Abstract
Rationale
Pharmacotherapies are often utilized to aid in smoking cessation and switching medication when treating nicotine dependence has become more commonplace. Although common, little is known about the impact of the initial therapeutic on the effects of the subsequent therapeutic.
Objectives
To begin to fill this gap in our understanding, this project determined how switching compounds that share stimulus elements with nicotine during extinction altered extinction responding and generalization of this extinction back to nicotine.
Methods
Rats were trained in a discriminated goal-tracking task where nicotine administration was followed by intermittent sucrose access; sucrose was withheld following saline administration. In Experiment 1, nornicotine supplanted nicotine in extinction sessions 1-3 and then a switch to varenicline on extinction sessions 4-6 was examined. In Experiment 2, the reverse was investigated; varenicline to start extinction and then a switch to nornicotine. Generalization of extinction back to the nicotine stimulus was then assessed by generating a cumulative dose-effect curve.
Results
Generalization of extinction back to the training nicotine stimulus was greater if nornicotine had been received at any point in extinction compared to only receiving varenicline. Whereas extinction with varenicline alone showed more generalization to lower doses of nicotine.
Conclusions
A switch in cessation pharmacotherapy during extinction did not impede or enhance generalization back to the nicotine training stimulus. The nornicotine stimulus appears to share more stimulus overlap with the 0.4 mg/kg nicotine stimulus and varenicline may share more overlap with lower nicotine doses.
Keywords: Nicotine, Nornicotine, Varenicline, Interoception, Learning, Extinction, Cumulative-Dose, Generalization, Rats, Smoking
Introduction
The first Surgeon General’s report regarding the deleterious effects of smoking on public health was published in 1964. In the subsequent 50 years, a population equivalent to the entire state of New York has died prematurely in the U.S. from smoking (ca. 20,830,000; USDHHS, 2014). Today, 18% of Americans smoke. This is a substantial reduction from the 43% that were smokers in 1964 (USDHHS, 2014). While this reduction represents significant progress, nicotine cessation remains highly challenging for these remaining individuals (Benowitz, 2010). This challenge comes from the complexity of nicotine dependence and the multifaceted causes of dependence that include cultural, social, behavioral, and biological factors.
One such factor, and our focus in this report, is learning which involves the interoceptive nicotine stimulus and its contribution to the tenacity of the smoking habit and nicotine dependence (Besheer et al, 2004; Bevins et al, 2012; Bevins and Murray, 2011; Charntikov et al, 2014; Glautier et al, 1996; Polewan et al, 2013; Troisi, 2006). To determine the role of learned associations with the nicotine stimulus, our lab utilizes a discriminated goal-tracking task (Besheer et al, 2004; Charntikov et al, 2014; Pittenger and Bevins, 2013a; Polewan et al, 2013). In this task, rats are administered nicotine prior to conditioning sessions in which there is intermittent access to sucrose. On intermixed days, rats receive saline injections prior to sessions in which sucrose is unavailable. After a number of training sessions, the nicotine stimulus comes to differentially evoke approach to the location the sucrose has occurred in the past (termed goal-tracking; Boakes, 1977; Farwell and Ayres, 1979).
Previous research has revealed that nicotine-evoked goal-tracking does not reflect state-dependent learning, nor does it result from non-associative stimulant effects of nicotine (Besheer et al, 2004; Bevins et al, 2007; Murray and Bevins, 2011; Reichel et al, 2007). Appetitive behavior controlled by nicotine is also sensitive to reinforcer devaluation (Pittenger and Bevins, 2013b) and extinction (Besheer et al, 2004; Charntikov et al, 2014; Murray et al, 2009b; Pittenger et al, 2013a; Polewan et al, 2013; Wilkinson et al, 2006). Extinction refers to the non-reinforced presentations of the nicotine stimulus that was previously paired with a reinforcer. Removal of the reinforcer produces a progressive decrease in conditioned responding evoked by the nicotine stimulus (Besheer et al, 2004; Charntikov et al, 2014; Murray and Bevins, 2007, 2009a; Polewan et al, 2013; Wilkinson et al, 2006).
Of interest here is the related finding that responding evoked by nicotine can be weakened when extinction is conducted with a compound that shares stimulus elements with nicotine (Bevins et al, 2012; Charntikov et al, 2014; Reichel et al, 2010). In one study, Reichel et al (2010) first trained rats in the nicotine discriminated goal-tracking task as described earlier. Rats were then switched to an extinction phase (no sucrose) and given varenicline, nornicotine, or ABT-418 across 6 extinction sessions; each session lasted 20 min. Notably, all three ligands prompted goal-tracking comparable to nicotine (i.e., full substitution) during “standard” 4-min substitution tests suggesting that the stimulus effects of these ligands were just like nicotine. However, across 20-min sessions, where there was more opportunity to learn about the absence of the reinforcer, the substitution pattern, and hence conclusions regarding similarity to the nicotine stimulus was quite different. ABT-418 only partially substituted for nicotine during the 1st extinction session, whereas nornicotine and varenicline partially substituted in all 6 extinction sessions. Finally, Reichel et al. then examined generalization of extinction back to the nicotine stimulus (termed “transfer of extinction learning”). Nicotine-evoked responding was unaffected in rats that had ABT-418 in the extinction phase. In contrast, extinction with nornicotine or varenicline at least partially generalized back to nicotine as evidenced by a partial reduction in goal-tracking evoked by the nicotine stimulus.
We use the term “nicotine stimulus” throughout this and other papers as a short-hand. Nicotine may be better labelled and conceptualized as an interoceptive context composed of many stimulus elements, each of which has its identifiable neurobiological processes (Bevins et al, 2011; Pittenger et al, 2013a). With this notion in mind, we take the difference between the brief 4-min tests versus the repeated, extensive 20-min extinction sessions and the transfer of extinction tests as revealing differences in the stimulus elements that comprise nicotine and the elements of nornicotine, varenicline, and ABT-418.
One goal of the present experiments was to determine how switching extinction stimuli that share stimulus elements with nicotine would alter transfer of extinction back to the nicotine stimulus. Recall that nornicotine and varenicline produce partial extinction and at least partial generalization back to the nicotine training stimulus. Given that nornicotine and varenicline differ somewhat in receptor mechanisms (Coe et al, 2005a; Coe et al, 2005b; Crooks and Dwoskin, 1997a; Damaj et al, 1998; Dwoskin et al, 1993; Gonzales et al, 2006; Middleton et al, 2007; Mihalak et al, 2006; Papke et al, 2007; Smith et al, 2007; Xu et al, 2001), we hypothesize that at least somewhat different stimulus elements are extinguished with the varenicline versus the nornicotine stimulus. What if we start extinction with one ligand, say nornicotine, and then switch to the other ligand partway through the extinction phase? Currently, it is unknown if the extinction pattern would be affected and whether transfer of extinction back to the nicotine stimulus would be enhanced because more of the elements of the nicotine stimulus had been under extinction. To fill this gap in our understanding, Experiment 1 examined the switch from nornicotine to varenicline and Experiment 2 assessed the opposite switch, varenicline to nornicotine.
Methods and materials
Subjects
Male Sprague-Dawley rats (N=120) were obtained from Harlan Industries (Indianapolis, IN, USA). Rats were housed individually in clear polycarbonate cages (48.3 × 26.7 × 20.3 cm; length × width × height) lined with TEK-Fresh cellulose bedding. Water access in home cages was ad libitum. Following acclimation to the colony, rats were handled for a minimum of 2 min per day for 3 consecutive days. At the end of handling, access to food (Harlan Teklad Rodent Diet) was restricted to maintain rats at 85% of their free-feeding body weight. The colony room was kept on a 12 h light/dark cycle (lights on at 6:00 A.M.). Experimental sessions were conducted during the light portion of the cycle. The colony room was temperature- and humidity-controlled. Protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
Ten conditioning chambers (ENV-008CT; Med Associates, Inc., Georgia, VT, USA) measuring 30.5 × 24.1 × 21.0 cm (length × width × height) were enclosed in sound- and light-attenuated cubicles. The cubicles were fitted with an exhaust fan to provide airflow and mask noise. The front, back, and ceiling of the chambers were clear polycarbonate; side walls were aluminum. One of the two side walls contained a recessed receptacle (5.2 × 5.2 × 3.8 cm; length × width × depth). A dipper arm raised a 0.1-ml cup of sucrose (26% w/v) into the receptacle. To record head entries into the dipper, the chambers were equipped with an emitter/detector unit placed 1.2 cm into the recessed receptacle and 3 cm above the rod floor of the chamber. A personal computer with Med Associates interface and software (Med-PC for Windows, version IV) controlled sucrose deliveries and recorded dipper entries.
Drugs
(−)-Nicotine hydrogen tartrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Varenicline tartrate was generously provided by NIDA (RTI, Research Triangle Park, NC, USA), and S-(−)-nornicotine fumarate was provided by Girindus America, Inc. (Cincinnati, OH, USA). Nicotine was dissolved in 0.9% saline and adjusted to a pH of 7.0 ± 0.2 using a dilute NaOH solution. Nicotine dose is reported as the free base. Varenicline and nornicotine were dissolved in 0.9% saline and doses are reported as a salts. All injections were subcutaneous (SC) in a volume of 1 ml/kg. Previous research suggests the doses utilized in this study and reported below produce robust discrimination and optimal transfer of extinction back to nicotine (Besheer et al, 2004; Polewan et al, 2013; Reichel et al, 2010).
Common Procedures
Acquisition
Rats received nicotine injections for 3 consecutive days before the start of training to minimize the initial locomotor suppressant effects of nicotine (Bevins et al., 2001). The experiment was conducted 7 days a week until completed. Rats were trained once daily across 32 consecutive days with 16 saline sessions intermixed with 16 nicotine sessions. The order of saline and nicotine sessions was pseudo-randomly assigned for each rat with the stipulation that no more than 2 days with the same type of session occur consecutively. During nicotine sessions, rats received nicotine 0.4 mg/kg 5 min before a 20-min session in which they received access to 36 deliveries of 26% sucrose (4 s each). Access to the first sucrose delivery ranged from 124 to 152 s with an average of 137 s from the start of the session; subsequent sucrose deliveries were presented on average every 25 s (range = 4 to 80 s). Saline sessions consisted of a saline injection 5 min prior to placement in the conditioning chamber, and no access to sucrose across the 20-min session.
Extinction
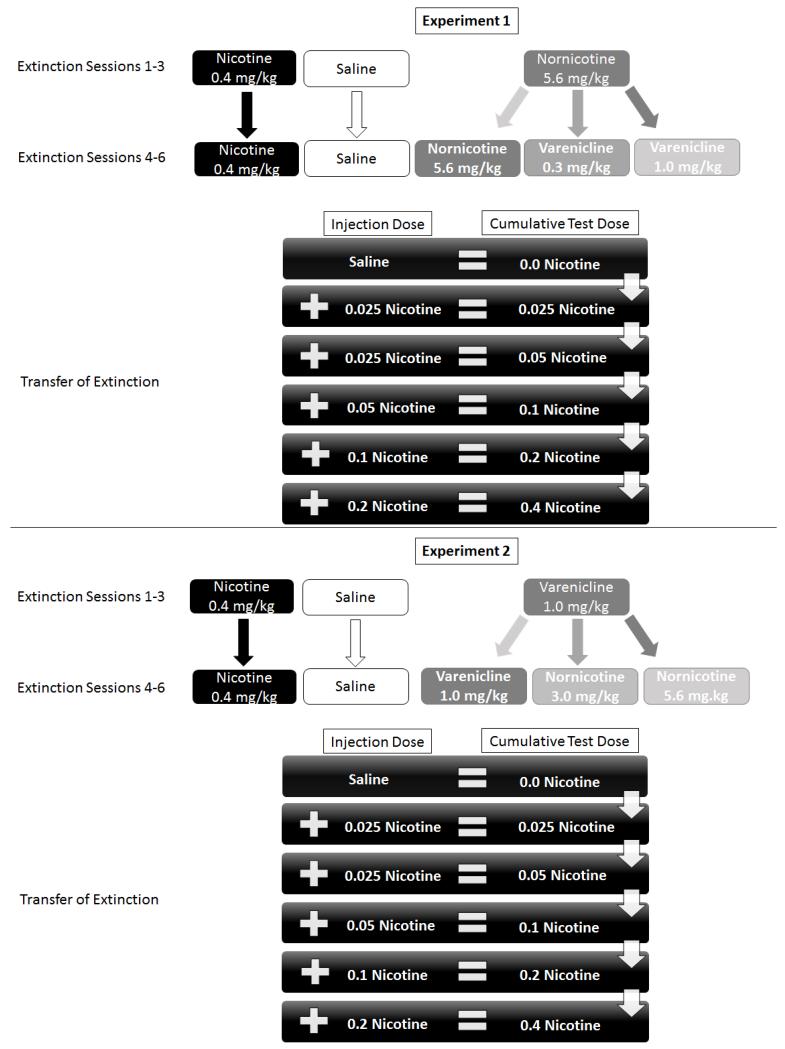
Daily extinction sessions followed 24 h after the last day of acquisition. Each extinction session consisted of an injection of the assigned ligand (see later description and Figure 1). The injection-to-placement interval (IPI) was 5 min for nicotine and saline, 15 min for nornicotine, and 30 min for varenicline (cf. Reichel et al, 2010). At the end of the IPI, there was a 20-min session in the chamber during which sucrose access was withheld. Extinction was separated into two phases, each lasting 3 days. There was a switch in assigned compound for some rats between the first and second half of extinction (see specifics of each experiment that follows).
Fig. 1.
Displays the experimental procedures for Experiment 1 (top) and Experiment 2 (bottom).
Transfer of Extinction Tests
Following 24 h after extinction, we investigated how extinction learning with the assigned compound(s) transferred back to the nicotine stimulus. For this testing, we used a cumulative dose-effect procedure that allowed us to examine a range of nicotine doses (see Figure 1). To start, rats were first administered saline 5 min prior to a 4-min test session in the chamber; sucrose was not available. Immediately after the test session, rats were administered 0.025 mg/kg of nicotine 5 min before the next 4-min transfer test. For the 5 min injection-to-placement interval, rats were held in a transport cage. We repeated this testing protocol 4 more times, administering the following doses of nicotine before each test: 0.025, 0.05, 0.1, and 0.2 mg/kg, respectively. Using this procedure, we were able to assess nicotine-evoked responding at the cumulative doses of 0.0 (saline), 0.025, 0.05, 0.1, 0.2, and 0.4 mg/kg.
Experiment 1
Experiment 1 was designed to determine the effect of switching from initial extinction training with nornicotine to subsequent extinction with varenicline. Following acquisition, rats (n= 60) were pseudo-randomly separated into 3 conditions with the stipulation that responding at the end of discrimination training did not differ statistically; 12 rats in the nicotine and in the saline condition and 36 rats in nornicotine condition. Following 3 days of extinction, rats in the nornicotine condition were further pseudo-randomly split into 3 separate groups with the stipulation that responding on the 3rd day of extinction did not differ. This approach created 5 separate groups in the second half of extinction. The 5 groups were as follows: 0.4 mg/kg nicotine→0.4 mg/kg nicotine (nic→nic); saline→saline (sal→sal); 5.6 mg/kg nornicotine→5.6 mg/kg nornicotine (nor→nor); 5.6 mg/kg nornicotine→0.3 mg/kg varenicline (nor→var0.3); 5.6 mg/kg nornicotine→1.0 mg/kg varenicline (nor→var1.0). The first part of group abbreviation denotes the drug administered during the first 3 extinction sessions, and the second part of the abbreviation designates the drug given in the last 3 extinction sessions (see Figure 1). Thus, the nic→nic group provided a benchmark of extinction with the training stimulus, whereas the sal→sal group demonstrated saline levels of responding (i.e., an exemplar of little to no extinction learning). The last 3 groups were tests of extinction with nornicotine throughout extinction (nor→nor) and the effect of early extinction with nornicotine on the later extinction with varenicline (nor→var0.3, nor→var1.0). Effects of the extinction learning on responding controlled by the nicotine stimulus was subsequently tested in the transfer of extinction test.
Experiment 2
Experiment 2 was designed to determine the opposite switch in extinction ligand from Experiment 1 (see Figure 1). That is, early extinction with varenicline followed by extinction with nornicotine. The 5 groups (n=60; 12/group) were as follows: 0.4 mg/kg nicotine→0.4 mg/kg nicotine (nic→nic); saline-saline (sal→sal); 1.0 mg/kg varenicline→1.0 mg/kg varenicline (var→var); 1.0 mg/kg varenicline→3.0 mg/kg nornicotine (var→nor3.0); 1.0 mg/kg varenicline→5.6 mg/kg nornicotine (var→nor5.6). Group abbreviations are structured as previously described. Similar to Experiment 1, transfer of extinction tests were conducted 24 h after the last extinction session.
Dependent Measures
Dipper entries per second before the first sucrose delivery in nicotine sessions and an equivalent time in saline sessions were used as the dependent measure during acquisition. Using a measure of responding before the first sucrose delivery prevents our index of acquisition from being biased by responding evoked by the sucrose and not the nicotine stimulus (see Besheer et al., 2004). A rate measure (i.e., dipper entries per sec) was used because the timing of the first sucrose delivery varied across sessions. Number of dipper entries in the 20-min extinction sessions and the 4-min cumulative dose-effect tests was used as the dependent measure because sucrose was never available.
Statistical Analyses
In acquisition, discrimination between saline and nicotine sessions was analyzed by a two-way within-subjects repeated measures analysis of variance (ANOVA) with Drug (nicotine vs. saline) as a within-subjects factor and Session as a repeated measure. Total dipper entries during the first phase of extinction (sessions 1-3) was analyzed by a two-way mixed measures ANOVA with Group (Experiment 1: nicotine, saline, and nornicotine; Experiment 2: nicotine, saline, and varenicline) as a between-subjects factor and Session as a repeated measure. Total dipper entries for the second half of extinction (sessions 4-6) was analyzed by a two-way mixed measures ANOVA with Group (Experiment 1: nic→nic, sal→sal, nor→nor, nor→var0.3, nor→var1.0; Experiment 2: nic→nic, sal→sal, var→var, var→nor3.0, var→nor5.6) as a between-subjects factor and Session as a repeated measure. Total dipper entries in the transfer of extinction tests was analyzed by a two-way mixed measures ANOVA with Group (Experiment 1: nic→nic, sal→sal, nor→nor, nor→var0.3, nor→var; Experiment 2: nic→nic, sal→sal, var→var, var→nor3.0, var→nor5.6) as a between-subjects factor and Nicotine Dose (0.0, 0.025, 0.05, 0.1, 0.2, 0.4) as a repeated measure. Statistical significance was declared at p<0.05. Bonferroni with alpha correction post-hoc tests were utilized; corrected p values are reported. All analyses were done using EZ package (Lawrence, 2013) in R, version 3.2.0.
Results
Experiment 1: Acquisition
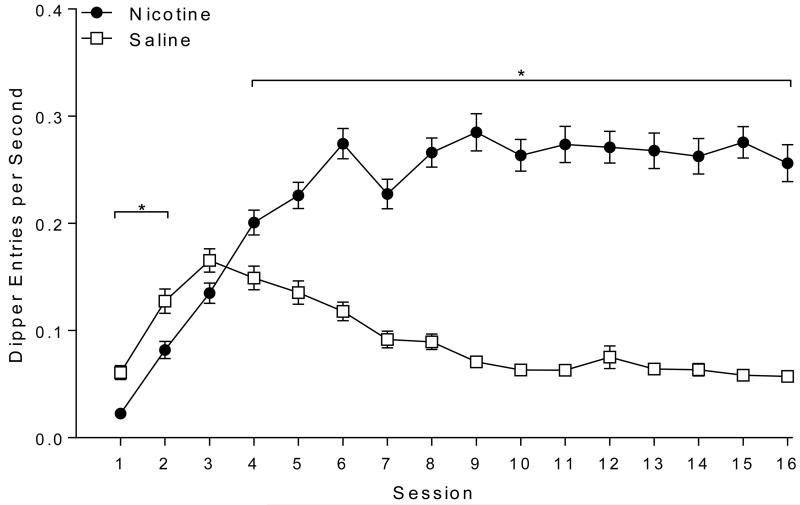
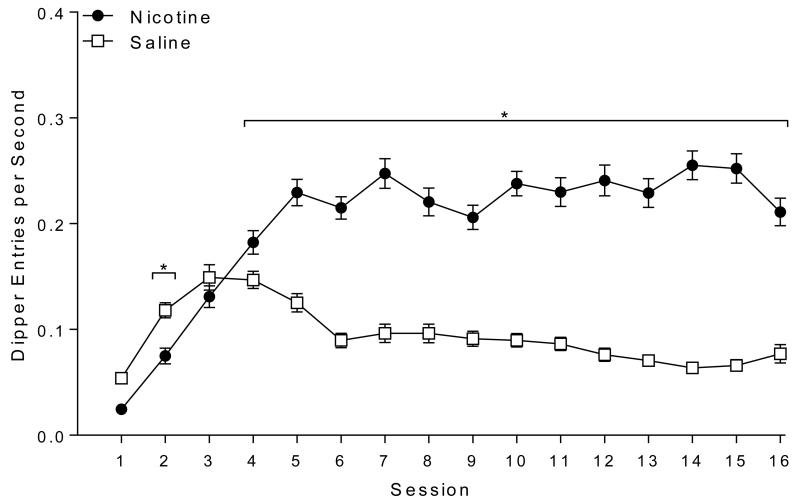
Rats demonstrated robust discrimination between saline and nicotine (Figure 2). There was a main effect of Drug [F(1,59)=324.03, p<0.001], a main effect of Session [F(15,885)=26.34, p<0.001], and a significant Drug × Session interaction [F(15,885)=61.96, p<0.001]. Post-hoc tests revealed that responding was lower on nicotine compared to saline days for sessions 1 and 2 (ps≤0.031). This effect was reversed on sessions 4 to 16 (ps≤0.001) with nicotine evoking more dipper entries than saline.
Fig. 2.
Shows dipper entries per second (±SEM) before the first sucrose delivery (nicotine sessions) or during a comparable time (saline sessions) for Experiment 1. * denotes significant differences (p<o.05) between the nicotine and saline days in a session.
Extinction
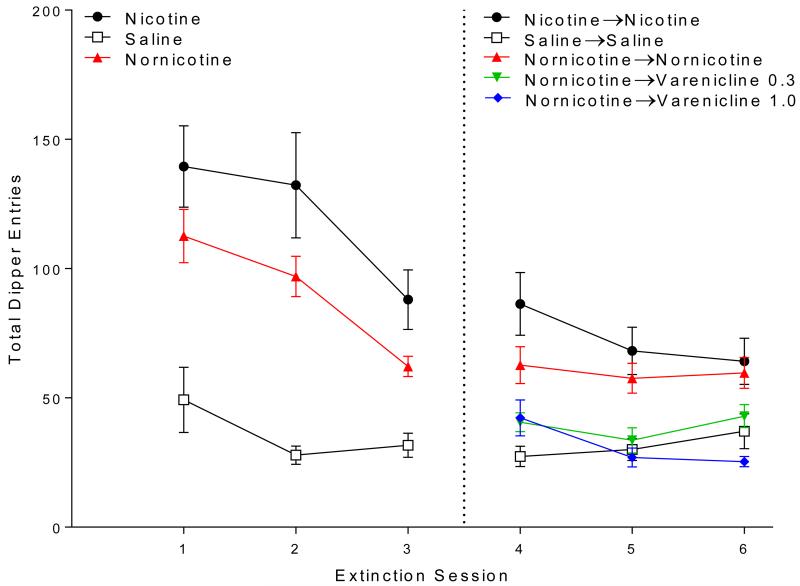
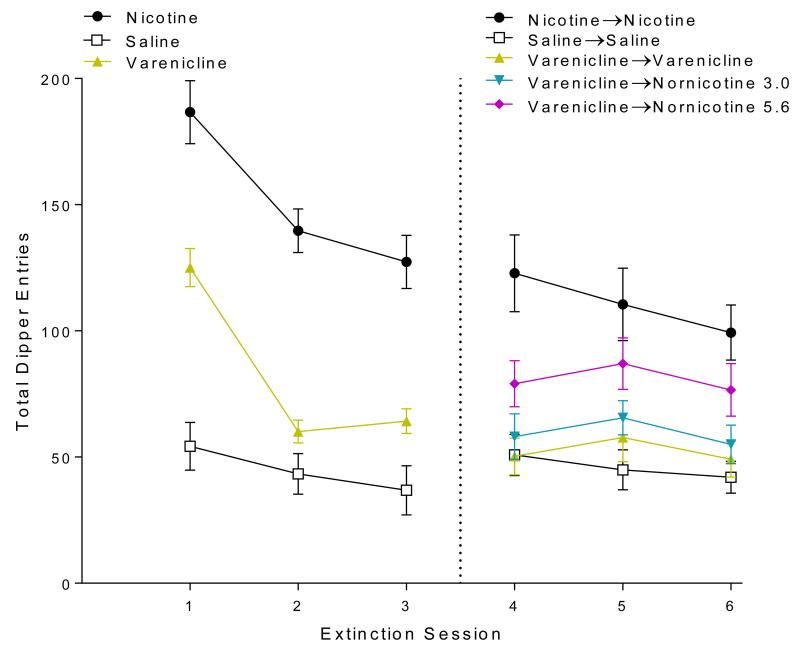
In early extinction (sessions 1-3; left side of Figure 3), there was a main effect of Group [F(2,57)=14.87, p<0.001] and a main effect of Session [F(2,114)=20.61, p<0.001]. The Group × Session interaction was also significant [F(4,114)=2.38, p=0.043]. Post-hoc tests revealed that nicotine-evoked responding was well above that of saline during all 3 sessions (ps≤0.009). Rats treated with nornicotine demonstrated responding statistically equivalent to the nicotine treated group during all early extinction sessions. Suggesting full substitution for the interoceptive stimulus effects of nicotine during early extinction. Nornicotine-evoked responding was significantly higher than saline during the 1st 2 extinction sessions (ps<0.001) and was not significantly different from saline on session 3. Analysis also revealed responding elicited by both nicotine and nornicotine was attenuated on session 3 from sessions 1 and 2 (ps≤0.002), demonstrating extinction of responding. Saline responding remained stable.
Fig. 3.
Shows total dipper entries during extinction sessions in Experiment 1. The left side displays extinction sessions 1-3 when rats received nicotine, saline, or nornicotine. The right side displays extinctions sessions 4-6 when rats receiving nornicotine were further split into groups that either continued to receive nornicotine (nornicotine→nornicotine) or were switch to varenicline (nornicotine→varenicline 1.0; nornicotine→varenicline 0.3)
In the second half of extinction (sessions 4-6; right side of Figure 3), there was a main effect of Group [F(4,55)=10.96, p<0.001], a main effect of Session [F(2,110)=6.135, p=0.003], and a significant Drug × Session interaction [F(8,110)=2.933, p=0.005]. Post-hocs prompted by the interaction revealed that nicotine evoked responding significantly higher than saline during late extinction (ps≤0.036). Rats that continued to receive nornicotine (nor→nor) remained responding at nicotine equivalent levels. Interestingly, rats switched from extinction with nornicotine to extinction with varenicline (nor→var0.3 and nor→var1.0) responded less than the nic→nic baseline rats on sessions 4 and 5 (ps≤0.002). Responding was lower on sessions 5 and 6 compared to session 4 in the nic→nic group (p≤0.005).
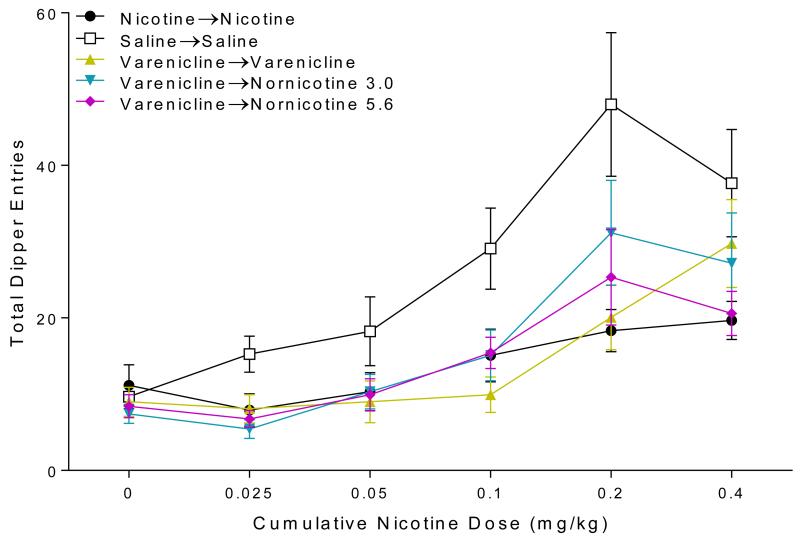
Transfer of Extinction Tests
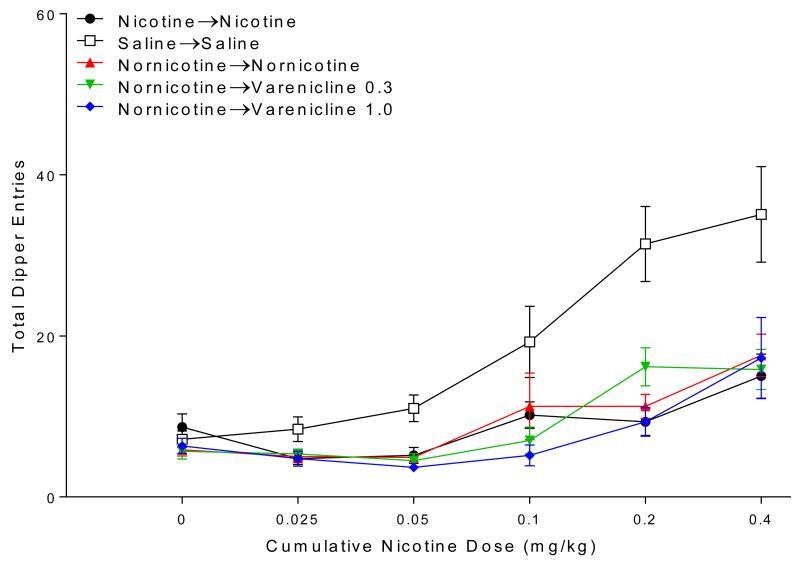
Responding evoked by nicotine was clearly attenuated by extinction learning (Figure 4). There was a main effect of Group [F(5,55)=8.63, p<0.001] and Nicotine Dose [F(5,275)=41.79, p<0.001], as well as a Group × Nicotine Dose interaction [F(20,275)=3.69, p<0.001]. Post-hoc analysis revealed rats treated with saline in extinction respond significantly more than all other groups when tested with a cumulative nicotine dose at or above 0.2 mg/kg (ps<0.001). Rats that received nornicotine throughout extinction (nor→nor) or started extinction with nornicotine and were subsequently switched to varenicline (nor→var0·3, nor→var1.0) responded at levels similar to rats that received nicotine throughout extinction (nic→nic) at all tested doses. Interestingly, both varenicline groups (nor→var0·3, nor→var1.0) responded significantly less than the saline baseline group when tested with 0.1 mg/kg nicotine (ps≤0.004), suggesting that extinction with varenicline may generalized to this lower dose of nicotine.
Fig. 4.
Shows total dipper entries during the cumulative-dose transfer of extinction tests in Experiment 1.
Experiment 2: Acquisition
Rats again displayed clear discrimination between saline and nicotine (Figure 5). The main effect of Drug [F(1,59)=392.4, p<0.001] and Session [F(15,885)=26.92, p<0.001] were significant, as was the Drug × Session interaction [F(15,885)=44.06, p<0.001]. Post-hoc analyses revealed responding following nicotine administration was lower than saline on session 2 (p=.003), with this pattern reversed on sessions 4 to 16 (ps≤0.034).
Fig. 5.
Shows dipper entries per second (±SEM) before the first sucrose delivery (nicotine sessions) or during a comparable time (saline sessions) for Experiment 2. * denotes significant differences (p<o.05) between the nicotine and saline days in a session.
Extinction
In early extinction (left side of Figure 6), there was a main effect of Group [F(2,57)=46.00, p<0.001] and Session [F(2,114)=45.54, p<0.001], as well as a significant Group × Session interaction [F(4,114)=6.094, p<0.001]. The nicotine group responded the most on all sessions (ps<0.001). Varenicline evoked significantly less responding than nicotine, yet more than saline on session 1 (ps<0.001). This data pattern supports the notion that initially varenicline partially substituted for nicotine during extinction. Responding evoked by varenicline was not significantly different from saline on sessions 2 and 3. Responding was reduced in sessions 2 and 3 from session 1 following administration of nicotine or varenicline (ps<0.001), while saline responding remained consistent across sessions.
Fig. 6.
Shows total dipper entries during extinction sessions in experiment 2. The left side displays extinction sessions 1-3 when rats received nicotine, saline, or varenicline. The right side displays extinctions sessions 4-6 when rats receiving varenicline were further split into groups the either continued to receive varenicline (varenicline→varenicline) or were switch to nornicotine (varenicline→nornicotine 5.6; varenicline→nornicotine 3.0)
In the second half of extinction (right side of Figure 6), there was a main effect of Group [F(4,55)=9.09, p<0.001] and a main effect of Session [F(2,110)=4.60, p<0.05]. However, the Group × Session interaction was not significant [F(8,110)=1.24, p=0.28]. Post-hoc analyses of the marginal group means revealed that nicotine evoked significantly more responding than the sal→sal (p<0.001), var→var (p<0.001), and the var→nor3.0 (p=0.001) groups. Notably, the group that initially received varenicline in extinction and was switched to the highest dose of nornicotine (var→nor5.6) displayed responding that was equivalent the nic→nic baseline group, suggesting the higher 5.6 mg/kg dose of nornicotine substituted for nicotine better than varenicline and the lower nornicotine dose. Analysis of the marginal means also revealed that responding was lower in session 6 compared to sessions 4 and 5 (ps≤0.046).
Transfer of Extinction Tests
Responding evoked by nicotine was clearly attenuated by extinction learning (Figure 7). There was a significant main effect of Group [F(4,55)=3.96, p=0.007] and Nicotine Dose [F(5,275)=36.95, p<0.001], and the Group × Nicotine Dose interaction was significant [F(20, 275)=2.08, p=0.005. Post-hocs revealed that rats treated with saline in extinction (sal→sal) responded significantly more than the rats that received extinction with nicotine (nic→nic) when tested with a cumulative nicotine dose at or above 0.2 mg/kg (ps≤0.016). Interestingly, the group that received varenicline throughout extinction (var→var) demonstrated responding significantly lower than the group that received no extinction (sal→sal) at lower doses of nicotine testing [0.1 (p=0.008) and 0.2 (p<0.001)]. The two groups that initially received varenicline and were switched to nornicotine (var→nor3.0 and var→nor5.6) also responded at levels significantly attenuated from saline baseline at the 0.2 mg/kg dose. At the 0.4 mg/kg training dose, the var→nor5.6 group was the only other group (aside from the nic→nic) that demonstrated attenuated responding compared to the saline baseline group (p=0.027), suggesting that this higher dose of nornicotine was required to sufficiently extinguish nicotine-evoked responding at the nicotine training dose.
Fig. 7.
Shows total dipper entries during the cumulative-dose transfer of extinction tests in Experiment 2.
Discussion
In the course of treatment for smoking cessation, a practitioner may switch medications when patients are struggling and vulnerable to a lapse (Ebbert et al, 2009a; Ebbert et al, 2009b; Ebbert et al, 2014; Issa et al, 2013; Karam-Hage et al, 2010; Koegelenberg et al, 2014; Piper et al, 2009; Rigotti, 2012; Rose and Behm, 2014; Steinberg et al, 2009). Although a common practice, little is known about the impact of switching pharmacotherapies. As detailed earlier, to address this gap in a preclinical animal model, we examined the effects of a switch from the tobacco alkaloid and N-demethylated nicotine metabolite, nornicotine (Crooks et al, 1997a; Crooks et al, 1997b; Green et al, 2001; Middleton et al, 2007) to the α4β2 partial and full α7 nAChR agonist, varenicline (Coe et al, 2005a; Coe et al, 2005b; Rollema et al, 2007), or vice versa.
In the present report, nicotine served as an interoceptive stimulus in a discriminated goal tracking task. As in our previous reports, rats in both experiments readily learned to goal-track when administered nicotine (Murray et al, 2009c; Struthers et al, 2009; Wilkinson et al, 2010). Following discrimination training, we examined the effects of early extinction with nornicotine or varenicline. Nornicotine evoked nicotine-like equivalent responding across all 6 extinction sessions. These findings extend previous work by Reichel et al (2010) that found partial responding in 6 repeated extinction sessions using a 3.0 mg/kg dose of nornicotine. The higher 5.6 mg/kg nornicotine dose used here in the nor→nor group may be more effective at substituting for 0.4 mg/kg nicotine than this lower dose. It should be noted, however that visual inspection of the data does reveal responding that appears lower than responding evoked by nicotine. Varenicline evoked partial nicotine-like responding, significantly lower than that of nicotine and significantly higher than saline evoked responding in early extinction, replicating the results of Reichel et al (2010).
Full substitution of nornicotine for nicotine in early extinction is concordant with the two-lever drug discrimination task that typically employs brief extinction test sessions to evaluate stimulus similarity. Nornicotine under these conditions evoked responding almost exclusively on the nicotine-appropriate lever (i.e., full substitution; Goldberg et al, 1989). Consistent with this outcome, if substitution of nornicotine for nicotine was evaluated in brief 4-min extinction sessions in the discriminated goal-tracking task, then full substitution was also seen (Reichel et al, 2010). Varenicline tested in the two-lever drug discrimination task has yielded mixed findings. Some researchers have reported partial substitution for nicotine similar to the repeated extinction tests here (LeSage et al, 2009; Smith et al, 2007), whereas others investigators have reported full substitution [>85% nicotine-appropriate responding; (Jutkiewicz et al, 2011; Paterson et al, 2010)]. Similar to these later studies, in the discriminated goal-tracking task, varenicline in brief substitution tests fully substituted for the nicotine stimulus (Reichel et al, 2010). These differences suggest that brief tests of stimulus similarity may be measuring something different than stimulus substitution tests that allow the rat to learn about non-reinforcement (Bevins et al, 2012). What these differences reflect and their importance awaits further investigation.
The switch in ligand midway through extinction provided a novel test of stimulus similarity. Recall that in Experiment 1, nornicotine substituted for nicotine over all 6 extinction sessions. Interestingly, when rats were switched from nornicotine to varenicline (nor→var0.3, nor→var1.0), those rats responded significantly less than nicotine baseline. This finding would not be expected if nornicotine and varenicline had distinct stimulus elements, sharing little overlap of mechanism with each other and with the nicotine training stimulus. This loss of goal-tracking with the switch to varenicline suggests that early extinction with nornicotine was sufficient to extinguish the stimulus elements of varenicline that overlapped with nicotine. That is, any overlap of mechanism shared by nicotine and varenicline, seemed to be shared by nornicotine and fully extinguished during early extinction with nornicotine. The findings of Experiment 2 provide additional support for this account. Rats that received varenicline in early extinction and subsequently switched to 5.6 mg/kg nornicotine demonstrated nicotine-like responding upon the switch. This finding suggests that the stimulus elements of nornicotine were not sufficiently extinguished by early extinction with varenicline.
To date, our lab has only tested generalization back to the training dose of nicotine, either in one 20-min transfer of extinction test 24 h after the last extinction session [Experiment 3 of Charntikov et al (2014); Polewan et al (2013); Reichel et al (2010)] or in 4 separate 20-min transfer tests repeated over 4 consecutive days [Experiment 1 and 2 of Charntikov et al (2014)]. These testing procedures were purposefully designed to provide an assessment of generalization to the stimulus of interest – the training dose of nicotine. In the present study, we extended this past work by determining how extinction learning generalized to a range of nicotine doses using a within-subjects design. One possibility was to modify the procedures utilized by Charntikov et al (2014) and conduct transfer tests with a set of doses over multiple days, eventually producing a dose-effect curve. This approach was unlikely to accomplish our goal given that each transfer test is itself an extinction session. In fact, Charntikov et al (2014) found that responding after the 1st transfer test was quickly attenuated, thus limiting the utility of the remaining 3 transfer tests. In an attempt to circumvent these issues in the present study, we decided to assess nicotine-evoked responding using a cumulative dose-effect protocol adapted from the drug discrimination literature (Bertalmio et al, 1982; Van Rossum, 1963; Wenger, 1980). In this form of the transfer test, each dose was administered and tested in a brief 4-min extinction session until all prescribed doses had been assessed (see Common Procedures). Notably, the work here has established the feasibility of using a cumulative dose-effect protocol within the discriminated goal-tracking task. In the rats that had received saline in the extinction phase, their nicotine-evoked responding was dose-dependent in a manner seen in previously published reports where each nicotine dose was tested in separate test sessions that included intervening discrimination training (Besheer et al, 2004; Pittenger et al, 2013a). Though the feasibility of using a cumulative-dose effect procedure was established in this report, whether this dose-effect function differs from the between-sessions approach awaits future research directly comparing them.
As expected, the transfer test revealed that nicotine-evoked responding in rats that had saline in extinction was higher relative to the rats that had nicotine as the extinction ligand. These two groups provided suitable comparisons with group sal→ sal serving as an exemplar of little to no extinction learning and the nic→nic group as a benchmark for extinction learning with the training stimulus. In Experiment 1, all groups that had a non-nicotine compound (nor→nor, nor→var0.3, nor→var1.0), regardless of whether there was switch in ligand or not, had a relatively comparable dose-effect function to rats that had nicotine in extinction. Further, in Experiment 2 the group that received the higher 5.6 mg/kg nornicotine (var→nor5.6), also had comparable responding to the group that had nicotine in extinction. This data pattern suggests that receiving extinction sessions with 5.6 mg/kg nornicotine alone, prior to, or followed by varenicline extinction, was sufficient to reduce conditioned responding to a level comparable to having had nicotine in extinction (cf. cumulative test dose of 0.4 mg/kg in Figure 4).
Interestingly, repeated extinction with varenicline alone (var→var) was more effective at reducing responding at the lower cumulative doses of nicotine than the group that received true nicotine extinction (nic→nic). However, extinction with varenicline alone did not attenuate responding when tested with the 0.4 mg/kg dose. With that said, the variance in the var→var group is quite large, indicating that responding is partially attenuated in some rats. These findings extend those of Reichel et al (2010) that also found only partial varenicline generalization to the 0.4 mg/kg training dose in transfer of extinction. The pattern of responding found in the present study suggests that the varenicline stimulus may be more similar to the lower nicotine doses and, that the stimulus elements that compose the 0.4 mg/kg training dose of nicotine differ somewhat from those lower doses in at least a subset of rats. Consistent with this latter suggestion, acquisition of two lever discrimination is slower and nicotine-appropriate responding was often lower with a lower training dose of nicotine (Chance et al, 1977; Gasior et al, 1999; Hirschhorn and Rosecrans, 1974; Stolerman et al, 1984). Further, Murray and Bevins (2007) found that extinction in the discriminated goal-tracking task was faster when the nicotine training dose was 0.1 mg/kg. It will be of interest to determine if substitution in repeated extinction sessions and transfer of extinction learning with varenicline would be more effective with a lower training dose of nicotine.
We consider the cumulative dose effect protocol used here as having limited utility – especially at the higher cumulative doses. Although this procedure allowed us to quickly assess within-subjects a set of nicotine doses, the 0.4 mg/kg training dose was not assessed until 5 other tests were completed. Each 4-min test was a brief extinction session, as the cumulative dose of nicotine increased toward the training dose. Given that these rats had already had an experimental history with non-reinforcement in these chambers, they may have been increasingly sensitive to the lack of sucrose deliveries. Such increased sensitivity would have further blunted responding as repeated testing occurred resulting in less sensitivity of the transfer tests at the higher cumulative doses. In addition, the metabolism of nicotine [approximate brain half-life of 52 minutes following peripheral administration (Ghosheh et al., 1999)] may also limit the utility of this cumulative dose effect test. While the cumulative dose transfer test has its limitations, our finding that animals trained only with varenicline displayed less generalization to the 0.4 mg/kg training dose of nicotine and enhanced generalization to lower doses of nicotine compared to animals that received nornicotine in extinction is still notable.
Future studies switching between other pharmacotherapies to enhance extinction learning and decrease appetitive behavior controlled by nicotine will be of interest. Does a switch between varenicline and bupropion, two commonly prescribed cessation aids (Chantix and Zyban, respectively), alter extinction and generalization back to the nicotine stimulus? Also of interest will be examination of how combining pharmacotherapies (e.g., varenicline + bupropion in repeated extinction) alters extinction learning (cf. Hall et al, [2015] for combination in self-administration). It is possible that a combination of these ligands may deepen extinction (i.e. attenuate further) and enhance nicotine generalization beyond that of any individual ligand. Such studies, like the one described in this report, will further increase our knowledge of interoceptive conditioning involving the nicotine stimulus. We hope that this increased understanding will be useful to practitioners when designing better smoking cessation strategies.
Acknowledgments
This research was supported by NIH research grant DA034389. All Med-PC programs used in this research are available upon request to Rick A Bevins, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE USA 68588-0308.
References
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertalmio AJ, Herling S, Hampton RY, Winger G, Woods JH. A procedure for rapid evaluation of the discriminative stimulus effects of drugs. J Pharmacol Methods. 1982;7(4):289–299. doi: 10.1016/0160-5402(82)90082-1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004;172(1):108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Barrett ST, Polewan RJ, Pittenger ST, Swalve N, Charntikov S. Disentangling the nature of the nicotine stimulus. Behav Processes. 2012;90(1):28–33. doi: 10.1016/j.beproc.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Murray JE. Internal stimuli generated by abused substances: Associative learining and conditioning and its implications for drug addiction. In: Schachtman TR, Reilly SS, editors. Associative Learning and Conditioning Theory: Human and Non-Human Applications. Oxford University Press; New York: 2011. [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal tracking task with rats. Behav Brain Res. 2007;177(1):134–141. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, NJ, US: 1977. pp. 67–101. [Google Scholar]
- Chance WT, Murfin D, Krynock GM, Rosecrans JA. A description of the nicotine stimulus and tests of its generalization to amphetamine. Psychopharmacology (Berl) 1977;55(1):19–26. doi: 10.1007/BF00432812. [DOI] [PubMed] [Google Scholar]
- Charntikov S, deWit NR, Bevins RA. Interoceptive conditioning with nicotine using extinction and re-extinction to assess stimulus similarity with bupropion. Neuropharmacology. 2014;86:181–191. doi: 10.1016/j.neuropharm.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005a;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Wirtz MC, Bashore CG, Bianco KE, Vetelino MG, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett. 2005b;15(22):4889–4897. doi: 10.1016/j.bmcl.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997a;54(7):743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP. Metabolites of nicotine in rat brain after peripheral nicotine administration. Cotinine, nornicotine, and norcotinine. Drug Metab Dispos. 1997b;25(1):47–54. [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther. 1998;284(3):1058–1065. [PubMed] [Google Scholar]
- Dwoskin LP, Buxton ST, Jewell AL, Crooks PA. S(−)-nornicotine increases dopamine release in a calcium-dependent manner from superfused rat striatal slices. J Neurochem. 1993;60(6):2167–2174. doi: 10.1111/j.1471-4159.1993.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res. 2009a;11(5):572–576. doi: 10.1093/ntr/ntp042. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009b;11(3):234–239. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311(2):155–163. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (‘goal tracking’) in rats. Learning and Motivation. 1979;10:295–312. [Google Scholar]
- Gasior M, Shoaib M, Yasar S, Jaszyna M, Goldberg SR. Acquisition of nicotine discrimination and discriminative stimulus effects of nicotine in rats chronically exposed to caffeine. J Pharmacol Exp Ther. 1999;288(3):1053–1073. [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence times and half-life of nicotine metabolites in rat brain after acute peripheral administration of [2′-14C] nicotine. Drug Metabolism & Disposition. 1999;27(12):1448–1455. [PubMed] [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7(2):144–154. [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 1989;97(3):295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Green TA, Crooks PA, Bardo MT, Dwoskin LP. Contributory role for nornicotine in nicotine neuropharmacology: nornicotine-evoked [3H]dopamine overflow from rat nucleus accumbens slices. Biochem Pharmacol. 2001;62(12):1597–1603. doi: 10.1016/s0006-2952(01)00838-3. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Slades S, Wells C, Rose JE, Levin ED. Bupropion-varenicline interaction and nicotine self-administration behavior in rats. Pharmacol Biochem Behav. 2015;130:84–89. doi: 10.1016/j.pbb.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn ID, Rosecrans JA. Studies on the time course and the effect of cholinergic and adrenergic receptor blockers on the stimulus effect of nicotine. Psychopharmacologia. 1974;40(2):109–120. doi: 10.1007/BF00421360. [DOI] [PubMed] [Google Scholar]
- Issa JS, Abe TO, Moura S, Santos PC, Pereira AC. Effectiveness of coadministration of varenicline, bupropion, and serotonin reuptake inhibitors in a smoking cessation program in the real-life setting. Nicotine Tob Res. 2013;15(6):1146–1150. doi: 10.1093/ntr/nts230. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther. 2011;339(1):194–202. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam-Hage M, Shah KR, Cinciripini PM. Addition of bupropion SR to varenicline alleviated depression and suicidal ideation: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(2) doi: 10.4088/PCC.09l00800blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegelenberg CF, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O’Brien JA, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155–161. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- Lawrence MA. EZ: Easy analysis and visualization of factorial experiments. 2013 R package version 4.2-2. http://CRAN.R-project.org/package=ez.
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91(3):461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LT, Rosecrans JA, Aceto MD, Harris LS. Discriminative stimulus properties of the optical isomers of nicotine. Psychopharmacology (Berl) 1980;68(3):283–286. doi: 10.1007/BF00428116. [DOI] [PubMed] [Google Scholar]
- Middleton LS, Crooks PA, Wedlund PJ, Cass WA, Dwoskin LP. Nornicotine inhibition of dopamine transporter function in striatum via nicotinic receptor activation. Synapse. 2007;61(3):157–165. doi: 10.1002/syn.20351. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol. 2007;18(8):707–716. doi: 10.1097/FBP.0b013e3282f14ec6. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Acquired appetitive responding to intravenous nicotine reflects a Pavlovian conditioned association. Behav Neurosci. 2009a;123(1):97–108. doi: 10.1037/a0013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Excitatory conditioning to the interoceptive nicotine stimulus blocks subsequent conditioning to an exteroceptive light stimulus. Behav Brain Res. 2011;221(1):314–319. doi: 10.1016/j.bbr.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Penrod RD, Bevins RA. Nicotine-evoked conditioned responding is dependent on concentration of sucrose unconditioned stimulus. Behav Processes. 2009b;81(1):136–139. doi: 10.1016/j.beproc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Wells NR, Lyford GD, Bevins RA. Investigation of endocannabinoid modulation of conditioned responding evoked by a nicotine CS and the Pavlovian stimulus effects of CP 55,940 in adult male rats. Psychopharmacology (Berl) 2009c;205(4):655–665. doi: 10.1007/s00213-009-1572-x. [DOI] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101(1):160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Min W, Hackett A, Lowe D, Hanania T, Caldarone B, et al. The high-affinity nAChR partial agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1455–1464. doi: 10.1016/j.pnpbp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Bevins RA. Interoceptive conditioning in rats: effects of using a single training dose or a set of 5 different doses of nicotine. Pharmacol Biochem Behav. 2013a;114-115:82–89. doi: 10.1016/j.pbb.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Bevins RA. Interoceptive conditioning with a nicotine stimulus is susceptible to reinforcer devaluation. Behavioral Neuroscience. 2013b Jun;(127(3)):465–473. doi: 10.1037/a0032691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polewan RJ, Savala SA, Bevins RA. Interoceptive conditioning with the nicotine stimulus: extinction learning as a method for assessing stimulus similarity across doses. Behav Pharmacol. 2013;24(1):45–54. doi: 10.1097/FBP.0b013e32835d5278. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Nicotine as a conditioned stimulus: impact of attention-deficit/hyperactivity disorder medications. Exp Clin Psychopharmacol. 2007;15(5):501–509. doi: 10.1037/1064-1297.15.5.501. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA. Extinction with varenicline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology. 2010;58(8):1237–1245. doi: 10.1016/j.neuropharm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA. Strategies to help a smoker who is struggling to quit. JAMA. 2012;308(15):1573–1580. doi: 10.1001/jama.2012.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am J Psychiatry. 2014;171(11):1199–1205. doi: 10.1176/appi.ajp.2014.13050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2014. Atlanta, GA. [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, et al. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190(2):157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Steinberg MB, Greenhaus S, Schmelzer AC, Bover MT, Foulds J, Hoover DR, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150(7):447–454. doi: 10.7326/0003-4819-150-7-200904070-00004. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Discriminative stimulus effects of nicotine in rats trained under different schedules of reinforcement. Psychopharmacology (Berl) 1989;97(1):131–138. doi: 10.1007/BF00443427. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84(3):413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Mecamylamine, dihydro-beta-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacol Biochem Behav. 2009;94(2):319–328. doi: 10.1016/j.pbb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi JR. Pavlovian-instrumental transfer of the discriminative stimulus effects of nicotine and ethanol in rats. The Psychological Record. 2006;56:499–512. [Google Scholar]
- Van Rossum JM. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Wenger GR. Cumulative dose-response curves in behavioral pharmacology. Pharmacol Biochem Behav. 1980;13(5):647–651. doi: 10.1016/0091-3057(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Carroll FI, Bevins RA. An investigation of bupropion substitution for the interoceptive stimulus effects of nicotine. J Psychopharmacol. 2010;24(6):817–828. doi: 10.1177/0269881109102518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, et al. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as a function of the number of conditioning trials and unpaired sucrose deliveries. Behav Pharmacol. 2006;17(2):161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]
- Xu R, Dwoskin LP, Grinevich VP, Deaciuc G, Crooks PA. Neuronal nicotinic acetylcholine receptor binding affinities of boron-containing nicotine analogues. Bioorg Med Chem Lett. 2001;11(9):1245–1248. doi: 10.1016/s0960-894x(01)00193-7. [DOI] [PubMed] [Google Scholar]