Abstract
Objective
To characterize tocolytic use and examine perinatal outcomes among women presenting very preterm with spontaneous labor and cervical dilation ≥4 cm.
Methods
This was a retrospective cohort study. Data from January 2000 to June 2011 in a single healthcare system were reviewed. Women with singleton, non-anomalous fetuses, and preterm labor with intact membranes between 23.0–32.0 weeks gestation who had cervical dilation ≥4 cm and <8 cm at admission were included. Women receiving one or more tocolytics (magnesium sulfate, indomethacin, or nifedipine) were compared to those who did not receive tocolysis. The primary outcome was composite major neonatal morbidity.
Results
Two hundred ninety seven women were included; 233 (78.5%) received at least one tocolytic. Women receiving tocolysis were slightly less dilated (median 5 vs. 6 cm, p<0.001) at presentation, and were more likely to receive at least a partial course of corticosteroids (88.4% vs. 56.3%, p<0.001). Initial composite severe neonatal morbidity rates were similar (41.6% vs. 43.8%, p=0.761) regardless of tocolytic administration. Those receiving tocolysis were significantly more likely to be pregnant at least 48 hours after admission (23.6% vs. 7.8%, p=0.005), but a similar proportion delivered within 7 days of admission (94.8% vs. 95.3%, p>0.99), and delivery gestational ages were similar (28.9 vs. 29.2 weeks, p=0.408). The incidence of chorioamnionitis and postpartum endometritis were similar between groups.
Conclusion
The majority of women presenting very preterm with advanced cervical dilation received tocolysis. Although tocolysis administration increased the likelihood of achieving at least 48 hours of latency, initial neonatal outcomes were similar.
Introduction
Preterm birth (< 37 weeks) is the leading cause of neonatal morbidity and mortality in the United States.1 Spontaneous preterm birth accounts for the majority of preterm birth. Very preterm neonates (< 33 weeks) are at highest risk for significant short and long term morbidity.2 Antenatal administration of corticosteroids (typically, intramuscular betamethasone) to women with threatened preterm delivery reduces neonatal morbidity and mortality.3–6 Neonates receive optimum benefit from corticosteroids when delivery is delayed for ≥48 hours, although incomplete courses provide some benefit.6–8
Treatment with tocolytics is associated with successful pregnancy prolongation for 48-hours, and permits administration of antenatal corticosteroids.9–13 Even so, providers may be reluctant to administer tocolysis due to a sense of futility and concerns for underlying intra-amniotic infection among women with spontaneous preterm labor and advanced cervical dilation. We sought to characterize tocolytic use, determine efficacy in pregnancy prolongation, and examine perinatal outcomes among women presenting very preterm with spontaneous preterm labor and advanced cervical dilation.
Materials and Methods
We studied a retrospective cohort of patients in a single healthcare system (Intermountain Healthcare) admitted to labor and delivery between January 2000 and June 2011 with a diagnosis of spontaneous preterm labor. Intermountain Healthcare is a not-for-profit system of 22 hospitals and more than 185 physician clinics, located primarily in the State of Utah. Administrative and clinical electronic medical record data are maintained in an ‘Enterprise Data Warehouse’ that captures data across multiple information systems, primarily entered during ‘point-of-care’ interactions with patients, as well as capturing billing and administrative/coding data. The Enterprise Data Warehouse was queried to identify women admitted to an Intermountain Healthcare facility with a diagnosis of spontaneous preterm labor, threatened preterm delivery, or cervical insufficiency (ICD9 diagnosis codes 644.0×, 644.2×, 654.5×, 658.1×, and 658.2×) between 23.0–31.9 weeks gestation. Women pregnant with a live singleton, non-anomalous fetus who had an initial digital cervical dilation of 4 centimeters or more but less than 8 centimeters at the time of presentation were included. Women with one or more of the following: ruptured membranes, polyhydramnios, intrauterine fetal demise, or evidence of chorioamnionitis at the time of presentation were excluded. Those with cervical dilation of 8 centimeters or greater on initial digital examination were also excluded from analysis due to expected imminent delivery. Women with medical indications for delivery (e.g., severe pre-eclampsia) were also excluded. For women who met inclusion criteria with more than one pregnancy during the study period, data were analyzed only for the first eligible pregnancy. All records identified from the Enterprise Data Warehouse as potentially meeting inclusion criteria were manually reviewed and updated by physician researchers to ensure data accuracy and study eligibility. This study was approved by the Intermountain Healthcare Institutional Review Board.
Decisions whether to administer tocolysis, which medication(s) to use, and medication dose(s) were made by individual physicians. Medications administered for tocolysis included magnesium sulfate, indomethacin, and nifedipine, used singly or in combination. Women receiving one or more tocolytics were compared to those who did not receive tocolysis. Labor management decisions were also made by individual physicians. In our healthcare system, magnesium sulfate was commonly used as a tocolytic agent prior to the widespread adoption of magnesium sulfate neuroprotection protocols in late 2008 and early 2009. Magnesium sulfate continues to be used as a tocolytic in some instances based on provider preference, and many providers view this medication as providing dual benefit. To ascertain the primary intent (neuroprotective agent vs. tocolytic agent) of magnesium sulfate administration, the history and physical examination, physician progress note, and discharge summary narratives were reviewed by physician researchers (T.A.M. and C.A.H.).
The primary outcome was composite major neonatal morbidity, defined as the diagnosis of at least one of the following prior to discharge: bronchopulmonary dysplasia, severe intraventricular hemorrhage (grades III or IV), periventricular leukomalacia, necrotizing enterocolitis, stillbirth, or neonatal death. Secondary outcomes included the incidence of delayed delivery for at least 48 hours after admission, delivery gestational age, individual neonatal morbidities, and specific tocolytic regimens in prolonging pregnancy for at least 48 hours after admission. All outcomes were determined by standardized clinical definitions, utilizing relevant radiographic and pharmacologic data as applicable, and were manually verified by chart abstraction.
Data were analyzed using Stata® version 13.1 (StataCorp, College Station, TX). Patients were grouped by whether or not they received tocolysis, and demographics were compared using chi-square, Student’s t-test, Fisher exact, and ANOVA as appropriate. All reported p-values are from a two-sided comparison. There was no adjustment for multiple comparisons and no imputation was made for missing data. Study outcomes were modeled using multivariable Poisson regression with robust variance estimates.20 In these models, the reported relative risk estimates represent ratios of incidence proportions, rather than ratios of rates with person-time denominators. Since tocolytic therapy is a short-term therapy with a goal of prolonging pregnancy for at least two days, the follow-up time was short enough to represent an equal time at risk for each patient, which justified modeling incidence proportions.
In the initial Poisson regression model examining factors associated with neonatal morbidity, variables included were Caucasian race, nulliparity, antenatal corticosteroid administration, initial cervical dilation, chorioamnionitis, pregnancy prolongation for at least 48 hours after admission, delivery gestational age, need for neonatal transfer of care, culture proven neonatal sepsis, and administration of tocolysis. In the initial regression model examining factors associated with prolongation of pregnancy for at least 48 hours after admission, variables included were gestational age at presentation, Caucasian race, prepregnancy body mass index, nulliparity, initial cervical dilation, chorioamnionitis, and administration of tocolysis.
The probability of remaining pregnant after admission (with and without tocolytic therapy) was displayed using a Kaplan-Meier graph. Survival curves were compared using the log-rank test for equality of survival functions.
Results
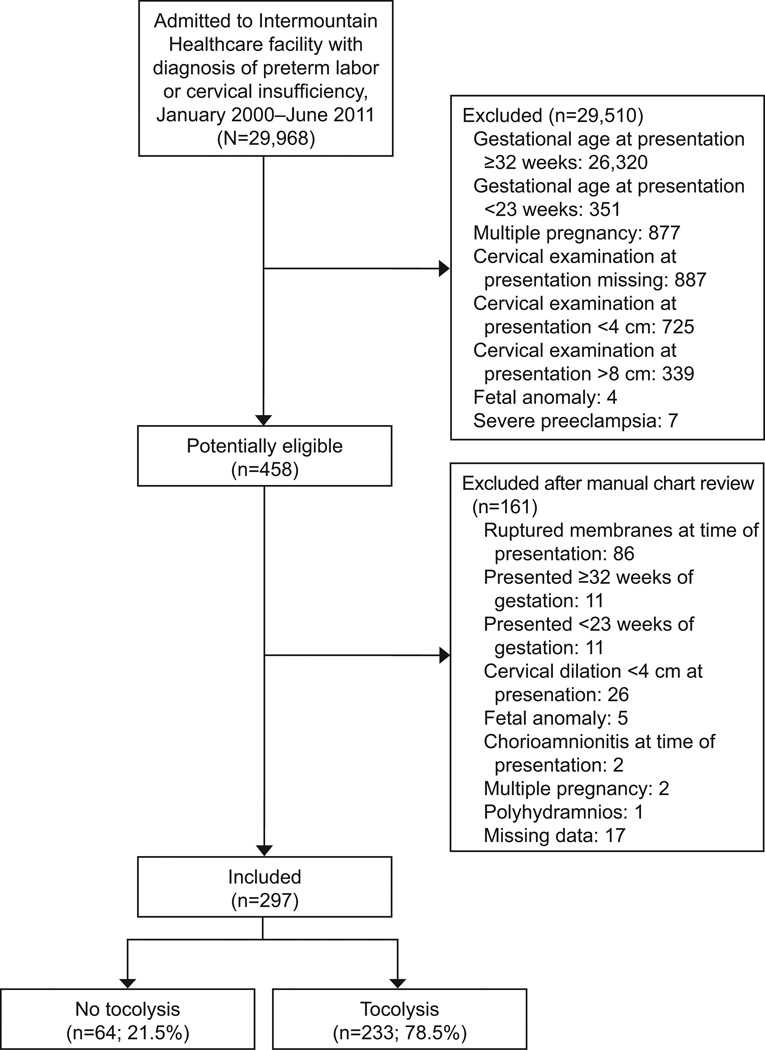
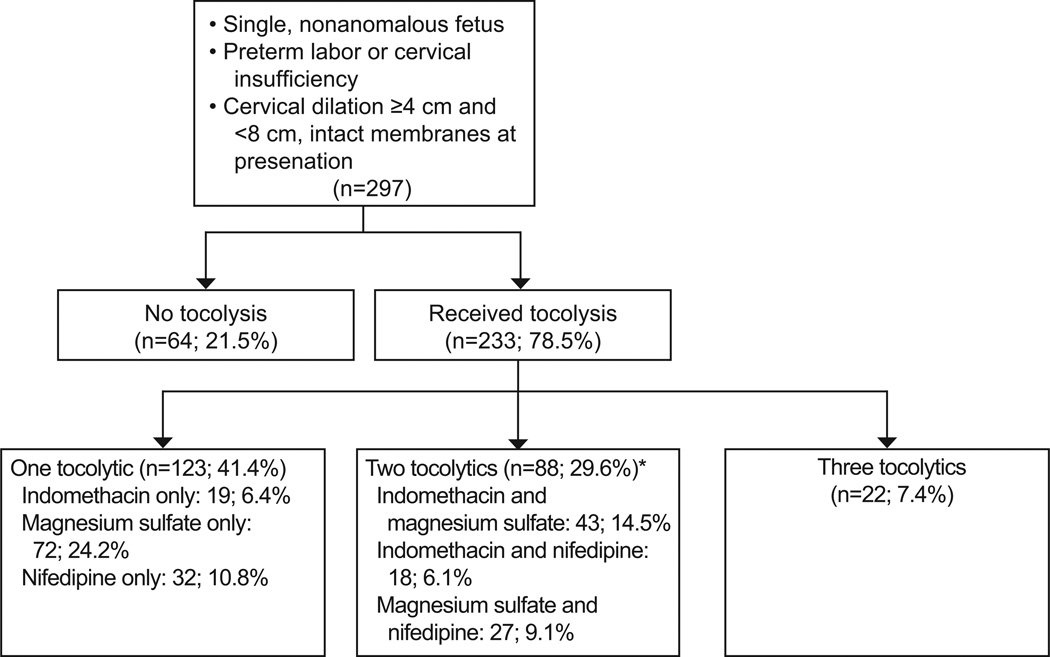
Two hundred ninety seven women met inclusion criteria, of whom 233 (78.5%) received at least one tocolytic (Figure 1). The most commonly used tocolytic was magnesium sulfate: 164 (55.2%) received it alone or in combination with other medications; 102 (34.4%) received indomethacin, and 99 (33.3%) received nifedipine. The majority of women who received tocolysis (123/233, 52.7%) were treated with only one tocolytic (Figure 2). Women who did not receive tocolysis had a higher mean cervical dilation, were less likely to receive at least one dose of betamethasone, and were less likely to be transferred to a tertiary care facility compared to those who received tocolysis; all other maternal baseline characteristics were similar between the two groups (Table 1).
Figure 1.
Study enrollment.
Figure 2.
Summary of tocolytics received among women included in the analysis. *Percentages may not equal 29.6% due to rounding.
Table 1.
Baseline characteristics, by tocolytic administration.
| Variable | Tocolysis N=233 |
No Tocolysis N=64 |
p-value |
|---|---|---|---|
| Mean maternal age at delivery (years) | 25.0 +/− 6.0 | 24.8 +/− 5.8 | 0.751 |
| White race (n, %) | 166 (71.2) | 45 (70.3) | 0.884 |
| Hispanic ethnicity (n, %) | 38 (16.3) | 11 (17.2) | 0.867 |
| Married (n, %) | 159 (68.2) | 47 (73.4) | 0.424 |
| Mean prepregnancy body mass index (kilograms/meter2) | 25.0 +/− 6.7 | 25.6 +/− 6.1 | 0.491 |
| Tobacco use during pregnancy (n, %) | 10 (4.3) | 2 (3.1) | 0.675 |
| Nulliparous (n, %) | 102 (43.8) | 20 (31.3) | 0.071 |
| One or more previous preterm deliveries (n, %) | 55 (23.6) | 20 (31.3) | 0.212 |
| Mean admission gestational age (weeks) | 28.4 +/− 2.6 | 29.0 +/− 2.7 | 0.128 |
| Median admission cervical dilation (centimeters, IQR) | 5 (4–5.5) | 6 (4.5–6.5) | <0.001 |
| Fetal malpresentation (n, %) | 56 (24.0) | 16 (25.0) | 0.873 |
| Preterm premature rupture of membranes occurred after admission but at least 24 hours prior to delivery (n, %) | 110 (47.2) | 37 (57.8) | 0.133 |
| Received one or more doses of antenatal corticosteroids (n, %) | 206 (88.4) | 36 (56.3) | <0.001 |
| Mother transferred from presenting hospital to another facility for higher level of care while pregnant | 46 (19.7) | 5 (7.8) | 0.025 |
Data are presented as n(%) unless otherwise noted.
Outcomes based on the number of tocolytics received and specific tocolytic regimens are detailed in Tables 2 and 3. Women receiving multiple tocolytics tended to be less dilated at the time of presentation, and were more likely to achieve 48 hours of latency between admission and delivery (Tables 2 and 3). However, there was no significant difference in number of tocolytics received based on gestational age at presentation, and the delivery gestational age also did not vary by tocolytic regimen. Women who did not receive tocolysis were also slightly more dilated at the time of presentation (median 6 cm, IQR 4.5–6.5 cm, compared to 5 cm, IQR 4.0–5.5, p<0.001). The vast majority of patients remained hospitalized until delivery (440/444, 99.1%). Labor and intrapartum characteristics were similar between groups (Table 4).
Table 2.
Presentation, latency, and neonatal morbidity by number of tocolytics received.
| Tocolytic Regimen |
n | Median (IQR) Gestational Age at Presentation (weeks) |
Median (IQR) Cervical Dilation at Presentation (cm) |
Median (IQR) Gestational Age at Delivery (weeks) |
Latency of >= 48 hours (n, %) |
Major neonatal morbidity (n, %) |
|---|---|---|---|---|---|---|
| No tocolysis | 64 | 29.8 (27.6 – 31.1) |
6 (4.5 – 6.5) |
29.9 (27.9 – 31.2) |
5 (7.8) | 28 (43.8) |
| Any one tocolytic* | 123 | 29.4 (27.6 – 31.1) |
5 (4 – 6)† |
29.9 (26.4 – 31.1) |
24 (19.5)* |
51 (41.5) |
| Any two tocolytics* | 88 | 28.0 (25.6 – 30.4)* |
4.5 (4 – 5)† |
28.9 (26.1 – 31.7) |
25 (28.4)† |
40 (45.5) |
| Three tocolytics* | 22 | 28.7 (26.5 – 30.4) |
4 (4 – 5)† |
29.0 (26.7 – 31.7) |
6 (27.3)† | 6 (27.3) |
| p-value | 0.087 | <0.001 | 0.566 | 0.014 | 0.482 |
Studied tocolytics: indomethacin, magnesium sulfate, nifedipine.
indicates p<0.05 in pairwise comparison with ‘no tocolysis’ group as referent.
Table 3.
Presentation, latency, and neonatal morbidity by specific tocolytic regimen.
| Tocolytic Regimen |
n | Median (IQR) Gestational Age at Presentation (weeks) |
Median (IQR) Cervical Dilation at Presentation (centimeters) |
Median (IQR) Gestational Age at Delivery (weeks) |
Latency of >= 48 hours (n, %) |
Major neonatal morbidity (n, %) |
|---|---|---|---|---|---|---|
| No tocolysis | 64 | 29.8 (27.6 – 31.1) |
6 (4.5 – 6.5) |
29.9 (27.9 – 31.2) |
5 (7.8) | 28 (43.8) |
| Nifedipine Only | 32 | 30.2 (28.4 – 31.1) |
5 (4 – 6.5) |
30.4 (28.5 – 31.9) |
4 (12.5) | 11 (34.4) |
| Magnesium Sulfate Only | 72 | 29.3 (26.1 – 31.0) |
5 (4 – 6) |
29.7 (26.1 – 31.1) |
17 (23.6)* | 30 (41.7) |
| Indomethacin Only | 19 | 28.3 (26.2 – 30.4) |
4.5 (4 – 6)* |
28.3 (26.3 – 30.4) |
3 (15.8) | 10 (52.6) |
| Magnesium Sulfate and Indomethacin | 43 | 28.4 (25.7 – 30.4) |
4.5 (4 – 5)* |
28.9 (25.7 – 30.9) |
10 (23.3)* |
22 (51.1) |
| Magnesium Sulfate and Nifedipine | 27 | 27.8 (25.3 – 30.3)* |
4 (4 – 5.5)* |
29.9 (26.0 – 31.9) |
11 (40.7)* |
8 (29.3) |
| Nifedipine and indomethacin | 18 | 27.7 (26.3 – 29.6) |
4 (4 – 5)* |
28.4 (26.3 – 30.7) |
4 (22.2) | 10 (55.6) |
| Nifedipine, indomethacin, and magnesium sulfate | 22 | 28.7 (26.5 – 30.4) |
4 (4 – 5)* |
29 (26.7 – 31.7) |
6 (27.3)* | 6 (27.3) |
| P-value | 0.161 | <0.001 | 0.348 | 0.023 | 0.317 | |
indicates p<0.05 in pairwise comparison with ‘no tocolysis’ group as referent.
Table 4.
Delivery characteristics and initial neonatal outcomes
| Variable | Tocolysis N=233 |
No Tocolysis N=64 |
p-value |
|---|---|---|---|
| Mean delivery gestational age (weeks) | 28.9 +/− 2.7 | 29.2 +/− 2.7 | 0.408 |
| Mean admission-to-delivery interval (days) | 1.8 +/− 4.3 | 1.2 +/− 4.9 | 0.326 |
| Pregnant for ≥48 hours after admission (n, %) | 55 (23.6) | 5 (7.8) | 0.005 |
| Pregnant for ≥7 days after admission (n, %) | 12 (5.2) | 3 (4.7) | 0.881 |
| Delivered ≥ 34.0 weeks gestation (n, %) | 4 (1.7) | 0 (0) | 0.291 |
| Delivered ≥ 37.0 weeks gestation (n, %) | 0 | 0 | - |
| Cesarean section (n, %) | 60 (25.8) | 21 (32.8) | 0.261 |
| Diagnosis of clinical chorioamnionitis (n, %) | 33 (14.2) | 7 (10.9) | 0.503 |
| Acute chorioamnionitis or funisitis on placental pathology* | 79/168 (47.0) | 18/49 (36.7) | 0.202 |
| Postpartum endometritis [in the absence of previous diagnosis of chorioamnionitis, (n, %)] | 4 (1.7) | 2 (3.1) | 0.478 |
| Median maternal length of stay (days, IQR) | 2 (2–5) | 2 (1–3) | 0.206 |
| Male infant (n, %) | 133 (57.1) | 37 (57.8) | 0.917 |
| Mean birthweight (grams) | 1357 +/− 491 | 1468 +/− 499 | 0.113 |
| Admission to neonatal intensive care unit (n, %) | 219 (94.0) | 56 (87.5) | 0.079 |
| Neonate transferred within 24 hours of birth to other facility for higher level of care | 10 (4.3) | 8 (12.5) | 0.015 |
| Major neonatal morbidity † | 97 (41.6) | 28 (43.8) | 0.761 |
| Neonatal death (n, %) | 19 (8.2) | 7 (10.9) | 0.485 |
| Intraventricular hemorrhage grade I or II (n, %) | 40 (17.2) | 7 (10.9) | 0.226 |
| Intraventricular hemorrhage grade III or IV (n, %) | 28 (12.0) | 11 (17.2) | 0.278 |
| Periventricular leukomalacia (n, %) | 11 (4.7) | 3 (4.7) | 0.991 |
| Neonatal bronchopulmonary dysplasia (n, %) | 60 (25.8) | 18 (28.1) | 0.702 |
| Neonatal respiratory distress (n, %) | 220 (94.4) | 54 (84.4) | 0.005 |
| Need for intubation (n, %) | 162 (69.5) | 39 (60.9) | 0.193 |
| Median days intubated (IQR) ‡ | 1 (0 – 10) | 2 (1 – 5) | 0.385 |
| Neonatal necrotizing enterocolitis (n, %) | 23 (9.9) | 5 (7.8) | 0.618 |
| Neonatal sepsis (culture proven) | 34 (14.6) | 7 (10.9) | 0.453 |
| Median neonatal length of stay (days, IQR) § | 42 (27–73) | 36 (14–59) | 0.080 |
placental pathology available for 217 pregnancies
defined as the diagnosis of at least one of the following prior to discharge: bronchopulmonary dysplasia, grade III or IV intraventricular hemorrhage, periventricular leukomalacia, necrotizing enterocolitis, stillbirth or neonatal death
among 201 intubated neonates
among neonates admitted to the neonatal intensive care unit
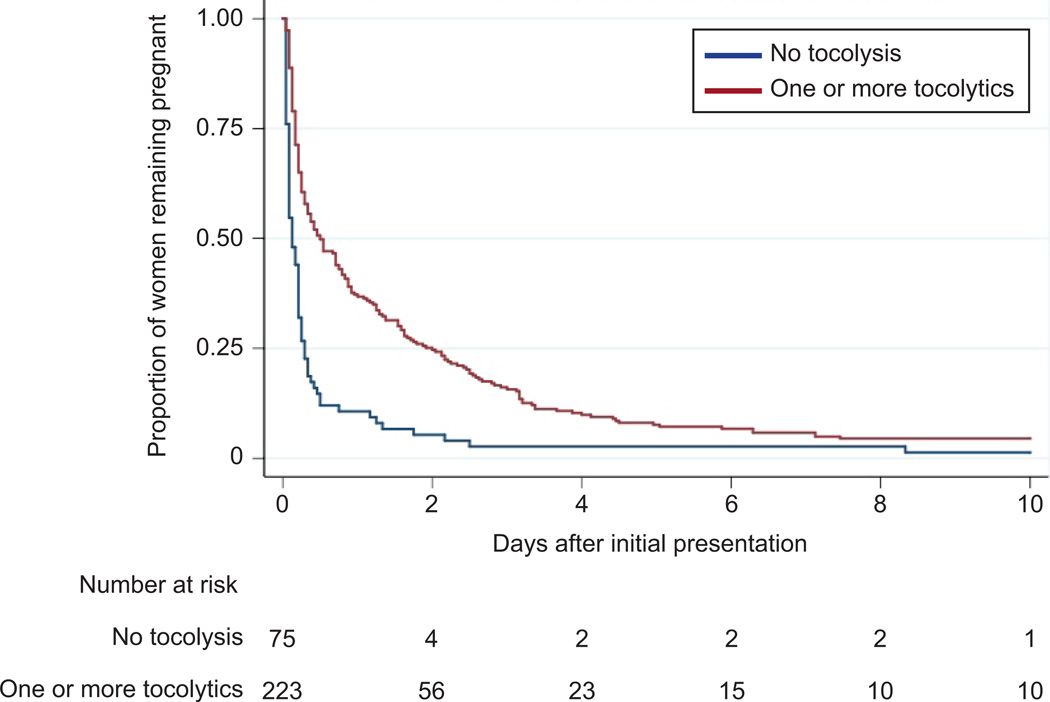
Initial perinatal outcomes were similar and did not appear to vary by tocolytic administration or specific regimen used; the rate of composite severe neonatal morbidity did not vary with tocolytic use (Table 4). Survival analysis examining the probability of remaining pregnant following presentation and hospitalization for advanced cervical dilation demonstrated a significant initial prolongation of pregnancy when tocolysis was used, p<0.001 (Figure 3). However, a similar proportion delivered within 7 days of admission (94.8% vs. 95.3%, p>0.99).
Figure 3.
Kaplan-Meier survival curve. A significant increase in the proportion of women remaining pregnant was observed among those receiving one or more tocolytics (P<.001, log-rank test) following admission for advanced cervical dilation.
There were two stillbirths; these babies were delivered at 23.3 weeks and 24.0 weeks; both mothers had received tocolysis. No mothers were admitted to the intensive care unit and there were no maternal deaths. The rates of chorioamnionitis and isolated postpartum endometritis (in the absence of a prior diagnosis of chorioamnionitis) were similar between those who did and did not receive tocolysis (Table 4). All but 4 women ultimately delivered preterm less than 34 weeks gestation, regardless of tocolytic status. All 4 of these women who delivered in the ‘late-preterm’ period between 34 and 37 weeks gestation received tocolysis (p=0.291, Table 4).
In multivariable Poisson regression models, the only factors associated with composite major neonatal morbidity included gestational age at delivery and a diagnosis of clinical chorioamnionitis (Table 5). Notably, corticosteroid use and tocolytic administration were not associated with a statistically significant reduction in major neonatal morbidity in this cohort after controlling for confounders.
Table 5.
Multivariable Poisson regression model with relative risk for composite major neonatal morbidity.
| Variable | Unadjusted RR (95% CI) |
p-value | Adjusted RR (95% CI) |
p-value |
|---|---|---|---|---|
| Delivery gestational age, per one week increase | 0.76 (0.73 – 0.80) | <0.001 | 0.77 (0.73 – 0.80) | <0.001 |
| Clinical chorioamnionitis | 1.77 (1.36 – 2.31) | <0.001 | 1.35 (1.09 – 1.68) | 0.006 |
| Caucasian race | 1.05 (0.78 – 1.41) | 0.759 | 1.25 (0.98 – 1.58) | 0.072 |
| Neonatal transfer in first 24 hours of life | 0.38 (0.13–1.08) | 0.070 | 0.50 (0.20 – 1.24) | 0.134 |
| Cervical dilation at presentation, per 1 centimeter increase | 1.05 (0.94 – 1.19) | 0.394 | 1.07 (0.97 – 1.17) | 0.163 |
| Any tocolysis | 0.95 (0.69 – 1.31) | 0.759 | 0.90 (0.70 – 1.16) | 0.420 |
Potential confounders dropped from the final model (p>0.20) included: nulliparity, antenatal corticosteroid administration, achieving at least 48 hours of latency, and culture proven neonatal sepsis.
A second regression model examined factors associated with achieving a latency period of 48 hours or longer between admission and delivery (Table 6). Tocolytic administration was associated with an increased likelihood of pregnancy prolongation of pregnancy for at least 48 hours when adjusting for confounders, including cervical dilation at presentation (RR 2.64, 95% CI 1.05–6.62, p=0.038, Table 6). Admission gestational age was also inversely associated with pregnancy prolongation for at least 48 hours; with each additional week of gestation, the likelihood of attaining at least 48 hours of latency between admission and delivery decreased (RR 0.89, 95% CI 0.82–0.96, p=0.003, Table 6).
Table 6.
Multivariable Poisson regression model for relative risk of pregnancy prolongation for ≥48 hours after admission.
| Variable | Unadjusted RR (95% CI) |
P- value |
Adjusted RR (95% CI) |
p-value |
|---|---|---|---|---|
| Admission gestational age, per one week increase | 0.87 (0.81 – 0.95) | 0.001 | 0.89 (0.82 – 0.96) | 0.003 |
| Received tocolysis | 3.02 (1.26 – 7.24) | 0.013 | 2.64 (1.05 – 6.62) | 0.038 |
| Caucasian race | 0.66 (0.42 – 1.04) | 0.070 | 0.68 (0.43 – 1.08) | 0.102 |
| Cervical dilation at presentation | ||||
| 4 centimeters | referent | referent | ||
| 5 centimeters | 0.65 (0.37 – 1.15) | 0.137 | 0.61 (0.35 – 1.06) | 0.081 |
| 6 centimeters | 0.42 (0.19 – 0.95) | 0.037 | 0.48 (0.22 – 1.05) | 0.066 |
| 7 centimeters | 0.59 (0.27 – 1.30) | 0.191 | 0.74 (0.35 – 1.59) | 0.445 |
Potential confounders dropped from the final model (p>0.20) included: maternal body mass index, nulliparity, and chorioamnionitis.
Of 164 women who received magnesium sulfate, 17 (10.4%) were given the drug primarily for the purposes of neuroprotection. The prescriber’s intent (tocolysis vs. neuroprotection) could not be ascertained by chart review in an additional 3 cases. When considering all 20 women who potentially received magnesium primarily for neuroprotection, 13 received at least one additional medication for the purposes of tocolysis. Changing the classification of the 7 women receiving only magnesium sulfate (with unknown intent) into the no tocolysis group did not alter the results (data not shown). Results also did not change if these individuals were excluded from analysis (data not shown).
Lastly, to evaluate for a time trend in outcomes, we divided the study cohort into two based on the calender years of patient presentation, 2000–2005 or 2006–2011. Outcomes in these two sub-groups were similar to outcomes in the whole cohort (data not shown).
Discussion
Among women with spontaneous preterm labor between 23 and 32 weeks of gestation and advanced cervical dilation, women who received tocolysis were more likely to achieve at least 48 hours of latency between admission and delivery. By 7 days after admission, more than 90% of women had delivered, and no differences were seen among those with and without tocolysis. Additionally, we did not appreciate an improvement in initial neonatal outcomes among women who received tocolysis.
This study was limited by the retrospective design. We suspect that the number of women who had indications for immediate delivery precluding tocolysis is low, given the proportion of operative deliveries was similar between those who did and did not receive tocolysis. Also, our findings may reflect the specific obstetric interventions employed at our institutions and the patient population studied, and outcomes may be different in other clinical settings and populations. In post-hoc power calculations, we had 58% power to detect a one-third reduction in major neonatal morbidity from 43.8% in the no-tocolysis group (2-sided test, alpha = 0.05).
We included a relatively large cohort of women with advanced preterm labor at early gestational ages. By limiting the study to those with advanced preterm labor, the risk of imminent and early delivery was high: 80% delivered within 48 hours of presentation and 95% delivered within 1 week; none delivered at term. With rare exception, medically necessary maternal (and fetal) transports for higher levels of care are made within our healthcare system; this allowed us to consider the vaginal exam at the time of initial presentation, regardless of where women initially presented.
Our results are similar to other limited, smaller reports. Previous reports of tocolysis for advanced preterm labor have included women with less advanced cervical dilation (3cm) and later gestational ages.21–23 Prior studies also demonstrated some efficacy in aggressive tocolysis among women with advanced cervical dilation. One recent secondary analysis of a randomized clinical trial examined 92 women presenting between 4–6 cm dilated at 24–32 weeks gestation and found, similar to our study, no differences in pregnancy prolongation by tocolytic regimen (indomethacin, magnesium, or nifedipine).24
Women receiving ≥1 tocolytic medication were more likely to remain pregnant for ≥48 hours after presentation. This increased latency period between admission and delivery increased the likelihood of antenatal corticosteroids administration. Although this prolongation was not accompanied by improvement in initial neonatal outcomes, previous studies have demonstrated the beneficial neonatal effects associated with antenatal corticosteroid administration. Although women who received tocolysis were more likely to achieve 48 hours of latency, only 60 neonates (55 with tocolysis, 5 without tocolysis) achieved ≥48 hours of latency. Thus, considering that the majority of neonates delivered to women receiving tocolysis failed to achieve 48 hours of latency, it is not unexpected that overall major neonatal morbidity or individual respiratory outcomes were similar between groups.
The rate of respiratory distress syndrome was high in this population and although statistically higher among those receiving tocolysis, we believe this was not clinically significant, given similar rates of bronchopulmonary dysplasia, need for intubation, and other neonatal morbidities. For those neonates delivered within 48 hours of antenatal steroid administration, the benefit of steroid administration is likely more difficult to quantify. Alternatively, the benefit may be more apparent in long-term childhood outcomes, which were unavailable. Additionally, prolongation of the very preterm pregnancy in the setting of advanced cervical dilation even for a few hours provides the benefit of allowing maternal transport. Birth in a hospital with neonatal intensive care units has been associated with improved neonatal outcomes compared to post-natal transport.25–27 There was no apparent short-term maternal or fetal harm noted in association with tocolytic administration. As previously discussed, the incidence of chorioamnionitis was high in this group, and was unrelated to tocolysis use.
In conclusion, use of indomethacin, magnesium sulfate, or nifedipine tocolysis among women presenting with preterm labor and advanced cervical dilation <32 weeks gestation may allow for maternal transfer and improve the likelihood of delaying delivery for at least 48 hours, without apparent adverse effects.
Acknowledgments
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development 5K23HD067224 (Dr. Manuck).
Source(s) of the work: University of Utah Department of Obstetrics and Gynecology and Intermountain Healthcare Department of Maternal Fetal Medicine.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2009. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 3.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525. [PubMed] [Google Scholar]
- 4.Robertson B. Corticosteroids and surfactant for prevention of neonatal RDS. Ann Med. 1993;25(3):285–288. doi: 10.3109/07853899309147876. [DOI] [PubMed] [Google Scholar]
- 5.Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990;97(1):11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 6.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 7.ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1308–1317. doi: 10.1097/AOG.0b013e31825af2f0. [DOI] [PubMed] [Google Scholar]
- 8.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 9.Flenady V, Wojcieszek AM, Papatsonis DN, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database Syst Rev. 2014;6:CD002255. doi: 10.1002/14651858.CD002255.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane database of systematic reviews. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Haas DM, Imperiale TF, Kirkpatrick PR, Klein RW, Zollinger TW, Golichowski AM. Tocolytic therapy: a meta-analysis and decision analysis. Obstet Gynecol. 2009;113(3):585–594. doi: 10.1097/AOG.0b013e318199924a. [DOI] [PubMed] [Google Scholar]
- 12.Klauser CK, Briery CM, Keiser SD, Martin RW, Kosek MA, Morrison JC. Effect of antenatal tocolysis on neonatal outcomes. J Matern Fetal Neonatal Med. 2012;25(12):2778–2781. doi: 10.3109/14767058.2012.714819. [DOI] [PubMed] [Google Scholar]
- 13.King J, Flenady V, Cole S, Thornton S. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2005;(2):CD001992. doi: 10.1002/14651858.CD001992.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. Bmj. 2012;345:e6226. doi: 10.1136/bmj.e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abramovici A, Cantu J, Jenkins SM. Tocolytic therapy for acute preterm labor. Obstet Gynecol Clin North Am. 2012;39(1):77–87. doi: 10.1016/j.ogc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Crowther CA, Brown J, McKinlay CJ, Middleton P. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2014;8:CD001060. doi: 10.1002/14651858.CD001060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkman ND, Thorp JM, Jr, Lohr KN, et al. Tocolytic treatment for the management of preterm labor: a review of the evidence. Am J Obstet Gynecol. 2003;188(6):1648–1659. doi: 10.1067/mob.2003.356. [DOI] [PubMed] [Google Scholar]
- 19.Vogel JP, Nardin JM, Dowswell T, West HM, Oladapo OT. Combination of tocolytic agents for inhibiting preterm labour. Cochrane Database Syst Rev. 2014;7:CD006169. doi: 10.1002/14651858.CD006169.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Psomiadis N, Goldkrand J. Efficacy of aggressive tocolysis for preterm labor with advanced cervical dilatation. J Matern Fetal Neonatal Med. 2005;18(1):47–52. doi: 10.1080/14767050500073142. [DOI] [PubMed] [Google Scholar]
- 22.de Veciana M, Porto M, Major CA, Barke JI. Tocolysis in advanced preterm labor: impact on neonatal outcome. Am J Perinatol. 1995;12(4):294–298. doi: 10.1055/s-2007-994478. [DOI] [PubMed] [Google Scholar]
- 23.Amon E, Midkiff C, Winn H, Holcomb W, Shumway J, Artal R. Tocolysis with advanced cervical dilatation. Obstet Gynecol. 2000;95(3):358–362. doi: 10.1016/s0029-7844(99)00570-0. [DOI] [PubMed] [Google Scholar]
- 24.Klauser CK, Briery CM, Tucker AR, et al. Tocolysis in women with advanced preterm labor: a secondary analysis of a randomized clinical trial. J Matern Fetal Neonatal Med. 2015:1–5. doi: 10.3109/14767058.2015.1018171. [DOI] [PubMed] [Google Scholar]
- 25.Nasr A, Langer JC, Canadian Pediatric Surgery N. Influence of location of delivery on outcome in neonates with congenital diaphragmatic hernia. J Pediatr Surg. 2011;46(5):814–816. doi: 10.1016/j.jpedsurg.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Hohlagschwandtner M, Husslein P, Klebermass K, Weninger M, Nardi A, Langer M. Perinatal mortality and morbidity. Comparison between maternal transport, neonatal transport and inpatient antenatal treatment. Arch Gynecol Obstet. 2001;265(3):113–118. doi: 10.1007/s004040100197. [DOI] [PubMed] [Google Scholar]
- 27.Towers CV, Bonebrake R, Padilla G, Rumney P. The effect of transport on the rate of severe intraventricular hemorrhage in very low birth weight infants. Obstet Gynecol. 2000;95(2):291–295. doi: 10.1016/s0029-7844(99)00528-1. [DOI] [PubMed] [Google Scholar]