Abstract
Objective
Although immune responses drive the pathogenesis of atherosclerosis, mechanisms that control antigen-presenting cell (APC)-mediated immune activation in atherosclerosis remain elusive. We here investigated the function of hypoxia-inducible factor (HIF)-1α in antigen presenting cells in atherosclerosis.
Approach and Results
We found upregulated HIF1α expression in CD11c+ APCs within atherosclerotic plaques of low-density lipoprotein receptor-deficient (Ldlr−/−) mice. Conditional deletion of Hif1a in CD11c+ APCs in high fat diet-fed Ldlr−/− mice accelerated atherosclerotic plaque formation and increased lesional T cell infiltrates, revealing a protective role of this transcription factor. HIF1α directly controls Stat3 transcription, and a reduced STAT3 expression was found in HIF1α-deficient APCs and aortic tissue, together with an upregulated IL-12 expression and expansion of Th1 cells. Overexpression of STAT3 in Hif1a-deficient APCs in bone marrow reversed enhanced atherosclerotic lesion formation and reduced Th1 cell-expansion in chimeric Ldlr−/− mice. Notably, deletion of Hif1a in LysM+ bone marrow cells in Ldlr−/− mice did not affect lesion formation or T cell activation. In human atherosclerotic lesions, HIF1α, STAT3 and IL-12 protein were found to co-localize with APCs.
Conclusions
Our findings identify HIF1α to antagonize APC-activation and Th1-polarization during atherogenesis in Ldlr−/− mice, and to attenuate the progression of atherosclerosis. These data substantiate the critical role of APCs in controlling immune mechanisms that drive atherosclerotic lesion development.
Keywords: atherosclerosis, inflammation, immune cells, leukocytes
Introduction
Atherosclerosis is a chronic and systemic inflammatory disease characterized by the accumulation of immune cells in the vessel wall.1, 2 Dendritic cells (DCs) localize to the intima and adventitia in healthy arteries in regions predisposed to atherosclerosis and accumulate in atherosclerotic lesions.3, 4 DCs are increasingly regarded to play important roles in immune mechanisms governing atherogenesis.4–6 Both local and systemic adaptive immune responses control atherogenesis, and pro- and anti-atherogenic CD4+ T helper cell subsets and their cytokines have been defined7. In particular, CD4+ type 1 T helper cells (Th1) and their cytokine IFN-γ promote atherosclerosis, whereas regulatory T cells (Tregs) inhibit vascular inflammation.7,8 The function of the Th17 subtype is still unclear, as contradicting reports have been published.7
The Hypoxia-inducible factor (HIF)-1α is among the primary transcription factors induced under hypoxic conditions, but can also be upregulated by inflammatory stimuli, such as oxLDL and TNFα in normoxia.9 In addition to regulating cell responses to hypoxia, e.g. glycolysis, and angiogenesis, HIF1α was identified to modulate adaptive and innate immune responses.10
Due to the high metabolic activity of inflammatory cells within lesions, and the reduced availability of oxygen in deeper plaque areas, atherosclerotic lesions harbor areas of hypoxia11, and HIF-1α can be detected in atherosclerotic lesions in both mice and humans.11–14 The direct cell-specific role of HIF1α in atherosclerosis in vivo, however, has not been addressed previously.
We here investigated the function of HIF1α in atherosclerosis in antigen-presenting cells (APCs). By deleting Hif1a specifically in CD11c+ cells, we here for the first time reveal a critical role of HIF1α in balancing APC-driven Th1-polarization during atherogenesis in Ldlr−/− mice, and to attenuate the progression of atherosclerosis in low-density lipoprotein receptor-deficient (Ldlr−/−) mice.
Materials and Methods
Materials and Methods are available online.
Results
HIF1α expression in atherosclerosis
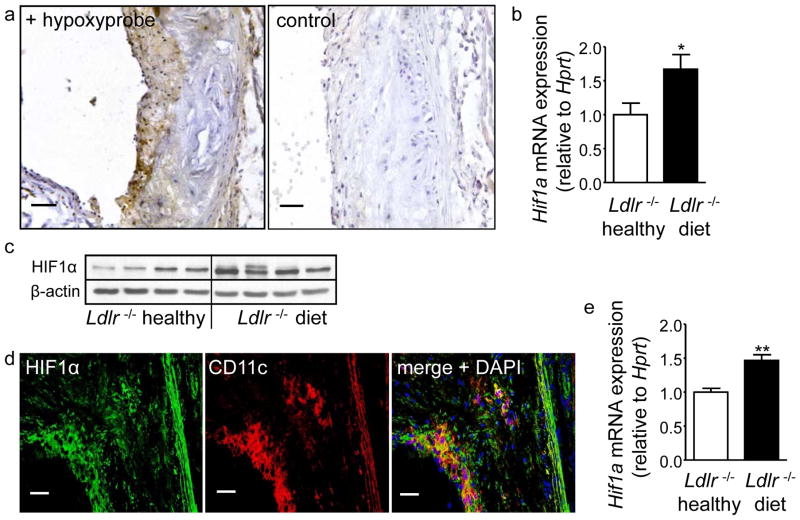
Hypoxic areas, as well as Hif1a expression have previously been demonstrated in human and murine atherosclerotic lesions.11, 14 Consistent with this, we detected hypoxic regions in atherosclerotic plaques in aortic roots of Ldlr−/− mice fed a HFD diet for 8 weeks (Figure 1a) but not in healthy 6 week old Ldlr−/− mice on normal chow by Hypoxyprobe staining (not shown); no staining was seen in Ldlr−/− mice without Hypoxyprobe performed as a negative control (Figure 1a). Likewise, Hif1a mRNA and protein expression were significantly upregulated in aortae of atherosclerotic Ldlr−/− mice compared to healthy Ldlr−/− controls (Figure 1b,c). Double immunofluorescence staining revealed abundant expression of HIF1α protein within lesions (Figure 1d), and the majority of CD11c+ cells showed co-localization with HIF1α (Figure 1d), indicating HIF1α expression in lesional APCs. Moreover, increased expression of Hif1α mRNA was observed in splenic APCs from atherosclerotic Ldlr−/− mice (Figure 1e), indicating systemic upregulation of HIF1α in addition to localized effects in aortic lesions.
Figure 1. Hypoxia and expression of HIF-1α in CD11c+ cells in atherosclerosis.
(a) Sections of the aortic root of Ldlr−/− mice after 8 weeks of HFD were stained with Hypoxyprobe (brown, scale bars: 50μm) to detect hypoxia. (b) Analyses of Hif1a mRNA expression by qPCR in aortic tissue of chow-fed Ldlr−/− mice and Ldlr−/− mice fed a HFD for 8 weeks, normalized to Hprt and relative to Ldlr −/− healthy mice (n=8 each). *p<0.05. (c) Analysis of HIF-1α protein expression by Western blot in aortic tissue of chow-fed Ldlr−/− mice and Ldlr−/− mice fed a HFD for 8 weeks. β-actin serves as a loading control. (d) Double-immunofluorescence staining of HIF1α (green) and CD11c (red) in the atherosclerotic aortic root plaque of a Ldlr−/− mouse fed a HFD for 8 weeks. Cell nuclei were counterstained with DAPI (blue) (scale bars: 50μm). (e) Analyses of Hif1a mRNA expression by qPCR in splenic APCs isolated from healthy chow-fed Ldlr−/− mice and Ldlr−/− mice fed a HFD for 8 weeks, normalized to Hprt and relative to Ldlr−/− healthy mice (n=4 each). **p<0.01.
Targeted deletion of Hif1a in CD11c+ APCs accelerates atherosclerotic lesion formation
To address the function of HIF1α in APCs, mice with a CD11c-specific deletion of Hif1a were generated, as confirmed by a marked deletion of Hif1a DNA (~80%) in isolated CD11c-cre+ Hif1aflox/floxLdlr−/− (Hif1a-CKO Ldlr−/−) compared with CD11c-cre+Hif1a+/+Ldlr−/− (Hif1a-WT Ldlr−/−) APCs. In CD4+ T cells only a marginal reduction was observed (~5%) (Supplementary Figure Ia). Likewise, Hif1a mRNA expression was reduced in isolated Hif1a-CKO Ldlr−/− APCs and Hif1a-CKO BMDCs15 compared to controls (Supplementary Figure Ib,c).
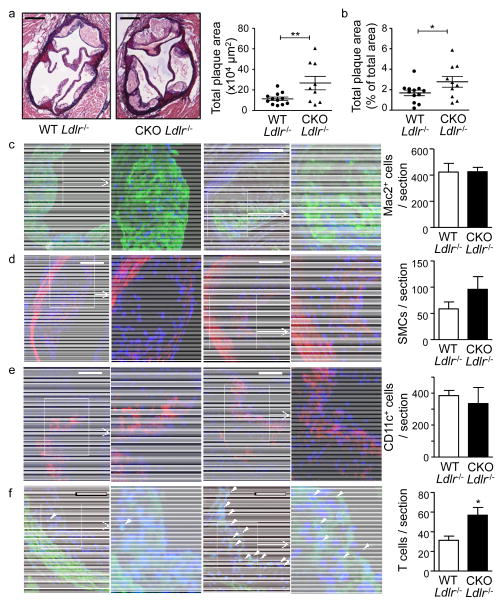
To study atherosclerotic lesion formation15, Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice were placed on a HFD for 8 weeks. Body weight (28.9±0.7 vs. 30.3±1.3 g), serum total cholesterol (12.7±1.0 vs. 12.9±1.8 × 1000 μg/ml) and triglyceride levels (6.1±0.2 vs. 5.6±0.1 mmol/L) did not differ between Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice. We observed a 2.3-fold increase in atherosclerotic plaque growth in the aortic root and a 1.6-fold increase in the aorta of Hif1a-CKO Ldlr−/− compared to Hif1a-WT Ldlr−/− mice (Figure 2a,b). Plaque cell density was unaltered between groups (4420±241.6 vs. 3763±382.6 cells/mm2 plaque area in Hif1a-CKO Ldlr−/− vs. Hif1a-WT Ldlr−/− mice, n.s.), and no differences in plaque Mac-2+ macrophages, SMC numbers or CD11c+ APCs were detected (Figure 2c–e). A marked increase in relative necrotic core area was observed in plaques of Hif1a-CKO Ldlr−/− mice versus Hif1a-WT Ldlr−/− mice (33.0±2.9 vs. 22.2±1.7% plaque area, p=0.0029), in line with a more advanced plaque phenotype. Notably, a 1.8-fold increase in numbers of CD3+ T cells was detected in lesions of Hif1a-CKO Ldlr−/− mice (Figure 2f), indicating that an enhanced accumulation of T cells within lesions was associated with an accelerated plaque growth due to deficiency of HIF1α in APCs.
Figure 2. Deficiency of HIF1α in CD11c+ APCs accelerates atherosclerotic plaque growth.
(a–b) Quantification of plaque area in Aldehyde Fuchsin-stained aortic roots in atherosclerotic Hif1a-WT Ldlr−/− (n=12) and Hif1a-CKO Ldlr−/− (n=9) mice (a) and Oil-Red-O stained aortae (b) of atherosclerotic Hif1a-WT Ldlr−/− (n=12) and Hif1a-CKO Ldlr−/− (n=10) mice fed a HFD for 8 weeks; representative sections of the aortic root are shown (scale bars: 250μm). Quantification of the number of Mac-2+ macrophages (green, c), α-smooth muscle actin+ smooth muscle cells (red, d), CD11c+ APCs (red, e), and CD3+ T cells (green, f); representative images of immunofluorescence staining and higher magnification images of boxed regions are shown; scale bars: 100μm; cell nuclei were counterstained with DAPI (blue); arrow heads indicate T cells. *p<0.05, **p<0.01.
HIF1a-deficient APCs promote T cell activation and Th1 polarization in atherosclerosis
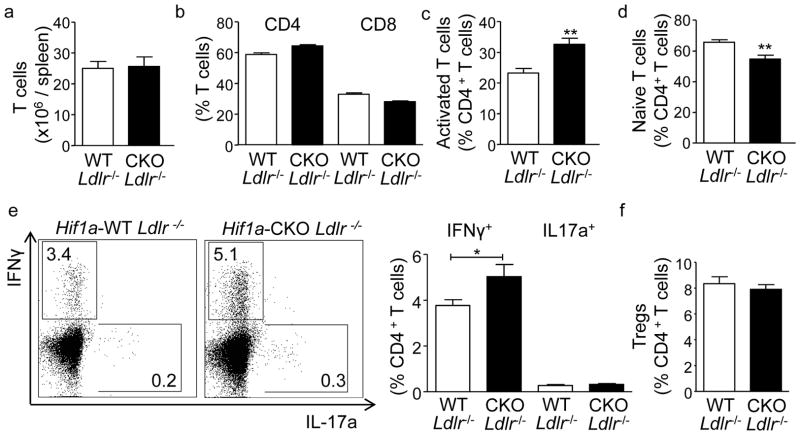
T cell activation and differentiation is governed by APCs, and drives atherosclerotic lesion development.4, 6, 15 When analyzing T cells in LNs, blood and spleen of atherosclerotic Hif1a-CKO Ldlr−/− compared to Hif1a-WT Ldlr−/− mice, increased frequencies of activated (CD44highCD62Llow) CD4+ T cells were observed in these organs. This was associated with an expansion of IFNγ+ Th1 cells in Hif1a-CKO Ldlr−/− mice. In contrast, naïve (CD44lowCD62Lhigh) CD4+ T cell frequencies were decreased, and IL-17+ Th17 or Foxp3+CD25+ regulatory CD4+ T cells showed no alterations (Figure 3c–f, Supplementary Figure II). No changes in CD3+ T cell numbers, the ratio of CD4+ and CD8+ T cells and organ weight were observed (Figure 3a,b, Supplementary Figure II). Furthermore, there were no differences in CD115+ monocytes or Gr1high and Gr1low monocyte subsets, CD11b+Gr1+CD115− neutrophils, CD11c+MHC-II+ APCs or CD19+ B cells in LNs, spleens and blood between groups (data not shown).
Figure 3. Ldlr−/− mice deficient in HIF1α in APCs display enhanced T cell activation.
(a–f) Flow cytometric analyses of T cell distributions in spleens from atherosclerotic Hif1a-WT Ldlr−/− (n=10) and Hif1a-CKO Ldlr−/− (n=7) mice fed a HFD for 8 weeks. Numbers of CD3+ T cells (a), frequencies of CD4+ and CD8+ T cells among CD3+ T cells (b), frequencies of activated CD44highCD62Llow (c) and naïve CD62LhighCD44low CD4+ T cells (d), IFNγ+CD4+ T cells, IL-17a+CD4+ T cells (e), and FoxP3+CD25+CD4+ Tregs (f). *p<0.05, **p<0.01 Representative dot plots showing intracellular IFNγ versus IL-17a expression are shown; values indicate gated events among CD4+ T cells.
Importantly, no changes in T cell distribution and phenotype, numbers of CD11c+ MHCII+ APCs, neutrophils, monocytes, and B cells were observed in 6 week old healthy Hif1a-CKO versus Hif1a-WT mice on normal chow (Supplementary Figure III). These data suggest that, HIF1α activation in APCs plays a crucial role in restraining T cell activation and Th1 cell differentiation under inflammatory conditions in atherosclerosis, while being dispensable under homeostatic conditions. Notably, an increased percentage of IFNγ+ CD4 T cells was also evidenced in LNs and spleens of Hif1a-CKO compared to Hif1a-WT mice after immunization with OVA protein as an artificial model antigen (Supplementary Figure IV), corroborating an important role of HIF1α in controlling APC-driven Th1 T cell polarization also under systemic inflammatory conditions unrelated to atherosclerosis.
Macrophages and DCs share phenotypic features.16 In particular, CD11c is expressed by both DCs and some macrophage subsets. To gain insight into the potential role of HIF1α in macrophages versus DCs among total APCs, atherosclerotic lesion formation was also assessed in Ldlr−/− mice reconstituted with bone marrow of LysM-cre+ Hif1aflox/flox mice.17 Efficient deletion of Hif1a mRNA expression (~70–80%) was confirmed in bone-marrow derived LysM-cre+ Hif1aflox/flox macrophages under normoxic and hypoxic conditions (Supplementary Figure Va). After 6 weeks of HFD, no differences in serum cholesterol, plaque size, and cellular plaque composition were noted. Moreover, no alterations in T cell distributions and activation were observed in spleens or LNs (Supplementary Figure Vb,c, Supplementary Table I). These data indicate that accelerated lesion formation in mice deficient in HIF1α in CD11c+ cells most likely originates from a defect in antigen-presenting and immune stimulatory functions.
An alternative approach furthermore supports the importance of HIF1α in APCs, as untreated and TNFα-stimulated bone marrow derived macrophages18 from Hif1a-CKO and Hif1a-WT mice did not display any consistent differences in pro-inflammatory Il12, Nos2, or anti-inflammatory Mrc1 and Igf1 mRNA expression (Supplementary Figure Vd).
HIF1α controls inflammatory IL-12 expression in APCs by regulating STAT3 expression
The migration of APCs is essential for efficient T cell activation and controlled by CCR7.19 However, unchanged Ccr7 expression in Hif1a-KO BMDCs or APCs from atherosclerotic Hif1a-CKO Ldlr−/− mice and BMDC migration towards CCL19 (Supplemental Figure VIa–c) point towards effects of HIF1α unrelated to CCR7-driven APC migration.
T cell activation and T helper cell polarization are shaped by co-stimulatory molecule engagement and exposure to a specific cytokine milieu, with Th1 cells critically depending on IL-12 secretion from DCs.20, 21 No significant changes in mRNA or surface protein expression of MHC-II, CD80 and CD86 were noted in TNF-α matured Hif1a-CKO versus Hif1a-WT BMDCs, as assessed by qPCR and flow cytometry (Supplementary Figure VIIa,c).However, a significant increase in the mRNA expression of Il12 together with elevated IL-12 protein levels in supernatants of Hif1a-CKO BMDCs were observed, whereas Il4, Il6, Il10, Tgfb or Tnfa were unaltered (Supplementary Figure VIIIa,b).
HIF1α has been shown to induce and to synergistically act with nuclear factor-κB (NF-κB).10 However, Nfkb1/p105 and Rela/p65 transcript or protein expression (Supplementary Figure IXa, and data not shown) were similar in mature Hif1a-WT versus Hif1a-CKO BMDC.
STAT3 has been shown to inhibit IL-12 cytokine production in DCs.22, 23 Notably, a significant reduction in Stat3 mRNA and protein expression could be detected in mature Hif1a-CKO versus Hif1a-WT BMDCs, as assessed by qPCR and flow cytometry (Supplementary Figure VIIIc,d), suggesting that HIF1α-dependent changes in STAT3 expression may regulate IL-12 production. Indeed, overexpression of STAT3 in BMDCs decreased Il12 mRNA, whereas overexpression of a dominant negative form of STAT3 (mutant in the DNA-binding domain24) enhanced Il12 expression independently of HIF1α, as similarly observed in both Hif1a-WT and Hif1a-CKO BMDCs (Supplementary Figure VIIIe). Most importantly, ChIP assays demonstrated that HIF1α directly interacts with predicted binding sites within the Stat3 promoter (Supplementary Figure VIIIf).
HIF1α controls inflammatory T cell responses in atherosclerosis
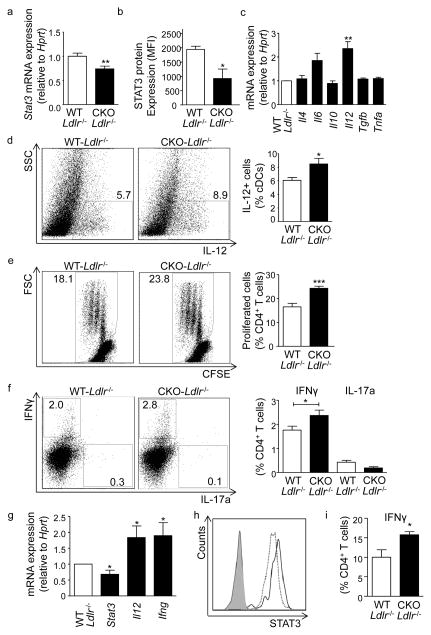
Importantly, HIF1α also functions to promote STAT3 expression in APCs in atherosclerosis in vivo, as witnessed by a significant reduction in both Stat3 transcript and protein levels in splenic APCs from atherosclerotic Hif1a-CKO Ldlr−/− versus Hif1a-WT Ldlr−/− mice (Figure 4a,b). Further recapitulating findings in vitro, no significant changes in Cd74, Cd80, Cd86 and Nfkb mRNA expression were noted (Supplementary Figure VIIb, IXb), but a significant increase in Il12 mRNA and IL-12 protein expression were observed in these APCs (Figure 4c,d).
Figure 4. Phenotype and functions of APCs from atherosclerotic mice deficient in HIF1α.
(a) Stat3 mRNA (n=14 mice each) and (b) intracellular protein expression (n=5 mice each), and (c) mRNA expression of indicated cytokines (3 independent experiments, n=3–4 mice per experiment) in APCs isolated from spleens of Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice fed a HFD for 8 weeks, analyzed by qPCR and flow cytometry. mRNA expression was normalized to Hprt and presented relative to WT controls. (d) Percent of IL-12+ cells among the APC population, analyzed by flow cytometry (n=6). Representative dot plots are shown (values indicate gated events among APCs). (e,f) APCs isolated from spleens of Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice fed a HFD for 8 weeks and pulsed with OVA323-339 peptide were co-cultured with naive CD4+ OT-II T cells for 3 days. T cell proliferation was analyzed by CFSE dilution (e) and polarization by intracellular staining for IFNγ and IL-17a (f). Quantification and representative dot plots are shown (values indicate gated events among CD4+ T cells, 3 independent experiments, n=3–5 mice per experiment). (g) mRNA expression of Stat3, Il12 and Ifng in whole aortae of Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice fed a HFD for 8 weeks (normalized to Hprt and expressed relative to WT controls. n=3 mice). (h) Intracellular STAT3 protein expression in CD11c+MHCII+ APCs in the aorta of Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice fed a HFD for 8 weeks (n=5 mice per group), analyzed by flow cytometry. Representative histograms for STAT3 fluorescence are shown (solid line - Hif1a-WT, dotted line - Hif1a-CKO, filled dark grey line - Hif1a-WT fluorescence minus one control (FMO), filled faint grey line Hif1a-CKO FMO). (i) Frequencies of IFNγ+CD4+ T cells in the aorta of Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice fed a HFD for 8 weeks (n=3-4 per group).*p<0.05, **p<0.01, ***p<0.001.
We further assessed the propensity of splenic APCs isolated from atherosclerotic Hif1a-WT Ldlr−/− and Hif1a-CKO Ldlr−/− mice to antigen-specifically activate OT-II T cells that express a T cell receptor specific for the model antigen OVA. While APCs of either genotype did not trigger noticeable activation of CFSE-labeled naïve CD4+ OT-II T cells in the absence of cognate antigen (not shown), significantly increased rates of T cell proliferation and an expansion in IFNγ-producing T cells were observed in co-cultures with OVA-loaded Hif1a-CKO Ldlr−/− compared to Hif1a-WT Ldlr−/− APCs (Figure 4e,f). In contrast, no alterations in IL-17+ Th17 and Foxp3+CD25+ regulatory CD4+ T cells were detected (Figure 4f, and data not shown), congruent with the T cell phenotype observed in atherosclerotic Hif1α-CKO Ldlr−/− mice. These data clearly indicate that APC-intrinsic deficiency in HIF1α promotes antigen-specific Th1-polarization. In line with known functions of IL-12 in T cell activation25, the presence of IL-12 blocking antibody significantly reduced IFNγ-producing T cell frequencies in co-cultures with Hif1a-CKO Ldlr−/− APCs (not shown).
Notably, a significant decrease in Stat3 mRNA expression was observed in aortic tissue and in STAT3 protein levels in lesional APCs of atherosclerotic Hif1a-CKO Ldlr−/− mice, associated with a significant increase in Il12 and Ifng transcript expression (Figure 4g,h). Furthermore, an increased frequency of IFNγ-producing T cells among CD4+ T cells was observed in the aorta of Hif1a-CKO Ldlr−/− mice (Figure 4i), suggesting that HIF1α may also control APC functions within lesions.
HIF1α-deficient APCs promote atherosclerosis due to reduced STAT3 expression
To further confirm that Hif1a-CKO APCs promote atherosclerosis in a STAT3-dependent manner, we used a Cre-dependent system for STAT3 expression (pLB2-Ubi-FLIP26). We generated a vector in which Stat3 cDNA was cloned in the reverse orientation and flanked by inverted loxP sequences, such that Cre-induced recombination irreversibly flipped Stat3 to a sense orientation, resulting in expression of STAT3 under the ubiquitin promoter in all Cre-expressing cells. The specificity of the Cre system was validated in vitro (Supplementary Figure Xa). Transduction of BM cells from Cd11c-cre+ mice with lentivirus27 carrying the pLB2-Ubi-FLIP-STAT3 vector, but not an empty control vector, confirmed significantly elevated Stat3 mRNA expression in differentiated BMDCs after 7 days (Supplementary Figure Xb).
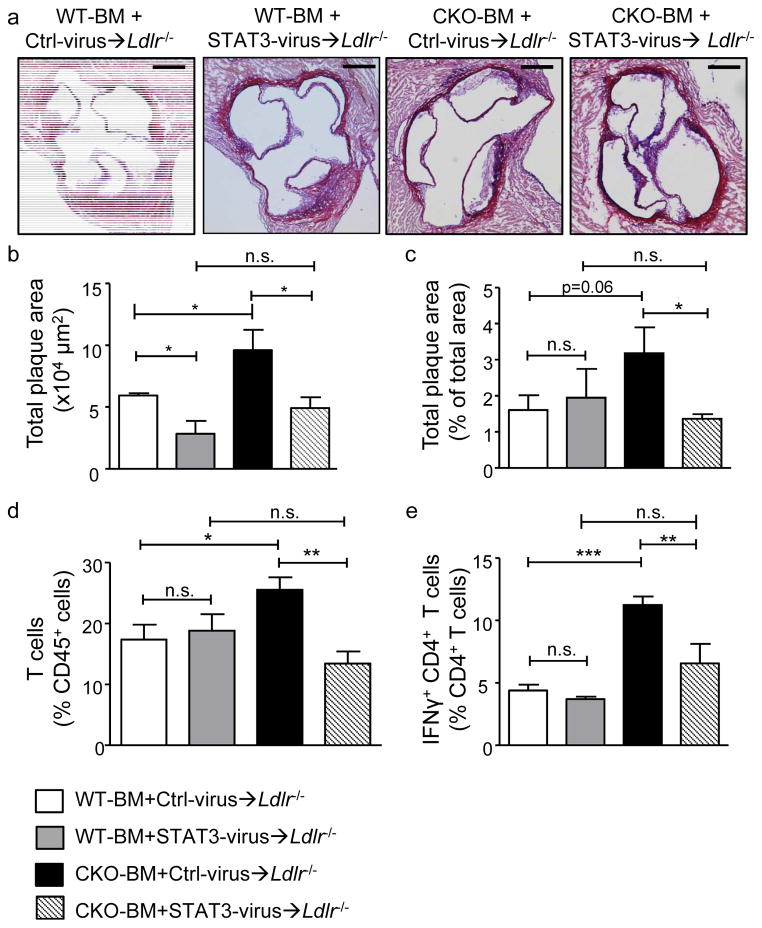
Hif1a-WT and Hif1a-CKO BM cells were transduced with lentivirus containing control or pLB2-Ubi-FLIP-STAT3 vector and transplanted into lethally irradiated Ldlr−/− mice. Notably, increased lesion formation in Ldlr−/− mice carrying control virus-transduced Hif1a-CKO BM (CKO-BM+Ctrl-virus→Ldlr−/−) versus Hif1a-WT BM (WT-BM+Ctrl-virus→Ldlr−/−) in the aortic root and aorta was completely prevented by transduction with the pLB2-Ubi-FLIP-STAT3 vector (CKO-BM+STAT3-virus→Ldlr−/−) after 4 weeks of HFD, and similar to levels seen in WT-BM+STAT3-virus→Ldlr−/− mice (Figure 5a–c). Moreover, this was paralleled by a reduction of the elevated total numbers of T cells in the aorta, and an abrogation of increased frequencies of IFNγ+ CD4+ T cells in spleens of these mice (Figure 5d, e), clearly indicating that diminished STAT3 entails pro-atherogenic effects of Hif1α-deficiency in APCs. WT-BM+Stat3-virus→Ldlr−/− mice displayed a reduction in atherosclerotic lesion formation in the aortic root but not in the aorta, no changes in aortic T cell frequencies and a small trend towards decreased Th1 cell-responses in the spleen when compared to WT-BM+Ctrl-virus→Ldlr−/− mice (Figure 5a,b), suggesting that prevailing actions of natural HIFα on STAT3 expression in WT APCs dampen effects of an additional overexpression in atherosclerosis. Splenic APC isolated from WT-BM+STAT3-virus→Ldlr−/− and CKO-BM+STAT3-virus→Ldlr−/− mice displayed an enhanced expression of Stat3 when compared to WT-BM+Ctrl-virus→Ldlr−/− or CKO-BM+Ctrl-virus→Ldlr−/− mice (Supplementary Figure Xc), confirming overexpression of Stat3 in APCs in vivo.
Figure 5. APC-intrinsic effects of HIF1α on plaque development are STAT3-mediated.
(a,b) Analysis of total plaque area in Aldehyde-Fuchsin-stained aortic roots, (c) relative plaque area in Oil-Red-O stained aortae, (d) CD3+ T cells, and (e) IFNγ+CD4+ T cells in spleens of Ldlr−/− mice transplanted with Hif1a-WT BM transduced with control lentivirus (WT-BM+Ctrl-virus→Ldlr−/−) or STAT3 overexpressing lentivirus (WT-BM+STAT3-virus→Ldlr−/−), or Hif1a-CKO BM transduced with control-lentivirus (CKO-BM+Ctrl-virus→Ldlr−/−) or STAT3 overexpressing lentivirus (CKO-BM+STAT3-virus→Ldlr−/−) and fed a HFD for 4 weeks (n=5–8 per group); representative sections of aortic roots are shown (a, scale bars, 250μm). *p<0.05, **p<0.01, ***p<0.001. n.s., non significant.
Expression of HIF1α, STAT3 and IL-12 in APCs in human atherosclerotic lesions
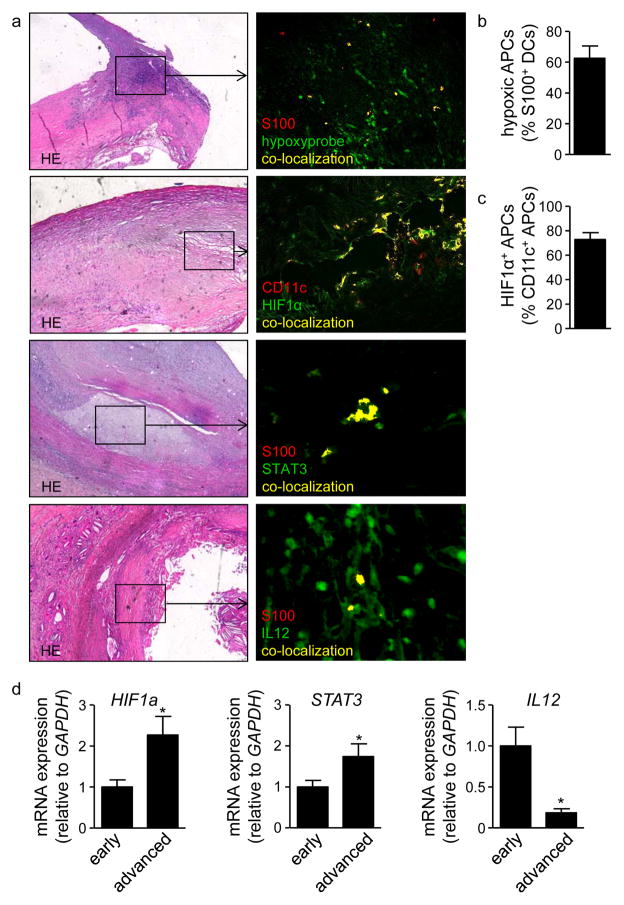
To finally assess whether these mechanisms may also be relevant to human disease, immunostaining of human atherosclerotic carotid artery plaques was performed. Similar to findings in mice and previous reports11–14, we detected hypoxia and abundant HIF1α protein expression in atherosclerotic carotid artery plaque tissue (Figure 6a). Co-staining for the APC markers S100 or CD11c revealed that the majority of APCs were hypoxic and expressed HIF1α, respectively (Figure 6a–c). We furthermore could detect both STAT3 and IL-12 protein in co-localization with S100+ APCs (Figure 6a). These data indicate that APCs express HIF1α, STAT3 and IL-12 in human atherosclerotic lesions. In addition, RT-PCR analyses of atherosclerotic plaques obtained from patients with high-grade carotid artery stenosis, histologically classified as early and advanced stages of atherosclerosis, demonstrated increased HIF-1α and STAT3 mRNA expression, but decreased IL12 transcript expression in advanced compared to early lesions (Figure 6d).
Figure 6. HIF1α, STAT3 and IL-12 in human atherosclerotic lesions.
(a) Hematoxylin and eosin stained sections, and double-immunofluorescence staining of S100 or CD11c (red) and Hypoxyprobe, HIF1α, IL-12 or STAT3 (green) in adjacent sections of advanced human atherosclerotic carotid artery plaques. (b) Quantification of Hypoxyprobe+ S100+ cells (n=10 plaques) and of (c) HIF1α+ CD11c+ cells (n=10 plaques). (d) mRNA expression of HIF1a, STAT3 and IL12 in human whole carotid artery plaques with early and advanced stages of atherosclerosis (n=10 per group). *p<0.05.
Discussion
Although T cell responses and in particular Th1-mediated immunity drive atherosclerotic lesion formation8, the pathways in APCs that control T cell activation remain largely elusive. The transcription factor HIF1α is known to modulate immune responses10 and to be found in atherosclerotic plaques.11–14 We here unveil that HIF-1α expression is upregulated in CD11c+ APCs in atherosclerotic Ldlr−/− mice. In order to assess the (patho-) physiological relevance of HIF-1α in this cell type, we used CKO mice with a deletion of Hif1a in CD11c+ APCs in Ldlr−/− mice. Importantly, an accelerated atherosclerotic lesion formation was observed in mice deficient in HIF-1α in APCs, together with an expansion of pro-inflammatory Th1 cells both locally within lesions, but also systemically, indicating that APC-expressed HIF-1α is of paramount importance in balancing uncontrolled Th1 cell responses and atherosclerosis in Ldlr−/− mice. Mechanistically, we could demonstrate that HIF1α directly binds the Stat3 promoter to control its transcription. Overexpression of Stat3 in Hif1a-deficient APCs in bone marrow reversed enhanced atherosclerotic lesion formation and reduced Th1 cell-expansion in chimeric Ldlr−/− mice. These findings offer unique insight into the regulatory function of HIF1α in APCs (Supplementary Figure XI), and substantiate the critical role of APCs in controlling immune mechanisms that drive atherogenesis.
In humans and apolipoprotein E-deficient mice, HIF1α expression was detected in atherosclerotic lesions and to increase from early to stable lesions.11,13, 14 In line, we were able to detect an upregulation of HIF1α expression in atherosclerotic aortae and aortic roots of Ldlr−/− mice when compared to healthy controls, and in advanced versus early human atherosclerotic lesions. Expressed in many cell types, HIF1α was also found to co-localize with hypoxic CD11c+ cells within lesions in both mice and humans.
Limited evidence on the role of HIF1α in atherosclerotic lesion formation exists. Mice lacking HIF1α in CD4+ T cells were previously shown to display increased T cell activation, associated with an augmented neointimal femoral artery hyperplasia after cuff placement.28 Systemic hydrodynamic injection of plasmids encoding constitutively active Hif1a into Apoe−/− mice, resulting in HIF1α overexpression predominantly in CD4+ T cells, lead to a reduction in lesion formation, associated with a shift towards an anti-inflammatory cytokine expression profile in CD4+ T cells.29 However, in contrast to these studies, which would be consistent with an induction of Foxp3 and Tregs by HIF1α30 and the demonstration of a protective function of Tregs in atherosclerosis,8 deficiency in HIF1α was more recently shown to diminish Th17 but to enhance Treg development in CD4+ T cells.31
In macrophages, HIF1α has been described to be critical for maintaining intracellular energy homeostasis, and Hif1a-deficient LysM-cre+ macrophages were shown to display normal cytokine production but an abrogated migratory capacity, preventing skin infiltration and inflammation.17 However, a reduced production of pro-inflammatory cytokines was demonstrated in Hif1a-deficient LysM-cre+ macrophages in response to LPS, together with a protection from LPS-induced sepsis.32 In the context of atherosclerosis, HIF1α was suggested to exert pro-atherogenic functions in cultured macrophages by promoting cholesterol accumulation.14 Variable effects of hypoxia-induced HIF1α expression have also been shown in DCs. For instance, a reduction in costimulatory molecule expression and of the stimulatory capacity for T-cell functions was observed in one study whereas increased expression of costimulatory molecules and an induction of allogeneic lymphocyte proliferation in response to LPS was noted in another report in vitro, whereas both studies described an up-regulated production of proinflammatory cytokines33, 34. Prior to our study, the direct in vivo role of HIF1α in APCs in atherosclerosis had not been addressed.
We here deleted Hif1a specifically in CD11c+ APCs, allowing a definite assessment of its role under physiological conditions and in atherosclerosis in vivo. APCs differentiated normally with no differences in their numbers or maturation in Hif1a-deficient mice. Moreover, no differences in APC phenotype and T cell activation were noted in young, healthy mice, indicating that HIF1α plays a subordinate role in maintaining homeostatic APC functions. In atherosclerotic Ldlr−/− mice, however, a significant increase in IL-12 was observed in Hif1a-deficient APCs, whereas other cytokines and the expression of MHC-II and co-stimulatory molecules were unaltered. Moreover, an enhanced activation of CD4+ T cells and increased frequencies of Th1 cells were observed in Hif1a-CKO Ldlr−/− versus Hif1a-WT Ldlr−/− mice in vivo in the aorta and spleen, and in co-cultures with Hif1a-deficient APCs isolated from atherosclerotic Ldlr−/− mice and loaded with OVA as a model antigen in vitro. These data suggest that Hif1a-deficiency in APCs drives T cell activation and Th1-differentiation, and that the effects of HIF1α deficiency are systemic.
Hypoxia can frequently be detected in atherosclerotic plaques. Hypoxyprobe (pimonidazole) is metabolized in living cells experiencing oxygen levels below 10mmHg (~1% O2). Cells positive for pimonidazole are thus viable and hypoxic, but do not experience a total lack of oxygen (anoxia). Both the thickness of the plaque exceeding the maximum oxygen diffusion distance, and more importantly, the high metabolic demand of cells within chronically inflamed tissue contribute to plaque hypoxia also within the oxygen-diffusion limit in symptomatic patients, rabbits and mice.11, 12, 35. In line, we detected hypoxic regions in atherosclerotic lesions of Ldlr−/− mice in luminal and intramural plaque cells. In addition to hypoxia, however, HIF1α expression can also be triggered and potentiated by oxLDL, lipopolysaccharides and pro-inflammatory cytokines9, 36, 37. Hence, HIF1α expression in hypoxic vascular APCs, known to ingest lipids and to be exposed to cytokines4, may arise from a combination of these factors. Likewise, increased HIF1α in splenic APCs may have been activated by systemically increased lipid mediators or atherogenic cytokines, possibly in combination with relative hypoxia due to higher oxygen consumption under conditions of splenic inflammation. In this regard it is interesting that similar changes in Th1-polarization were observed upon systemic immunization with OVA in otherwise healthy CKO mice, providing further evidence that HIF1α controls APC-driven T cell responses in inflammation also unrelated to atherosclerosis.
Increased atherosclerotic lesion size in Hif1a-CKO Ldlr−/− mice was accompanied by an increased necrotic core area. It was recently shown that silencing of HIF1α provokes a loss in viability with increased rates of apoptosis and necrosis in cultured human macrophages, potentiated in the presence of oxLDL or under hypoxic conditions.38 Although the potential impact of reduced monocyte/macrophage viability in atherosclerotic plaques is unclear and may depend on plaque stage, an increased apoptosis/necrosis of HIF1α-deficient APCs may have contributed to the expansion of the necrotic core in our model, warranting further investigations of this mechanism and its impact on atherogenesis in the future.
Notably, Ldlr−/− mice carrying LysM-cre+ Hif1aflox/flox bone marrow did not display any differences in plaque size or T cell activation. This may appear counterintuitive as a substantial proportion of CD11c+ APCs, e.g. monocyte-derived DCs and CD11c+ macrophages, would also lose expression of HIF1α in this model. However, HIF1α may have pro-atherogenic functions in CD11c− myeloid cell subsets that promote atherosclerosis, such as Ly6Chigh monocytes2, macrophages and neutrophils39 that contrast with its protective role in CD11c+ APCs. For instance, several reports have described a pro-inflammatory function of HIF1α in neutrophils17, 40 or macrophages.38 Hence, the loss of protective HIF1α signaling in some CD11c+ cells may have been counterbalanced by the loss of its pro-atherogenic functions in other cell types in LysM-cre+ Hif1aflox/flox mice. Alternatively, the phenotype observed in CD11c-cre+ Hif1aflox/flox mice may be preferentially related to atheroprotective functions of HIF1α in classical DCs. In the future, lineage-specific deletion of HIF1α in novel models may provide a clearer picture of its role in these various cell populations.
We did not detect any alterations in NF-κB, IKKα or IκBα expression in APCs deficient in HIF1α, similar to Hif1α-deficient LysM-cre+ macrophages,32 indicating that the deletion of Hif1a does not directly affect the NF-κB pathway per se. In agreement with the identification of binding sites in silico, ChIP analyses demonstrated direct binding of HIF1α to the Stat3 promoter, and Hif1a-deficient APCs to display a reduction in Stat3 mRNA and protein expression. These findings for the first time reveal HIF1α as an important regulator of STAT3 expression. Interestingly, STAT3 is known to exert immune-suppressive and anti-inflammatory functions in myeloid cells,41 and mice with Stat3–deficient APCs were previously shown to produce significantly more IL-12 in response to LPS, associated with an increased capacity to stimulate T cell proliferation and IFNγ secretion.22 Accordingly, overexpression of STAT3 reduced Il12 transcript levels in BMDCs, whereas a dominant negative mutant of STAT3 elevated Il12 expression, corroborating evidence that STAT3 interferes with Il12 transcription.22, 42 Importantly, these effects occurred down-stream of HIF1α, as also evidenced in Hif1a-deficient APCs. Lentiviral transduction of Hif1a-CKO BM with overexpression of STAT3 in APCs reversed the enhanced atherosclerotic lesion formation, decreased T cell infiltrates and reduced Th1-cell polarization in chimeric Ldlr−/− mice, confirming that increased levels of HIF1α and STAT3 in APCs are pivotal in controlling atherosclerotic plaque formation. In line with a clear but non-significant trend towards increased STAT3 expression in splenic APCs, marginal effects on plaque size and unaffected aortic T cell accumulation and Th1 cell responses were observed in WT-BM+Ctrl-virus→Ldlr−/− vs. WT-BM+STAT3-virus→Ldlr−/− mice. This may indicate that reduced STAT3 availability in CKO APCs rather than its additional supplementation in WT APCs that already inherently display increased HIF1α and STAT3 levels in atherosclerosis determines disease development in this setting.
Interestingly, human APCs exposed to hypoxia that display increased levels of HIF1α showed a reduced secretion of IL-1243 and induced lower T cell IFNγ-production,34, 43 suggesting an HIF1α-triggered pathway restraining Th1 responses in human APCs. Notably, extending previous findings describing the presence of hypoxia and HIF1α in human atherosclerotic lesions,11–14 we here demonstrate that APCs express HIF1α, STAT3 and IL-12 in human atherosclerotic lesions. Furthermore, an increased HIF1α and STAT3 but a decreased IL-12 mRNA expression was observed in advanced versus early carotid artery plaques. These data suggest that the regulatory signaling axis revealed in our study in APCs in mice may also be operative in human disease. Interestingly, IL-12 expression in plasma and plaque tissue was previously shown to correlate with IFN-γ expression, with T cells being the principal source of IFN-γ in the arterial wall in humans,44 in line with the notion that IL-12-controlled Th1 T cell responses are of primary importance during plaque development.
The bidirectional effects of HIF1α-deficiency in APCs on other lesional cell types and their contribution to lesion formation remains to be addressed. For instance, mast cells are present in atherosclerotic plaques and are considered to promote lesion growth and plaque destabilization.45 Mast cell-derived cytokines, via an induction of HIF1α in APCs, may have led to an attenuated pro-atherogenic APC phenotype balancing overshooting inflammation in atherosclerosis, and be in line with mast cells often ensuing Th2-type inflammatory responses.45, 46 However, pro-inflammatory mediators released by mast cells, as induced by the contact with T cells, which showed an increased activation in CKO Ldlr−/− mice, may have also contributed to enhanced inflammation45, 46 and plaque progression in our study.
Although it is widely acknowledged that Th1-mediated immune responses drive atherosclerotic lesion formation,6 still little is known about the pathways and transcription factors in APCs that drive T cell polarization in atherosclerosis. Moreover, although HIF-1α can be detected in atherosclerotic lesions in both mice and humans11–14 and can modulate immune responses,10 its cell-specific role in atherosclerosis had not been addressed previously. While dispensable under homeostatic conditions, our findings for the first time demonstrate that HIF1α balances APC activation and Th1-polarization during atherogenesis in Ldlr−/− mice and attenuates disease progression.
Supplementary Material
Significance.
Atherosclerosis remains the number one cause of death in the Western world. Insights into the mechanisms of disease development are still limited. The transcription factor Hypoxia-inducible factor (HIF)-1α is induced under hypoxic conditions, but can also be upregulated by inflammatory stimuli. We here show that atherosclerotic lesion formation is associated with an upregulated expression of HIF1α in atherosclerotic lesions and antigen-presenting cells (APCs) in atherosclerosis-prone mice. By conditionally deleting Hif1α in CD11c+ cells we reveal that HIF1α balances excessive APC-mediated pro-atherogenic T cell proliferation and Th1 polarization. In contrast, deletion of Hif1a in LysM+ bone marrow cells in Ldlr−/− mice did not affect lesion formation or T cell activation. These findings offer unprecedented insights into the function of HIF1α in APCs in atherosclerosis, and provide the first evidence that this transcription factor restrains DC-driven T-cell responses in atherosclerosis.
Acknowledgments
We thank Melanie Schott for excellent technical assistance, Dr. Peilin Zheng for help with lentivirus production, and Dr. Eliana Ribechini for help with the immunization assay.
Sources of Funding
This work was supported in part by the VENI fellowship of the Netherlands Organization of Scientific research (016.116.017) to J.C. Sluimer, the Deutsche Forschungsgemeinschaft (ZE827/1-2, SFB688 TPA22) to A. Zernecke, by a Career Development Award from Juvenile Diabetes Research Foundation (2-2010-383) to S. Kissler, and by DRC grant from NIH (P30DK036836) to the Joslin Diabetes Center.
Non standard abbreviations
- APC
antigen presenting cell
- BM
bone marrow
- BMDC
bone marrow derived DC
- BMDM
bone marrow derived macrophage
- CKO
conditional knock out
- DC
dendritic cell
- HIF
hypoxia-inducible factor
- IFN
interferon
- IL
interleukin
- Ldlr
low-density lipoprotein receptor
- Stat
Signal Transducers and Activators of Transcription
- Th
T helper cell
- Treg
regulatory T cell
- WT
wild type
Footnotes
Disclosures
None.
References
- 1.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 2.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zernecke A. Dendritic cells in atherosclerosis: Evidence in mice and humans. Arterioscler Thromb Vasc Biol. 2015 doi: 10.1161/ATVBAHA.114.303566. [DOI] [PubMed] [Google Scholar]
- 5.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 7.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: Mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 8.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 9.Shatrov VA, Sumbayev VV, Zhou J, Brune B. Oxidized low-density lipoprotein (oxldl) triggers hypoxia-inducible factor-1alpha (hif-1alpha) accumulation via redox-dependent mechanisms. Blood. 2003;101:4847–4849. doi: 10.1182/blood-2002-09-2711. [DOI] [PubMed] [Google Scholar]
- 10.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999;19:870–876. doi: 10.1161/01.atv.19.4.870. [DOI] [PubMed] [Google Scholar]
- 13.Vink A, Schoneveld AH, Lamers D, Houben AJ, van der Groep P, van Diest PJ, Pasterkamp G. Hif-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis. 2007;195:e69–75. doi: 10.1016/j.atherosclerosis.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, Gassmann M, Gardner LB, Fisher EA. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber C, Meiler S, Doring Y, et al. Ccl17-expressing dendritic cells drive atherosclerosis by restraining regulatory t cell homeostasis in mice. J Clin Invest. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. Hif-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutgens E, Lievens D, Beckers L, et al. Deficient cd40-traf6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. Ccr7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 20.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce il-12 and direct the development of th1 cells from naive cd4+ t cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 21.Gutcher I, Becher B. Apc-derived cytokines and t cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, Berin C, Reizis B, Schindler C. Dendritic cell (dc)-specific targeting reveals stat3 as a negative regulator of dc function. J Immunol. 2010;184:2638–2645. doi: 10.4049/jimmunol.0902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nefedova Y, Cheng P, Gilkes D, Blaskovich M, Beg AA, Sebti SM, Gabrilovich DI. Activation of dendritic cells via inhibition of jak2/stat3 signaling. J Immunol. 2005;175:4338–4346. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for stat3 in il-6-induced regulation of growth and differentiation in m1 leukemia cells. Embo J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 25.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of il-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 26.Stern P, Astrof S, Erkeland SJ, Schustak J, Sharp PA, Hynes RO. A system for cre-regulated rna interference in vivo. Proc Natl Acad Sci U S A. 2008;105:13895–13900. doi: 10.1073/pnas.0806907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kissler S, Stern P, Takahashi K, Hunter K, Peterson LB, Wicker LS. In vivo rna interference demonstrates a role for nramp1 in modifying susceptibility to type 1 diabetes. Nat Genet. 2006;38:479–483. doi: 10.1038/ng1766. [DOI] [PubMed] [Google Scholar]
- 28.Kurobe H, Urata M, Ueno M, et al. Role of hypoxia-inducible factor 1alpha in t cells as a negative regulator in development of vascular remodeling. Arterioscler Thromb Vasc Biol. 2010;30:210–217. doi: 10.1161/ATVBAHA.109.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Shoshan J, Afek A, Maysel-Auslender S, Barzelay A, Rubinstein A, Keren G, George J. Hif-1alpha overexpression and experimental murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:665–670. doi: 10.1161/ATVBAHA.108.183319. [DOI] [PubMed] [Google Scholar]
- 30.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of foxp3 drives regulatory t-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of t(h)17/t(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 33.Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, Volke M, Glasner J, Warnecke C, Wiesener MS, Eckardt KU, Steinkasserer A, Hensel M, Willam C. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 34.Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–3734. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- 35.Marsch E, Theelen TL, Demandt JA, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–2553. doi: 10.1161/ATVBAHA.114.304023. [DOI] [PubMed] [Google Scholar]
- 36.Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- 37.Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: Implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 38.Tawakol A, Singh P, Mojena M, et al. Hif-1alpha and pfkfb3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol. 2015;35:1463–1471. doi: 10.1161/ATVBAHA.115.305551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C. Protective role of cxc receptor 4/cxc ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 40.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by hif-1alpha-dependent nf-kappab activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivashkiv LB, Hu X. Signaling by stats. Arthritis Res Ther. 2004;6:159–168. doi: 10.1186/ar1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoentjen F, Sartor RB, Ozaki M, Jobin C. Stat3 regulates nf-kappab recruitment to the il-12p40 promoter in dendritic cells. Blood. 2005;105:689–696. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Ma C, Liu S, Sun J, Shao Q, Gao W, Zhang Y, Li Z, Xie Q, Dong Z, Qu X. Hypoxia skews dendritic cells to a t helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology. 2009;128:e237–249. doi: 10.1111/j.1365-2567.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranjbaran H, Sokol SI, Gallo A, Eid RE, Iakimov AO, D’Alessio A, Kapoor JR, Akhtar S, Howes CJ, Aslan M, Pfau S, Pober JS, Tellides G. An inflammatory pathway of ifn-gamma production in coronary atherosclerosis. J Immunol. 2007;178:592–604. doi: 10.4049/jimmunol.178.1.592. [DOI] [PubMed] [Google Scholar]
- 45.Chavez-Sanchez L, Espinosa-Luna JE, Chavez-Rueda K, Legorreta-Haquet MV, Montoya-Diaz E, Blanco-Favela F. Innate immune system cells in atherosclerosis. Arch Med Res. 2014;45:1–14. doi: 10.1016/j.arcmed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.