitude of changes in the absorption increase depended on the concentration of SF. Fig. 4B shows the absorption difference at 280 nm observed after 60 min for SF concentrations ranging from 7.4 to 740 μM. The observed absorption changes are consistent with the interaction of the isothiocyanate (-N=C=S) group of SF with one or more cysteine thiol group(s) of urease with the formation of dithiocarbamates that absorb more strongly in this wavelength region [33,34]:
Figure 4.
(A) Time-course of difference spectra of partially purified H. pylori urease (9 μg/mL) treated with 740 μM SF for 12, 30, 76, and 120 min. The control cuvette contained the equivalent concentration of sulforaphane. (B) Change in absorption at 280 nm in 60 min when sulforaphane (7.4 – 740 μM) was added to partially purified H. pylori urease (30 μg/mL).
The difference spectrum between urease and the urease-SF complex has two absorption maxima (at 280 nm and near 320 nm). The latter is consistent with the formation of a dithiocarbamate. The presence of two isosbestic points (near 250 and 330 nm) in the difference spectra suggests that the reactions responsible for these spectral changes involve relatively specific chemical reactions (Fig. 4A).
3.4. Inactivation potencies of other isothiocyanates on H. pylori urease
Treatment of H. pylori urease for 2 h with 5 mM concentrations of a number of SF analogs disclosed a wide range of inhibitory (inactivational) potencies (Table 1). Thus, whereas the natural product iberin (methylsulfinylpropyl-NCS) was equally potent to SF, methylthiopentyl-NCS, methylthiobutyl-NCS, methylsulfinyloctyl-NCS, and methylsulfinylpentyl-NCS were inactive, as were n-hexyl-NCS, benzyl-NCS, phenylethyl-NCS, and the rhamnosyloxybenzyl-NCS derived from the Moringa tree. An unexpected finding was the very high inactivator potency of benzoyl-ITC which has not been isolated as a natural product, but which bears substantial structural similarities to the clinically tested urease inhibitor N-(diaminophosphinyl)-4-fluorobenzamide [31].
Table 1.
Inactivation of partially purified H. pylori urease by various isothiocyanates (ITC). Substrate concentration was 20 mM urea, and 80 μg urease was used for each test.
| Isothiocyanate | Structure | Conc (mM) | Remaining activity (%) at:
|
|
|---|---|---|---|---|
| 30′ | 120′ | |||
| hexyl- |
|
5 | 100 | 100 |
| 5-(methylthio)pentyl-[berteroin] |
|
5 | 100 | 100 |
| 4-rhamnopyranosyl-oxy(benzyl)- |
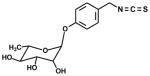
|
5 | 100 | 100 |
| 8-(methylsulfinyl)octyl-[hirsutin] |
|
5 | -- | 100 |
| 5-(methylsulfinyl)pentyl-[alyssin] |
|
5 | -- | 100 |
| 2-phenylethyl-[phenethyl] |
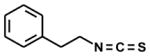
|
5 | -- | 100 |
| benzyl-[tropaeolin] |
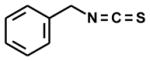
|
5 | -- | 100 |
| 4-(methylthio)butyl-[erucin] |
|
5 | 100 | -- |
| 3-(methylsulfinyl)propyl-[iberin] |
|
5 | 43 | 5 |
| 4-(methylsulfinyl)butyl-[sulforaphane] |
|
5 | 36 | 0 |
| benzoyl- |
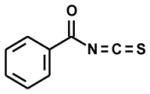
|
5 | 0 | 0 |
| 1 | 12 | -- | ||
| 0.1 | 77 | -- | ||
| 0.01 | 100 | -- | ||
3.5. Relation of bactericidal activity of isothiocyanates to urease inhibition potency
The minimum bactericidal concentration (MBC) of SF for various strains of H. pylori has been previously reported to be in the low microgram per mL range [7]. A more detailed examination of the MBC for a number of commonly-used H. pylori strains is shown in Table 2. The values are relatively uniform (2.8 – 5.6 μg/mL) and comparable to our previous measurements obtained by somewhat different methods (mean 2 – 4 μg/mL; [7]).
Table 2.
Minimum Bactericidal Concentration (MBC) of sulforaphane (SF) against several H. pylori strains.
| Strain of H. pylori
|
Properties | MBC (μg/mL) | |
|---|---|---|---|
| ATCC No. | Common Designation | ||
| 49503 | 60190 | a cytotoxin-producing strain | 2.8 |
| 51110 | ure- 60190 variant | near-isogenic urease-minus variant of 60190 | 5.6 |
| 700824 | J99 | genome-sequenced type-strain; cagA+, vacA+ | 2.8 |
| 700392 | 26695 | genome-sequenced type-strain | 5.6 |
| -- | SS-1 | mouse-adapted human strain | 2.8 |
Very importantly, SF is potently bactericidal against the urease-negative and therefore noninfective strain (ATCC 51110), whereas the potent urease inhibitor benzoyl-ITC does not have bactericidal activity against several strains of H. pylori that we tested (data not shown). Furthermore, a number of ITC inducers of cytoprotective enzymes which were previously shown [35] to be bactericidal to H. pylori (e.g., 5-(methylthio)pentyl-ITC (berteroin), benzyl-ITC 8-(methylsulfinyl)octyl-ITC (hirsutin), phenethyl-ITC, 5-(methylsulfinyl)pentyl-ITC (alyssin), and 4-(methylthio)butyl-ITC (erucin) were not inhibitors of H. pylori urease when tested under identical conditions to SF, benzoyl-ITC, and iberin (Table 1). Similar observations were made with the ellagitannin tellimagrandin and the triterpenoid TP-225 in that they had antibiotic activity against H. pylori, but were inactive as urease inhibitors (data not shown).
Interestingly, and in marked contrast to the very high levels of SF accumulation in cultured mammalian cells [36], H. pylori did not concentrate SF appreciably, from culture medium (data not shown). Clearly, since SF kills both virulent (ure+; urease –containing) H. pylori, and a mutant strain that lacks a functional urease (ure−), the bactericidal/bacteriostatic effects of SF are not due primarily to urease inactivation.
Urease is involved in directly increasing the inflammation associated with H. pylori infections in a manner that has been postulated to increase tight junction permeability, and is not associated with the catalytic activity of the enzyme [37,38]. Whether inhibition of this mode of urease action by ITCs could alter the pathogenicity of H. pylori without killing or inhibiting the organism directly, requires elucidation in appropriate model systems.
In conclusion, it is intriguing to reflect on the unforeseen roles that urease has played in the history of biological science: (i) urease was the first enzyme to be crystallized (from jack bean) leading J.B. Sumner to attribute a specific biological activity to a pure protein, and thereby setting off one of the bitterest scientific polemics of the twentieth century; (ii) Urease was the first enzyme shown to require two nickel atoms at the active site; (iii) Urease is synthesized in large quantities by several important human infective agents in addition to H. pylori, including Proteus mirabilis, Mycobacterium tuberculosis, Cryptococcus neoformans, and contributes in important ways to the inflammatory consequences of these infections; (iv) A large number of urease inhibitors have been developed for agricultural purposes to prevent soil nitrogen depletion by denitrification (and subsequent evaporation of ammonia). The present study raises the possibility that in addition to-, or as an alternative mode of action -- ITC inactivation of H. pylori urease may reduce gastric colonization by this organism and/or the inflammation associated with it. Whether its inhibition can play a significant role in reducing the consequences of H. pylori colonization of half of the world’s population warrants further exploration.
Highlights.
Sulforaphane, which is bactericidal to H. pylori, also inactivates its urease
Kinetic and spectral evidence consistent with cysteine thiol covalent modification
Urease inactivation has potential to reduce consequences of Helicobacter infection
Acknowledgments
The authors are grateful to Dr. Patricia Gravitt for generously sharing her laboratory space for the bacterial culture work and to Yolanda Eby and Roslyn Howard for their assistance. Primary funding for these studies was provided by the Lewis B. and Dorothy Cullman Foundation. Supplementary funds came from NIH grant R01 CA093780-05A2 and Prevent Cancer Foundation grant 90034316.
Abbreviations
- ITC
isothiocyanate(s)
- MBC
minimum bactericidal concentration
- SF
sulforaphane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jed W. Fahey, Email: jfahey@jhmi.edu.
Katherine K. Stephenson, Email: kstephen@jhmi.edu.
Kristina L. Wade, Email: kwade@jhmi.edu.
Paul Talalay, Email: ptalalay@jhmi.edu.
References
- 1.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobacter pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894–1901. doi: 10.1053/j.gastro.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Ha N, Oh S, Sung JY, Cha KA, Lee M, Oh B. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nature Structure Biol. 2001;8(6):505–509. doi: 10.1038/88563. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Perez GI, Olivares AZ, Cover TL, Blaser MJ. Characteristics of Helicobacter pylori variants selected for urease deficiency. Infect Immun. 1992;60:3658–3663. doi: 10.1128/iai.60.9.3658-3663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mobley HLT. Urease. In: Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: Physiology and Genetics. Chapter 16. ASM Press; Washington D.C: 2001. http://www.ncbi.nlm.nih.gov/books/NBK2417/ [PubMed] [Google Scholar]
- 6.Hu LT, Mobley HLT. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990;58(4):992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Nat Acad Sci USA. 2002;99(11):7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res. 2009;2(4):353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 9.Sumner JB. Isolation and crystallization of the enzyme urease. J Biol Chem. 1926;69:435–441. [Google Scholar]
- 10.Balasubramanian A, Ponnuraj K. Crystal structure of the first plant urease from jack bean: 83 Years of journey from its first crystal to molecular structure. J Mol Biol. 2010;400:274–283. doi: 10.1016/j.jmb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Desnuelle P, Rovery M. Sur l’inactivation de l’uréase par l’isocyanate de phenyl. Biochim Biophys Acta. 1949;3:26–33. [Google Scholar]
- 12.Riddles PW, Andrews RK, Blakeley RL, Zerner B. Jack bean urease VI. Determination of thiol and disulfide content. Reversible inactivation of the enzyme by the blocking of the unique cysteine residue. Biochim Biophys Acta. 1983;743:115–120. [Google Scholar]
- 13.Dixon NE, Gazzola C, Watters JJ, Blakeley RL, Zerner B. Inhibition of jack bean urease (EC 3.5.1.5) by acetohydroxamic acid and by phosphoramidate. An equivalent weight for urease. J Amer Chem Soc. 1975;97(14):4130–4131. doi: 10.1021/ja00847a044. [DOI] [PubMed] [Google Scholar]
- 14.Dixon NE, Gazzola C, Asher CJ, Lee DSW, Blakeley RL, Zerner B. Jack bean urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can J Biochem. 1980;58:474–480. doi: 10.1139/o80-063. [DOI] [PubMed] [Google Scholar]
- 15.Dixon NE, Riddles PW, Gazzola C, Blakeley RL, Zerner B. Jack bean urease (EC 3.5.1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, N-methylurea, and related compounds. Can J Biochem. 1980;58:1335–1344. doi: 10.1139/o80-181. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava PK, Kayastha AM. Significance of sulfhydryl groups in the activity of urease from pigeonpea (Cajanus cajan L.) seeds. Plant Sci. 2000;159:149–158. doi: 10.1016/s0168-9452(00)00343-5. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Kawase M, Tani S. α-Hydroxyketones as inhibitors of urease. Bioorg Med Chem. 2004;12:501–505. doi: 10.1016/j.bmc.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Kot M, Bicz A. Inactivation of jack bean urease by N-ethylmaleimide: pH dependence, reversibility and thiols influence. J Enzyme Inhib Med Chem. 2008;23(4):514–520. doi: 10.1080/14756360701674264. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Kaysatha AM. Soybean (Glycine max) urease: Significance of sulfhydryl groups in urea catalysis. Plant Physiol Biochem. 2010;48:746–750. doi: 10.1016/j.plaphy.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Krajewska B. Hydrogen peroxide-induced inactivation of urease: Mechanism, kinetics and inhibitory potency. J Molec Catalysis B: Enzymatic. 2011;68:262–269. [Google Scholar]
- 21.Krajewski B, Zaborska W. Jack bean urease: The effect of active-site binding inhibitors on the reactivity of enzyme thiol groups. Bioorgan Chem. 2007;35(5):355–365. doi: 10.1016/j.bioorg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Kawase M, Tani S. Urease inhibitory activity of simple α,β-unsaturated ketones. Life Sci. 2003;73(23):2985–2990. doi: 10.1016/s0024-3205(03)00708-2. [DOI] [PubMed] [Google Scholar]
- 23.Fahey JW, Kensler TW, Talalay P. Notes from the field: “Green” chemoprevention as frugal medicine. Cancer Prev Res. 2012;5(2):179–188. doi: 10.1158/1940-6207.CAPR-11-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rokita E, Makristathis A, Hirschl AM, Rotter ML. Purification of surface-associated urease from Helicobacter pylori. J Chromatorgr B. 2000;737:203–212. doi: 10.1016/s0378-4347(99)00374-6. [DOI] [PubMed] [Google Scholar]
- 25.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 26.Dixon NE, Blakeley RL, Zernerm B. Jack bean urease (EC 3.5.1.5). III. The involvement of active-site nickel ion in inhibition by β-mercaptoethanol, phosphoramidate, and fluoride. Can J Biochem. 1980;58:481–488. doi: 10.1139/o80-064. [DOI] [PubMed] [Google Scholar]
- 27.Dixon NE, Hinds JA, Fihelly AK, Gazzola C, Winzor DJ, Blakeley RL, Zerner B. Jack bean urease (EC 3.5.1.5). IV. The molecular size of the mechanism of inhibition by hydroxamic acids. Spectrophotometric titration of enzymes with reversible inhibitors. Can J Biochem. 1980;58:1323–1334. doi: 10.1139/o80-180. [DOI] [PubMed] [Google Scholar]
- 28.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin IS, Masuda H, Naohide K. Bactericidal activity of wasabi (Wasabia japonica) against Helicobacter pylori. Int J Food Microbiol. 2004;94(3):255–261. doi: 10.1016/S0168-1605(03)00297-6. [DOI] [PubMed] [Google Scholar]
- 30.Van Slyke DD, Archibald RM. Manometric, titrimetric, and colorimetric methods for measurement of urease activity. J Biol Chem. 1944;154:623–642. [Google Scholar]
- 31.Krajewska B. Ureases I. Functional, catalytic and kinetic properties: A review. J Molec Catalysis B: Enzymatic. 2009;59(1–3):9–21. [Google Scholar]
- 32.Kitz R, Wilson IB. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962;237(10):3245–3249. [PubMed] [Google Scholar]
- 33.Zhang Y, Cho C-G, Posner GH, Talalay P. Spectroscopic quantitation of organic isothiocyanates by cyclocondensation with vicinal dithiols. Anal Biochem. 1992;205(1):100–107. doi: 10.1016/0003-2697(92)90585-u. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal Biochem. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 35.Haristoy X, Fahey JW, Scholtus I, Lozniewski A. Evaluation of antimicrobial effect of several isothiocyanates on Helicobacter pylori. Planta Med. 2005;71:326–330. doi: 10.1055/s-2005-864098. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 37.Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM., Jr Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136:236–246. doi: 10.1053/j.gastro.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapointe TK, O’Connor PM, Jones NL, Menard D, Buret AG. Interleukin-1 receptor phosphorylation activates Rho kinase to disrupt human gastric tight junctional claudin-4 during Helicobacter pylori infection. Cell Microbiol. 2010;12(5):692–703. doi: 10.1111/j.1462-5822.2010.01429.x. [DOI] [PubMed] [Google Scholar]