Abstract
Social buffering, the phenomenon by which the presence of a familiar individual reduces or even eliminates stress- and fear-induced responses exists in different animal species, and has been examined in the context of the mother-infant relationship in addition to adults. Although it is a well-known effect, the biological mechanisms, which underlie it, as well as its developmental impact are not well understood. Here we provide a review of evidence of social and maternal buffering of stress reactivity in nonhuman primates, and some data from our group suggesting that when the mother-infant relationship is disrupted maternal buffering is impaired. This evidence underscores the critical role that maternal care plays for proper regulation and development of emotional and stress responses of primate infants. Disruptions of the parent-infant bond constitute early adverse experiences associated with increased risk for psychopathology. We will focus on infant maltreatment, a devastating experience not only for humans, but for nonhuman primates as well. Taking advantage of this naturalistic animal model of adverse maternal caregiving we have shown that competent maternal care is critical for the development of healthy attachment, social behavior and emotional and stress regulation, as well as of neural circuits underlying these functions.
Keywords: social buffering, nonhuman primates, stress reactivity, HPA axis
Stress, the Hypothalamic-Pituitary-Adrenal (HPA) Axis and Early Experiences
Animal models of early adverse experience, primarily involving disruption of the mother-infant relationship, have played a critical role in understanding how early social experiences “get under the skin” and change developmental trajectories through the identification of biological mechanisms by which the mother regulates brain, behavioral, and physiological development of the infant. Recently identified biological mechanisms go beyond the effects of stress hormones and stress-induced inflammation, and include alterations in the gut microbiome that lead to neurotransmitter changes in the infant’s brain (Borre et al., 2014; Cryan & Dinan, 2012), as well as epigenetic modifications that translate experiences into molecular changes in DNA that can be transmitted from one generation to another (Champagne, 2008; Roth et al., 2009; Weaver et al., 2004). In this paper we will focus on an additional regulatory mechanism of maternal care on infant development: maternal buffering of stress and fear responses. We will first describe the HPA stress response and its development, as well as the control of the stress and fear response by the central nervous system. From there we will review our current understanding of social buffering, both peer and maternal, in nonhuman primates. We will conclude with a review of the impact that disruptions of the mother-infant relationship have on neurodevelopment of the stress and fear response.
The presence of a sensitive and responsive caregiver serves as a potent external regulator of the infant’s physiology, buffering its fear and stress responses early in life through sensory, nutritional, motor and thermal pathways (Hofer, 1984; Howell & Sanchez, 2011; Kuhn et al., 1991; Raineki, Lucion, & Weinberg, 2014; Rincón-Cortés & Sullivan, 2014). Thus, maternal behaviors could potentially control the maturational rates of emotion and stress self-regulatory strategies and the underlying brain circuits in the offspring. This ability of early experience and the environment to modulate development may have evolutionary roots in which early experiences and environments program physiological and brain development to support behaviors, cognitive, and biological processes that help the offspring (and future generations) adapt to that particular environment. However, the early critical or sensitive periods of increased neuroplasticity that serve as windows of opportunity for adaptation to the environment may also serve as windows of vulnerability.
Much of the literature examining the biological mechanisms linking early adversity (including mother-infant relationship disruption) and developmental psychopathology has focused on the HPA axis because it is activated in response to early stressful experiences (Heim, Ehlert, & Hellhammer, 2000; Sánchez et al., 2005) and can cause long-term neurobiological changes via genomic mechanisms (Barr et al., 2003; Kinnally et al., 2011). Stressful stimuli activate stressor-specific pathways that converge onto and activate neurons in the hypothalamic paraventricular nucleus (PVN) triggering the release of corticotropin-releasing factor (CRF) into the anterior pituitary that in turn releases adrenocorticotropic hormone (ACTH) into the systemic blood circulation. ACTH provokes the synthesis and release of glucocorticoids (GCs: cortisol in primates), which are highly catabolic steroid hormones. Limbic regions such as the amygdala and hippocampus, as well as the ventromedial prefrontal cortex (vmPFC), regulate HPA axis activity through indirect projections to the PVN, and they also express glucocorticoid receptors (GR), mediating GC negative feedback (Herman et al., 2003; Herman, Ostrander, Mueller, & Figueiredo, 2005; Ulrich-Lai & Herman, 2009). Stressors, including mother-infant relationship disruption, activate the HPA axis and associated brain areas, including the cortico-limbic structures mentioned above (vmPFC, amygdala, hippocampus). Other neurobiological systems, such as the sympathetic nervous system, are also activated during threatening situations. Chronic stress or traumatic experiences early in life can impact the way these stress-response systems mature, leading to extreme emotional and stress reactivity and other psychopathologies. Much of the evidence supporting a role for variations in maternal care in stress response function comes from studies in rodent and nonhuman primate animal models, making it essential to understand how the stress response matures in these species across development.
A stress hyporesponsive period (SHRP) has been reported in rodent pups during which the HPA axis is less responsive to stress, with an inability to mount a corticosterone (GC produced by the adrenal cortex in rodents) response to stressors that can effectively activate the axis at later ages (Gunnar & Vazquez, 2006; Sanchez, Ladd, & Plotsky, 2001). Given the wide effects of GCs on homeostasis and gene expression, the role of the SHRP is thought to be to keep GC levels low to avoid massive effects on normative brain growth and development (Sapolsky & Meaney, 1986). Although the SHRP involves an immature HPA axis, there is also strong evidence that the dam’s presence plays a major role in maintaining low GC levels during the SHRP, and that she also regulates the development of the pup HPA axis and fear systems during the transition out of the SHRP (Moriceau & Sullivan, 2006; Stanton, Wallstrom, & Levine, 1987; Stanton, Gutierrez, & Levine, 1988). In humans and nonhuman primates, although there is also some evidence of an early period of relative stress hyporesponsivity, it is (1) primarily dependent on the buffering effect of maternal (or paternal) presence and not on the immaturity of the HPA axis (unlike the rodent SHRP), (2) highly dependent on quality of parental care and (3) may extend through childhood (Gunnar & Fisher, 2006; Hostinar, Sullivan, & Gunnar, 2014; McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009). Although the underlying mechanisms of this period of stress hyporesponsivity are different for rodents and primates, the evidence from both models supports the hypothesis that GC levels need to be tightly regulated during brain development to facilitate typical maturation.
The development of the HPA axis in nonhuman primates follows a similar pattern as that observed in humans, making them ideal model organisms when attempting to investigate complex sequelae related to stress physiology. The presence of a basal HPA diurnal rhythm has been reported in infant rhesus monkeys, but it still appears to be immature at 5 months of age (comparable to 18–24 months in humans (Raper, Bachevalier, Wallen, & Sanchez, 2013). By the juvenile stage (starting around 12 months, comparable to 4 years in humans) rhesus monkeys show an adult-like diurnal pattern of cortisol secretion (Barrett et al., 2009; Raper et al., 2014; Sánchez et al., 2005). Although there is no solid evidence of a true SHRP in rhesus monkeys, socially familiar cues, specifically the presence of a nurturing mother, do buffer the HPA axis stress responses in infant rhesus macaques (McCormack et al., 2009; Sanchez, 2006). Thus, as described for humans above, nonhuman primate mothers also function as external regulators of infant HPA axis activity, serving as strong social buffers that prevent stress-induced activations to potential threats. This highlights the importance of early social experiences, in particular the role of nurturing caregiving, on the development of the HPA axis across different mammalian species, as well as the utility of animal models to study the role of adverse caregiving on the etiology of early life stress-induced developmental psychopathology.
Multiple neural pathways transmit information about specific types of threat to the mammalian PVN (Herman et al., 2003; Herman et al., 2005; Ulrich-Lai & Herman, 2009). Psychological and emotional stressors are evaluated and transmitted by brain regions including cortico-limbic structures such as the vmPFC, amygdala, and hippocampus, as described above. These regions change drastically, and over relatively long periods of time, during both pre- and postnatal development in mammals, which is thought to make them particularly sensitive and vulnerable to early social experiences (Andersen, 2003). Recent research has elucidated a critical role of the development of vmPFC-amygdala circuits for proper development of the HPA axis and emotional regulation. Lesion studies represent a successful approach when attempting to describe the complex relationships that exist between these highly interconnected structures during development. Recent studies by our group have shown that when the amygdala is lesioned around 2 weeks of age both HPA axis reactivity and basal function are altered. Lesions of the amygdala during this neonatal period are associated with heightened –not blunted- basal and stress-induced HPA axis reactivity during infancy and the juvenile period (Raper et al., 2013; Raper et al., 2013; Raper, Wilson, Sanchez, Machado, & Bachevalier, 2013; Raper et al., 2014), despite a well established role of the amygdala in stimulating the HPA axis in adult animals, supported by adult lesions of the amygdala resulting in blunted HPA axis reactivity (Kalin, Shelton, & Davidson, 2004; Machado & Bachevalier, 2008), without effects on basal HPA axis activity (Kalin et al., 2004; Machado & Bachevalier, 2008; Norman & Spies, 1981; Sapolsky, Zola-Morgan, & Squire, 1991). These findings suggest that the influence of the amygdala on the HPA axis may change across development, and may have seemingly paradoxical roles, inhibiting HPA axis activity in primates early in development, and then switching later into a stimulatory role on cortisol release. Interestingly, adult and neonatal lesions of these limbic circuits result in similar alterations in emotional reactivity,(Kalin et al., 2004; Machado & Bachevalier, 2008; Machado et al., 2008; Machado, Kazama, & Bachevalier, 2009; Raper et al., 2013; Raper, Wilson et al., 2013), which combined with the differential effects of lesion timing on the HPA axis described above highlights the need for further experiments to determine the underlying brain-behavior relationships.
Social Buffering of Stress Responses in Nonhuman Primate Species
As defined above, social buffering is the phenomenon in which the presence of another animal, or group of animals, can reduce or even eliminate the HPA activation of another individual when exposed to stressful stimuli (Levine, Johnson, & Gonzalez, 1985). This phenomenon has been observed in several species, including humans and non-human animals, and has been reported during development in the context of the mother-infant relationship, as well as in familiar adults.
The first descriptions of social buffering in nonhuman primates were reported in studies examining mother-reared and surrogate-reared infants. Hill, McCormack, & Mason (1973) examined the HPA axis response of surrogate-reared rhesus monkeys when exposed to novelty, and reported that the HPA response was significantly lower when the infant was in the presence of their surrogate mother compared to when alone. Mendoza, Smotherman, Miner, Kaplan, & Levine (1978) also examined the HPA responses of mother-reared and surrogate-reared squirrel monkey infants in response to separation and reunion with the mother/surrogate. All infants demonstrated heightened levels of plasma cortisol in response to the separation from the mother, and all infants demonstrated a return to baseline cortisol levels when reunited with their mothers/surrogates. These findings indicate that infants not only developed an attachment to their mother-figure, but that both mothers and surrogate mothers helped dampen the infant stress response at reunion.
Following the comparison studies on the bonds between monkey infants and their mothers/surrogates, research began to focus specifically on the HPA axis response of squirrel monkey (a New World primate species) infants under a variety of stressful situations, and the ability of the mother to buffer the stress response. Many of these landmark studies were conducted by Seymour Levine. In one of the first studies, Coe, Mendoza, Smotherman, & Levine, (1978) examined the plasma cortisol levels of infant squirrel monkeys under 4 conditions: 1) baseline, 2) 30 minutes after a brief separation and reunion from the mother, 3) 30 minutes after the infant was removed from the social group and housed alone in a cage, and 4) 30 minutes after the mother was removed from the group, while the infant remained in the group. Infants did not demonstrate an elevation in cortisol levels after the brief separation and reunion from the mother, suggesting that the resumption of contact with the mother eliminated an HPA axis reaction to the brief separation. Compared to baseline cortisol values, infants showed a significant increase in cortisol levels when they were separated from the group, as well as when the mother was separated from the group (and the infant was left in the social group). However, infant cortisol levels were not as high following maternal separation when they remained in the group, suggesting that the social group may have buffered the stress response of the infant while the mother was removed. Additional support for a social buffering effect on infant HPA reactivity was later reported (Coe, Wiener, & Levine, 1983; Wiener, Johnson, & Levine, 1987), such that squirrel monkey infants showed a lower HPA axis response to maternal separation when the infant remained in the social group compared to being removed from the social group. In another study, Levine, Wiener, & Coe (1993) demonstrated that not only physical contact, but even olfactory and auditory cues from the mother were able to reduce the HPA axis response of a separated squirrel monkey infant compared to complete isolation from the mother. Altogether these studies suggest that squirrel monkey infants can use social companions to buffer their HPA stress response in response to challenges (e.g. the face of novelty stress), with direct contact with mother having the strongest ability to buffering effect on the HPA axis stress activations (Levine, 2000).
Additional studies conducted on the rhesus monkey mother-infant relationship confirmed findings reported in squirrel monkeys. Gonzalez, Gunnar, & Levine (1981) examined both mother and infant HPA axis responses to several separation conditions, including being removed from the social group but housed together, and being removed and housed separately. The cortisol levels of the infants when separated with the mother were moderately low compared to when housed during complete separation. In addition, the infants also appeared to dampen the stress response of the mothers, suggesting that social buffering can be bi-directional between mothers and infants during stressful situations. Additional studies in rhesus macaques further validated the finding that in the presence of the mother, rhesus infants showed small to no elevations in cortisol when exposed to novelty (Gunnar, Gonzalez, & Levine, 1980; Levine et al., 1985).
Several studies further manipulated the degree of maternal presence when exposing rhesus infants to novelty to determine what specific maternal cues were necessary to elicit social buffering. Levine et al. (1985) reported that infant cortisol was buffered when the infant was removed from the social group while in contact with the mother, even if they were just in visual contact. Interestingly, the behavioral profile was very different, in that the infants were more likely to vocalize toward the mother when she was only accessible visually. Bayart, Hayashi, Faull, Barchas, & Levine (1990) also reported that infants who had visual and auditory access to their mother during a separation from their social group showed lower cortisol responses compared to those that were in complete isolation, although the behavioral profiles were more reactive than during the alone condition. They also reported increased 3-methoxy-4-hydroxyphenylglycol (MHPG), a norepinephrine metabolite, levels in the complete isolation condition compared to separation in the presence of the mother. Levine et al. (1985) suggested that the inverse relationship that is observed between behavioral reactivity and physiological reactivity when rhesus infants are isolated in the presence of the mother may be explained as a coping mechanism. Specifically, the vocalizations may serve as a coping strategy to the stressful situation, allowing for reduction of physiological arousal in the infant, in addition to serving as solicitations for aid.
Finally, several studies have examined the extent to which the social group can help buffer infants’ HPA axis response in the face of a stressor. Coe, Wiener, Rosenberg, & Levine, (1985) examined the response of rhesus infants during two forms of separation: infant removal from the mother and the social group, or mother removal from the social group while the infant remained in the group. They found that when the infants remained in the social group while their mother was removed, their cortisol reactivity was blunted in comparison to when they themselves were removed from the group, suggesting that the social group acted as a social buffer by helping reduce the infant stress response to the mother being removed from the group. It is also possible that the first form of separation (maternal and group) combined with exposure to novelty more strongly activated the HPA response than just removing the mother from the social group (i.e. no exposure to a novel environment). Further experiments that systematically address the effect of each condition would need to be conducted to clarify this point.
More recently Winslow, Noble, Lyons, Sterk, & Insel (2003) examined the effect that social partners have on buffering the stress response of 3 year old rhesus macaques who were either mother-reared or peer-reared during the first year of life. Social partners were able to buffer the stress response of the mother-reared animals, but the same degree of buffering was not observed in the nursery-reared animals, suggesting long-term impact of the mother-infant relationship on the ability of primates to benefit from social buffering effects.
Taken together, these results suggest that, during normative development, separation from the social group does not strongly activate the HPA axis of monkey infants when the infants are left in contact with the mother. When the infant is exposed to the stress of group separation, and allowed visual, auditory, or olfactory access to the mother, behavioral reactivity increases, yet cortisol levels are maintained at approximately baseline levels. Finally, when the mother is removed from the infant, but the infant remains in the social group, its HPA axis is not activated as strongly as when the infant is removed from the group alone, suggesting that familiar conspecifics may also act as social buffers. These results collectively suggest that the mother, and in some cases other animals, play an important role in reducing arousal in infants (Gunnar et al., 1980). It appears that physical contact with the mother helps reduce both the physiological and the behavioral responses to stressful stimuli in infants (Gunnar et al., 1980). According to Levine (2000) the mother is the primary source of security for an infant, and by being able to maintain proximity to her in the face of uncertainty, the infant is able to use her to control its arousal level.
Social buffering has also been examined in adult animals. The first studies were conducted in squirrel monkeys, and found that the presence of several group members had the strongest effect at reducing the stress response in the face of a stressor. When adult male squirrel monkeys were placed in a novel environment with visual access to a snake, they showed increased levels of cortisol when tested alone or with a partner; however, when tested with their group, they did not show cortisol elevations (Coe, Franklin, Smith, & Levine, 1982; Vogt, Coe, & Levine, 1981). Likewise when adult male squirrel monkeys were exposed to fear conditioning (light-shock pairing), their cortisol levels were elevated when tested alone, while cortisol elevations were attenuated when tested with a social partner, and returned to baseline when several social partners were present (Stanton, Patterson, & Levine, 1985). These results suggest that the presence of several social partners is most effective for reducing the HPA axis activation of adult squirrel monkeys when exposed to a stressor.
Social buffering has also been reported in marmosets, another New World primate species. Smith, McGreer-Whitworth, & French (1998) found that marmosets placed in a novel environment exhibited lower levels of urinary cortisol when exposed with their heterosexual pair mate compared to when tested alone. Rukstalis & French (2005) examined whether or not the vocalizations of a pair mate would have an effect on an isolated adult marmoset’s HPA response. Adult marmosets who were separated from their group, and who heard the vocalizations of their pair mate throughout the separation, had significantly lower levels of urinary cortisol compared to when tested alone or when tested with the sounds of an unfamiliar animal. This demonstrated the effect referred to as “vocal buffering”.
Social buffering has also been reported in adult macaques. Gust, Gordon, Brodie, & McClure (1994) examined the cortisol and immunological response of adult female rhesus monkeys exposed to a 96 hour separation from their social group. Compared to baseline values, the immunological response to the separation was significantly reduced when the separated animals had a social companion with them, compared to being alone. However, the presence of another animal did not buffer the cortisol responses of the separated animals. In a more naturalistic design, Young, Majolo, Heistermann, Schulke, & Ostner (2014) examined the role that social support had on the stress response of wild male Barbary macaques. The researchers evaluated levels of fecal cortisol in these animals during 2 stressful events: when exposed to low temperatures, and when exposed to group aggression. They found that the animals who had stronger social ties had lower levels of fecal cortisol compared to those with weaker social bonds. This suggests that even when faced with naturalistic stressors, social buffering is a powerful phenomenon that influences HPA axis responses.
Taken together, these findings suggest that the presence of social partners can also attenuate the stress response of adult nonhuman primates when exposed to different stressor types. However, it appears that there are species-specific differences in whether or not one animal can have such an effect, or whether it takes several known social partners. Galvao-Coelho, Silva, & De Sousa (2012) further suggested that there are sex differences in the effect that one or more social members have to buffer the stress response of adult animals.
Social Buffering Following Disruption of the Mother-Infant Relationship
For primates the mother-infant relationship is arguably the most salient early experience. This has been demonstrated by the strong link between disruption of this relationship and poor behavioral and physiological outcomes in the infant. The effects of this form of early adversity are thought to be due in part to a disruption in the mother’s ability to buffer her infant’s stress response, as described previously. This could thus lead to exposure to elevated levels of cortisol and alterations in HPA function. This is supported by studies in several nonhuman primate species and models of mother-infant relationship disruption, discussed below.
Disruption of the mother-infant relationship results in alterations in HPA axis function, including the ability of familiar conspecifics to buffer the stress response, i.e. social buffering. One very potent, albeit severe, method of disrupting the mother-infant relationship is to prevent the formation of that bond/relationship as in the case of nursery-rearing infant monkeys. This manipulation causes a broad range of behavioral and HPA axis alterations, including altered circadian cortisol rhythms (Boyce, Champoux, Suomi, & Gunnar, 1995), increased basal HPA function (Higley, Suomi, & Linnoila, 1992), and decreased basal HPA function (Clarke, 1993) (for review, see Sanchez et al., 2001). In contrast to mother-reared infant rhesus monkeys, who are able to use familiar conspecifics to buffer their responses to stress (Coe et al., 1978; Levine, 2000; Mendoza et al., 1978; Wiener et al., 1987), nursery-reared animals do not show this effect and show alterations in salient social behaviors including excessive allogrooming and intermale mounting (Winslow et al., 2003). As mentioned above, Harry Harlow’s group also investigated the cortisol responses of surrogate- and mother-reared infants to a stressor during separation from the attachment figure (i.e. the surrogate or the mother). They demonstrated that mother-reared infants showed similar pretest cortisol levels and cortisol reactivity prior to a separation period as compared to the surrogate-reared group while the surrogate was present; however after separation, while neither the surrogate- or mother-reared animals showed differences in cortisol increases between pretest and posttest measures, mother-reared animals showed increased pretest –baseline- cortisol levels (Meyer, Novak, Bowman, & Harlow, 1975). The authors further explained that this effect was driven by an order-effect in the mother-reared infants in which the second infant to be tested each day had elevated pretest cortisol levels (Meyer et al., 1975). The results of this study suggest alterations in the surrogate-reared animals’ ability to use social information to modulate their stress physiology. All of this evidence combined suggests that the species typical mother-infant relationship plays a unique role in modulating the infant’s ability to use other conspecifics to buffer their stress response, and that replacing the mother with a cloth surrogate, although this has been shown to represent an attachment relationship, is not enough to normalize this aspect of HPA axis function. Further evidence of this effect comes from a recent study that reported elevated levels of cortisol accumulated in hair (a measure of chronic GC exposure) that were related to anxious behavior in animals raised in a nursery with access to same age peers, suggesting that peers are not able to buffer stress and emotional reactivity like mothers do (Dettmer, Novak, Suomi, & Meyer, 2012).
Maternal separation is another experimental manipulation of the mother-infant relationship that has been used to investigate the role of the mother in modulating the developing HPA axis. As described above, both New and Old World nonhuman primates, including rhesus monkeys, squirrel monkeys (Samiri sciureus), and common marmosets (Callithrix jacchus) have been studied using variations of this paradigm. In rhesus monkeys, short maternal separations result in infants’ increased activity, frequency of vocalizations, and HPA axis activations (Bayart et al., 1990; Harlow, Harlow, & Suomi, 1971). Repeated maternal separation in infancy leads to increased cortisol reactivity and flattened diurnal rhythms in females later in life, during the juvenile period (Sánchez et al., 2005). These effects, though, are dependent on the timing of the separation as demonstrated by elegant work by Lyons and colleagues. There is an interesting body of literature supporting “stress inoculation” effects, the concept that certain types of stress at specific times during development actually increase resilience when the individual faces challenges later in life. Most of the evidence for this phenomenon in nonhuman primates comes from studies of squirrel monkeys exposed to mild intermittent stress consisting of separation from the mother and social group at developmentally distinct times during developmental (i.e. weaning, when they are no longer dependent on their mothers). This stress exposure appears to be protective, allowing the animal to better adapt to environmental challenges later in life (Lyons & Parker, 2007; Lyons, Parker, & Schatzberg, 2010; Parker, Buckmaster, Schatzberg, & Lyons, 2004). In these animals there is also evidence that the HPA stress response is better calibrated to handle challenges, as demonstrated by reduced basal levels of stress hormones while mounting comparable neuroendocrine responses to moderate stressors as compared to unexposed animals (Parker, Buckmaster, Lindley, Schatzberg, & Lyons, 2012). In this model, the distribution of the glucocorticoid receptor (GR) in the brain was also changed as a result of stress exposure (Patel, Katz, Karssen, & Lyons, 2008). The GR has also been implicated in models of care disruption using marmosets (a biparental primate species) separated from their parents daily between days 2 and 28 of life. These repeated separations resulted in reductions in GR mRNA in the hippocampus in adolescent marmosets (Dettling, Feldon, & Pryce, 2002a; Dettling, Feldon, & Pryce, 2002b). Altogether, these GR effects suggest that maternal (or parental) presence at certain points in development can lead to specific cellular changes that may be partly responsible for the ability of the mother to modulate the HPA stress response.
The variable foraging demand (VFD) model contrasts with the models described above in that it is an ethologically valid model of early life adversity that exploits the species typical behavior of foraging for food. Leonard Rosenblum and colleagues first developed this model in bonnet macaques (Macaca radiata), which consists of variable and unpredictable food availability (e.g. periods of intense foraging alternated with periods of free access to food) (Rosenblum & Paully, 1984). This manipulation results in disruption of the early mother-infant relationship by altering maternal behavior (i.e. reducing the amount of time spent responding to infant solicitations for care) (Rosenblum & Paully, 1984). Infants exposed to the high foraging demand condition showed physiological hyper-responsiveness to stressful stimuli, as well as elevated levels of CRF in cerebrospinal fluid (CSF), but reduced levels of cortisol as compared to those raised under low foraging demand (Coplan et al., 2001; Coplan et al., 1996). Just as the models described above that involved more invasive manipulations of maternal care, the more ecologically and ethologically valid VFD model points to similar alterations in the HPA stress response associated with changes in mother-infant interactions that may be related to the mechanisms involved in social buffering of the stress response.
Another ethologically valid model of mother-infant relationship disruption is naturally occurring infant maltreatment in nonhuman primates. Infant maltreatment is not a uniquely human phenomenon, but has also been reported in both wild and captive populations of nonhuman primate species, including macaques, baboons, and marmosets (Brent, Koban, & Ramirez, 2002; Johnson, Kamilaris, Calogero, Gold, & Chrousos, 1996; Maestripieri & Carroll, 1998; Troisi, D'Amato, Fuccillo, & Scucchi, 1982). Our group has studied this phenomenon in rhesus monkeys and defines maltreatment as an adverse early experience that includes two comorbid types of maternal behavior that both result in overt signs of infant distress (vocalizations, tantrums, etc.): (1) physical abuse consisting of aberrant violent behaviors exhibited toward the infant causing pain and distress (drags, crushes, sits on, or roughly grooms the infant), typically occurring during the first 2–3 months of life, and (2) high rates of infant rejection beginning early in life, consisting of pushing the infant away when it solicits maternal contact (Maestripieri & Carroll, 1998; McCormack, Sanchez, Bardi, & Maestripieri, 2006). Several alterations in the HPA axis have been associated with this disruption in species typical mother-infant behavior, including increases in both basal and stress levels of cortisol, and blunted ACTH responses to a CRF pharmacological challenge, reflecting down regulation of CRF receptors in the pituitary as a consequence of chronic CRF overactivity by the hypothalamic PVN, which further supports stress-induced activation of central components of the HPA axis –i.e. the “H”ypothalamus and the “P”ituitary- (Koch, McCormack, Sanchez, & Maestripieri, 2014; Sanchez, 2006; Sanchez et al., 2010). Altogether these findings suggest that maltreatment is a stressful experience that results in elevated activity of the HPA axis, likely including overproduction of hypothalamic CRF which leads to pituitary down regulation of CRF receptors.
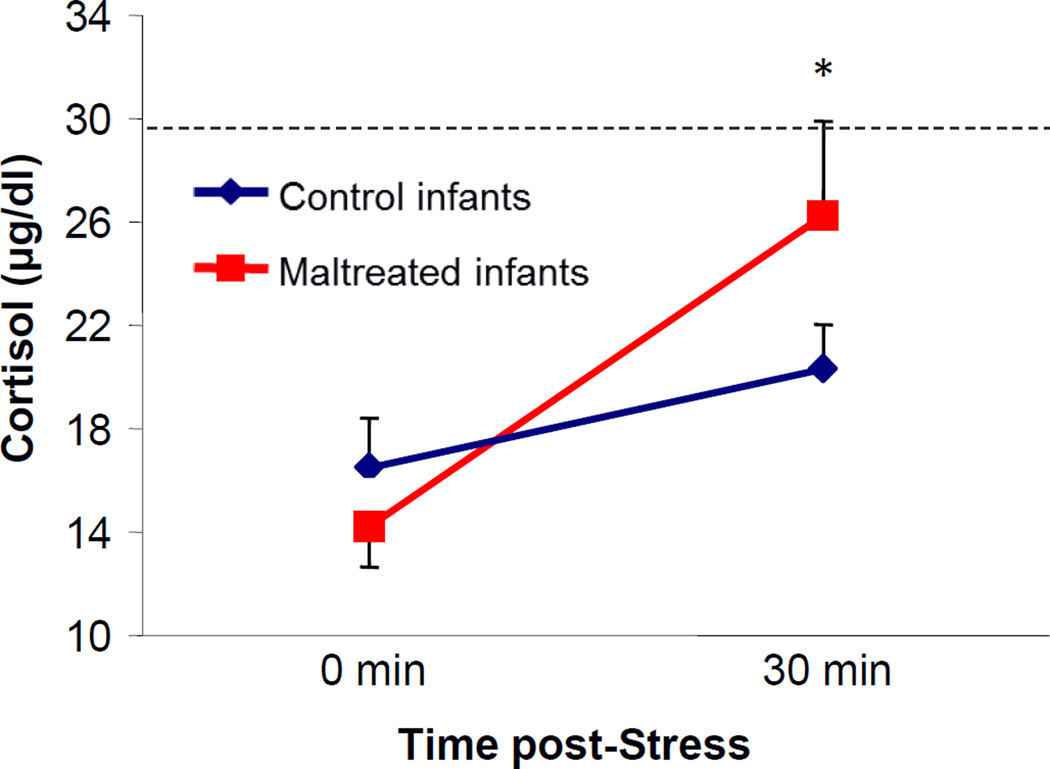
This early infant maltreatment experience is more complicated than just being stressful for the infants. Additional findings from our lab suggest that the ability of the mother to buffer the infant’s stress reactivity is also impaired in maltreating mothers, which deepens the developmental impact of this poor caregiving experience. Figure 1 shows an impaired ability of the mother’s presence to buffer stress-induced cortisol increases in maltreated infants (t(18)=−1.936, p=0.034, one-tailed). In that study, mother-infant pairs (10 control, 10 maltreated) were exposed together to a novel stress test when the infant was 2 months old (see (McCormack et al., 2006) for a description of the selection of subjects and characterization of early maternal care). A baseline blood sample was collected from both mother and infant, and another blood sample was collected 30 min following exposure to the novel cage and room (post-stress, 30 min) for measures of plasma cortisol concentrations by radioimmunoassay (RIA; Diagnostic Systems Lab commercial kit, Webster, TX). Infants’ plasma cortisol concentrations (in µg/dl) are plotted in reference to the average stress-induced cortisol levels reached when infants are exposed to a novel stress test alone (without the mother) at similar ages. Thus, while the presence of a sensitive/responsive caregiver (control/competent, mother) prevents stress-induced cortisol elevations in the infant, the presence of maltreating mothers did not buffer the stress responses in their offspring. Of course, one possibility is that this is due to maltreating mothers exhibiting negative behaviors towards the infants during testing. However, analysis of mother-infant interactions from the video-recorded sessions did not show any significant differences in the behaviors exchanged during the test in comparison to control mother-infant pairs. We also examined whether higher stress responses in the maltreating mothers could account for the elevated cortisol responses of their infants, but did not find significant differences between control and maltreating mothers (both groups showed significant cortisol elevations in response to the novelty test: t(18)=−1.58, n.s.). No correlations were found between the stress-induced cortisol increases in moms and infants, either (r(18)=0.26, n.s.).
Figure 1.
Impaired ability of the mother’s presence to buffer stress-induced cortisol increases in maltreated infants. Mother-infant pairs were exposed together to a novel stress test when the infant was 2 months old. A baseline (0 min) blood sample was collected from both mother and infant, and another blood sample was collected 30 min following exposure to the novel cage and room (post-test, 30 min). Infants’ plasma cortisol concentrations (µg/dl) are plotted in comparison to the average stress-induced cortisol levels reached when the infants were exposed to a novel stress test alone (without the mother). * Significant difference between the stress-induced increase in cortisol (delta: stress-baseline) of control and maltreated infants (t(18)=−1.936, p=0.034, one-tailed).
Long-term Impact of Maternal Care on Development of Emotional and Stress Regulation Brain Circuits
Although some of the adverse caregiving experiences and stress activations described above are limited to the infant period, their long-term impact can be significant. For example, in the infant maltreatment model recent neuroimaging studies have begun to map the long-term consequences for structural brain development. Among other ongoing studies, our group has reported associations between elevated basal cortisol levels in maltreated infant macaques at 1 month of age, when abuse rates were highest, and reduced white matter integrity in tracts important for behavioral and emotional regulation, visual processing, somatosensory, and motor integration during adolescence (Howell et al., 2013). But this is just one example, and we will review below evidence of long-term impact of maternal care on the development of neurocircuits involved in stress and emotion regulation.
Adverse caregiving experiences such as those reviewed above happen at a time of rapid neurodevelopmental changes, creating windows of high plasticity in which early experiences are encoded (Andersen, 2003; Knudsen, 2004; Rice & Barone, 2000). The developmental trajectories and sensitive periods are regionally dependent, with cortical maturation happening first in low order sensory cortices (such as visual and somatosensory processing areas), followed by the association cortices that integrate those sensory inputs (e.g. temporal and prefrontal areas) (Giedd, 2004; Giedd & Rapoport, 2010; Gogtay et al., 2004; Shaw et al., 2008). This literature suggests that brain regions with extended development (PFC, association cortices, amygdala, hippocampus) are particularly sensitive and vulnerable to early experiences, especially maternal care. Studies in humans (Bremner et al., 1997; De Bellis et al., 1999; De Bellis, Keshavan et al., 1999; De Bellis, 2005; Drevets, 2008; Teicher et al., 2003; Tottenham & Sheridan, 2010) and animal models (Arabadzisz et al., 2010; Bale et al., 2010; Coplan et al., 1998; Coplan et al., 2001; Coplan et al., 2010; Coplan et al., 1996; Howell et al., 2014; Jackowski et al., 2011; Law et al., 2009; Law, Pei, Feldon, Pryce, & Harrison, 2009; Mathew et al., 2003; O’Connor & Cameron, 2006; Pryce et al., 2005; Sanchez et al., 2001; Spinelli et al., 2009) have provided compelling evidence that the link between adverse caregiving and problems with emotional and stress regulation is, indeed, greatly due to alterations in the typical development of these cortico-limbic circuits. As an example, recent neuroimaging studies by our group in the nonhuman primate model of infant maltreatment demonstrated an association between abuse rates received by infant rhesus monkeys and bigger amygdala volumes and emotional reactivity during adolescence (Howell et al., 2014). These findings parallel some long-term effects in maltreated humans, including structural differences in amygdala, orbitofrontal cortex, anterior cingulate cortex among other regions (Dannlowski et al., 2012; McCrory, De Brito, & Viding, 2012; Teicher, Anderson, Ohashi, & Polcari, 2014).
The plasticity of PFC-amygdala circuits in infants and their sensitivity to early maternal care may have an adaptive role. A main goal of the infant brain is to bond to the caregiver at any cost. Elegant rodent studies (reviewed in another paper in this issue by Regina Sullivan and colleagues), have mapped some of the neurobiological mechanisms underlying the regulation of maternal behaviors on the pup’s brain development (Eghbal-Ahmadi, Avishai-Eliner, Hatalski, & Baram, 1999; Howell & Sanchez, 2011; Korosi & Baram, 2009; Sanchez et al., 2001). Examples of these mechanisms include the regulation of the pup’s physiology through effects of milk on the gastrointestinal tract, stimulation of sensory pathways by the mother that regulate the development of PFC-amygdala circuits and recent work demonstrating that the presence of the mother increases cortical synchronization in pups (Sarro, Wilson, & Sullivan, 2014). Seminal work by Sullivan and collegues has also characterized the neurobiological systems that allow rat pups to attach to the dam (Moriceau & Sullivan, 2005; Sullivan & Lasley, 2010) and that are particularly vulnerable to poor maternal care (Rincón-Cortés & Sullivan, 2014). These studies have shown that the mammalian infant brain is wired to form and maintain strong bonds with the mother, though may use different sensory systems in different species (e.g. olfactory, in rats). Their initial studies showed that pups use a different learning circuit (brainstem noradrenergic) than adults, which helps them quickly learn a robust preference for their mother, while at the same time fear/avoidance brain circuits are “off”. These results explain why the strong bond with the mother is preserved even when she is physically abusive, as shown in humans (Helfer, Kempe, & Krugman, 1999), nonhuman primates (Harlow & Harlow, 1965; Sanchez et al., 2010), and even dogs (Fisher, 1955). Once the pups start exploring the environment, that fear/avoidance learning system is turned “on” to keep them away from danger and the attachment/approach automatic system becomes less automatic and more regulated (Rincón-Cortés & Sullivan, 2014). Similar behavioral switches towards increased fear are present in primates when they start exploring the environment (e.g. around 7–9 months in humans when we start walking; (Sroufe, 1977)). The parallel developmental switches in approach/avoidance strategies across rodent and primate species seem supported by developmental switches reported in the amygdala’s role regulating fear and stress responses, as well as in its connectivity with the PFC both in humans (Gee et al., 2013) and nonhuman primates (Raper et al., 2014). Although the emerging evidence is compelling, we need to better understand the tempo and role of these normative developmental switches before we can map the impact of poor caregiving.
Conclusions and Future Directions
The evidence for social buffering of primate stress and emotional responses, particularly from the presence of a responsive mother, is quite compelling. We are just beginning to understand the underlying neurobiology of this phenomenon. Using nonhuman primate models, we have identified critical brain circuits (i.e. prefrontal-limbic circuits) and developmental periods during which the formation of strong mother-infant bonds seems to be particularly important for proper development of stress and emotional regulation. Prefrontal-limbic circuits are sensitive to adversity early in life (Howell et al., 2013; Howell et al., 2014; Jackowski et al., 2011) and have been implicated in poor health outcomes associated with adverse early experience (Howell et al., 2014). Recent investigations into the impact of early life adversity in human children have been guided by and are consistent with these findings from animal models, reporting alterations in both PFC and limbic brain regions (Ansell et al., 2012; Dannlowski et al., 2012; Frodl et al., 2012; Pechtel & Pizzagalli 2011; Wang et al., 2014). Given the worldwide prevalence (Kessler et al., 2010) and negative social and economic impact of early adversity (Saul et al., 2014), particularly of parent-infant relationship disruption, it is critical to further elucidate its neurobiological underpinnings, and the biological processes that translate the mother’s presence into infant’s physiological and behavioral regulatory responses so that tractable targets for prevention and intervention can be identified. Further nonhuman primate work is needed to dissect the critical biological pathways of maternal communication (olfactory, visual, tactile, auditory signals) and how they provoke neural, behavioral, and physiological changes in their infants. This will explain the relationships suggested by human work. We will also need to consider other developmental periods such as adolescence. By utilizing nonhuman primate models we have the opportunity to parse out the neurobiological and physiological mechanisms of social buffering during development, which would help provide the basis for efficacious treatments and preventative strategies in humans.
Acknowledgments
This work was supported by NIMH grants P50 MH078105 and F31 MH086203 (to BRH), and NICHD grants HD055255 and HD077623. The content is solely the responsibility of the authors and does not represent the official views of the NIMH, NICHD or the NIH. The project was also supported by the Office of Research Infrastructure Programs/OD (ORIP/OD) P51OD11132 (YNPRC Base grant, formerly NCRR P51RR000165). The YNPRC is fully accredited by the Association for the Assessment and Accreditation of Laboratory Care, International. We also want to thank Anne Graff, Alison Grand and Richelle Scales for technical assistance with experimental procedures used to generate some of the findings presented here.
Footnotes
Disclosure: Neither Mar Sanchez, Brittany Howell nor Kai McCormack have any relevant financial or nonfinancial conflicts to disclose.
Contributor Information
Mar M. Sanchez, Dept. of Psychiatry & Behavioral Sciences, Emory University School of Medicine, Yerkes National Primate Research Center, Center for Translational Social Neuroscience and the Silvio O. Conte Center for Oxytocin and Social Cognition, Emory University, 954 Gatewood Rd NE, Atlanta, GA 30329, mmsanch@emory.edu
Kai M. McCormack, Dept. of Psychology, Spelman College, 350 Spelman Lane Southwest, Atlanta, GA 30314, kmccormack@spelman.edu
Brittany R. Howell, Institute of Child Development, University of Minnesota, 51 East River Parkway, Minneapolis, MN 55455, brhowell@umn.edu
References
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews. 2003;27(1):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological psychiatry. 2012;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Pryce CR. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biological Psychiatry. 2010;67(11):1106–1109. doi: 10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Susser ES. Early life programming and neurodevelopmental disorders. Biological Psychiatry. 2010;68(4):314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch K, Higley J. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes, Brain and Behavior. 2003;2(6):336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. Early adverse rearing experiences alter sleep–wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psychoneuroendocrinology. 2009;34(7):1029–1040. doi: 10.1016/j.psyneuen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (macaca mulatta) Behavioral Neuroscience. 1990;104(1):98. [PubMed] [Google Scholar]
- Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends in Molecular Medicine. 2014;20(9):509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: Reactivity to peer interactions and altered circadian activity. Developmental Psychobiology. 1995;28(5):257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biological Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent L, Koban T, Ramirez S. Abnormal, abusive, and stress-related behaviors in baboon mothers. Biological Psychiatry. 2002;52(11):1047–1056. doi: 10.1016/s0006-3223(02)01540-8. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in neuroendocrinology. 2008;29(3):386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Developmental Psychobiology. 1993;26(8):433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiology & Behavior. 1982;29(6):1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- Coe CL, Mendoza SP, Smotherman WP, Levine S. Mother-infant attachment in the squirrel monkey: Adrenal response to separation. Behavioral Biology. 1978;22(2):256–263. doi: 10.1016/s0091-6773(78)92305-2. [DOI] [PubMed] [Google Scholar]
- Coe CL, Wiener SG, Levine S. Symbiosis in parent-offspring interactions. Springer; 1983. Psychoendocrine responses of mother and infant monkeys to disturbance and separation; pp. 189–214. [Google Scholar]
- Coe CL, Wiener SG, Rosenberg LT, Levine S. Endocrine and immune responses to separation and maternal loss in nonhuman primates. The Psychobiology of Attachment and Separation. 1985:163–199. [Google Scholar]
- Coplan JD, Abdallah CG, Tang CY, Mathew SJ, Martinez J, Hof PR, Pantol G. The role of early life stress in development of the anterior limb of the internal capsule in nonhuman primates. Neuroscience Letters. 2010;480(2):93–96. doi: 10.1016/j.neulet.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Smith E, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Rosenblum LA. Variable foraging demand rearing: Sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biological Psychiatry. 2001;50(3):200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Trost RC, Owens MJ, Cooper TB, Gorman JM, Nemeroff CB, Rosenblum LA. Cerebrospinal fluid concentrations of somatostatin and biogenic amines in grown primates reared by mothers exposed to manipulated foraging conditions. Archives of General Psychiatry. 1998;55(5):473–477. doi: 10.1001/archpsyc.55.5.473. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(4):1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews. Neuroscience. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Bauer J. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Ryan ND. Developmental traumatology part I: Biological stress systems. Biological Psychiatry. 1999;45(10):1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey B, Giedd JN, Boring AM, Ryan ND. Developmental traumatology part II: Brain development. Biological Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. The psychobiology of neglect. Child Maltreatment. 2005;10(2):150–172. doi: 10.1177/1077559505275116. doi:10/2/150 [pii] [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacology Biochemistry and Behavior. 2002a;73(1):259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (callithrix jacchus, primates) and analysis of its effects on early development. Biological Psychiatry. 2002b;52(11):1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37(2):191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W. Neuroimaging studies of mood. Neurobiology of Mental Illness. 2008:461. [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1999;19(10):3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AE. The Effects of Differential Early Treatment on the Social and Exploratory Behavior of Puppies. 1955 [Google Scholar]
- Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. Journal of psychiatry & neuroscience: JPN. 2012;37(1):37. doi: 10.1503/jpn.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao-Coelho NL, Silva HP, De Sousa MB. The influence of sex and relatedness on stress response in common marmosets (callithrix jacchus) American Journal of Primatology. 2012;74(9):819–827. doi: 10.1002/ajp.22032. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021(1):77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CA, Gunnar MR, Levine S. Behavioral and hormonal responses to social disruption and infant stimuli in female rhesus monkeys. Psychoneuroendocrinology. 1981;6(1):53–64. doi: 10.1016/0306-4530(81)90048-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18(03):651–677. [PubMed] [Google Scholar]
- Gunnar MR, Gonzalez CA, Levine S. The role of peers in modifying behavioral distress and pituitary-adrenal response to a novel environment in year-old rhesus monkeys. Physiology & Behavior. 1980;25(5):795–798. doi: 10.1016/0031-9384(80)90387-x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. In: Stress neurobiology and developmental psychopathology. Cicchetti D, Cohen DJ, editors. Hoboken, NJ, US: John Wiley & Sons Inc; 2006. pp. 533–577. [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiology & Behavior. 1994;55(4):681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. In: Schrier AM, Harlow HF, Stollnitz F, editors. Behavior of nonhuman primates. 2. ed. New York: Academic Press; 1965. [Google Scholar]
- Harlow HF, Harlow MK, Suomi SJ. From thought to therapy: Lessons from a primate laboratory. American Scientist. 1971 [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Helfer ME, Kempe RS, Krugman RD. The battered child. University of Chicago Press; 1999. [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32(2):127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hill SD, McCormack SA, Mason WA. Effects of artificial mothers and visual experience on adrenal responsiveness of infant monkeys. Developmental Psychobiology. 1973;6(5):421–429. doi: 10.1002/dev.420060506. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: A psychobiologic perspective on bereavement*. Psychosomatic Medicine. 1984;46(3):183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Grand AP, McCormack KM, Shi Y, LaPrarie JL, Maestripieri D, Sanchez MM. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: Relation to amygdala volume. Developmental Psychobiology. 2014;56(8):1735–1746. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Sanchez MM. Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Development and Psychopathology. 2011;23(04):1001–1016. doi: 10.1017/S0954579411000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, McCormack KM, Grand AP, Sawyer NT, Zhang X, Maestripieri D, Sanchez MM. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: Associations with high cortisol during infancy. Biology of Mood & Anxiety Disorders. 2013;3(1) doi: 10.1186/2045-5380-3-21. 21-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski A, Perera TD, Abdallah CG, Garrido G, Tang CY, Martinez J, Smith EL. Early-life stress, corpus callosum development, hippocampal volumetrics, and anxious behavior in male nonhuman primates. Psychiatry Research: Neuroimaging. 2011;192(1):37–44. doi: 10.1016/j.pscychresns.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Calogero AE, Gold PW, Chrousos GP. Effects of early parenting on growth and development in a small primate. Pediatric Research. 1996;39(6):999–1005. doi: 10.1203/00006450-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Williams DR. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry. 2010;197(5):378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Feinberg C, Kim D, Ferguson K, Leibel R, Coplan JD, Mann JJ. DNA methylation as a risk factor in the effects of early life stress. Brain, Behavior, and Immunity. 2011;25(8):1548–1553. doi: 10.1016/j.bbi.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E. Sensitive periods in the development of the brain and behavior. Cognitive Neuroscience, Journal of. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Koch H, McCormack K, Sanchez MM, Maestripieri D. The development of the hypothalamic–pituitary–adrenal axis in rhesus monkeys: Effects of age, sex, and early experience. Developmental Psychobiology. 2014;56(1):86–95. doi: 10.1002/dev.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother's love to baby's future. Frontiers in Behavioral Neuroscience. 2009;3:27. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM, Field T, Symanski R, Zimmerman E, Scafidi F, Roberts J. Tactile-kinesthetic stimulation effects on sympathetic and adrenocortical function in preterm infants. The Journal of Pediatrics. 1991;119(3):434–440. doi: 10.1016/s0022-3476(05)82059-1. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Feldon J, Pryce CR, Harrison PJ. Gene expression in the anterior cingulate cortex and amygdala of adolescent marmoset monkeys following parental separations in infancy. International Journal of Neuropsychopharmacology. 2009;12(6):761–772. doi: 10.1017/S1461145708009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, Harrison PJ. Early parental deprivation in the marmoset monkey produces long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder. Neuropsychopharmacology. 2009;34(6):1381–1394. doi: 10.1038/npp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic–pituitary–adrenal axis. European Journal of Pharmacology. 2000;405(1):149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S, Johnson DF, Gonzalez CA. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behavioral Neuroscience. 1985;99(3):399. doi: 10.1037//0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Levine S, Wiener SG, Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology. 1993;18(4):297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of Traumatic Stress. 2007;20(4):423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: Implications for understanding resilience. Developmental Psychobiology. 2010;52(7):616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33(7):926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (macaca mulatta): Consistent pattern of behavior across different social contexts. Behavioral Neuroscience. 2008;122(2):251. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9(2):147. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psychological Science. 1998;9(2):143–145. [Google Scholar]
- Mathew SJ, Shungu DC, Mao X, Smith EL, Perera GM, Kegeles LS, Gorman JM. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biological Psychiatry. 2003;54(7):727–735. doi: 10.1016/s0006-3223(03)00004-0. [DOI] [PubMed] [Google Scholar]
- McCormack K, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Developmental Psychobiology. 2006;48(7):537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman T, Higley J, Maestripieri D, Sanchez M. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: A review of neurobiological and genetic research. Journal of the Royal Society of Medicine. 2012;105(4):151–156. doi: 10.1258/jrsm.2011.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SP, Smotherman WP, Miner MT, Kaplan J, Levine S. Pituitary-adrenal response to separation in mother and infant squirrel monkeys. Developmental Psychobiology. 1978;11(2):169–175. doi: 10.1002/dev.420110209. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, Bowman RE, Harlow HF. Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Developmental Psychobiology. 1975;8(5):425–435. doi: 10.1002/dev.420080507. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Developmental Psychobiology. 2005;47(3):230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RL, Spies HG. Brain lesions in infant female rhesus monkeys: Effects on menarche and first ovulation and on diurnal rhythms of prolactin and cortisol. Endocrinology. 1981;108(5):1723–1729. doi: 10.1210/endo-108-5-1723. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Cameron JL. Translating research findings on early experience to prevention: Animal and human evidence on early attachment relationships. American Journal of Preventive Medicine. 2006;31(6):175–181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Lindley SE, Schatzberg AF, Lyons DM. Hypothalamic-pituitary-adrenal axis physiology and cognitive control of behavior in stress inoculated monkeys. International Journal of Behavioral Development. 2012;36(1):45–52. doi: 10.1177/0165025411406864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Archives of General Psychiatry. 2004;61(9):933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33(3):360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience & Biobehavioral Reviews. 2005;29(4):649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Raineki C, Lucion AB, Weinberg J. Neonatal handling: An overview of the positive and negative effects. Developmental Psychobiology. 2014;56(8):1613–1625. doi: 10.1002/dev.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Bachevalier J, Wallen K, Sanchez M. Neonatal amygdala lesions alter basal cortisol levels in infant rhesus monkeys. Psychoneuroendocrinology. 2013;38(6):818–829. doi: 10.1016/j.psyneuen.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wallen K, Sanchez MM, Stephens SB, Henry A, Villareal T, Bachevalier J. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Hormones and Behavior. 2013;63(4):646–658. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wilson M, Sanchez M, Machado CJ, Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinology. 2013;38(7):1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Stephens SB, Henry A, Villarreal T, Bachevalier J, Wallen K, Sanchez MM. Neonatal amygdala lesions lead to increased activity of brain CRF systems and hypothalamic-pituitary-adrenal axis of juvenile rhesus monkeys. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2014;34(34):11452–11460. doi: 10.1523/JNEUROSCI.0269-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. doi:sc271_5_1835 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, Sullivan RM. Early life trauma and attachment: Immediate and enduring effects on neurobehavioral and stress axis development. Frontiers in Endocrinology. 2014;5 doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum LA, Paully GS. The effects of varying environmental demands on maternal and infant behavior. Child Development. 1984:305–314. [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: Exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Hormones and Behavior. 2005;47(1):1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13(03):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Mccormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Development and Psychopathology. 2010;22(01):45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57(4):373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11(1):65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Zola-Morgan S, Squire LR. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1991;11(12):3695–3704. doi: 10.1523/JNEUROSCI.11-12-03695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Wilson DA, Sullivan RM. Maternal regulation of infant brain state. Current Biology. 2014;24(14):1664–1669. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul J, Valle LA, Mercy JA, Turner S, Kaufmann R, Popovic T. CDC grand rounds: creating a healthier future through prevention of child maltreatment. MMWR Morb Mortal Wkly Rep. 2014;63(12):260–263. [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (callithrix kuhli) Hormones and Behavior. 1998;34(3):211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry. 2009;66(6):658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA. Wariness of strangers and the study of infant development. Child Development. 1977:731–746. [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavioral Neuroscience. 1988;102(5):692. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Patterson JM, Levine S. Social influences on conditioned cortisol secretion in the squirrel monkey. Psychoneuroendocrinology. 1985;10(2):125–134. doi: 10.1016/0306-4530(85)90050-2. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Developmental Psychobiology. 1987;20(2):131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Lasley EN. Fear in love: Attachment, abuse, and the developing brain. Paper presented at the Cerebrum: The Dana Forum on Brain Science, 2010. 2010 [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 2003;27(1):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: Altered network centrality of cingulate, precuneus, temporal pole and insula. Biological Psychiatry. 2014;76(4):297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, D'Amato FR, Fuccillo R, Scucchi S. Infant abuse by a wild-born group-living japanese macaque mother. Journal of Abnormal Psychology. 1982;91(6):451. doi: 10.1037//0021-843x.91.6.451. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt JL, Coe CL, Levine S. Behavioral and adrenocorticoid responsiveness of squirrel monkeys to a live snake: Is flight necessarily stressful? Behavioral and Neural Biology. 1981;32(4):391–405. doi: 10.1016/s0163-1047(81)90826-8. [DOI] [PubMed] [Google Scholar]
- Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, Li L. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Human brain mapping. 2014;35(4):1154–1166. doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wiener SG, Johnson DF, Levine S. Influence of postnatal rearing conditions on the response of squirrel monkey infants to brief perturbations in mother-infant relationships. Physiology & Behavior. 1987;39(1):21–26. doi: 10.1016/0031-9384(87)90339-8. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Young C, Majolo B, Heistermann M, Schulke O, Ostner J. Responses to social and environmental stress are attenuated by strong male bonds in wild macaques. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(51):18195–18200. doi: 10.1073/pnas.1411450111. [DOI] [PMC free article] [PubMed] [Google Scholar]