Abstract
Obesity and type 2 diabetes are associated with increased risk of breast cancer incidence and mortality. Common features of obesity and type 2 diabetes are insulin resistance and hyperinsulinemia. A mammary tumor promoting effect of insulin resistance and hyperinsulinemia was demonstrated in the transgenic female MKR mouse model of pre-diabetes inoculated with mammary cancer cells. Interestingly, in MKR mice, as well as in other diabetic mouse models, males exhibit severe hyperglycemia, while females display insulin resistance and hyperinsulinemia with only a mild increase in blood glucose levels. This gender-specific protection from hyperglycemia may be attributed to estradiol, a key player in the regulation of the metabolic state, including obesity, glucose homeostasis, insulin resistance, and lipid profile. The aim of this study was to investigate the effects of ovariectomy (including the removal of endogenous estradiol) on the metabolic state of MKR female mice and subsequently on the growth of Mvt-1 mammary cancer cells, inoculated into the mammary fat pad of ovariectomized mice, compared with sham-operated mice. The results showed an increase in body weight, accompanied by increased fat mass, elevated blood glucose levels and hypercholesterolemia in ovariectomized MKR mice. In addition, mammary tumor growth was significantly higher in these mice. The results suggest that ovarian hormone deficiency may promote impaired metabolic homeostasis in the hyperinsulinemic MKR female mice, which in turn is associated with increased growth of mammary tumors.
Keywords: insulin resistance, hyperinsulinemia, ovariectomy, obesity, breast cancer
Introduction
Numerous studies and meta-analyses confirm the association between type 2 diabetes mellitus (T2D) and an increased risk of breast cancer incidence and mortality in postmenopausal women (Coughlin, et al. 2004; Larsson, et al. 2007; Michels, et al. 2003). Hyperglycemia, insulin resistance and subsequently hyperinsulinemia, key features of T2D, have been suggested as potential molecular mechanisms underlying this association (Novosyadlyy and LeRoith 2010; Xu, et al. 2014). The mammary tumor promoting effect of hyperinsulinemia was demonstrated in the transgenic female MKR mouse model of T2D inoculated with mammary cancer cells, by Novosyadlyy et al, (Novosyadlyy, et al. 2010). Interestingly, female MKR mice exhibit marked hyperinsulinemia and insulin resistance, but only a mild increase in blood glucose levels, compared with male MKR mice, which manifest severe diabetes (Fernandez, et al. 2001). This gender-specific phenomenon was observed in Zucker diabetic fatty rats (Clark, et al. 1983), as well as in obese Agouti yellow mice (Klebig, et al. 1995). In streptozotocin (STZ)-induced diabetes, male mice were susceptible to STZ, while female mice are resistant, unless they are ovariectomized (Paik, et al. 1982; Puah and Bailey 1985). Therefore, the gender-specific protection from hyperglycemia may be attributed to ovarian hormones.
It is now evident that estradiol plays an important role in several physiological and pathophysiological states, including obesity, glucose homeostasis and insulin resistance (Barros and Gustafsson 2011; Louet, et al. 2004). Supporting the beneficial effects of estradiol on metabolic homeostasis are studies showing greater insulin sensitivity in premenopausal women compared with age-matched men (Donahue, et al. 1997; Nuutila, et al. 1995), as well as a reduced incidence of T2D in postmenopausal women subjected to hormone-replacement therapy (HRT) (Margolis, et al. 2004). In addition, it was shown that estradiol deficiency in postmenopausal women leads to increased body weight, which was associated with an increase in abdominal fat (Lovejoy, et al. 2008). The metabolic effects of estradiol are suggested to be mediated by estrogen receptor (ER)α, as both male and female ERα-knockout mice exhibit glucose intolerance and insulin resistance, along with increased weight gain and fat mass (Heine, et al. 2000).
In this study, we investigated whether removal of ovarian hormones (including estradiol) by ovariectomy could exacerbate the mild diabetic features observed in female MKR mice, compared with male MKR mice. In addition, we investigated the effects of ovariectomy and the consequent alterations in the metabolic phenotype on the growth of Mvt-1 mammary cancer cells, inoculated into the mammary fat pad of these ovariectomized female MKR mice (MKR-OVX). Our results indeed show increased weight gain in MKR-OVX mice compared with sham-operated MKRs (MKR-Sham), which was accompanied by increased inguinal fat, as well as elevated fasting and non-fasting blood glucose levels, and hypercholesterolemia. In addition, mammary tumor growth was significantly higher in MKR-OVX mice compared with MKR-Sham mice, associated with higher activation of the phosphoinositide 3 kinase (PI3K)/Akt signaling pathway in tumor cells of MKR-OVX group. These results suggest that ovarian hormones may have a protective effect on the metabolic state in MKR female mice. Impairments in metabolic homeostasis caused by ovariectomy are associated with increased mammary tumor growth.
Material and methods
Animals
MKR mice were used in this study. The generation of MKR transgenic mice (on the FVB/N background), carrying a dominant-negative insulin-like growth factor (Igf)1 receptor (Igf1r) specifically targeted to the skeletal muscle, has been described previously (Fernandez et al. 2001). Mice were fed standard chow and water ad libitum, and were kept on a 12 h light/dark cycle. Mice studies were performed according to the protocol approved by the Technion Animal Inspection Committee. The Technion holds a National Institutes of Health (NIH) animal approval (license number A5026-01). MKR mice were subjected to sham surgery (MKR-Sham) or ovariectomy (MKR-OVX) at 6 weeks of age. Briefly, mice were anesthetized with isoflurane and buprenorphine (50 μl of 0.03 mg/ml solution) was administered S.C. as analgesic. A short dorsal midline incision was made, and ovaries and oviducts were exteriorized through muscle wall incisions bilateral to the spine. Oviduct was ligated and the ovary was removed by a single cut. Remaining tissue was replaced into the peritoneal cavity and skin was sutured.
Food was weighed every 3-4 days. Food intake is presented as daily intake (g) per 100 g mice.
Cell culture
Mvt-1 mouse mammary cancer cells were derived from MMTV-c-Myc/Vegf transgenic mice tumor explants, as previously described (Pei, et al. 2004). Cells were cultured in Dulbecco's modified Eagle's medium (Biological Industries, Beit Haemek, Israel), supplemented with 10% v/v fetal bovine serum (Biological Industries) and 1% v/v penicillin:streptomycin (Biological Industries). Cells were maintained at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air.
Syngeneic orthotopic tumor models
Mvt-1 cells were detached with trypsin solution (Biological Industries) into single cells and suspended in PBS at a concentration of 0.5×106 cells/ml. 100 μl (50,000 cells) were then injected into the left inguinal mammary fat pads (no. 4) of MKR-Sham or MKR-OVX mice, 35 days after ovariectomy (11 weeks old).
Tumor volume was monitored once a week using calipers and calculated in mm3 by the formula: width2×length×0.5. At sacrifice, tumors were removed and weighed.
Blood glucose measurement
Blood glucose levels were measured weekly in a non-fasting state. Blood samples were taken from the tail vein and glucose levels were determined using an automated glucometer (Accu-Check Preforma System, Roche Diagnostics, Mannheim, Germany).
Body composition
Body composition was measured every two weeks, and was determined in non-anesthetized mice using the NMR analyzer (Minispec LF50/mq7.5 analyzer, Bruker Optic GmbH, Germany). Results are presented as the ratio between fat mass and lean mass.
At sacrifice, inguinal fat was collected and weighed. Results are presented as inguinal fat mass percent of total body weight.
Glucose-tolerance test (GTT) and Insulin-tolerance test (ITT)
Glucose- and insulin-tolerance tests were performed during daylight time, after 4 h fasting. Glucose (2 g/kg body weight), or insulin (12 IU/kg, NovoRapid® Vial, Novo Nordisk, Bagsvaerd, Denmark) were administered I.P. Blood glucose levels were measured at the indicated time points.
Serum analysis
At sacrifice, blood samples were taken from the heart of anesthetized mice. Blood was allowed to clot and was then centrifuged for 10 min at 8,000 rpm, 4°C. Supernatant (serum) was collected into a fresh tube and maintained in −80°C for analysis. Serum insulin, leptin and adiponectin levels were measured with Rat/Mouse Insulin 96-Well Plate Assay Kit, Mouse Leptin 96-Well Plate Assay Kit, and Mouse Adiponectin 96-Well Plate Assay Kit, respectively (Millipore, Billerica, MA) according to manufacturer's instructions. Data acquisition was performed using the PowerWave XS2 (BioTek, Winooski, VT) and Gen5 software. Cholesterol and triglycerides were measured by Siemens Dimension® analyzer (Siemens Healthcare, Germany).
Protein extraction and Western blot analysis
Tumors were homogenized in lysis buffer (10 mmol l−1 Tris-HCl pH 7.5, 150 mmol l−1 sodium chloride, 10 mmol l−1 sodium pyrophosphate, 1 mmol l−1 β-glycerolphosphate, 1 mmol l−1 sodium orthovanadate, 50 mmol l−1 sodium fluoride, 1.25% v/v CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), and Complete® Protease Inhibitor Cocktail (Roche Diagnostics). Lysates were rotated at 4°C for 1 h and centrifuged at 13,000 rpm for 10 min. Supernatants were collected and protein concentrations were determined with Protein Assay Kit (Bio-Rad, Richmond, California). Protein (12 μg) was electrophoresed through a 10% polyacrilamide gel and transferred to a nitrocellulose membrane. The membranes were immunoblotted with the desired antibody, followed by an appropriate secondary antibody conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania). Immunoreactivity was detected by enhanced chemiluminescence (WesternBright™ Quantum Western blotting detection kit, Advansta, Melano Park, California), using luminescent image analyzer LAS-4000 (Fujifilm, Tokyo, Japan). Densitometry analysis was performed using ImageQuant software (GE Healthcare Bio-Sciences, Pittsburgh, Pennsylvania).
The following antibodies were used: Phospho-Akt (Thr308), total-Akt, and β-actin (Cell Signaling Technology, Danvers, Massachusetts).
Statistical analysis
Results are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism (Version 5; GraphPad Software Inc.). Student's T-test and two-way analysis of variance (ANOVA), followed by Bonferroni multiple comparison post-test, were used to determine the statistical significance of differences between groups. P <0.05 was considered statistically significant.
Results
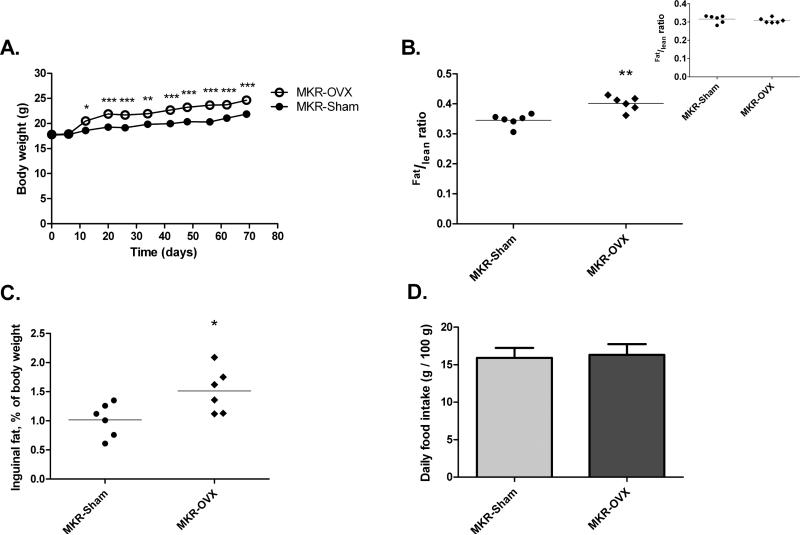
Sham surgery and ovariectomy of MKR mice (MKR-Sham and MKR-OVX, respectively) were performed at 6 weeks of age. At the beginning of the experiment, body weights of all mice were similar (Fig 1A). However, two weeks after surgery, an increased weight gain was observed in MKR-OVX group compared with MKR-Sham mice. Maximum difference in body weight of 3 g was reached three weeks after surgery and remained constant until the end of the experiment. Body composition results, presented as the ratio between fat mass and lean mass, were also similar between the two groups at the beginning of the experiment (panel of Fig 1B). However, at the end of the experiment, fat to lean ratio of ovariectomized mice was significantly higher by 16% than the ratio calculated for sham-operated mice (Fig 1B). To support this result, inguinal fat was weighed at sacrifice, and presented as percent of total body weight (Fig 1C). Indeed, the percentage of inguinal fat mass in MKR-OVX mice was higher by 1.5 fold than in MKR-Sham mice. Daily food intake was similar for both groups (Fig 1D).
Fig 1. Ovariectomy leads to increased body weight and fat mass in MKR mice.
(A) Body weight was followed on a weekly basis from before surgery (day 0) until sacrifice. Results are representatives of two independent experiments and presented as mean ± SEM, n=10. (B) Body composition was analyzed every two weeks. Results are representatives of three last analyses before sacrifice and presented as the ratio between fat mass and lean mass for each mouse + mean, n=6. Panel: body composition results before surgery. (C) Inguinal fat was collected and weight at sacrifice. Results are presented as inguinal fat mass percent of total body weight for each mouse + mean, n=6. (D) Food was weighed every 3-4 days. Food intake is presented as mean ± SEM of daily intake (g) per 100 g mice, n=6. Two-way ANOVA, followed by Bonferroni multiple comparison post-test (A), or student's T-test (B-D) were used to determine statistical significance; *P<0.05, **P<0.01, ***P<0.001 vs. MKR-Sham.
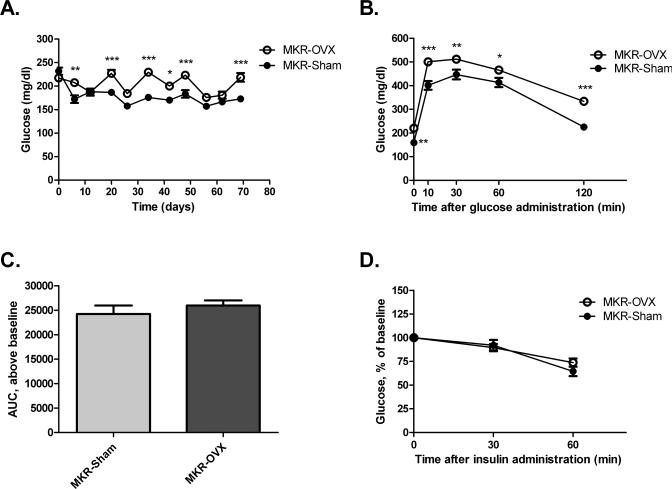
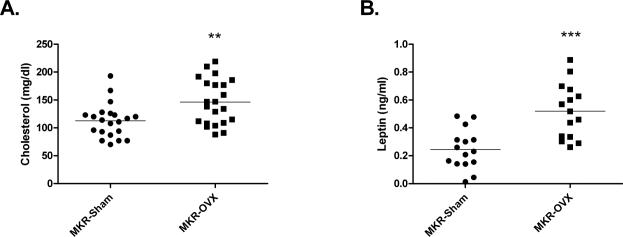
Blood glucose levels on the day of the surgery and two weeks after surgery were similar for both MKR groups (Fig. 2A). In parallel to the increase in body weight, blood glucose levels three weeks after surgery were significantly higher for MKR-OVX mice compared with MKR-Sham mice. Overall, glucose levels remained elevated by about 20% in MKR-OVX group until the end of the experiment. Glucose tolerance and insulin sensitivity were also studied in MKR-OVX and MKR-Sham mice. Fasting glucose (blood glucose at time 0, before glucose load) was significantly higher in MKR-OVX mice compared with MKR-Sham groups (Fig. 2B). Blood glucose levels remained elevated for MKR-OVX group throughout the assay. However, calculation of the area under the curve (AUC) from baseline of each group resulted in similar values (Fig 2C). These data suggest that the elevated glucose levels observed after glucose load emanate from higher baseline glucose levels and that glucose tolerance in ovariectomized MKR mice is similar to sham-operated mice. Insulin resistance (Fig 2D), as well as serum levels of insulin, triglycerides and adiponectin (Table 1) were also similar between the two MKR groups. Serum cholesterol levels were significantly higher by 30% in MKR-OVX mice compared with MKR-Sham group (Fig 3A). Interestingly, MKR-OVX mice showed a two-fold increase in serum leptin levels compared to MKR-Sham mice (Fig 3B).
Fig 2. Ovariectomy leads to increased blood glucose levels under fasting and non-fasting conditions.
(A) Blood glucose levels under non-fasting conditions were followed on a weekly basis from before surgery (day 0) until sacrifice. (B) GTT: glucose (2g/kg) was administered I.P. to fasting mice, and blood glucose was measured at the indicated times. (C) Area under the curve for each group was calculated above the respective baseline using GraphPad Prism software. (D) ITT: insulin (12 IU/kg) was administered I.P. to fasting mice, and blood glucose was measured at the indicated times. Results are representatives of Three independent experiments and presented as mean ± SEM, n=10. Two-way ANOVA, followed by Bonferroni multiple comparison post-test (A,B,D), or student's T-test (C) were used to determine the statistical significance; *P<0.05, **P<0.01, ***P<0.001 vs. MKR-Sham.
Table 1.
Serum analysis of metabolic parameters
| MKR-Sham |
MKR-OVX |
|
|---|---|---|
| Insulin (ng/ml) | 5.35 ± 0.68 | 6.40 ± 0.57 |
| Triglycerides (mg/dl) | 314 ± 50 | 313 ± 55 |
| Adiponectin (μg/ml) | 3.74 ± 0.27 | 4.10 ± 0.26 |
Data represent the mean ± SEM, n≥13.
Student's T-test was used to determine the statistical significance.
Fig 3. Ovariectomy leads to increased cholesterol and leptin levels in serum of MKR mice.
At the end of the experiment, blood samples were taken from the heart of anesthetized mice, and serum was collected and used for (A) cholesterol, and (B) leptin measurements. Results are the sum of data from three independent experiments and presented as raw data + mean, (A) n=21, (B), n=15. Student's T-test was used to determine the statistical significance; **P<0.01, ***P<0.001 vs. MKR-Sham.
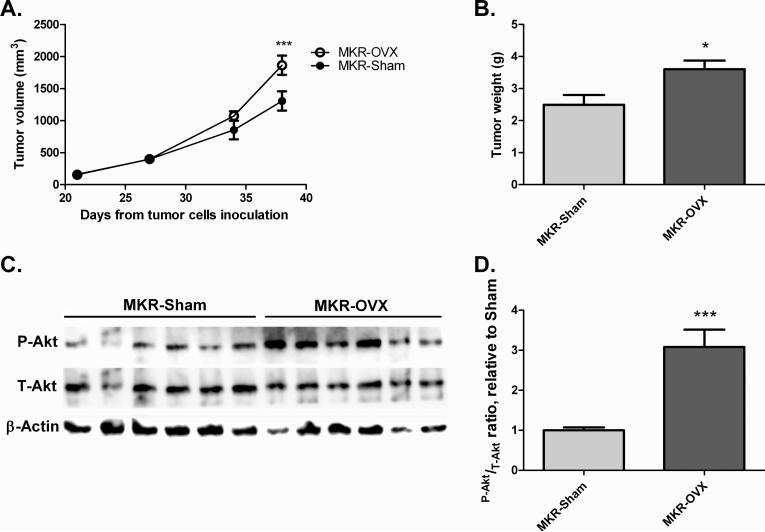
Mvt-1 mammary tumor cells were inoculated into the inguinal mammary fat pad of MKR-OVX and MKR-Sham groups (50,000 cells per mouse). At the time of inoculation, the differences in body weight and glucose homeostasis between MKR-OVX and MKR-Sham mice had stabilized, indicating an existing effect of ovariectomy on the metabolic state of MKR mice. Tumor volume assessments commenced as tumors were detected, and continued on a weekly basis until sacrifice. A gradual increase in the rate of tumor growth was observed in ovariectomized MKR mice (Fig. 4A). At the time of sacrifice (38 days after cells inoculation), tumors of MKR-OVX mice were significantly larger (by ~40%) than MKR-Sham tumors. Also, tumor weights were higher in MKR-OVX mice compared with MKR-Sham mice (Fig. 4B). Western blot analysis of protein extracted from tumors at the end of the experiment showed an increase in phospho-Akt (Thr308) levels by three fold in MKR-OVX mice compared with MKR-Sham mice (Fig 4C and D), indicating that the tumorigenic outcome of ovarian hormone deficiency in MKR mice are mediated through activation of the PI3K/Akt pathway signaling pathway.
Fig 4. Ovariectomy leads to increased mammary tumor growth in MKR mice and activated Akt signaling in inoculated tumor cells.
Mvt-1 mammary tumor cells (50,000 cells) were inoculated into the mammary fat pad of ovariectomized and sham-operated MKR mice. (A) After detection of tumors, tumor volume was assessed at the indicated times. (B) Tumor weight was measured at sacrifice. (C) Protein was extracted from tumors and Western blot analysis was performed using antibodies directed against phospho-Akt (Thr308, P-Akt), total-Akt (T-Akt), and beta-actin. (D) Densitometry analysis was performed using ImageQuant software. Results are representatives of two independent experiments and presented as mean ± SEM, n=6. Two-way ANOVA, followed by Bonferroni multiple comparison post-test (A), or student's T-test (B,D) were used to determine the statistical significance; *P<0.05, ***P<0.001 vs. MKR-Sham.
4. Discussion
The results from this study suggest that ovarian hormones may have a protective effect on the metabolic state and consequently on mammary tumor development in MKR female mice. The effects of ovariectomy in these transgenic mice were evident by the increased body weight and fat mass, elevated blood glucose levels (in both fasting and non-fasting conditions), and in increased mammary tumor growth in ovariectomized mice compared with sham-operated mice.
It is known that estradiol plays a role in the regulation of obesity and fat accumulation both in human and in animal models. The prevalence of obesity markedly increases with the decline in estradiol levels following menopause (Carr 2003). Ovariectomized mice fed a high fat diet (HFD), showed increased body weight and fat mass compared with sham-operated mice (Camporez, et al. 2013). In the present study, we show increased body weight in ovariectomized hyperinsulinemic MKR female mice (fed regular chow) compared with sham-operated mice, which was accompanied by increased body fat mass. The effects of estradiol on body weight are suggested to be mediated by ERα-expressing cells in the hypothalamus, which are involved in the regulation of energy intake and expenditure (Xu, et al. 2011). In the present study, we hypothesize that the differences in body weight and fat mass between MKR-OVX and MKR-Sham mice were due to decreased energy expenditure of ovariectomized mice, since daily food intake was similar for groups. Our hypothesis is supported by previous reports, showing similar effects (Camporez et al. 2013; Rogers, et al. 2009).
The MKR transgenic mouse model has been previously shown to have insulin resistance and elevated insulin levels in serum compared with wild-type mice (Fernandez et al. 2001). We verified these characteristics in both MKR-OVX and MKR-Sham mice compared with age-matched wild-type mice (data not shown). It has been shown that estradiol deficiency leads to hyperglycemia and insulin resistance in postmenopausal women and mouse models (Carr 2003; Heine et al. 2000; Yakar, et al. 2006). In the present study, fasting and non-fasting blood glucose levels were elevated in ovariectomized MKR mice, suggesting that estradiol may be involved in glucose homeostasis in hyperinsulinemic MKR mice. Glucose and insulin tolerance tests, as well as serum insulin levels, were similar for both MKR groups. However, since insulin sensitivity is markedly impaired in both MKR groups, it may be reasonable to assume that ovariectomy would not have further effects on insulin resistance. There are several mechanisms by which estradiol affects glucose homeostasis. These include: (1) increasing the insulin production capacity of β-cells in the pancreas (Choi, et al. 2005); (2) increasing hepatic insulin sensitivity by suppressing liver enzymes involved in gluconeogenesis and glycogenolysis pathways (Bryzgalova, et al. 2006), and (3) inducing expression of glucose transporters, mainly GLUT4, in skeletal muscles, thereby increasing glucose uptake by the tissue (Barros, et al. 2006). Since serum insulin levels and insulin resistance are similar for both MKR groups, we suggest that the increased baseline glucose levels observed in MKR-OVX mice are mainly due to increased glucose output from the liver.
As in the case of energy and glucose homeostasis, estradiol is known to have beneficial effects on lipid profile. Treatment of mice with an ERα- and ERβ-selective agonists resulted not only in reduced weight and fat mass, but also in decreased plasma cholesterol (Lemieux, et al. 2005; Yepuru, et al. 2010). Also, female aromatase knockout mice show hypercholesterolemia (but not hypertriglyceridemia) (Jones, et al. 2000). Moreover, postmenopausal women undergoing HRT showed a significant reduction in total serum cholesterol levels (Espeland, et al. 1998). In the present study, ovariectomy indeed led to increased cholesterol levels. A direct link between estradiol protective effects and cholesterol homeostasis was proposed by De Marinis et al. The authors suggested that the presence of estradiol leads to reduced expression and activity of the enzyme 3-hydroxy 3-methylglutaryl coenzyme A reductase, the rate-limiting enzyme in the synthesis process of cholesterol (De Marinis, et al. 2008).
Ovariectomy in MKR mice led to increased mammary tumor volume and weight. To understand the possible mechanisms underlying the changes in tumor measurements, we investigated the activation of the PI3K/Akt pathway. The PI3K/Akt pathway is activated by hormones and growth factors and has been well established to play a significant role in cell proliferation and survival, thereby leading to tumor growth (Paplomata and O'Regan 2014). Indeed, Akt protein was activated (phosphorylated) to a higher degree in tumor cells inoculated into ovariectomized MKR mice. The mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) pathway, also involved in cell proliferation processes (Makin and Dive 2001), showed similar degree of activation in tumor cells of both MKR groups (data not shown). In general, estradiol is thought to promote breast cancer development by inducing transformation, proliferation, and DNA damage (Preston-Martin, et al. 1990; Russo, et al. 2006) by both ER-dependent and independent mechanisms (Yue, et al. 2013). However, anti-cancer actions of estradiol have also been reported (Anderson, et al. 2004; LaCroix, et al. 2011). These data give rise to an ongoing debate on whether HRT increases or decreases the risk of breast cancer (Chlebowski and Anderson 2012). On the other hand, the association of HRT with reduced risk of diabetes and an overall improvement in metabolic parameters, such as insulin sensitivity, blood glucose levels, obesity and lipids profile is less controversial (Bhupathiraju and Manson 2014; Margolis et al. 2004; Salpeter, et al. 2006). In light of these observations, we suggest that ovariectomy does not affect mammary tumor growth directly, but rather indirectly, through changes in the environment of the tumor cells. The metabolic impairments induced by ovarian hormone deficiency, i.e. increased body weight, accumulation of fat mass, hyperglycemia, and hypercholesterolemia, are good candidates for mediating the tumorigenic outcome of ovariectomy, as they all favor tumor growth (as discussed below). Specifically, Mvt-1 cells do not express ER (data not shown), indicating that mammary tumor growth in our model is independent on the presence of estradiol, further supporting our suggestion.
Studies claim that obesity is a risk factor for breast cancer (Petrelli, et al. 2002; Pierobon and Frankenfeld 2013; Robinson, et al. 2014). Obesity may contribute to mammary tumor development through several mechanisms. Systemically, obesity is associated with insulin resistance and hyperinsulinemia and an increased bioavailability of several growth factors and adipokines, such as leptin, which is known to activate the PI3K/Akt signaling pathway and promote tumor growth, as reviewed by Hursting et al (Hursting, et al. 2012). In addition, obesity leads to subclinical inflammation in adipose tissue, which is characterized by macrophages surrounding necrotic adipocytes and forming crown-like structures (CLS) (Cinti, et al. 2005). The local production of pro-inflammatory mediators in breast tissue, as evident by the increased numbers of CLS in mammary glands of obese mice and women (Morris, et al. 2011; Subbaramaiah, et al. 2011), is known to promote angiogenesis and to increase cancer progression (Sundaram, et al. 2013). Hypercholesterolemia is also associated with risk of breast cancer (Kitahara, et al. 2011). ApoE knockout mice, which demonstrate high cholesterol and triglycerides levels in serum, were shown to have enhanced mammary tumor growth. Cholesterol-induced activation of the PI3K/Akt signaling pathway in tumor cells was suggested as the molecular mechanism linking hypercholesterolemia and tumor growth (Alikhani, et al. 2013). Hyperglycemia, can promote cancer growth by simply providing the energy necessary for cell proliferation and neoangiogenesis (Sciacca, et al. 2013).
Although ovariectomy led to impairments in metabolic homeostasis in MKR female mice, the changes in metabolic parameters were moderate and did not reach the drastic levels of hyperglycemia and glucose intolerance reported previously for male MKR mice (Fernandez et al. 2001), suggesting that other factors, aside from estradiol, may play a role in metabolic control in female MKR model. An important candidate may be progesterone, which has also been implicated in regulation of metabolic homeostasis. However, while estradiol plays a protective role in metabolic control, progesterone promotes metabolic impairments, such as insulin resistance and hyperglycemia (Gonzalez, et al. 2000; Picard, et al. 2002). Thus, it is possible that the moderate metabolic impairments observed in MKR-OVX mice are the result of the interplay between estradiol and progesterone withdrawal caused by removal of ovaries.
Insulin resistance and, subsequently, increased levels of insulin, with its cell proliferation effect, often stand at the basis of the association between T2D and breast cancer progression (Novosyadlyy et al. 2010). Interestingly, both ovariectomized and sham-operated mice in this study are insulin resistant and hyperinsulinemic. Nonetheless, the changes in metabolic parameters observed in ovariectomized MKR mice were able to drive mammary tumor growth at a higher rate than in sham-operated MKR mice. These results suggest that in the face of previously existing insulin resistance and hyperinsulinemia, other metabolic impairments, i.e. obesity, hyperglycemia and hypercholesterolemia, may promote growth of mammary tumors.
Taken together, we suggest a protective role of ovarian hormones on the metabolic state of female MKR transgenic mouse model for T2D. Ovariectomy leads to impaired metabolic homeostasis in MKR mice, manifested by increased body weight and fat mass, as well as hypercholesterolemia and hyperglycemia, which in turn is associated with increased growth of mammary tumors in the face of insulin resistance and hyperinsulinemia in MKR mice. These results highlight the importance of close monitoring and of maintaining balanced body weight, as well as glucose and cholesterol levels, specifically in diabetic postmenopausal women, as impairments in these parameters may favor breast cancer outcome and/or worsening of breast cancer prognosis.
Acknowledgments
Funding
This work was supported by grants from the Diabetes and Metabolism Clinical Research Center of Excellence, Clinical Research Institute at Rambam (to D.L.R.), the Israel Science Foundation grant (to D.L.R.), NCI (grant number 2R01CA12879906A1 to D.L.R.) and NIH/NCI (grant number 1K08CA190770 grant to E.J.G.).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Alikhani N, Ferguson RD, Novosyadlyy R, Gallagher EJ, Scheinman EJ, Yakar S, LeRoith D. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene. 2013;32:961–967. doi: 10.1038/onc.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006;103:1605–1608. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhupathiraju SN, Manson JE. Menopausal Hormone Therapy and Chronic Disease Risk in the Women's Health Initiative: Is Timing Everything? Endocr Pract. 2014;20:1201–1213. doi: 10.4158/EP14205.RA. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Camporez JP, Jornayvaz FR, Lee HY, Kanda S, Guigni BA, Kahn M, Samuel VT, Carvalho CR, Petersen KF, Jurczak MJ, et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154:1021–1028. doi: 10.1210/en.2012-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104:517–527. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SB, Jang JS, Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology. 2005;146:4786–4794. doi: 10.1210/en.2004-1653. [DOI] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker fatty rat. Proc Soc Exp Biol Med. 1983;173:68–75. doi: 10.3181/00379727-173-41611. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- De Marinis E, Martini C, Trentalance A, Pallottini V. Sex differences in hepatic regulation of cholesterol homeostasis. J Endocrinol. 2008;198:635–643. doi: 10.1677/JOE-08-0242. [DOI] [PubMed] [Google Scholar]
- Donahue RP, Bean JA, Donahue RA, Goldberg RB, Prineas RJ. Insulin response in a triethnic population: effects of sex, ethnic origin, and body fat. Miami Community Health Study. Diabetes Care. 1997;20:1670–1676. doi: 10.2337/diacare.20.11.1670. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Marcovina SM, Miller V, Wood PD, Wasilauskas C, Sherwin R, Schrott H, Bush TL. Effect of postmenopausal hormone therapy on lipoprotein(a) concentration. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Circulation. 1998;97:979–986. doi: 10.1161/01.cir.97.10.979. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15:1926–1934. doi: 10.1101/gad.908001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Alonso A, Alvarez N, Diaz F, Martinez M, Fernandez S, Patterson AM. Role of 17beta-estradiol and/or progesterone on insulin sensitivity in the rat: implications during pregnancy. J Endocrinol. 2000;166:283–291. doi: 10.1677/joe.0.1660283. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, Kakarala M, Brodie A, Berger NA. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila) 2012;5:1260–1272. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc Natl Acad Sci U S A. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- Lemieux C, Phaneuf D, Labrie F, Giguere V, Richard D, Deshaies Y. Estrogen receptor alpha-mediated adiposity-lowering and hypocholesterolemic actions of the selective estrogen receptor modulator acolbifene. Int J Obes (Lond) 2005;29:1236–1244. doi: 10.1038/sj.ijo.0803014. [DOI] [PubMed] [Google Scholar]
- Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc Natl Acad Sci U S A. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6:180–185. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin G, Dive C. Modulating sensitivity to drug-induced apoptosis: the future for chemotherapy? Breast Cancer Res. 2001;3:150–153. doi: 10.1186/bcr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE, Nurses' Health S. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care. 2003;26:1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi PA, Molinolo AA, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novosyadlyy R, LeRoith D. Hyperinsulinemia and type 2 diabetes: impact on cancer. Cell Cycle. 2010;9:1449–1450. doi: 10.4161/cc.9.8.11512. [DOI] [PubMed] [Google Scholar]
- Nuutila P, Knuuti MJ, Maki M, Laine H, Ruotsalainen U, Teras M, Haaparanta M, Solin O, Yki-Jarvinen H. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes. 1995;44:31–36. doi: 10.2337/diab.44.1.31. [DOI] [PubMed] [Google Scholar]
- Paik SG, Michelis MA, Kim YT, Shin S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes. 1982;31:724–729. doi: 10.2337/diab.31.8.724. [DOI] [PubMed] [Google Scholar]
- Paplomata E, O'Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6:154–166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei XF, Noble MS, Davoli MA, Rosfjord E, Tilli MT, Furth PA, Russell R, Johnson MD, Dickson RB. Explant-cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In Vitro Cell Dev Biol Anim. 2004;40:14–21. doi: 10.1290/1543-706X(2004)40<14:ECOPMT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- Picard F, Wanatabe M, Schoonjans K, Lydon J, O'Malley BW, Auwerx J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta -cell proliferation. Proc Natl Acad Sci U S A. 2002;99:15644–15648. doi: 10.1073/pnas.202612199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–314. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–7421. [PubMed] [Google Scholar]
- Puah JA, Bailey CJ. Insulinotropic effect of ovarian steroid hormones in streptozotocin diabetic female mice. Horm Metab Res. 1985;17:216–218. doi: 10.1055/s-2007-1013496. [DOI] [PubMed] [Google Scholar]
- Robinson WR, Tse CK, Olshan AF, Troester MA. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993-2001. Cancer Causes Control. 2014;25:1101–1117. doi: 10.1007/s10552-014-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Vigneri R, Tumminia A, Frasca F, Squatrito S, Frittitta L, Vigneri P. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr Metab Cardiovasc Dis. 2013;23:808–815. doi: 10.1016/j.numecd.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog. 2013;12:19. doi: 10.4103/1477-3163.119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CX, Zhu HH, Zhu YM. Diabetes and cancer: Associations, mechanisms, and implications for medical practice. World J Diabetes. 2014;5:372–380. doi: 10.4239/wjd.v5.i3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Nunez NP, Pennisi P, Brodt P, Sun H, Fallavollita L, Zhao H, Scavo L, Novosyadlyy R, Kurshan N, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147:5826–5834. doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, Miller DD, Dalton JT, Narayanan R. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem. 2010;285:31292–31303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–170. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]