Abstract
OBJECTIVES
To identify independent risk factors for cesarean delivery after induction of labor (IOL) and to develop a nomogram for predicting cesarean delivery among nulliparous women undergoing IOL at term.
METHODS
This is a retrospective cohort study including nulliparous women with singleton, term (≥37 0/7 weeks of gestation), cephalic pregnancies undergoing IOL from July 1, 2006, through May 31, 2012, at a tertiary care academic center. Inductions were identified using ICD-9 codes. Demographic, delivery, and outcome data were abstracted manually from the medical record. Women with a contraindication to vaginal delivery (malpresentation, abnormal placentation, prior myomectomy) were excluded. Independent risk factors for cesarean delivery were identified using logistic regression.
RESULTS
During the study period, there were 785 nulliparous inductions who met study criteria; 231 (29.4%) underwent cesarean delivery. Independent risk factors associated with an increased risk of cesarean delivery included older maternal age, shorter maternal height, greater body mass index, greater weight gain during pregnancy, older gestational age, hypertension, diabetes mellitus, and initial cervical dilation <3 cm. A nomogram was constructed based on the final model with a bias-corrected c-index of 0.709 (95% CI 0.671–0.750).
CONCLUSION
We identified independent risk factors which can be utilized to predict cesarean delivery among nulliparous women undergoing IOL at term. If validated in other populations, the nomogram could be useful for individualized counseling of women with a combination of identifiable antepartum risk factors.
INTRODUCTION
Induction of labor (IOL) is the use of pharmacological, mechanical, or both methods to initiate labor (1). The goal of IOL is to achieve vaginal delivery, and IOL may be indicated for various conditions that may adversely affect maternal or fetal well-being during pregnancy (2).
In the United States (U.S.), more than 22% of women undergo IOL (2). Nulliparous women are more likely to undergo IOL and are at higher risk of cesarean delivery after IOL as compared to multiparous women (3, 4). However, the effect of IOL on cesarean delivery rates is an ongoing debate as it depends on the comparison group (spontaneous labor or expectant management) (5). For uncomplicated pregnancies, IOL at term has not been associated with increased risk of cesarean delivery compared to expectant management (6, 7).
Cesarean delivery after IOL has been reported to be associated with greater body mass index (BMI) and age, shorter height, diabetes mellitus, and hypertension (8–11). Obstetrical factors including the findings from pelvic exam at the start of IOL (an “unfavorable” Bishop score, variably defined as <6 or <8) and IOL for fetal or postterm indications have been associated with increased risk of cesarean delivery (12–16).
Given the increased risks of complications for mother and fetus associated with intrapartum as opposed to antepartum cesarean delivery (17), predicting with accuracy the rate of cesarean delivery after IOL may prevent additional complications for those at highest risk. In this study, we develop a tool incorporating antepartum factors to predict the likelihood of cesarean delivery after IOL among nulliparous women at term.
MATERIALS AND METHODS
The protocol for this retrospective cohort study was approved by the Mayo Clinic Institutional Review Board. All nulliparous women with singleton, term (≥37 0/7 weeks of gestation), cephalic pregnancies who underwent IOL at Mayo Clinic Rochester from July 1, 2006 to May 31, 2012 were identified. Only women who provided research authorization were eligible for inclusion. Women with a contraindication to vaginal delivery (malpresentation, abnormal placentation, prior myomectomy) were excluded. Women with premature rupture of membranes were excluded if it was unclear whether induction or augmentation of labor occurred and if the cervical dilation at the time of admission was ≥4 cm. This article is written in accordance with The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies (18).
Inductions were identified using ICD-9 codes. Data on maternal characteristics and neonatal outcomes were collected from the institution’s obstetrics database which is obtained via electronic medical review and manual entry by data technicians. Data was confirmed and missing data were abstracted from the medical record by two obstetricians (MCT and MRH).
Based on a combination of time period–specific recommendations from the American College of Obstetricians and Gynecologists’ Committee Opinions and Practice Bulletins, inductions were categorized as “medically indicated” or “nonmedically indicated,” taking into account both the indication for delivery and the timing of delivery. Prior to the ACOG Committee Opinion, “Medically Indicated Late-Preterm and Early-Term Deliveries” published in April 2013 (19), there was not clear guidance on timing of delivery for many indications. Therefore, we used expert opinion and consensus to resolve unclear indications where evidence or national recommendations were lacking utilizing the Delphi method. The expert opinion group consisted of the first author (obstetrician), five Maternal-Fetal Medicine specialists, and three Maternal-Fetal Medicine fellows.
During the study period, testing for fetal lung maturity based on amniocentesis between 37 0/7–38 6/7 weeks of gestation was an acceptable practice, most notably for indications including diabetes mellitus and prior stillbirth (20, 21). We considered inductions performed at or beyond 37 0/7 weeks of gestation that were preceded with a mature amniocentesis as medically indicated due to historic acceptance of this practice during the study period that predated the current wealth of evidence that neonatal morbidity is increased prior to 39 0/7 weeks of gestation (22–24).
The admission cervical exam is the 3-component modified Bishop score (which is a sum of points based on cervical dilation, effacement, and fetal station with a total range of points 0–9). The modified Bishop score has been shown to predict vaginal delivery equally as well as the full 5-component Bishop score (cervical dilation, effacement, position, consistency, and fetal station with total range of points 0–13) (25). A modified Bishop score of <6 is considered unfavorable.
At our teaching institution, we have no “private” patients or providers. All providers are salaried Mayo Clinic employees who deliver patients at a single Mayo Clinic-owned hospital. All women are Mayo patients cared for under the supervision of obstetricians including a minority of Midwifery and Family Medicine patients. We utilize standard order sets for cervical ripening and oxytocin induction. All women with a Bishop score <6 receive 1 of 2 methods for cervical ripening: Foley catheter or vaginal misoprostol 25 mcg every 4 hours. While dinoprostone is also included in the standardized induction of labor order sets, it is rarely used due to cost. The choice of Foley or vaginal misoprostol and timing of artificial rupture of membranes is based on patient and provider preference.
In addition to the order sets for induction of labor, patients scheduled for induction of labor or planned cesarean delivery are presented and reviewed during the preceding week at a meeting attended by Obstetrics and Maternal-Fetal Medicine attendings. The process of scheduling an induction includes a phone call to the labor and delivery charge nurse who is empowered to decline scheduling and report a nonindicated induction. Finally, during the study period, we followed ACOG guidelines for diagnosing active phase of labor arrest (4 hours) and arrest of the second stage of labor (2 hours for multiparas with regional analgesia or 1 hour without; 3 hours for nulliparas with regional analgesia or 2 hours without) (26).
The primary outcome in this analysis was cesarean delivery after IOL. Statistical analyses were performed using the SAS version 9.3 software package (SAS Institute, Inc.; Cary, NC) and version 3.0.2 of the R package (27). Standard descriptive statistics (mean and standard deviation (SD) or median and interquartile range (IQR)) were used to summarize continuous variables and frequency and percentage for categorical variables. Univariable and multivariable logistic regression models were fit to identify factors associated with cesarean delivery. To develop a prediction model, we first used stepwise and backward variable selection methods considering all variables with a P<0.20 based on univariable analysis; variables with a P<0.05 were retained in the final model. In addition, variable selection was explored by fitting a likelihood cross-validated penalized logistic regression model with a lasso penalty using the R ‘glmnet’ package (28). The lasso approach performs variable selection by shrinking the parameter coefficients for associated variables while setting the parameter coefficients of unassociated variables to zero. These approaches identified the same subset of variables, and a final multivariable logistic model was refit using this subset of variables. Associations were summarized using odds ratios (OR) and corresponding 95% confidence intervals (CIs) estimated from the final multivariable model. The model was further refined by incorporating restricted cubic splines to model the potential non-linear relationship for some of the continuous variables. A nomogram was created using the R ‘design’ package. Calibration was assessed graphically by examining how far the predicted probabilities are from the actual proportion of patients who underwent cesarean delivery based on grouping patients into deciles according to their predicted probability. Discrimination was assessed using the c-index, a measure of a model’s predictive accuracy that is equivalent to the area under receiver operating characteristic curve for the final model. An optimism corrected, nearly unbiased estimate of c-index estimate was derived using 1000 bootstrap resamples as a method of internal validation (29). Sensitivity analyses were performed excluding all women with premature rupture of membranes as the indication for induction.
RESULTS
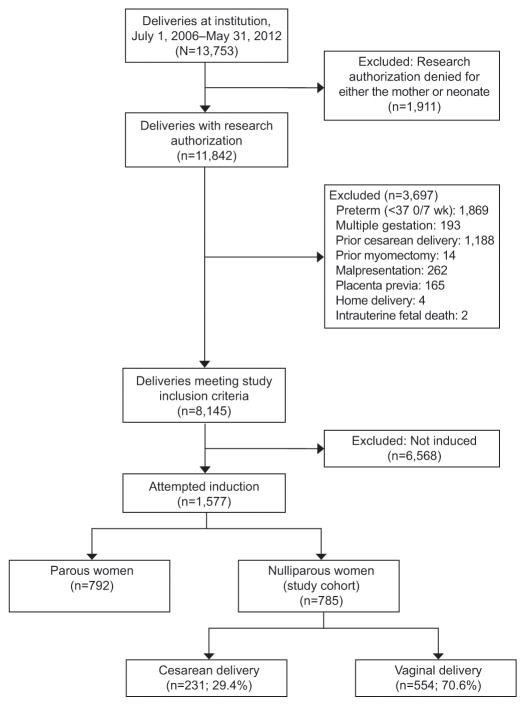
During the study period, 13,753 deliveries occurred at our institution, of which 8,145 met inclusion criteria. Of the 8,145 deliveries, 1,577 (19.4%) were inductions, and 785 (49.8%) were nulliparous inductions (Figure 1). Of the 785 nulliparous inductions, 231 (29.4%) underwent cesarean delivery. Nonreassuring fetal heart rate status was the most common indication for cesarean delivery (84/231, 36.4%) followed by arrest of dilation (69/231, 29.9%) and arrest of second stage of labor (61/231, 26.4%). Of women who delivered vaginally, 83.2% (461/554) were spontaneous, 5.6% (31/554) vacuum-assisted, and 11.2% (62/554) forceps-assisted.
Figure 1.
Identification of study cohort.
Maternal antepartum and intrapartum characteristics are contrasted between women who delivered by cesarean delivery and women who delivered vaginally (Table 1). The majority (96.4%) of the inductions were medically indicated with a cesarean delivery rate of 29.3% compared to 32.1% (P=0.75) among those with nonmedically indicated induction (Table 2).
Table 1.
Antepartum and intrapartum characteristics for nulliparous women undergoing IOL at term.
| Characteristic | Cesarean delivery N=231 |
Vaginal delivery N=554 |
P† |
|---|---|---|---|
| Maternal age (years), Mean (SD) | 28.0 (5.4) | 27.2 (4.7) | 0.04 |
| Maternal race, N (%) | 0.42 | ||
| White | 195/227 (85.9) | 473/541 (87.4) | |
| Black or African American | 14/227 (6.2) | 21/541 (3.9) | |
| Asian | 7/227 (3.1) | 26/541 (4.8) | |
| American Indian/Alaska Native | 0 | 1/541 (0.2) | |
| Other | 11/227 (4.8) | 20/541 (3.7) | |
| Hispanic, N (%) | 12/213 (5.6) | 19/507 (3.7) | 0.26 |
| Baseline weight (kg), Mean (SD) | 76.9 (21.9) | 71.7 (18.0) | <0.001 |
| Baseline height (cm), Mean (SD) | 163.3 (7.1) | 165.4 (6.7) | <0.001 |
| Baseline BMI (kg/m2), Mean (SD) | 28.7 (7.7) | 26.1 (6.0) | <0.001 |
| Weight change during pregnancy (kg), Mean (SD) | 15.9 (6.6) | 14.9 (5.9) | 0.03 |
| Positive GBS status, N (%) | 45/227 (19.8) | 132/533 (24.8) | 0.14 |
| Gestational age at delivery, Median (IQR) | 40 (39, 41) | 40 (39, 41) | 0.13 |
| Medically indicated induction, N (%) | 222 (96.1) | 535 (96.6) | 0.75 |
| Hypertension, N (%) | 57 (24.7) | 96 (17.3) | 0.02 |
| Diabetes mellitus, N (%) | <0.001 | ||
| None | 192 (83.1) | 508 (91.7) | |
| Gestational | 27 (11.7) | 42 (7.6) | |
| Pregestational | 12 (5.2) | 4 (0.7) | |
| Initial cervical dilation, N (%) | <0.001 | ||
| Closed (0 points) | 57/228 (25.0) | 85/536 (15.9) | |
| 1–2 cm (1 point) | 142/228 (62.3) | 318/536 (59.3) | |
| 3–4 cm (2 points) | 27/228 (11.8) | 133/536 (24.8) | |
| 5–6 cm (3 points) | 2/228 (0.9) | 0 | |
| Initial cervical effacement, N (%) | 0.002 | ||
| 0–30% (0 points) | 74/226 (32.7) | 107/532 (20.1) | |
| 40–50% (1 point) | 80/226 (35.4) | 216/532 (40.6) | |
| 60–70% (2 points) | 12/226 (5.3) | 33/532 (6.2) | |
| 80–100% (3 points) | 60/226 (26.5) | 176/532 (33.1) | |
| Initial station, N (%) | 0.006 | ||
| −3 (0 points) | 37/219 (16.9) | 50/527 (9.5) | |
| −2 (1 point) | 69/219 (31.5) | 160/527 (30.4) | |
| −1 or 0 (2 points) | 112/219 (51.1) | 307/527 (58.3) | |
| +1 or +2 (3 points) | 1/219 (0.5) | 10/527 (1.9) | |
| Modified Bishop score, Median (IQR) | 4.0 (2.0, 5.0) | 4.0 (3.0, 6.0) | <0.001 |
| Modified Bishop score <6, N (%) | 170/219 (77.6) | 361/525 (68.8) | 0.01 |
| Artificial rupture of membranes, N (%) | 139 (60.2) | 310 (56.0) | 0.28 |
| Meconium*, N (%) | 72 (31.2) | 103 (18.6) | <0.001 |
| Method of induction, other than AROM*, N (%) | <0.001 | ||
| Prostaglandin only | 15 (6.5) | 52 (9.4) | |
| Foley only | 1 (0.4) | 2 (0.4) | |
| Prostaglandin + Foley | 2 (0.9) | 3 (0.5) | |
| Oxytocin alone | 52 (22.5) | 238 (43.0) | |
| Prostaglandin + oxytocin | 78 (33.8) | 178 (32.1) | |
| Foley + oxytocin | 36 (15.6) | 42 (7.6) | |
| Prostaglandin + Foley + oxytocin | 46 (19.9) | 36 (6.5) | |
| None | 1 (0.4) | 3 (0.5) | |
| Male infant sex*, N (%) | 139 (60.2) | 269 (48.6) | 0.003 |
| Fetal position*, N (%) | <0.001 | ||
| Occiput anterior | 145/217 (66.8) | 521/553 (94.2) | |
| Occiput posterior | 41/217 (18.9) | 26/553 (4.7) | |
| Occiput transverse | 31/217 (14.3) | 6/553 (1.1) |
Abbreviations: BMI, body mass index; GBS, group B streptococcus; AROM, artificial rupture of membranes; SD, standard deviation; IQR, interquartile range.
Meconium, method of induction, infant sex, and fetal position were not considered in the multivariable modeling as they are not reliably known antepartum.
Two-sided P based on the chi-square or Fisher’s exact for categorical variables, Wilcoxon rank-sum test for gestational age, Bishop score, and the three components of the Bishop score, and the t-test for all remaining continuous variables.
Table 2.
Indications for induction among nulliparous women undergoing IOL at term.
| Indication* | Cesarean delivery N=231 |
Vaginal delivery N=554 |
|---|---|---|
| Nonmedically indicated | 9 (3.9) | 19 (3.4) |
| Late-term (<41 weeks of gestation) | 1 (11.1) | 1 (5.3) |
| Diabetes mellitus† | 2 (22.2) | 0 |
| Elective | 6 (66.7) | 17 (89.5) |
| Other | 0 | 1 (5.3) |
| Medically indicated | 222 (96.1) | 535 (96.6) |
| Late-term (≥41 weeks of gestation) | 82 (36.9) | 194 (36.3) |
| PROM | 30 (13.5) | 107 (20.0) |
| Gestational hypertension | 28 (12.6) | 49 (9.2) |
| Preeclampsia/HELLP | 15 (6.8) | 32 (6.0) |
| Diabetes mellitus | 20 (9.0) | 26 (4.9) |
| Chronic hypertension | 6 (2.7) | 8 (1.5) |
| Cholestasis | 1 (0.5) | 6 (1.1) |
| Psychosocial | 1 (0.5) | 3 (0.6) |
| Other | 10 (4.5) | 25 (4.7) |
| Fetal indication | 29 (13.1) | 85 (15.9) |
| Oligohydramnios | 19/29 (65.5) | 43/85 (50.6) |
| FGR | 5/29 (17.2) | 22/85 (25.9) |
| Non-reassuring antenatal testing (NST, BPP, etc.) | 4/29 (13.8) | 12/85 (14.1) |
| Anomalies | 0 | 8/85 (9.4) |
| Other | 1/29 (3.4) | 0 |
Abbreviations: NST, non-stress test; BPP, biophysical profile; HELLP, hemolysis, elevated liver enzyme levels, and low platelet levels; FGR, fetal growth restriction; PROM, premature rupture of membranes.
Type of indication (nonmedically indicated compared to medically indicated) was not significantly associated with cesarean delivery (p=0.75).
Diabetes indication deemed nonmedically indicated depending on pregestational or gestational and control and/or comorbidities.
In univariable analysis, the following antepartum variables had a P<0.20 and were considered in multivariable modeling: maternal age, baseline height, weight, BMI, weight change during pregnancy, group B Streptococcus status, gestational age, hypertension, diabetes (none, gestational, pregestational), initial cervical dilation, initial cervical effacement, initial fetal station, and modified Bishop score. The presence of meconium, method of induction, male fetal sex, and fetal malposition were also significantly associated with cesarean delivery after IOL but were not considered in multivariate modeling as these variables are not antepartum factors or reliably known at the start of induction.
Table 3 summarizes the factors identified as independently associated with cesarean delivery after IOL. The following 8 variables remained significantly associated with cesarean delivery in multivariate modeling: maternal age, baseline height, weight, BMI, weight change during pregnancy, gestational age, hypertension, diabetes, and initial cervical dilation. For every 5-year increase in maternal age, there was a 26% increase in the odds of cesarean delivery (OR 1.26, 95% CI 1.05–1.51). Decreasing height was also associated with cesarean delivery (OR 1.37, 95% CI 1.20–1.56 per 5 cm decrease in height). A 5 kg/m2 increase in BMI, 5 kg increase in weight during pregnancy, and 1 week increase in gestational age were associated with a 41% (OR 1.41, 95% CI 1.23–1.63), 29% (OR 1.29, 95% CI 1.11–1.50), and 40% (OR 1.40, 95% CI 1.20–1.63), increase in the odds of cesarean delivery, respectively. Hypertension was also associated with a nearly 2-fold risk of cesarean delivery (OR 1.72, 95% CI 1.05–2.81). Both gestational and pregestational diabetes were associated with an increased risk of cesarean delivery with pregestational diabetes increasing the risk of cesarean delivery by 8-fold (OR 1.81, 95% CI 1.00–3.30 and OR 8.35, 95% CI 2.37–29.48, respectively). Finally, for initial cervical dilation, women who were closed had a greater than 4-fold risk of cesarean delivery (OR 4.03, 95% CI 2.22–7.33) and women who were 1–2 cm dilated had over a 2-fold risk of cesarean delivery (OR 2.74, 95% CI 1.64–4.58) compared to women who were ≥3 cm dilated. Sensitivity analysis excluding 137 women with premature rupture of membranes resulted in the same set of variables included in the final multivariable model with similar odds ratios for each of the variables; therefore, the final reported analyses included the full cohort of 785 nulliparous inductions.
Table 3.
Final model including independent risk factors for cesarean delivery among nulliparous women undergoing IOL at term.
| Characteristic | Unadjusted OR (95% CI) |
P | Adjusted OR (95% CI) |
P |
|---|---|---|---|---|
| Maternal age (years)* | 1.17 (1.01–1.37) | 0.04 | 1.26 (1.05–1.51) | 0.01 |
| Height (cm)* | 1.25 (1.11–1.40) | <0.001 | 1.37 (1.20–1.56) | <0.001 |
| BMI (kg/m2)* | 1.33 (1.18–1.49) | <0.001 | 1.41 (1.23–1.63) | <0.001 |
| Weight change during pregnancy (kg)* | 1.15 (1.01–1.31) | 0.04 | 1.29 (1.11–1.50) | 0.001 |
| Gestational age (weeks)* | 1.12 (1.00–1.25) | 0.06 | 1.40 (1.20–1.63) | <0.001 |
| Hypertensive disorder | 1.56 (1.08–2.27) | 0.02 | 1.72 (1.05–2.81) | 0.03 |
| Diabetes mellitus | <0.001 | 0.001 | ||
| Gestational vs. none | 1.70 (1.02–2.84) | 1.81 (1.00–3.30) | ||
| Pregestational vs. none | 7.94 (2.53–24.91) | 8.35 (2.37–29.48) | ||
| Initial cervical dilation | <0.001 | <0.001 | ||
| Closed (0 points) vs. 3–6 cm (2–3 points) | 3.08 (1.82–5.19) | 4.03 (2.22–7.33) | ||
| 1–2 cm (1 point) vs. 3–6 cm (2–3 points) | 2.05 (1.31–3.21) | 2.74 (1.64–4.58) |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio
OR per 5 year increase in age, 5 cm decrease in height, 5 kg/m2 increase in BMI, 5 kg increase in weight change during pregnancy, and 1 week increase in gestational age
All variables listed in this table were retained in the final multivariable model.
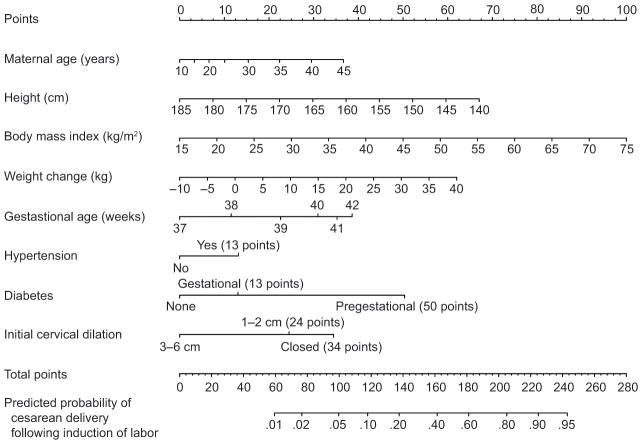
After incorporating restricted cubic splines to model the potential non-linear relationship for maternal age and gestational age, a nomogram was constructed from the final multivariable logistic model (Figure 2). As an example, based on our nomogram, a woman who is 34 years old (22 points), 164 cm tall (31 points), has a baseline BMI of 27 kg/m2 (20 points) and gained 15 kg during pregnancy (31 points), at 38 weeks of gestation (12 points), has hypertension (13 points), is without diabetes (0 points), and is 1 cm dilated on admission (24 points), has 153 total points which corresponds to a predicted probability of cesarean delivery of 32% (95% CI 22–43%). Alternatively, a 26 year old (11 points) who is 172 cm tall (20 points), has a baseline BMI of 35 kg/m2 (33 points) and gained 29 kg during pregnancy (49 points), at 39 weeks of gestation (23 points), has hypertension (13 points), has pregestational diabetes (50 points), and is 1 cm dilated on admission (24 points), has 223 total points which corresponds to a predicted probability of cesarean delivery of 89% (95% CI 67–97%). A histogram of the predicted probabilities for the study cohort is presented in Figure 3; the values ranged from 0.01 to 0.98 with a median (IQR) of 0.27 (0.16–0.40).
Figure 2.
Nomogram for predicting the probability of cesarean delivery after induction of labor among nulliparous women. For a given woman, points are assigned to each of the variables using the points axis at the top of the figure and a total summated score is derived. The total points score corresponds to a predicted probability of cesarean delivery after induction of labor at term.
Figure 3.
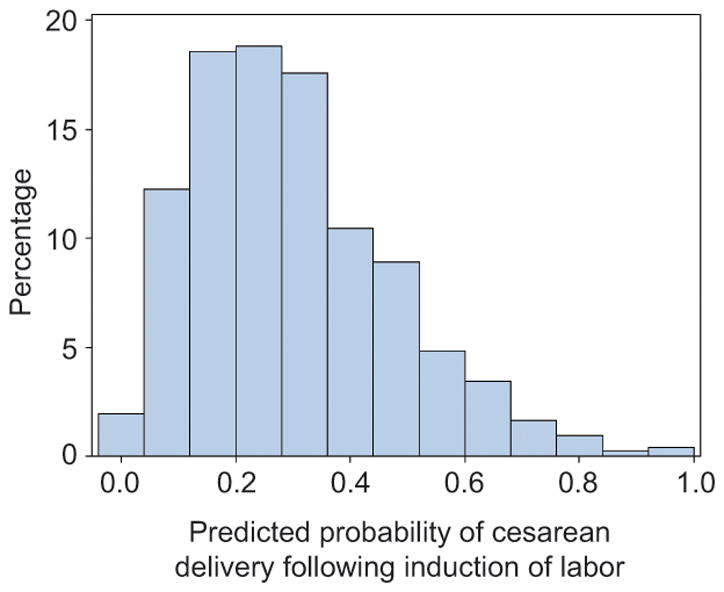
Histogram of the predicted probability of cesarean delivery after induction of labor among nulliparous women.
The overall predictive ability of the final model displayed in the nomogram, as measured by the c-index derived from the original data (biased estimate), was 0.729 (95% CI 0.688–0.770). Using bootstrap resampling as a method for internal validation, a bias- or optimism-corrected c-index of 0.709 (95% CI 0.671–0.750) was obtained. The model had reasonable calibration as depicted in Figure 4.
Figure 4.
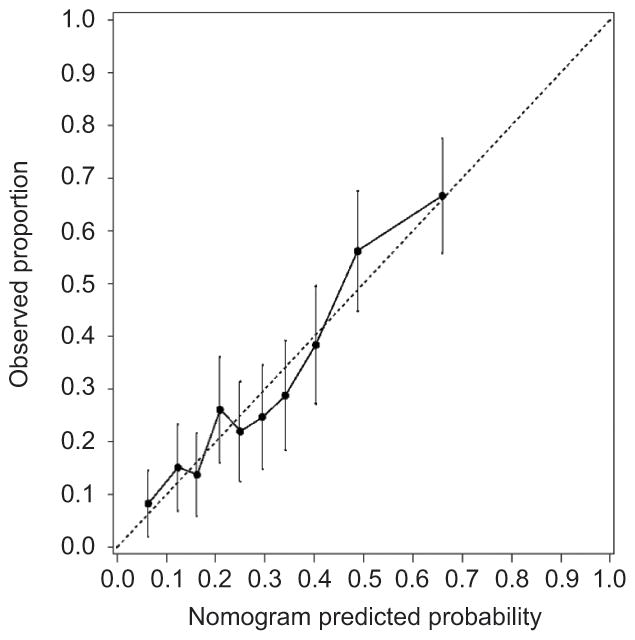
Calibration plot for the final multivariable model depicted in the nomogram. The dashed line indicates the ideal reference line where the predicted probabilities estimated from the model would match the observed proportion that underwent cesarean delivery after induction of labor. Patients were grouped into deciles according to their predicted probability estimated by the final model and the mean predicted probability within each decile is depicted along the x-axis. Within each decile, the observed proportion that underwent cesarean delivery after induction of labor was determined and is depicted along the y-axis along with corresponding 95% confidence intervals (vertical bars).
DISCUSSION
Our nomogram is intended to improve counseling and individualize the risks of cesarean delivery after IOL. Risk factors for cesarean delivery in our model included older maternal age, shorter maternal height, greater BMI, greater weight gain during pregnancy, older gestational age, hypertension, diabetes mellitus, and initial cervical dilation <3 cm. Many of the identified factors (BMI, weight change during pregnancy, and gestational age at delivery) are “modifiable” risk factors.
Previous efforts to validate two models utilizing cervical length by transvaginal ultrasound and maternal characteristics including age, height, BMI, parity, gestational age, and Bishop score have shown moderate predictive capacities with areas under the ROC curve of 0.76 and 0.67 (30). However, their models differed from ours in that they included cervical length and parity as predictors. Pelvic examination with calculation of a Bishop score is the current standard of care for induction planning rather than sonographic assessment of cervical length. We chose to exclude multiparous women as efforts to reduce primary cesarean delivery rates focus on nulliparous women given their contribution to cesarean delivery rates and the effect of the first cesarean delivery on future pregnancy outcomes (31, 32).
The results of our multivariable logistic regression of known predictors of success of nulliparous IOL may be used to counsel women facing IOL similarly to the nomogram developed for vaginal birth after cesarean (VBAC) (33). Such a tool may be useful for deciding IOL versus prelabor cesarean delivery for women at very high risk of cesarean delivery and for counseling women in general. For example, a woman who has a predicted success of vaginal delivery after induction of <20% may elect to avoid the potential excessive risks of intrapartum cesarean delivery. However, the threshold at which the risks of attempted IOL outweigh the risks of prelabor cesarean delivery is unknown. We recommend that once the decision to induce is made, regardless of probability of success, providers should follow guidelines for diagnosing failed induction of labor as proposed by Spong and colleagues (32).
Our model included only antepartum factors that are reliably known prior to the start of IOL. We found a significantly increased risk of cesarean delivery with other factors including meconium, male fetal sex, fetal malposition, and higher birthweight. The presence of meconium, male fetal sex, and fetal occiput posterior position have been previously associated with cesarean delivery (34–36). Although birthweight is a known predictor of cesarean delivery after IOL (13), this was not included in our model as it is a variable that is only known accurately post-delivery and estimated fetal weight antenatally both sonographically and clinically is weak (37, 38).
Although method of induction differed between the two groups, we felt that this represented the actual method of induction and perhaps not the intended method and, thus, was not known at the start of the induction. Previous randomized trials have found no difference in the rate of cesarean delivery between women undergoing cervical ripening with prostaglandins as compared to Foley catheter (39, 40).
Our study is not without limitations. It is possible that not all inductions performed during the study period were identified using ICD-9 codes. Our study population is majority Caucasian and the mean BMI in both groups was <30 kg/m2, and we do not have data on socioeconomic or insurance status. Although the nomogram has been internally validated, this nomogram has not been externally validated in other populations and is only applicable to nulliparous women at term. We do not know what effects using such a nomogram would have on primary cesarean delivery rates. The affects are likely to vary across institutions and patient populations as the risk of cesarean delivery varies widely across U.S. hospitals (41). Additionally, induction of labor practices differ across institutions, likely resulting in varying rates of cesarean delivery after induction.
Areas for future study include predictors of cesarean delivery after induction in preterm pregnancies and those complicated by obesity. Limited data suggest that risk of cesarean delivery after IOL decreases with advancing gestational age at preterm gestations (42, 43). Obese women are more likely to undergo a cesarean delivery during labor and experience post-cesarean complications including infection (44, 45). It is worth considering then if obese women, particularly women with a BMI ≥50 kg/m2, should be delivered by prelabor cesarean to allow a controlled delivery, perhaps in a center with adequate anesthesia personnel and equipment. A recent retrospective cohort study compared prelabor cesarean delivery to IOL for 661 women with a BMI ≥40 kg/m2 with singleton, term pregnancies (46). They found no difference in maternal or neonatal morbidity composite outcomes. More evidence on how to manage this increasingly obese population of women is needed.
Because IOL will continue to be a therapeutic tool and alternative to cesarean delivery for pregnancies at risk of maternal or neonatal morbidity, it is worth studying further whether induction is the safest option for all women. Based on our nomogram, women at the highest for cesarean delivery after induction may be identified with the potential to avoid the additional risks of prolonged labor and intrapartum cesarean delivery. This nomogram should be validated in other populations and a threshold of increased risk of IOL compared to prelabor cesarean delivery established prior to use in clinical practice.
Acknowledgments
Supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.American College of Obstetricians and Gynecologists. Patient Safety and Quality Improvement. [Accesssed April 2015];reVITALize Obstetric Data Definitions. http://www.acog.org/About-ACOG/ACOG-Departments/Patient-Safety-and-Quality-Improvement/reVITALize-Obstetric-Data-Definitions.
- 2.ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 Pt 1):386–97. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 3.Laughon SK, Zhang J, Grewal J, Sundaram R, Beaver J, Reddy UM. Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol. 2012;206(6):486e1–9. doi: 10.1016/j.ajog.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouse DJ, Owen J, Hauth JC. Criteria for failed labor induction: prospective evaluation of a standardized protocol. Obstet Gynecol. 2000;96(5 Pt 1):671–7. doi: 10.1016/s0029-7844(00)01010-3. [DOI] [PubMed] [Google Scholar]
- 5.Darney BG, Caughey AB. Elective induction of labor symposium: nomenclature, research methodological issues, and outcomes. Clin Obstet Gynecol. 2014;57(2):343–62. doi: 10.1097/GRF.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 6.Darney BG, Snowden JM, Cheng YW, Jacob L, Nicholson JM, Kaimal A, et al. Elective induction of labor at term compared with expectant management: maternal and neonatal outcomes. Obstet Gynecol. 2013;122(4):761–9. doi: 10.1097/AOG.0b013e3182a6a4d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015 Apr 13; doi: 10.1016/j.ajog.2015.04.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Beckmann M. Predicting a failed induction. Aust N Z J Obstet Gynaecol. 2007;47(5):394–8. doi: 10.1111/j.1479-828X.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 9.Ennen CS, Bofill JA, Magann EF, Bass JD, Chauhan SP, Morrison JC. Risk factors for cesarean delivery in preterm, term and post-term patients undergoing induction of labor with an unfavorable cervix. Gynecol Obstet Invest. 2009;67(2):113–7. doi: 10.1159/000166307. [DOI] [PubMed] [Google Scholar]
- 10.Gunatilake RP, Smrtka MP, Harris B, Kraus DM, Small MJ, Grotegut CA, et al. Predictors of failed trial of labor among women with an extremely obese body mass index. Am J Obstet Gynecol. 2013;209(6):562e1–5. doi: 10.1016/j.ajog.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Michelson KA, Carr DB, Easterling TR. The impact of duration of labor induction on cesarean rate. Am J Obstet Gynecol. 2008;199(3):299e1–4. doi: 10.1016/j.ajog.2008.06.072. [DOI] [PubMed] [Google Scholar]
- 12.Crane JM. Factors predicting labor induction success: a critical analysis. Clin Obstet Gynecol. 2006;49(3):573–84. doi: 10.1097/00003081-200609000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Crane JM, Delaney T, Butt KD, Bennett KA, Hutchens D, Young DC. Predictors of successful labor induction with oral or vaginal misoprostol. J Matern Fetal Neona. 2004;15(5):319–23. doi: 10.1080/14767050410001702195. [DOI] [PubMed] [Google Scholar]
- 14.Parkes I, Kabiri D, Hants Y, Ezra Y. The indication for induction of labor impacts the risk of cesarean delivery. J Matern Fetal Neona. 2014:1–5. doi: 10.3109/14767058.2014.993965. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz KM, Feldman D. Induction of labor for fetal growth restriction: risk factors for primary cesarean delivery. Obstet Gynecol. 2014;123 (Suppl 1):56S. doi: 10.3109/14767058.2014.980807. [DOI] [PubMed] [Google Scholar]
- 16.Gulmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. The Cochrane database of systematic reviews. 2012;6:CD004945. doi: 10.1002/14651858.CD004945.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen VM, O’Connell CM, Baskett TF. Maternal morbidity associated with cesarean delivery without labor compared with induction of labor at term. Obstet Gynecol. 2006;108(2):286–94. doi: 10.1097/01.AOG.0000215988.23224.e4. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 19.ACOG committee opinion no. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–10. doi: 10.1097/01.AOG.0000428648.75548.00. [DOI] [PubMed] [Google Scholar]
- 20.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994) Gestational diabetes Obstet Gynecol. 2001;98(3):525–38. [PubMed] [Google Scholar]
- 21.ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748–61. doi: 10.1097/AOG.0b013e31819e9ee2. [DOI] [PubMed] [Google Scholar]
- 22.Clark SL, Miller DD, Belfort MA, Dildy GA, Frye DK, Meyers JA. Neonatal and maternal outcomes associated with elective term delivery. Am J Obstet Gynecol. 2009;200(2):156e1–4. doi: 10.1016/j.ajog.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 23.Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–33. doi: 10.1097/AOG.0b013e3182255999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–20. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laughon SK, Zhang J, Troendle J, Sun L, Reddy UM. Using a simplified Bishop score to predict vaginal delivery. Obstet Gynecol. 2011;117(4):805–11. doi: 10.1097/AOG.0b013e3182114ad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102(6):1445–54. doi: 10.1016/j.obstetgynecol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Accessed March 2015]. http://www.R-project.org/ [Google Scholar]
- 28.James GWD, Hastie T, Tibshirani R. An Introduction to Statistical Learning: with Applications in R. New York: 2013. [Google Scholar]
- 29.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Verhoeven CJ, Oudenaarden A, Hermus MA, Porath MM, Oei SG, Mol BW. Validation of models that predict Cesarean section after induction of labor. Ultrasound Obstet Gynecol. 2009;34(3):316–21. doi: 10.1002/uog.7315. [DOI] [PubMed] [Google Scholar]
- 31.Brennan DJ, Murphy M, Robson MS, O’Herlihy C. The singleton, cephalic, nulliparous woman after 36 weeks of gestation: contribution to overall cesarean delivery rates. Obstet Gynecol. 2011;117(2 Pt 1):273–9. doi: 10.1097/AOG.0b013e318204521a. [DOI] [PubMed] [Google Scholar]
- 32.Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the First Cesarean Delivery: Summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181–93. doi: 10.1097/aog.0b013e3182704880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grobman WA, Lai Y, Landon MB, Spong CY, Leveno KJ, Rouse DJ, et al. Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109(4):806–12. doi: 10.1097/01.AOG.0000259312.36053.02. [DOI] [PubMed] [Google Scholar]
- 34.Schuit E, Kwee A, Westerhuis ME, Van Dessel HJ, Graziosi GC, Van Lith JM, et al. A clinical prediction model to assess the risk of operative delivery. BJOG. 2012;119(8):915–23. doi: 10.1111/j.1471-0528.2012.03334.x. [DOI] [PubMed] [Google Scholar]
- 35.Ghi T, Maroni E, Youssef A, Morselli-Labate AM, Paccapelo A, Montaguti E, et al. Sonographic pattern of fetal head descent: relationship with duration of active second stage of labor and occiput position at delivery. Ultrasound Obstet Gynecol. 2014;44(1):82–9. doi: 10.1002/uog.13324. [DOI] [PubMed] [Google Scholar]
- 36.Gawade P, Markenson G, Bsat F, Healy A, Pekow P, Plevyak M. Association of gestational weight gain with cesarean delivery rate after labor induction. J Reprod Med. 2011;56(3–4):95–102. [PubMed] [Google Scholar]
- 37.Goetzinger KR, Odibo AO, Shanks AL, Roehl KA, Cahill AG. Clinical accuracy of estimated fetal weight in term pregnancies in a teaching hospital. J Matern Fetal Neona. 2014;27(1):89–93. doi: 10.3109/14767058.2013.806474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melamed N, Yogev Y, Meizner I, Mashiach R, Bardin R, Ben-Haroush A. Sonographic fetal weight estimation: which model should be used? J Ultrasound Med. 2009;28(5):617–29. doi: 10.7863/jum.2009.28.5.617. [DOI] [PubMed] [Google Scholar]
- 39.Jozwiak M, Oude Rengerink K, Ten Eikelder ML, van Pampus MG, Dijksterhuis MG, de Graaf IM, et al. Foley catheter or prostaglandin E2 inserts for induction of labour at term: an open-label randomized controlled trial (PROBAAT-P trial) and systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):137–45. doi: 10.1016/j.ejogrb.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Jozwiak M, ten Eikelder M, Oude Rengerink K, de Groot C, Feitsma H, Spaanderman M, et al. Foley catheter versus vaginal misoprostol: randomized controlled trial (PROBAAT-M study) and systematic review and meta-analysis of literature. Am J Perinatol. 2014;31(2):145–56. doi: 10.1055/s-0033-1341573. [DOI] [PubMed] [Google Scholar]
- 41.Kozhimannil KB, Arcaya MC, Subramanian SV. Maternal clinical diagnoses and hospital variation in the risk of cesarean delivery: analyses of a National US Hospital Discharge Database. PLoS Med. 2014;11(10):e1001745. doi: 10.1371/journal.pmed.1001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nassar AH, Adra AM, Chakhtoura N, Gomez-Marin O, Beydoun S. Severe preeclampsia remote from term: labor induction or elective cesarean delivery? Am J Obstet Gynecol. 1998;179(5):1210–3. doi: 10.1016/s0002-9378(98)70133-4. [DOI] [PubMed] [Google Scholar]
- 43.Wing DA, Tran S, Paul RH. Factors affecting the likelihood of successful induction after intravaginal misoprostol application for cervical ripening and labor induction. Am J Obstet Gynecol. 2002;186(6):1237–40. doi: 10.1067/mob.2002.123740. discussion 40–3. [DOI] [PubMed] [Google Scholar]
- 44.Stamilio DM, Scifres CM. Extreme obesity and postcesarean maternal complications. Obstet Gynecol. 2014;124(2 Pt 1):227–32. doi: 10.1097/AOG.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe KB, Rossi RA, Warshak CR. The effect of maternal obesity on the rate of failed induction of labor. Am J Obstet Gynecol. 2011;205(2):128e1–7. doi: 10.1016/j.ajog.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 46.Subramaniam A, Jauk VC, Goss AR, Alvarez MD, Reese C, Edwards RK. Mode of delivery in women with class III obesity: planned cesarean compared with induction of labor. Am J Obstet Gynecol. 2014;211(6):700e1–9. doi: 10.1016/j.ajog.2014.06.045. [DOI] [PubMed] [Google Scholar]