Abstract
Background
The effectiveness of systemic antimonial (sodium stibogluconate, Pentostam, SSG) treatment versus local heat therapy (Thermomed) for cutaneous leishmaniasis was studied previously and showed similar healing rates. We hypothesized that different curative immune responses might develop with systemic and local treatment modalities.
Methods
We studied the peripheral blood immune cells in a cohort of 54 cutaneous Leishmania major subjects treated with SSG or TM. Multiparameter flow cytometry, lymphoproliferative assays and cytokine production were analyzed in order to investigate the differences in the immune responses of subjects before, on and after treatment.
Results
Healing cutaneous leishmaniasis lead to a significant decline in circulating T cells and NKT-like cells, accompanied by an expansion in NK cells, regardless of treatment modality. Functional changes involved decreased antigen specific CD4+ T cell proliferation (hyporesponsiveness) seen with CD8+ T cell depletion. Moreover, the healing (or healed) state was characterized by fewer circulating regulatory T cells, reduced IFN-γ production and an overall contraction in polyfunctional CD4+ T cells.
Conclusion
Healing from cutaneous Leishmaniasis is a dynamic process that alters circulating lymphocyte populations and subsets of T, NK and NKT-like cells. Immunology of healing, through local or systemic treatments, culminated in similar changes in frequency, quality, and antigen specific responsiveness with immunomodulation possibly via a CD8+ T cell dependent mechanism. Understanding the evolving immunologic changes during healing of human leishmaniasis informs protective immune mechanisms.
Author Summary
Globally, leishmaniasis treatment relies on the use of antimonial drugs (i.e. SSG). In an earlier study we showed that skin lesions due to L. major treated by the ThermoMed (TM) device healed at a similar rate and with less associated systemic toxicity than lesions treated with intravenous SSG. The current study compared the immune responses of these two therapeutic groups before, during and after therapy which may be relevant to resistance to reinfection and also in consideration for the development of local (versus systemic) therapy. Antimonials have immune effects on both the host and parasite while heat treatment locally kills the parasite and induces inflammation from a secondary burn. We demonstrated that healing from cutaneous leishmaniasis is a dynamic process associated with a modulation of immune responses independent of treatment modalities.
Introduction
Leishmaniasis, a vector-borne parasitic disease, remains a pressing global health concern with 12 million persons infected, 2 million new infections each year, limited therapeutic options and no effective vaccine [1].
Healing cutaneous leishmaniasis (CL) relies on the development of an effective and balanced protective immune response. The intracellular parasite needs to be contained, while the pathologic immune response needs to be controlled. The murine model for L. major substantially contributed to our understanding of protective immunity and helped establish the T helper 1 (Th1)/Th2 paradigm that explained resistance and susceptibility to Leishmania infection [2,3]. This model demonstrated that T lymphocytes are key for the generation of this protective response through their IFN-γ production which activates macrophages to produce toxic nitrogen and oxygen metabolites to kill the intracellular amastigotes [4]. The Th1 cytokine profile, i.e. IFN-γ, TNF-α and IL-12, is crucial to eliminate Leishmania [5], while the development of a Th2 immune response with the production of IL-4, TGF-β and IL-10 favors parasite multiplication and fails to control the infection [6]. The quality of a T cell response, defined by the pattern of cytokine production at the single cell level, underscores the importance of polyfunctional CD4+T cells specifically producing IFN-γ, TNF-α and IL-2 for protection [7,8]. Additionally, immunoregulatory mechanisms involving regulatory and memory T cells can significantly influence leishmaniasis outcome [9].
The precise role of human CD4+T cell subsets, their cytokine patterns and the immune response pathways engaged during and after effective leishmaniasis therapy are incompletely understood. While pentavalent antimonial drugs (i.e. SSG, meglumine antimoniate) have been used to treat CL for decades [10], they are toxic, require extended duration of treatment, and drug resistant parasites have emerged as a problem [11,12]. The mechanism of action of SSG includes effects on both the host macrophage and parasite [13]. Thermotherapy is an alternative treatment for CL [14,15], delivering localized radiofrequency waves into skin lesions to physically destroy the temperature sensitive parasites. Thermomed (TM, Thermosurgery Technologies, Phoenix AZ), cleared by the Food and Drug Administration, is one of the most studied devices in use [15]. Clinical trials comparing local heat to systemic antimonial therapy showed similar CL cure rates [14,16–20].
We previously reported that subjects treated with the TM device showed similar healing by 2 and 12 months follow-up, with less associated systemic toxicity than those treated with intravenous SSG [21]. We hypothesized that an immunomodulatory systemic therapy would induce a different immune response compared to a locally applied physical treatment, though both methods were ultimately curative. This work comparatively evaluated the immune response profile over time in the participants treated with SSG or TM. We showed a modulation of immune response occurs during healing from cutaneous leishmaniasis independent of either treatment modality.
Materials and Methods
Ethics statement
All participants provided written informed consent and study protocols were approved by Institutional Review Boards at both WRAMC and the Walter Reed Army Institute of Research.
Study population
All participants were U.S. military personnel referred to the Walter Reed Army Medical Center (WRAMC) for treatment of parasitologically confirmed L. major infection (Table 1). Details of the clinical trial are published [21]. Seven healthy uninfected control subjects were recruited under a separate protocol.
Table 1. Demographic characteristics and outcome presented by treatment arm.
| Characteristic | SSG (n = 20) | TM (n = 19) | p value |
|---|---|---|---|
| Median age in years (range) | 24.5 (18–57) | 25 (20–41) | 0.204* |
| Male gender (%) | 95 | 100 | 1** |
| Race (%) | 0.85*** | ||
| White | 65 | 57 | |
| Black | 15 | 26.3 | |
| Hispanic | 15 | 10.5 | |
| Filipino/Asian | 5 | 5.2 | |
| Median number of lesions | 3 (1–17) | 3 (1–14) | 0.99* |
| Total area of lesions (mm) | 531.5 (100–3230) | 691.5 (81–2533) | 0.95* |
| Median time since onset (days) | 137.5 | 145.5 | 0.7* |
| Number healed at 6 months (%) | 18/20 | 15/19 | 0.407** |
* Mann-Whitney
** Fisher exact
***Vassarstats
Sample collection and storage
Whole blood subjects were drawn at time points designated “pre-treatment” (PRE), “on-treatment” (ON) and “post-treatment” (POST) (Days 0, 9±1 and 219±68 following treatment initiation, respectively). For pre- and on-treatment subjects, blood was drawn at WRAMC and processed fresh. At POST, blood was drawn at alternate medical facilities and shipped via overnight carrier for processing. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood as previously described [22].
Antibodies for flow cytometry
The following fluorescence-conjugated antibodies were used for multiparameter flow cytometry: CD3 (SK7), CD4 (SK3), CD8 (SK1), CD14 (M5E2), CD19 (HIB19) CD25 (2A3), IL-10 (JES3-19F1), TNF-α (Mab11), IL-2 (5344.111), γδ TCR (B1) (BD Biosciences, San Jose, CA); CD4 (SFCI12T4D11) (Beckman Coulter, Fullerton, CA); IL-17 (eBio64DEC17) and αβ TCR (IP26) (BioLegend, San Diego, CA); IFN-γ (4S.B3) (eBioscience, San Diego, CA). All antibodies were titrated prior to use to determine optimal staining concentrations. Flow cytometry data was acquired either on a FACS Calibur or LSR-II flow cytometer (BD Biosciences) and data analyzed using FlowJo software (TreeStar, Ashland OR).
Fresh cell population phenotyping
Prior to cryopreservation, a PBMC aliquot was stained for cell surface markers and analyzed by flow cytometry. Markers included the BD SimulTEST (CD45, CD14) and BD MultiTEST (CD3, CD16, CD56, CD45, CD19) reagents. T cell populations were further analyzed by staining with CD3, CD4, CD8, and CD25. Following staining, cells were fixed in 2% paraformaldehyde, data collected with a FACS Calibur flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland OR).
Lymphocyte proliferation assay (LPA) and quantitation of secreted cytokines
Cryopreserved PBMC were thawed in complete media. A portion of the PBMC was depleted of CD8+T cells (CD8depl PBMC) using the Dynal CD8 Positive Isolation Kit (Invitrogen, Carlsbad CA). Total PBMC or CD8depl PBMC were plated in the presence of soluble Leishmania antigens from L. major parasites (SLA, 2.5 μg/mL, generous gift of Dr. Frank Neva) for 6 days at 37°C, 5% CO2. Pokeweed mitogen (PWM, 5 μg/mL, Sigma) was used as a positive control. Cell-free supernatant was collected from each well, triplicate subjects pooled, and used to quantify cytokines using the Q-Plex Human Cytokine–IR Array (Quansys Biosciences, Logan, UT) according to manufacturer’s protocol [23]. For LPA, cells were pulsed as previously reported [24].
CFDA-SE labeling of PBMC
Cryopreserved PBMC were thawed and labeled with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE, Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions [25].
Intracellular cytokine staining
Cryopreserved PBMC were thawed and incubated overnight at 37°C, 5% CO2. Cells were plated at 1 x 106 per well and stimulated with L. major whole lysate (1μg/mL, generous gift from David Sacks) for 24 hours at 37°C, 5% CO2. Brefeldin A (10μg/mL, Sigma) was added to all wells at 18 hours. All cells were costimulated with 1 μg/mL CD28 and CD49d antibodies (BD Biosciences). Following stimulation, cells were stained for population identification markers (CD3, CD4, CD8, CD14 and CD19) and intracellular cytokine expression (TNF-α, IFN-γ, and IL-2). T cell receptor (TCR) phenotyping antibodies were included for the αβ TCR and γδ TCR.
Statistics
All statistics were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
Results
Sample cohort description
Fifty-four U.S soldiers (98% male) with CL were enrolled and randomized to either local heat therapy (TM) or 10 days of intravenous SSG (Table 1). To evaluate the immune response profiles in these subjects, PBMC were isolated from whole blood at three time points. Pre-treatment (PRE) cells were collected upon enrollment into the study (n = 54, 100%). The on-treatment cells were collected on 9±1 treatment day (n = 54, 100%), and post-treatment (POST) subjects collected at a mean of 7 months (range 4.7–9.2 months), after treatment (n = 39, 72%). Because 39/54 participants provided cells at all time points, the majority of our analysis is restricted to this subcohort (Table 1). No significant differences were noted between treatment arms or subcohort and cohort regarding demographic characteristics, disease burden and therapy outcome.
Lymphocyte populations differ between disease and healed state
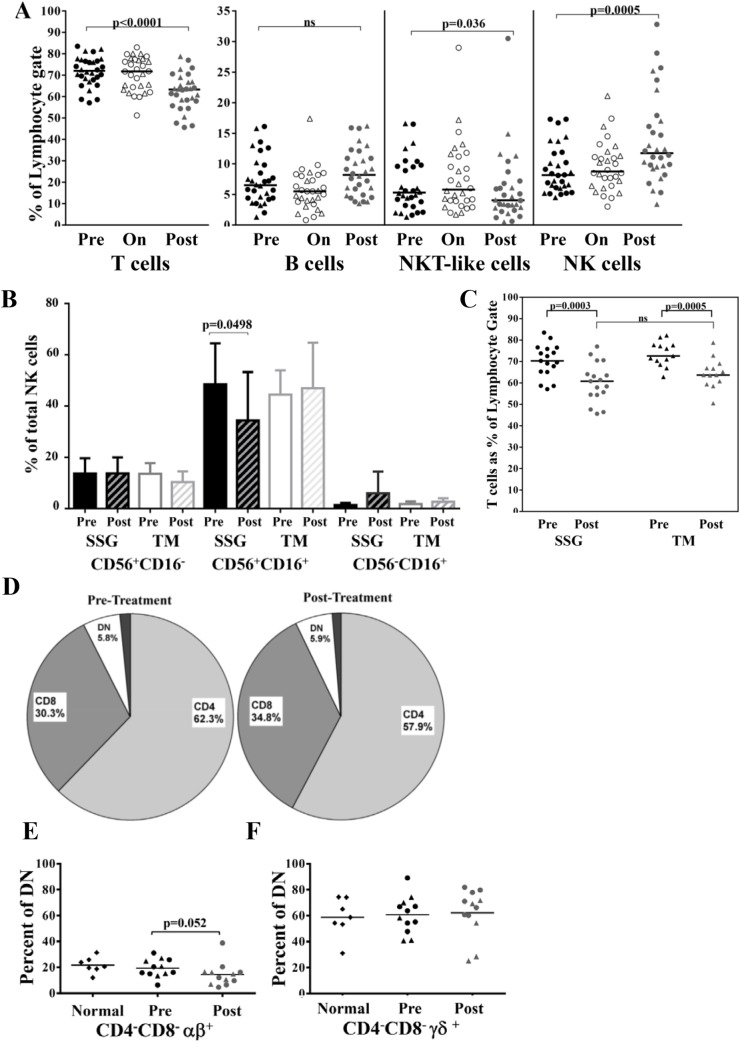
Freshly isolated cells were stained and analyzed by flow cytometry to characterize the circulating lymphocyte populations. Data from 30 subjects for which there were adequate numbers of cells for all time points is shown (Fig 1). The distribution of lymphocyte populations, including T cells, B cells, NKT-like and NK cells, was unchanged from pre-treatment through the first ten days of treatment (Fig 1A). At POST we observed a significant decrease in circulating T cells (pre, 73%; post, 63%; p< 0.0001), and a concomitant increase in circulating NK cells (pre, 8%; post, 12%; p = 0.0005). The proportion of B cells was unchanged while NKT-like cells showed a modest yet significant decrease (p = 0.036). Results were not affected by removing the few treatment failures from each group (S1 Fig). The observed changes did not correlate with the severity of disease in terms of lesion size (S2 Fig). Analysis of NK subsets based on CD56 and CD16 markers showed a significant decrease in CD16+CD56+ cells at POST in the SSG group (Fig 1B).
Fig 1. Characterization of lymphocyte populations by flow cytometry.
Data is presented from 30 subjects (17 in the SSG arm and 13 in TM arm represented in circles and triangles respectively) for which cells from all three time points were available. (A) Percentage of lymphocytes positive for surface expression of CD3 (T cells), CD19 (B cells), CD16/CD56 (CD3-: NK cells; CD3+: NKT-like cells). (B) Distribution of subpopulations of NK cells based on CD16 and CD56 expression. (C) T cell phenotype stratified by treatment arm. (D) Distribution of T cells into CD4+, CD8+ and CD4−CD8− (double negative, DN) populations pre- and post-treatment. (E-F) Identification of TCR expression within the T cell populations. Aggregate data from 12 study subjects compared to data from 7 healthy controls for (E) αβ and (F) γδ respectively. Bars represent medians. P values were derived using the Wilcoxon matched pairs test.
The subjects were stratified and reanalyzed to determine if the observed changes in cell populations in POST correlated with treatment arm. Similar declines in circulating T cells were seen in both the SSG and TM subjects. Surprisingly, there was no difference when comparing the percentage of T cells in POST between treatment groups (Fig 1C). Similar population changes for NK cells and NKT-like cells were observed in both treatment arms (S3 Fig). We next investigated CD4+ and CD8+T cells subsets before and after treatment. There was a marked decrease in the median percentage of CD4+T cells (pre, 62.3; post, 57.9; p = 0.0089) and a proportionate increase in CD8+T cells (pre, 30.3; post, 34.8; p = 0.0128) post-treatment, with no changes in the CD4-CD8- (double negative, DN) population (Fig 1D). We determined the TCR distribution in CL caused by L. major, using flow cytometry to profile the TCR repertoire of each of the four subsets of T cells (based on CD4 and/or CD8 expression) in our subjects and in healthy donors (n = 7). Here the αβ TCR was exclusively expressed on single-positive CD4+T cells and double-positive CD4+CD8+T cells, and predominantly on the single-positive CD8+T cells (representative donor shown, S4 Fig). The DNT cells, on the other hand, were a mixture of αβ expressors, γδ expressors and a population that was negative for both of these TCR. Surprisingly, our results for the αβ and γδ TCR align in healthy and L. major infected subjects. A decrease in αβ expression (p = 0.052) (Fig 1E) and trend in increase of γδ was observed in POST (Fig 1F) while the overall percentage of DNT cells remained unchanged during the course of the study.
Both CD4+ and CD8+T cells play a role in proliferative responses
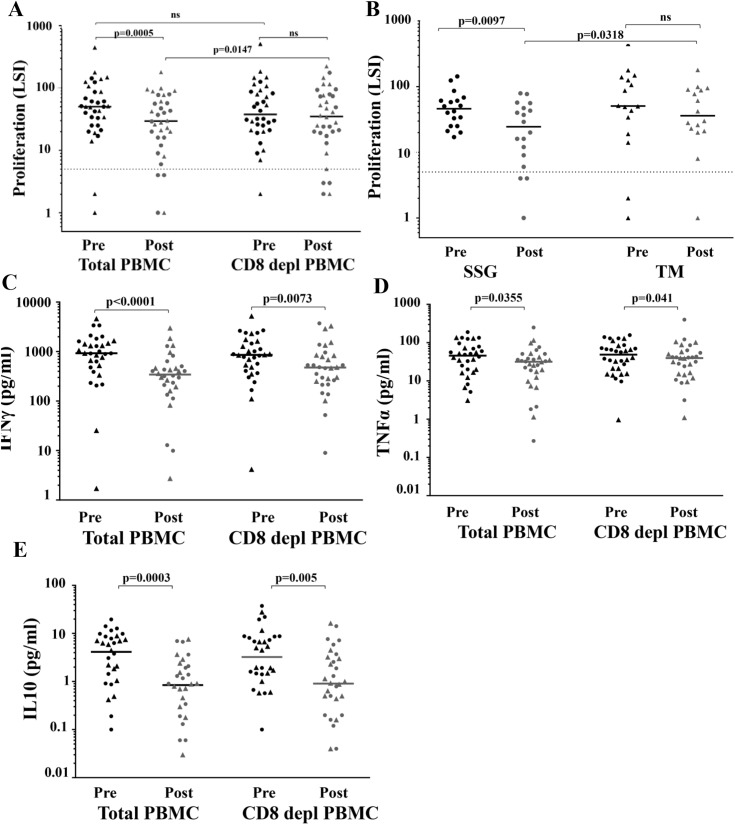
The lymphoproliferative response in 34 evaluable subjects was analyzed at different time points with concurrent cytokine production. Interestingly, a significant decrease in Leishmania antigen-specific T cell proliferation against SLA (p = 0.0005) was seen in POST subjects of total PBMC (Fig 2A). These differences persisted when analyzed without the few treatment failures in each group (S5 Fig). However, when analyzed by treatment arm, this decrease in proliferation after therapy was only observed in the SSG but not TM treatment (Fig 2B).
Fig 2. Lymphoproliferative response and cytokine production.
(A) Whole PBMC or CD8+T cell-depleted PBMC (CD8 depl PBMC) from 18 subjects (circles) and 16 subjects (triangles) treated respectively with SSG and TM at pre-treatment (black) and post-treatment (grey) stages were stimulated with SLA for 6 days followed by an 8 hour pulse with [3H]-thymidine. Lymphocyte stimulation index (LSI) was determined as fold-increase in mean cpm from triplicate wells over unstimulated wells. An LSI ≥ 5 (dotted line) is considered a positive response. (B) L. major antigen responses in whole PBMC stratified by treatment arm. (C-E) Cytokine production following stimulation with SLA. (C) IFN-γ, (D) TNF-α and (E) IL-10 production by total PBMC or CD8+ cell-depleted PBMC was quantified from supernatants sampled on day 6 of the lymphoproliferation assays. Bars represent medians. P values were derived using the Wilcoxon matched pairs test.
Recent reports suggest that CD8+T cells play a regulatory role in immunity to leishmaniasis [26]. In testing the role of CD8+ cells in proliferation responses PRE and POST, we depleted CD8+ cells from the bulk PBMC prior to stimulation. The proliferation differences between PRE and POST responses were abrogated with CD8+T cell depletion pointing to a potential immunomodulatory or regulatory role for CD8+T cells (Fig 2A). Cytokines were quantified to determine if the suppressive effect of the CD8+T cells involved soluble mediators. Interestingly, IFN-γ, IL-10 and TNF-α were produced at significantly lower levels in POST, whether the CD8+T cells were present or not (Fig 2C, 2D and 2E) which restricts the CD8+T cell effects to modulation of lymphocyte proliferation independent of cytokines tested here.
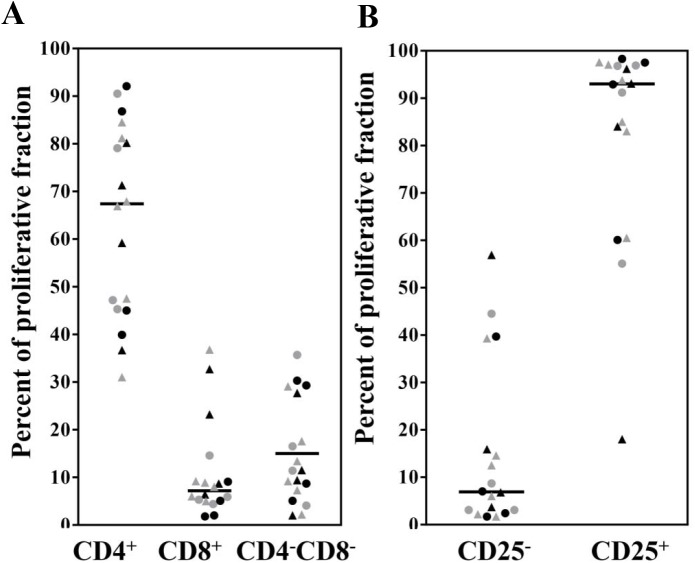
We next used CFSE labeling to identify antigen-specific proliferating cell subsets in both bulk and CD8+T cell depleted PBMC. Aggregate data is shown in Fig 3Aand 3B and a representative gating example in S6 Fig. While the predominant proliferative fraction consisted of CD4+T cells (68%), there was a modest expansion of CD8+T cells (7%) and CD4-CD8- DNT cells (15%) (Fig 3A). As expected, the vast majority (>90%) of responding cells were activated, as assessed by CD25 expression (Fig 3B).
Fig 3. Identification of responding populations by CFDA-SE labeling and flow cytometry analysis.
Identification of proliferating lymphocytes based on expression of (A) CD4 and CD8 or (B) CD25. Circles represent SSG subjects and triangles represent TM subjects. Black and grey are for PRE and POST respectively. Bars represent medians.
Reduced circulating regulatory CD4+T cells after healing
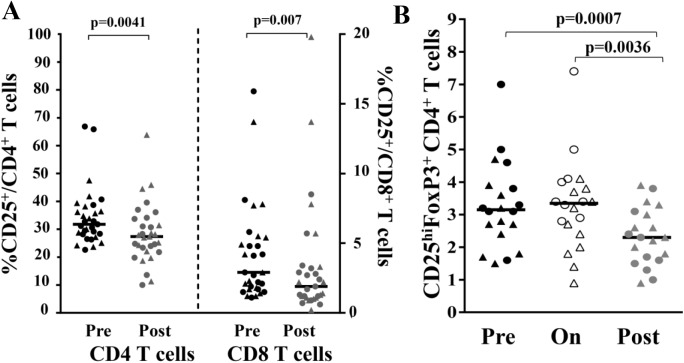
Based on CD25 expression and the observed modulation of proliferative immune response, we investigated the role of T regulatory (Treg) cells in the healing process. PBMC were analyzed by flow cytometry to determine the levels of activated T cells, identified by CD25 expression. At POST, we observed a decrease in the percentages of circulating activated T cells in both the CD4 and CD8 compartments (Fig 4A). We identified Treg as those cells within the CD4+T cell compartment that expressed the highest levels of CD25 (CD25+ bright) and FoxP3 (S7 Fig). Aggregate data from n = 20 sets of subjects shows that while there was no effect on the Treg population during treatment, there was a marked reduction in circulating CD4+ Treg cells in POST (pre, 3.1%; on, 3.3%; post, 2.3%; p-values = 0.0007 and 0.0036) (Fig 4B).
Fig 4. Identification of activated and regulatory T cell populations by flow cytometry.
(A) Freshly isolated cells were stained for CD3, CD4, CD8 and CD25 for identification of activated T cells. Data obtained from 31 subjects (circles for SSG subjects and triangles for TM subjects) for which pre-treatment (black) and post-treatment (grey) cells were available. Bars represent medians. (B) Identification of Treg cells from thawed PBMC. Aggregate data from 20 donors for CD4+CD25high Foxp3+. P values derived using the Wilcoxon matched pairs test.
Decreased cytokine production in post-treatment polyfunctional CD4+ T cells
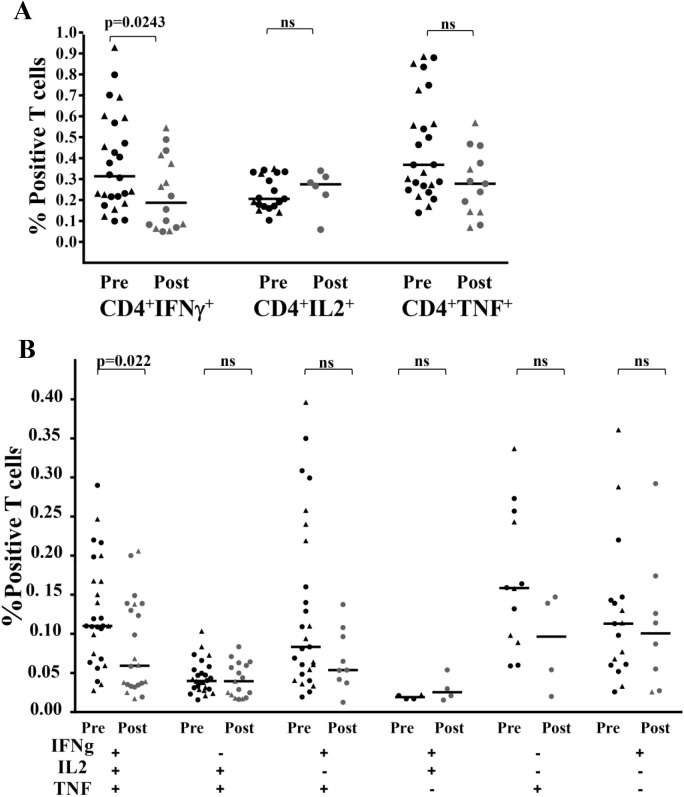
The degree of protection against various infections including leishmaniasis [7] is predicted by the frequency of polyfunctional CD4+ memory T cells that produce IFN-γ, TNF-α, and IL-2. We assessed intracellular cytokine production by CD4+T cells PRE and POST using multiparameter flow cytometry. First, we were able to independently quantify production of IFN-γ, TNF-α and IL-2 by the CD4+ cells, and observed a significant decrease in production of IFN-γ at POST (Fig 5A). Next, we used Boolean gating to analyze the polyfunctionality of these SLA-specific CD4+T cell responses and found a significant decrease in the frequency of triple positive CD4+ T cells expressing IFN-γ, IL-2 and TNF-α at POST also (Fig 5B). For the subjects that failed to meet the validation criteria in the Boolean gating (minimum 50 events), no values are reported which explains the fewer numbers of points in certain subsets.
Fig 5. Characterization of the cytokine production capacity of responding T cells.
Multiparameter flow cytometry was used to determine (A) IFN-γ, IL-2, and TNF-α production in CD4+ T cells (B) the frequency of cells expressing each of the seven possible combinations of IFN-γ, IL-2, and TNF-α. Circles represent SSG subjects and triangles represent TM subjects. P values derived using the Mann-Whitney test for unpaired samples.
Discussion
Little is known about the cellular phenotypic profile and immune response of humans prior-to and following treatment with different leishmaniasis therapeutic regimens. In this study, we compared the immune response profile in a cohort of L. major infected subjects treated with intravenous SSG or locally applied heat therapy (TM) [21]. The mechanism of actions of these two treatment modalities and the nature, location and distribution of therapy are markedly different. Although both treatments resulted in clinical healing, we hypothesized that an immunomodulating systemic therapy might act through different immune mechanisms compared to a localized, physical, direct parasite-killing therapy.
In this study, we report two important findings with functional immunologic underpinnings. First, downmodulation of Leishmania antigen-specific CD4+T cell proliferative responses possibly through a CD8+T-cell dependent mechanism was observed after therapy. Second, we report that Leishmania-specific polyfunctional CD4+T cells also decrease after therapy.
Since clinical cure from leishmaniasis is classically and primarily dependent on T cell subtypes and relevant cytokine production profiles [27,28], cells were phenotyped from subjects before and after treatment. After treatment and independent of the treatment modality, circulating T cells and NKT-like cells were decreased with a concomitant increase in circulating NK cells highlighting the relevance of the innate immune system for Leishmania control. NK and T cells seemed to have reciprocal effects; wherein NK cell-produced IFN-γ which resulted in T cell activation and the T cell derived IL-2 lead to NK triggering [29]. Similarly, an association between the increased frequency of NK cells and lesion healing is reported after immunotherapy with BCG/Leishmania antigens [30].
NKT-like cells share several characteristics with NK cells [31] and serve as frontline innate immune effectors and potential regulators of adaptive immune responses against microorganisms [32]. Although only a trend, the increase of NKT-like cells observed during treatment could be explained by their ability to serve as an early source of regulatory cytokines and their degranulation-related killing function.
In our T cell subset analysis, we showed a high percentage of CD4+T cells in the early treatment phase, suggesting their association with disease progression [33]; while the percentage of CD8+T cells increased post treatment. This could reflect the down-modulation of the immune response, as a means to mitigate immunopathology, consistent with other studies linking CD8+T cell subset induction with the healing process [26] and lesion resolution during antimonial therapy [34]. Contraction of CD4+T cells and expansion of CD8+T cells during healing suggests CD4 modulation after cure [35]. CD8+T cells were also increased in healed Brazilian CL subjects suggesting potential modulation of the activity of CD4+ cells by direct cytolytic effect of infected macrophages, or by other regulatory effects [33]. Our results confirm that a balance between the proportion of CD4+ and CD8+T cells is important for leishmaniasis healing [33,36–38]. We also analyzed DN T cells, and in particular the αβ subpopulation, a highly activated T cell subset producing cytokines to activate monocytes and macrophages [39]. DN lymphocytes are the second most prevalent cell type producing IFN-γ in human CL [40] and contribute to a leishmanicidal immune environment [39]. DN T cells were recently described as important players in effective and protective primary and secondary anti L. major immunity in experimental cutaneous leishmaniasis [41]. Leishmania-reactive DN T cells express predominantly αβ TCR, are restricted by MHC class II molecules, lack immunoregulatory properties and display transcriptional profile distinct from conventional CD4+ T cells. Current dogma that DN T cells are CD4 and CD8 T cells that have lost their co-receptors is being challenged by the emerging theory that Fas-mediated apoptosis actively removes normally existing DN T cells from the periphery. Impaired Fas-mediated apoptosis may lead to accumulation of these cells rather than de novo generation of DN T cells from activated CD4 or CD8 T cells [42].
In our study, both αβ and γδ subpopulations were similarly represented in the L. major and uninfected control subjects and remained stable during the course of treatment. DN T cell population changes were previously described in human infection with L.(V) braziliensis. In that study, 75% of DN T cells from subjects expressed the αβ TCR compared to uninfected persons where 80% of DN T cells express the γδ TCR [39]. This discordance was not observed here and this may be attributed to different Leishmania species with differing disease patterns and/or genetic backgrounds of the individuals studied.
Leishmania induced immunity is based upon the generation of memory T cells that recognize cognate Leishmania antigens and proliferate after exposure thus activating the effector cells [43]. In our study, responses to SLA were consistently diminished in the post treatment phase. Surprisingly, the proliferative responses were significantly decreased only for subjects receiving systemic treatment but not subjects receiving local treatment. This could in part be explained by the higher numbers of treatment failures at 6 months in TM (4/19 in TM group versus 2/20 in SSG group) causing LPA due to parasite persistence. Similarly to our findings, others also report a decline of the lymphoproliferative response after therapy [28,36–38].
The CD8+T cell-dependent decrease in CD4+T cell proliferation suggested a post treatment, curative type counter-regulatory mechanism. In contrast, in a BALB/c mouse model, CD8 T cell depletion did not interfere with the proliferative ability of draining lymph node CD4 T cells and was associated with an increase in parasite load [44]. As demonstrated for CD4 T cells [45], CD8 immunomodulation maybe due, for example, to up- regulation of Fas expression on CD4 to induce their apoptotic death. We know that CD8 T cells play a role in the healing process and resistance to reinfection in New World human CL. Conversely, other studies associate CD8+ to tissue injury [46]. Recently, it was hypothesized that changes in the frequency of effector CD8+ T cells, during and after antimonial therapy is a critical step to generate an efficient immune response either for by triggering or resolving the lesion [34]. In vivo experiments with human cells showed that CD8 T cells produce IFN-γ and drive Th1 differentiation [47]. However in our study, after treatment, all subjects showed decreased IFN-γ, IL-10 and TNF-α levels, with or without CD8+ depletion. This indicated that CD8+T cell mediated regulation of the CD4+T cell response was not attributable to the soluble mediators studied here. The high IFN-γ production observed pre-treatment suggests that the subjects have initiated an immune response to eliminate the parasite [48]. Additionally, during effective treatment, gradual parasite destruction by macrophages is expected with a diminishing parasite load. Overall, our results add evidence that local heat therapy of CL elicits a systemic cytokine response similar to that of systemic pentavalent antimony. In fact, a decrease in IFN-γ, IL-5 and TNF-α in both groups was seen at day 28 post treatment with meglumine antimoniate in a previous study [49]. These results indicate that proinflammatory responses were progressively downmodulated after therapy and that the cytokine profile produced after cure is shaped during the active phase of disease [50].
Our results were contrary to our hypothesis, as the subjects in the both treatment arms generally exhibited similar cellular immune response profiles. This may be explained, in part, by the tendency of CL to eventually self-heal so cure processes may have occurred despite therapy [51]. Another potential limitation of our study is that there were fewer subjects collected at the 6 month time point, however this was similar between treatment arms. A local immune analysis in the skin may have provided additional clues to immune response alterations induced by different treatments, as might an earlier post timepoint.
Taken together, our findings highlight the existence of regulatory mechanisms that counterbalance early immune responses without altering the CL healing outcome. The magnitude of effector T cell responses can be controlled by regulatory T cells at the lesion site by suppressing lymphocyte proliferation [52]. These mechanisms are important to maintain the host tissue integrity against a subsequent or persistent inflammatory response. Induction of Tregs during chronic infections results from antigen presentation in a particular cytokine environment [53,54]. Interestingly, we found that the percentage of CD25hiCD4+Foxp3+ cells decreased after treatment suggesting that Tregs may be responsible for the suppression that was associated with healing and that their drop is not an artifact of CD4 decrease demonstrated earlier. Tregs have been shown to substantially contribute to tissue repair by providing regulation at sites of healing [55].
To gain a better understanding of the complex immunopathogenesis of CL, study of the quality of a Th1 response, not solely its magnitude, was recently adopted [7,8]. Our analysis evaluated polyfunctional CD4+T cells in response to treatment. Overall, we observed a contraction in polyfunctional CD4+T cells in the post-treatment group, both in terms of number of responding cells and production of multiple cytokines.
In conclusion, healing of CL is a dynamic but consistent process. Similar changes in frequency, quality, and antigen specific responses were observed in both treatment arms and may represent a signature for curative responses.
Supporting Information
Data is presented from 27 subjects (15 in the SSG arm and 12 in TM arm represented in circles and triangles respectively) for which cells from all three time points were available. Percentage of lymphocytes positive for surface expression of CD3 (T cells), CD19 (B cells), CD16/CD56 (CD3-: NK cells; CD3+: NKT-like cells).
(TIF)
Data is presented from 30 subjects (17 in the SSG arm and 13 in TM arm represented in circles and triangles respectively, red represents subjects for whom lesion size was above 1000mm) for which cells from all three time points were available. Percentage of lymphocytes positive for surface expression of CD3 (T cells), CD19 (B cells), CD16/CD56 (CD3-: NK cells; CD3+: NKT-like cells).
(TIF)
(TIF)
Representative donor showing flow cytometry analysis.
(TIF)
Whole PBMC or CD8+T cell-depleted PBMC (CD8 depl PBMC) from 16 subjects (circles) and 14 subjects (triangles) treated respectively with SSG and TM at pre-treatment (black) and post-treatment (grey) stages were stimulated with SLA for 6 days followed by an 8 hour pulse with [3H]-thymidine. Lymphocyte stimulation index (LSI) was determined as fold-increase in mean cpm from triplicate wells over unstimulated wells. An LSI ≥ 5 (dotted line) is considered a positive response.
(TIF)
Representative sample showing gating strategy and identification of the proliferative fraction of lymphocytes.
(TIF)
Gating example showing Treg cells were identified as viable lymphocytes positive for CD3 and CD4 expressing high levels of CD25 and positive for the transcription factor FoxP3.
(TIF)
Acknowledgments
The authors thank the Thermomed study participants, research nurses and physicians; these data would not be available without their participation. The laboratory assistance of Tina Libby-Tong, Maureen Stefaniak, and Doris Thelian was invaluable. We appreciate the critical reviews of Dr. Andréa Teixeira de Carvalho and the assistance of Dr. Cara Olsen with the statistical analysis. We acknowledge the support of the U.S. Army Medical Materiel Development Activity as study sponsor.
Disclaimer
The content and views expressed in this publication are the sole responsibility of the authors and do not necessarily reflect the views or policies of the Department of Defense, Department of Health and Human Services, or the U.S. Government.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Department of Defense Global War on Terrorism funds and by a cooperative agreement [W81XWH-07-2-0067] between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. and the U.S. Department of Defense (DOD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, et al. (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357. [PubMed] [Google Scholar]
- 3. Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM (1989) Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med 169: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Assis Souza M, de Castro MC, de Oliveira AP, de Almeida AF, de Almeida TM, et al. (2013) Cytokines and NO in American tegumentary leishmaniasis patients: profiles in active disease, after therapy and in self-healed individuals. Microb Pathog 57: 27–32. 10.1016/j.micpath.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 5. Kaye P, Scott P (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 9: 604–615. 10.1038/nrmicro2608 [DOI] [PubMed] [Google Scholar]
- 6. Castellano LR, Filho DC, Argiro L, Dessein H, Prata A, et al. (2009) Th1/Th2 immune responses are associated with active cutaneous leishmaniasis and clinical cure is associated with strong interferon-gamma production. Hum Immunol 70: 383–390. 10.1016/j.humimm.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 7. Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, et al. (2007) Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13: 843–850. [DOI] [PubMed] [Google Scholar]
- 8. Macedo AB, Sanchez-Arcila JC, Schubach AO, Mendonca SC, Marins-Dos-Santos A, et al. (2012) Multifunctional CD4(+) T cells in patients with American cutaneous leishmaniasis. Clin Exp Immunol 167: 505–513. 10.1111/j.1365-2249.2011.04536.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexander J, Brombacher F (2012) T helper1/t helper2 cells and resistance/susceptibility to leishmania infection: is this paradigm still relevant? Front Immunol 3: 80 10.3389/fimmu.2012.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minodier P, Parola P (2007) Cutaneous leishmaniasis treatment. Travel Med Infect Dis 5: 150–158. [DOI] [PubMed] [Google Scholar]
- 11. Vanaerschot M, Dumetz F, Roy S, Ponte-Sucre A, Arevalo J, et al. (2014) Treatment failure in leishmaniasis: drug-resistance or another (epi-) phenotype? Expert Rev Anti Infect Ther 12: 937–946. 10.1586/14787210.2014.916614 [DOI] [PubMed] [Google Scholar]
- 12. Ameen M (2007) Cutaneous leishmaniasis: therapeutic strategies and future directions. Expert Opin Pharmacother 8: 2689–2699. [DOI] [PubMed] [Google Scholar]
- 13. McGwire BS, Satoskar AR (2014) Leishmaniasis: clinical syndromes and treatment. QJM 107: 7–14. 10.1093/qjmed/hct116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navin TR, Arana BA, Arana FE, de Merida AM, Castillo AL, et al. (1990) Placebo-controlled clinical trial of meglumine antimonate (glucantime) vs. localized controlled heat in the treatment of cutaneous leishmaniasis in Guatemala. Am J Trop Med Hyg 42: 43–50. [DOI] [PubMed] [Google Scholar]
- 15. Velasco-Castrejon O, Walton BC, Rivas-Sanchez B, Garcia MF, Lazaro GJ, et al. (1997) Treatment of cutaneous leishmaniasis with localized current field (radio frequency) in Tabasco, Mexico. Am J Trop Med Hyg 57: 309–312. [DOI] [PubMed] [Google Scholar]
- 16. Bumb RA, Prasad N, Khandelwal K, Aara N, Mehta RD, et al. (2013) Long-term efficacy of single-dose radiofrequency-induced heat therapy vs. intralesional antimonials for cutaneous leishmaniasis in India. Br J Dermatol 168: 1114–1119. 10.1111/bjd.12205 [DOI] [PubMed] [Google Scholar]
- 17. Safi N, Davis GD, Nadir M, Hamid H, Robert LL Jr., et al. (2012) Evaluation of thermotherapy for the treatment of cutaneous leishmaniasis in Kabul, Afghanistan: a randomized controlled trial. Mil Med 177: 345–351. [DOI] [PubMed] [Google Scholar]
- 18. Lopez L, Robayo M, Vargas M, Velez ID (2012) Thermotherapy. An alternative for the treatment of American cutaneous leishmaniasis. Trials 13: 58 10.1186/1745-6215-13-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sadeghian G, Nilfroushzadeh MA, Iraji F (2007) Efficacy of local heat therapy by radiofrequency in the treatment of cutaneous leishmaniasis, compared with intralesional injection of meglumine antimoniate. Clin Exp Dermatol 32: 371–374. [DOI] [PubMed] [Google Scholar]
- 20. Reithinger R, Mohsen M, Wahid M, Bismullah M, Quinnell RJ, et al. (2005) Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled trial. Clin Infect Dis 40: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 21. Aronson NE, Wortmann GW, Byrne WR, Howard RS, Bernstein WB, et al. (2010) A randomized controlled trial of local heat therapy versus intravenous sodium stibogluconate for the treatment of cutaneous Leishmania major infection. PLoS Negl Trop Dis 4: e628 10.1371/journal.pntd.0000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marovich MA, Mascola JR, Eller MA, Louder MK, Caudrelier PA, et al. (2002) Preparation of clinical-grade recombinant canarypox-human immunodeficiency virus vaccine-loaded human dendritic cells. J Infect Dis 186: 1242–1252. [DOI] [PubMed] [Google Scholar]
- 23. Valcour VG, Ananworanich J, Agsalda M, Sailasuta N, Chalermchai T, et al. (2013) HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One 8: e70164 10.1371/journal.pone.0070164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Currier JR, Ngauy V, de Souza MS, Ratto-Kim S, Cox JH, et al. (2010) Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 5: e13983 10.1371/journal.pone.0013983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eller MA, Slike BM, Cox JH, Lesho E, Wang Z, et al. (2011) A double-blind randomized phase I clinical trial targeting ALVAC-HIV vaccine to human dendritic cells. PLoS One 6: e24254 10.1371/journal.pone.0024254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stager S, Rafati S (2012) CD8(+) T cells in leishmania infections: friends or foes? Front Immunol 3: 5 10.3389/fimmu.2012.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Da-Cruz AM, Bertho AL, Oliveira-Neto MP, Coutinho SG (2005) Flow cytometric analysis of cellular infiltrate from American tegumentary leishmaniasis lesions. Br J Dermatol 153: 537–543. [DOI] [PubMed] [Google Scholar]
- 28. Coutinho SG, Da-Cruz AM, Bertho AL, Santiago MA, De-Luca P (1998) Immunologic patterns associated with cure in human American cutaneous leishmaniasis. Braz J Med Biol Res 31: 139–142. [DOI] [PubMed] [Google Scholar]
- 29. Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, et al. (2003) CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101: 3052–3057. [DOI] [PubMed] [Google Scholar]
- 30. Reis LC, Brito ME, Souza MA, Medeiros AC, Silva CJ, et al. (2009) Cellular immune response profile in patients with American tegumentary leishmaniasis prior and post chemotherapy treatment. J Clin Lab Anal 23: 63–69. 10.1002/jcla.20291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, et al. (2011) NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol 41: 1913–1923. 10.1002/eji.200940278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang Y, Cui X, Cui C, Zhang J, Zhou F, et al. (2014) The function of CD3+CD56+ NKT-like cells in HIV-infected individuals. Biomed Res Int 2014: 863625 10.1155/2014/863625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brelaz-de-Castro MC, de Almeida AF, de Oliveira AP, de Assis-Souza M, da Rocha LF, et al. (2012) Cellular immune response evaluation of cutaneous leishmaniasis patients cells stimulated with Leishmania (Viannia) braziliensis antigenic fractions before and after clinical cure. Cell Immunol 279: 180–186. 10.1016/j.cellimm.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 34. Ferraz R, Cunha CF, Gomes-Silva A, Schubach AO, Pimentel MI, et al. (2015) Apoptosis and frequency of total and effector CD8+ T lymphocytes from cutaneous leishmaniasis patients during antimonial therapy. BMC Infect Dis 15: 74 10.1186/s12879-015-0799-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Da-Cruz AM, Bittar R, Mattos M, Oliveira-Neto MP, Nogueira R, et al. (2002) T-cell-mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol 9: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Da-Cruz AM, Conceicao-Silva F, Bertho AL, Coutinho SG (1994) Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun 62: 2614–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coutinho SG, Oliveira MP, Da-Cruz AM, De Luca PM, Mendonca SC, et al. (1996) T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol 84: 144–155. [DOI] [PubMed] [Google Scholar]
- 38. Toledo VP, Mayrink W, Gollob KJ, Oliveira MA, Costa CA, et al. (2001) Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem Inst Oswaldo Cruz 96: 89–98. [DOI] [PubMed] [Google Scholar]
- 39. Gollob KJ, Antonelli LR, Faria DR, Keesen TS, Dutra WO (2008) Immunoregulatory mechanisms and CD4-CD8- (double negative) T cell subpopulations in human cutaneous leishmaniasis: a balancing act between protection and pathology. Int Immunopharmacol 8: 1338–1343. 10.1016/j.intimp.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bottrel RL, Dutra WO, Martins FA, Gontijo B, Carvalho E, et al. (2001) Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun 69: 3232–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mou Z, Liu D, Okwor I, Jia P, Orihara K, et al. (2014) MHC class II restricted innate-like double negative T cells contribute to optimal primary and secondary immunity to Leishmania major. PLoS Pathog 10: e1004396 10.1371/journal.ppat.1004396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martina MN, Noel S, Saxena A, Rabb H, Hamad AR (2015) Double negative (DN) alphabeta T cells: misperception and overdue recognition. Immunol Cell Biol 93: 305–310. 10.1038/icb.2014.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carvalho EM, Correia Filho D, Bacellar O, Almeida RP, Lessa H, et al. (1995) Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg 53: 273–277. [DOI] [PubMed] [Google Scholar]
- 44. Herath S, Kropf P, Muller I (2003) Cross-talk between CD8(+) and CD4(+) T cells in experimental cutaneous leishmaniasis: CD8(+) T cells are required for optimal IFN-gamma production by CD4(+) T cells. Parasite Immunol 25: 559–567. [DOI] [PubMed] [Google Scholar]
- 45. Conceicao-Silva F, Hahne M, Schroter M, Louis J, Tschopp J (1998) The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur J Immunol 28: 237–245. [DOI] [PubMed] [Google Scholar]
- 46. Santos Cda S, Boaventura V, Ribeiro Cardoso C, Tavares N, Lordelo MJ, et al. (2013) CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNgamma(+)-mediated parasite killing in human cutaneous leishmaniasis. J Invest Dermatol 133: 1533–1540. 10.1038/jid.2013.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pompeu MM, Brodskyn C, Teixeira MJ, Clarencio J, Van Weyenberg J, et al. (2001) Differences in gamma interferon production in vitro predict the pace of the in vivo response to Leishmania amazonensis in healthy volunteers. Infect Immun 69: 7453–7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Souza MA, Castro MC, Oliveira AP, Almeida AF, Reis LC, et al. (2012) American tegumentary leishmaniasis: cytokines and nitric oxide in active disease and after clinical cure, with or without chemotherapy. Scand J Immunol 76: 175–180. 10.1111/j.1365-3083.2012.02717.x [DOI] [PubMed] [Google Scholar]
- 49. Lobo IM, Soares MB, Correia TM, de Freitas LA, Oliveira MI, et al. (2006) Heat therapy for cutaneous leishmaniasis elicits a systemic cytokine response similar to that of antimonial (Glucantime) therapy. Trans R Soc Trop Med Hyg 100: 642–649. [DOI] [PubMed] [Google Scholar]
- 50. Nogueira RS, Gomes-Silva A, Bittar RC, Silva Mendonca D, Amato VS, et al. (2014) Antigen-triggered interferon-gamma and interleukin-10 pattern in cured mucosal leishmaniasis patients is shaped during the active phase of disease. Clin Exp Immunol 177: 679–686. 10.1111/cei.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carvalho AM, Magalhaes A, Carvalho LP, Bacellar O, Scott P, et al. (2013) Immunologic response and memory T cells in subjects cured of tegumentary leishmaniasis. BMC Infect Dis 13: 529 10.1186/1471-2334-13-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, et al. (2006) CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis 193: 1313–1322. [DOI] [PubMed] [Google Scholar]
- 53. Belkaid Y (2003) The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert Opin Biol Ther 3: 875–885. [DOI] [PubMed] [Google Scholar]
- 54. Wohlfert E, Belkaid Y (2008) Role of endogenous and induced regulatory T cells during infections. J Clin Immunol 28: 707–715. 10.1007/s10875-008-9248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodriguez-Pinto D, Navas A, Blanco VM, Ramirez L, Garcerant D, et al. (2012) Regulatory T cells in the pathogenesis and healing of chronic human dermal leishmaniasis caused by Leishmania (Viannia) species. PLoS Negl Trop Dis 6: e1627 10.1371/journal.pntd.0001627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data is presented from 27 subjects (15 in the SSG arm and 12 in TM arm represented in circles and triangles respectively) for which cells from all three time points were available. Percentage of lymphocytes positive for surface expression of CD3 (T cells), CD19 (B cells), CD16/CD56 (CD3-: NK cells; CD3+: NKT-like cells).
(TIF)
Data is presented from 30 subjects (17 in the SSG arm and 13 in TM arm represented in circles and triangles respectively, red represents subjects for whom lesion size was above 1000mm) for which cells from all three time points were available. Percentage of lymphocytes positive for surface expression of CD3 (T cells), CD19 (B cells), CD16/CD56 (CD3-: NK cells; CD3+: NKT-like cells).
(TIF)
(TIF)
Representative donor showing flow cytometry analysis.
(TIF)
Whole PBMC or CD8+T cell-depleted PBMC (CD8 depl PBMC) from 16 subjects (circles) and 14 subjects (triangles) treated respectively with SSG and TM at pre-treatment (black) and post-treatment (grey) stages were stimulated with SLA for 6 days followed by an 8 hour pulse with [3H]-thymidine. Lymphocyte stimulation index (LSI) was determined as fold-increase in mean cpm from triplicate wells over unstimulated wells. An LSI ≥ 5 (dotted line) is considered a positive response.
(TIF)
Representative sample showing gating strategy and identification of the proliferative fraction of lymphocytes.
(TIF)
Gating example showing Treg cells were identified as viable lymphocytes positive for CD3 and CD4 expressing high levels of CD25 and positive for the transcription factor FoxP3.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.