Abstract
Objective
Prevalence of vitamin D-deficiency and its association with the risk of cardiovascular disease prompted us to evaluate the effect of vitamin D status on lipid metabolism and atherosclerosis in hypercholesterolemic microswine.
Approach and Results
Yucatan microswine were fed with vitamin D-deficient (0IU/d), vitamin D-sufficient (1,000IU/d) or vitamin D-supplemented (3,000IU/d) high cholesterol diet for 48 weeks. Serum lipids and 25(OH)-cholecalciferol levels were measured biweekly. Histology and biochemical parameters of liver and arteries were analyzed. Effect of 1,25(OH)2D3 on cholesterol metabolism was examined in human HepG2 and THP-1 macrophage-derived foam cells. Vitamin D-deficiency decreased plasma HDL levels, expression of liver-X-receptors (LXRs), ATP binding cassette transporter A1 (ABCA1) and ABCG1, and promoted cholesterol accumulation and atherosclerosis in hypercholesterolemic microswine. Vitamin D promoted nascent HDL formation in HepG2 cells via ABCA1-mediated cholesterol efflux. CYP27B1 and VDR were predominantly present in the CD206 + M2 macrophage foam cell-accumulated cores in coronary artery plaques. 1,25(OH)2D3 increased the expression of LXRs, ABCA1, ABCG1, and promoted cholesterol efflux in THP-1 macrophage-derived foam cells. 1,25(OH)2D3 decreased intracellular free cholesterol and polarized macrophages to M2-phenotype with decreased expression of TNF-α, IL-1β, IL-6 under LPS-stimulation. 1,25(OH)2D3 markedly induced CYP27A1 expression via a VDR-dependent JNK1/2 signaling pathway and increased 27-hydroxycholesterol levels, which induced LXRs, ABCA1 and ABCG1 expression, stimulated cholesterol efflux that was inhibited by VDR antagonist and JNK1/2 signaling inhibitor in THP-1 macrophage-derived foam cell.
Conclusion
Vitamin D protects against atherosclerosis in hypercholesterolemic swine via controlling cholesterol efflux and macrophage polarization via increased CYP27A1 activation.
Keywords: Atherosclerosis, Cholesterol efflux, Macrophage polarization, Vitamin D
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by accumulation of macrophages in the arterial intima, which along with an associated inflammatory response in blood vessel walls initiates the formation and progress of atherosclerotic lesions [1]. In atherosclerotic plaques, macrophage-derived foam cell formation is a hallmark of the progression of atherosclerosis that incites inflammatory rupture of plaques and leads to life threatening cardiovascular complications [2]. It is well established that an atherogenic lipid profile, including increased serum low-density lipoprotein (LDL), promotes the massive accumulation of cholesterol in macrophages, while high-density lipoprotein (HDL) inhibits macrophages-derived foam cell formation via promoting cholesterol efflux and transporting it to the liver for excretion, a process termed reverse cholesterol transport [3]. In atherosclerotic lesions, macrophages are submitted to micro-environmental factors, including cytokines and lipid signals, which might differentiate these cells into morphologically and functionally distinct phenotypes that are usually characterized by the classical pro-inflammatory M1 macrophages and alternatively activated anti-inflammatory M2 macrophages[4]. Several ligand-activated nuclear hormone receptors, such as liver X receptor-α (LXR-α) and peroxisome proliferator activated receptor-β, are important lipid sensors that not only regulate expression of genes involved in lipid metabolism but also regulate macrophage polarization[5].
Vitamin D deficiency has been rising in the general population. Approximately 50% of the population worldwide has low levels of plasma 25-hydroxyvitamin D (25(OH)D3<20 ng/mL), a stable marker of vitamin D status[6]. Beyond its well-defined role in calcium homeostasis, vitamin D has recently been identified as an important factor in cardiovascular health [7,8]. Clinical studies have indicated that plasma 25(OH) D3 level below 20 ng/mL are associated with increased risk for coronary heart disease [9]. Several mechanisms including protection of endothelial function, modulation of immune response, inhibition of vascular smooth muscle cell growth, have been proposed to account for the anti-atherosclerotic effect of vitamin D 7. Recently, several large retrospective studies demonstrated that vitamin D deficiency is associated with atherogenic lipid profile including increased serum LDL and decreased HDL levels [10,11]. High 25(OH)D3 concentrations in the elderly are associated with low prevalence of metabolic syndrome (MetS) with more beneficial HDL-C levels[12]. 1,25(OH)2D3, the biologically active form of vitamin D, suppresses foam cell formation by reducing oxidized LDL uptake in diabetic subjects [13]. However, repletion of 25-hydroxyvitamin D levels in the short-term has been reported not to improve the lipid profile in human [14]. Thus, further studies are warranted to define the exact role of vitamin D on the lipid metabolism and atherosclerosis.
In the present study, we found that vitamin D-deficiency decreased plasma HDL levels and promoted the progression of atherosclerosis in hypercholesterolemic swine via impaired LXRs/ATP-binding membrane cassette transporter A1 (ABCA1) pathway, a crucial regulator in the formation and function of HDL. Our experiments revealed that 1,25(OH)2D3 promotes nascent HDL formation in HepG2 cells via ABCA1-mediated cholesterol efflux. In THP-1 macrophages-derived foam cell, 1,25(OH)2D3 increased the expression of LXRs, ABCA1, ABCG1, and promoted cholesterol efflux, and polarized macrophages to an M2-phenotype with decreased expression of inflammatory cytokines under LPS-stimulation. 1,25(OH)2D3 causes upregulation of CYP27A1 via a VDR-dependent JNK1/2 signaling, which plays a crucial role in activation of LXRs/ ABCA1/ABCG1 pathway.
MATERIALS AND METHODS
Materials and methods are available in the online-only Data Supplement.
RESULTS
1. Vitamin D deficiency decreases plasma HDL level and promotes atherosclerosis in hypercholesterolemic swine
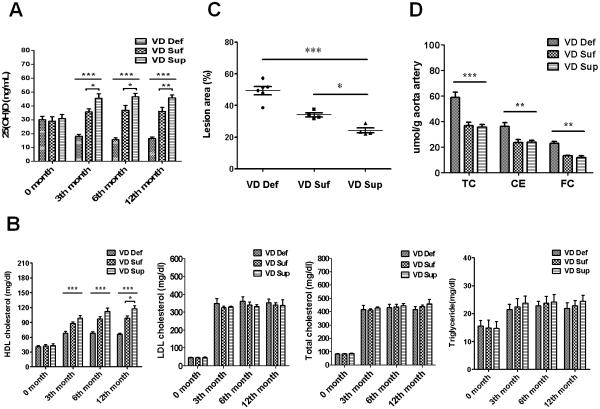
To investigate the effect of vitamin D on the lipid metabolism and the progression of atherosclerosis, microswine were fed on vitamin D-deficient (VD Def), vitamin D- sufficient (VD Suf), and vitamin D-supplemented (VD Sup) high cholesterol diets. As shown in Fig. 1A, VD Def diet produced significant vitamin D deficiency, while VD Sup diet increased the serum level of 25(OH)D3, a stable marker of vitamin D status. Lipid levels of animals fed with high cholesterol diets were increased with time. Compared with VD Suf group, a significant decrease in HDL-C was observed as early as the end of the 3th month in VD Def group (VD Def group, 68.4±7.9 mg/dL; VD Suf group, 87.8±6.2 mg/dL; VD Sup group, 98.5±10.3 mg/dL; p<0.01). Furthermore, the VD Sup group had significantly higher level of HDL-C at month 12 compared with the VD Suf group (VD Sup group, 117.3±12.8 mg/dL; VD Suf group, 98.3±9.6 mg/dL; p<0.05). (Fig. 1B). Compared with VD Suf group, vitamin D deficiency increased while vitamin D supplementation decreased the levels of LDL-C. Nevertheless, there was no statistically significant difference among these groups (Fig. 1B).
Figure 1. Vitamin D deficiency decreases plasma HDL level and promotes atherosclerosis in hypercholesterolemic swine.
Fig. 1A: Plasma 25(OH)-cholecalciferol (D) and Fig.1B: lipid levels in vitamin D deficient (VD Def, n=6), vitamin D sufficient (VD Suf, n=4) and vitamin D supplemented (VD Sup, n=4) hypercholesterolemic swine. Fig.1C: Scatter plot showing lesion areas on the portion of the thoracic aorta of swine fed with the VD def (circle), VD Suf (square), and VD Sup (triangles) diet. Fig. 1D: Chemical analysis of cholesterol and cholesteryl ester in the common carotid arteries of swine. Data are expressed as means ± SEM. Statistical differences between groups were detected by one-way ANOVA with post-hoc analysis using Tukey's test. (*** p< 0.01, VD Def compared to VD Suf or VD Sup; ** p< 0.01, VD Sup compared to VD Suf; * p< 0.05, VD Sup compared to VD Suf).
To investigate the effect of vitamin D status on the atheroma progression in hypercholesterolemic swine, the histological analyses of fatty streak in thoracic aorta from different treatment groups were compared. As shown in Fig. 1C, relative aortic fatty streak lesion area was 49.35±6.17% for the VD Def group, 34.11±2.56% for the VD Suf group, and 24.28±3.05% for the VD Sup group (p <0.01, VD Def compared to VD Suf or VD Sup; p <0.05, VD Sup compared to VD Suf), suggesting that vitamin D deficiency resulted in a more remarkable progress in aortic fatty streak lesions formation under high-cholesterol diet. To further evaluate the extent of atherosclerosis, common carotid arteries from three dietary groups were analyzed for total cholesterol and cholesteryl ester deposition (Fig. 1D). Total cholesterol contents of the arteries were significantly higher in the VD Def group than in swine from the VD Suf or VD Sup group (p <0.01, 58.9±9.81 vs 36.8±5.28 μmol/g artery or 58.9±9.81 vs 35.5±4.44 μmol/g artery). Free cholesterol content in the common carotid artery was significantly increased by 73.2% or 93.2% in the VD Def group compared to VD Suf or VD Sup groups, respectively; further suggesting that vitamin D deficiency exacerbates the lipid accumulation and atherosclerosis in hypercholesterolemic microswine.
2. Vitamin D deficiency decreases 27-hydroxycholesterol (HOC) level in liver of hypercholesterolemic swine
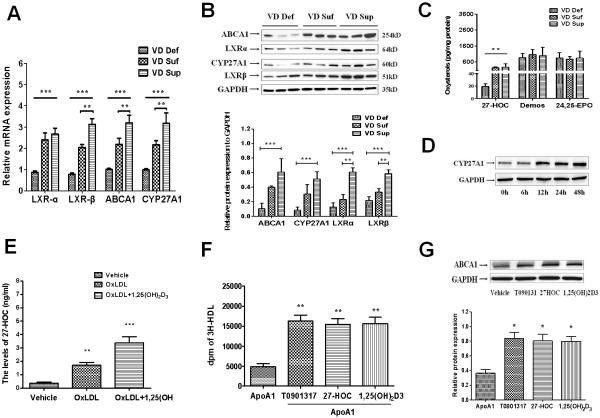
To examine the molecular signaling targets that could explain the difference in HDL levels response to vitamin D status, we first performed microarray analysis to examine differentially expressed genes in liver, which is the major organ for the synthesis of HDL. A total of 24,124 genes were examined using the porcine GeneChip for liver of pigs (n=4 for each treatment groups). A total of 342 mRNAs were identified as being up- or down-regulated more than 2-fold in the VD Def group compared with VD Suf and VD Sup groups. Of these genes, 38 genes were regulated in a VitD dose-dependent manner. Analysis of these genes using Gene Ontology Biological Process revealed differential expression of genes primarily regulates the following biological functions: oxidative stress, lipid metabolism and inflammatory response, and cell signaling and interactions (Table I in the online-only Data Supplement). These results support the concept that in addition to its established role in regulating calcium homeostasis, vitamin D appears to play an important role in the modulation of metabolic inflammatory diseases, including atherosclerosis. Prominent effects of vitamin D on the genes in the lipid metabolism were noted. Interestingly, the transcription of genes encoding oxysterols receptor LXRs pathway, ligand-activated transcription factors that raise HDL-C levels, were significantly decreased in the VD Def group (Table I in the online-only Data Supplement and Fig. 2A). After further validation by Western-blot, the key proteins in the LXRs pathway, including LXR-α and LXR-β, its target gene ABCA1 and steroid catabolic gene CYP27A1 were decreased in VD Def group compared to VD Suf and VD Sup groups (Fig. 2B). CYP27A1 plays a crucial role in the biogenesis of oxysterols, crucial ligands for the activation of LXRs. To clarify whether the regulated effect of vitamin D on the HDL metabolism is related to the oxysterol metabolism, the levels of steroid catabolic intermediates, including 27-hydroxycholesterol (HOC), desmosterol, and 24,25-epoxycholesterol in the liver were measured in the three groups. Compared with VD Suf and VD Sup groups, vitamin D-deficiency decreased the levels of 27-HOC. However, there was no significant difference in the levels of desmosterol and 24,25- epoxycholesterol in the three groups (Fig. 2C). In the cultured HepG2 cell, 1,25(OH)2D3 (10nM) treatment substantially upregulated the expression of CYP27A1 in a time-dependent manner (Fig. 2D) and increased 27-HOC concentrations in oxLDL-loaded HepG2 cells (Fig. 2E).
Figure 2. Vitamin D deficiency decreases 27 hydroxycholesterol (HOC) level in liver of hypercholesterolemic swine.
Fig. 2A: The mRNA expression of LXR-α, LXR-β, ABCA1, and CYP27A1 in liver were determined by quantitative real-time PCR. Fig. 2B: The expression of LXR-α, LXR-β, ABCA1, and CYP27A1 in liver were determined by Western-blot. Fig. 2C: The oxysterol levels in liver of hypercholesterolemic swine were determined by HPLC. Statistical differences between groups were detected by one-way ANOVA and Student's t test (***p < 0.01, VD Def compared to VD Suf or VD Sup; **p < 0.05, VD Sup compared to VD Suf, n=3). Fig. 2D: HepG2 cells were cultured on six-well plates. Cells were treated with either vehicle (ethanol) or 1,25(OH)2D3 (10nM) for 0, 3h, 6h, 12h and 24h. The protein was isolated for western-blot analysis of CYP27A1 expression. Fig. 2E: Cells were treated with either vehicle (ethanol), oxLDL (50μg/ml) and oxLDL (50μg/ml) + 1,25(OH)2D3 (10nM) for 24h. The 27-HOC level in HepG2 cells was determined by HPLC (***p < 0.01, oxLDL+1,25(OH)2D3 compared to oxLDL only or vehicle; **p < 0.05, 1,25(OH)2D3 compared to vehicle, n=3). Fig. 2F: HepG2 cells were treated with LXR agonist T0901317 (5μM), 27-HOC (1μM), and 1,25(OH)2D3 (10nM), respectively, for 18h and then incubated with lipid free apoA-I (25μg/ml) for 12h. The radioactivity in [3H]-HDL fraction was determined by liquid scintillation counting (** p < 0.05, compared to apoA-I alone treatment, n=3). Fig. 2G: The expression of ABCA1 in HepG2 cells was assessed by Western blot analysis. Values are means ± SEM for n=3 per group. (* p < 0.05, compared to apoA-I alone treatment, n=3).
The activation of LXRs by oxysterol ligands induces the expression of ABCA1, which interacts with apoA-I and promotes the cholesterol efflux that plays a crucial role in the biogenesis of nascent HDL [15, 16]. Since 1,25(OH)2D3 increases 27-HOC production in HepG2 cells, we investigated whether 1,25(OH)2D3 plays a role in nascent HDL formation. After labeling overnight with [1,2-3H(N)]cholesterol, HepG2 cells were treated with LXR agonist T0901317 (5μM), 27-HOC (1μM), and 1,25(OH)2D3 (10nM), respectively, for 18h and then incubated with lipid-free apoA-I (25μg/ml) for 12h. The medium was collected and filtered through a 0.45 μm PVDF membrane to separate non-HDL and HDL. The radioactivity in [3H]-HDL fraction was determined by liquid scintillation counter. We found that the 1,25(OH)2D3 as well as LXR agonist T0901317 and 27-HOC significantly increased cholesterol efflux to apoA-I (Fig. 2F). Following the incubation, ABCA1 protein expression in HepG2 cells was markedly increased by T0901317, 27-HOC and 1,25(OH)2D3 (Fig. 2G). Overall, these data suggest that 1,25(OH)2D3 promotes the nascent HDL formation in HepG2 cells by upregulating CYP27A1 activity and ABCA1 expression.
3. Vitamin D increases cholesterol efflux and M2 polarization in coronary artery of hypercholesterolemic swine
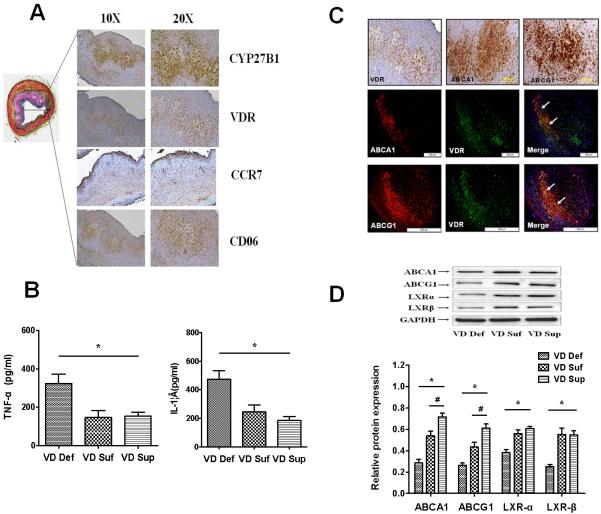
To determine the exact mechanism of vitamin D on the inhibition of atherosclerotic progress, we stained coronary plaques from hypercholesterolemic swine. As shown in Fig. 3A, vitamin D metabolism, signal-related proteins including CYP27B1 and VDR were predominantly present in the macrophage foam cell- accumulated cores of plaques in coronary artery. Vitamin D3 and its biologically active form, 1,25(OH)2D3, have been confirmed to have crucial effects on macrophage function via binding to its receptor VDR[7]. Therefore, this data suggest that vitamin D- mediated inhibition of atherosclerosis may be the result from the direct effect of vitamin D on arterial wall besides its effect on HDL levels. Macrophages, with extreme polarization phenotypes M1 and M2 macrophages, are the major cell components in atherosclerotic lesion that contribute to the foam cell formation and secretion of inflammatory factors. In order to clarify the exact role of vitamin D in macrophage function, we compared the staining intensity between M1 marker CCR7 and M2 marker mannose receptor (CD206) in the regions of CYP27B1-positive and VDR-positive cores in plaques. We found significantly greater staining of M2 macrophage marker, CD206, in VDR-positive area, while pro-inflammatory M1 macrophage marker CCR7 showed minimal immunoreactivity in this area (Fig. 3A), suggesting that vitamin D may play a role in the regulation of macrophage differentiation and inflammatory response in atherosclerotic lesion. To verify this hypothesis, we examined the expression of pro-inflammatory cytokines (TNF-α and IL-1β) in the left common carotid arteries. As shown in Fig. 3B, there was significantly increased level of pro-inflammatory cytokines in the VD Def group compared to VD Suf or VD Sup groups (p <0.05, VD Def vs VD Suf or VD Sup).
Figure 3. Vitamin D increases cholesterol efflux and M2 macrophage polarization in artery of hypercholesterolemic swine.
Fig.3A: Vitamin D signal-related CYP27B1 and VDR are expressed at sites of M2 macrophage (CD206 positive) foam cells in coronary atherosclerotic lesions of hypercholesterolemic swine (Left= Movat stain, Right = Immunohistochemistry); Fig. 3B: Proteins from the common carotid arteries of different treatment groups of swine were isolated and analyzed to determine the levels of inflammatory cytokines TNF-α and IL-1β by ELISA. Fig. 3C: ABCA1/ABCG1 are co-expressed with VDR at sites of M2 macrophage foam cells in coronary atherosclerotic lesions of hypercholesterolemic swine; Fig. 3D: Western-blot analysis of ABCA1, ABCG1, LXRα and LXR β expression in the common carotid arteries of different hypercholesterolemic swine. Values are means ± SEM for n=3 animals per group. Statistical differences between groups were detected by one-way ANOVA (*p < 0.05, VD Def compared to VD Suf or VD Sup; #p < 0.05, VD Sup or VD Suf, n=3)
As shown in Fig. 3C, ABCA1 and ABCG1, another LXRα target genes primarily expressed in macrophages, are mainly co-expressed with VDR at the sites of M2 macrophage foam cells in coronary atherosclerotic lesions of hypercholesterolemic swine, suggesting that the ABCA1/ABCG1 in macrophage might be regulated targets for the vitamin D treatment. Therefore, we next examined the effect of vitamin D on the expression of LXRα/β, ABCA1, and ABCG1 in macrophages. Western-blot results in Fig. 3D showed that the vitamin D level positively correlated to the expression of ABCA1, ABCG1 and LXRα/β in artery of hypercholesterolemic swine, suggesting that the enhanced effect of vitamin D deficiency on atherosclerosis might be related to the impaired cholesterol efflux in the arterial wall.
4. 1,25(OH)2D3 promotes cholesterol efflux and M2 polarization in macrophage- derived foam cells via upregulating 27HOC
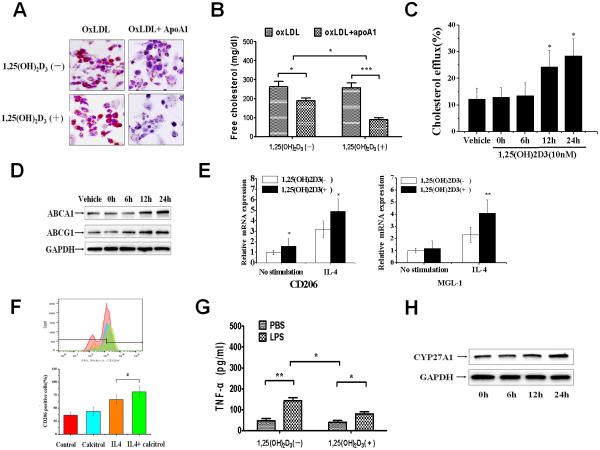
To further determine whether 1,25(OH)2D3 altered cholesterol efflux in macrophages, we analyzed the effect of 1,25(OH)2D3 on ABCA1-mediated cholesterol efflux in THP-1 macrophage-derived foam cells. As shown in Fig. 4A, oxLDL-induced macrophage-derived foam cells cultured in 1,25(OH)2D3 deficient media exhibited a significantly greater staining in oil red after treatment with apoA-I compared with macrophage-derived foam cells cultured in 1,25(OH)2D3-supplemented media. Next, we examined the effect of 1,25(OH)2D3 on cholesterol content and cholesterol efflux in THP-1 macrophage-derived foam cells. As shown in Fig. 4B–C, the ability of apoA-I to decrease cholesterol content and to promote cholesterol efflux in THP-1 macrophage-derived foam cell was significantly increased in 1,25(OH)2D3- supplemented media compared with 1,25(OH)2D3-deficient media. To determine whether the increased ability of apoA-I to promote cholesterol efflux in 1,25(OH)2D3- supplemented media is related to cholesterol transporters, we analyzed the expression of ABCA1 and ABCG1 in these cells. Analysis of protein levels by Western blotting demonstrated a 1,25(OH)2D3-induced upregulation in the levels of ABCA1, and ABCG1 expression in THP-1 macrophage-derived foam cells (Fig.4D).
Figure 4. 1,25(OH)2D3 promotes cholesterol efflux and M2 polarization in macrophage-derived foam cells via upregulating 27HOC.
Fig. 4A: THP-1 macrophages were treated with oxLDL (50 μg/ml) for 48 h, then cultured with apoA1(25 μg/ml) under 1,25(OH)2D3-deficient medium (Top) or 1,25(OH)2D3 (10nM)-supplement medium (bottom) for 24h. Macrophages were stained with Oil Red O. Fig. 4B: The intercellular free cholesterol (FC) was determined by the cholesterol quantitation kit. n = 3; ***p<0.001, **p<0.01, *p<0.05, two-way analysis of variance (ANOVA) with Bonferroni's post-hoc test. Fig. 4C: Liquid scintillation counting was performed to determine the intercellular cholesterol efflux. Fig. 4D: THP-1 macrophage-derived foam cells were treated with 1,25(OH)2D3 (10nM) for 0, 6h, 12h and 24h, the expression of ABCA1 and ABCG1 were determined by western-blot. Fig. 4E: After cultured with 1,25(OH)2D3 (10 nM) for 24h, THP-1 macrophage-derived foam cells were treated with IL-4 (20ng/ml) for 6h. The M2 marker (CD206 and MGL-1) mRNA levels in cells were determined by quantitative real-time PCR. Fig. 4F: Representative histograms of CD206/mannose receptor (MR) in THP-1 macrophage-derived foam cells exposed to vehicle (Ctrl), 1,25(OH)2D3 (10 nM), IL-4 (20ng/ml), or IL-4+1,25(OH)2D3. Fig. 4G: After cultured with 1,25(OH)2D3 (10nM) for 24h, THP-1 macrophage-derived foam cells were treated with LPS (10ng/ml) for 12h. The level of TNFα was quantified by ELISA. n = 3; **p<0.01, *p<0.05, two-way analysis of variance (ANOVA) with Bonferroni's post-hoc test. Fig. 4H: THP-1 macrophage derived foam cells were treated with either vehicle (ethanol) or 1,25(OH)2D3 (10 nM) for 0, 6, 12, 24h. The expression of CYP27A1 was determined by Western-blot.
Macrophages-derived foam cells from atherosclerotic subjects have been shown to exhibit classic (M1) pro-inflammatory phenotype [5,17]. LXR-α activation and increasing ABCA1 levels have been found to inhibit inflammation and induce expression of IL-10, a marker of the M2 macrophage phenotype [18,19]. To investigate whether the increased activation of LXR-α induced by 1,25(OH)2D3 results in the macrophage phenotypic polarization, we compared the expression of M1 or M2 marker in basal, interleukin (IL)-4 or LPS condition. Compared with 1,25(OH)2D3-deficient media, the macrophages from 1,25(OH)2D3-supplemented media have a significant increase in IL-4 stimulated mRNA expression of CD206 and MGL-1 (Fig. 4E). Cells were analyzed by fluorescence activated cell sorter using anti-mannose receptor CD206 antibody. As shown in Fig. 4F, 1,25(OH)2D3 robustly increased IL-4-induced expression of CD206, suggesting 1,25(OH)2D3 promotes the M2 polarization of macrophage- derived foam cells. In vitro stimulation with LPS demonstrated that macrophages from 1,25(OH)2D3-supplemented media produced significantly less pro-inflammatory cytokines, including TNF-α and IL-1β, compared with macrophages from 1,25(OH)2D3-deficient media (Fig. 4G). These results support the concept that 1,25(OH)2D3 in macrophage is anti-atherogenic via promotion of cholesterol efflux and anti-inflammatory macrophage polarization by upregulating LXR-α pathway.
To clarify whether the regulated effect of 1,25(OH)2D3 on macrophage is dependent on 27HOC, we investigated the effect of 1,25(OH)2D3 on macrophage CYP27A1 expression. 1,25(OH)2D3 treatment substantially increased the expression of CYP27A1 in THP-1 macrophage-derived foam cells (Fig. 4H). We also examined the effect of 27-HOC on the expression of ABCA1 and ABCG1, and found that 27-HOC induced ABCA1 and ABCG1 expression in a dose-dependent manner in THP-1 macrophage-derived foam cells (Figure I in the online-only Data Supplement). These results suggest that the 27-HOC/LXR-α pathway plays a crucial role in the regulation of macrophage cholesterol efflux and anti-inflammatory effect of 1,25(OH)2D3.
5. 1,25(OH)2D3 induces 27-hydroxycholesterol in a VDR-dependent JNK signal manner
CYP27A1 expression is controlled by activated JNK signal [20,21]. In multiple cell types, the JNK pathway has been demonstrated to be activated by 1,25(OH)2D3[20–22]. After treatment with 1,25(OH)2D3 for 30 min, the phosphorylation of JNK1/2 (p-JNK1/2) in THP-1 macrophage-derived foam cells was detected, and it was further enhanced up to 3h (Fig. 5A). We further used JNK signal inhibitor SP600125 to determine whether 1,25(OH)2D3 is exerting its CYP27A1 induction effects through activated JNK signal. THP-1 macrophage-derived foam cells were exposed to SP600125 and/or 1,25(OH)2D3. SP600125 almost completely blocked 1,25(OH)2D3-induced expression of p-JNK1/2 in THP-1 macrophages (Fig. 5B). The ability of 1,25(OH)2D3 to induce CYP27A1, ABCA1 and ABCG1 mRNA expression in THP-1 macrophage-derived foam cells was significantly impaired by SP600125 (Fig. 5C), suggesting that 1,25(OH)2D3-induced CYP27A1 expression is mediated by the JNK signaling pathway.
Figure 5. 1,25(OH)2D3 induces CYP27A1 in a VDR-dependent JNK/SAPK signal manner.
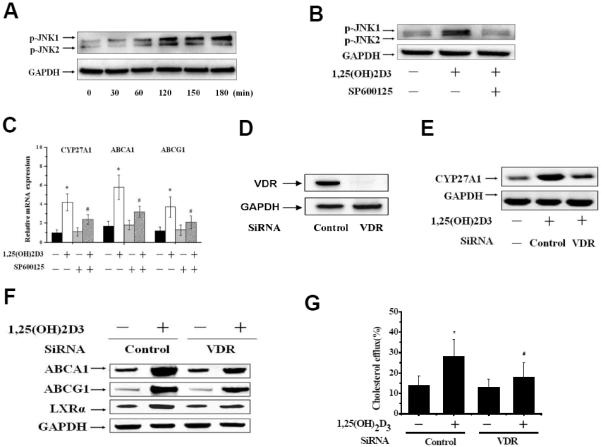
Fig. 5A: THP-1 macrophage-derived foam cells were cultured with either 1,25(OH)2D3 (10nM) or vehicle at the indicated time point. Cells were harvested and subjected to Western blot analysis to monitor the levels of p-JNK1/2. Fig. 5B: THP-1 macrophages were treated with 1,25(OH)2D3 (10nM) or JNK inhibitor SP600125 (2μM) for 3h. Total proteins were subjected to immunoblot analyses with antibody against p-JNK1/2. Fig. 5C: THP-1 macrophage-derived foam cells were treated with SP600125 (2μM) for 30 min, followed by stimulation with 1,25(OH)2D3 (10nM) for 24 h. Total RNA were subjected to real-time PCR for CYP27A1, ABCA1 and ABCG1(*p<0.01 compared to untreated groups, #p<0.01 compared to 1,25(OH)2D3 groups,. Fig. 5D: THP-1 macrophage-derived foam cells were transfected with control or VDR siRNA for 48h, protein samples were immunoblotted with anti-VDR antibody. Fig. 5E: THP-1 macrophage-derived foam cells transfected with control (WT) or VDR siRNA (VDR−/−) were treated with 1,25(OH)2D3 for 24h. CYP27A1 was analyzed by Western-blot. Fig. 5F: ABCA1, ABCG1, and LXR-α expression were analyzed by Western-Blot; Fig. 5G: The intercellular cholesterol efflux was determined by liquid scintillation counting. *p<0.01 compared to other groups. The data represent the mean ± SE from three separate experiments with triplicate samples.
To test the role of VDR on the effect of 1,25(OH)2D3 on CYP27A1 in THP-1 macrophage-derived foam cells, we first incubated cells with 100 nM VDR siRNA, and the expression of VDR was almost completely inhibited by siRNA (Fig. 5D). The ability of 1,25(OH)2D3 to induce expression of CYP27A1 in THP-1 macrophage- derived foam cells was also significantly inhibited in VDR siRNA-treated cells (Fig. 5E). We then confirmed a role of VDR in the 1,25(OH)2D3-mediated increase of cholesterol efflux in VDR knockdown cells. The effect of 1,25(OH)2D3 on ABCA1, ABCG1 and LXRα expression in VDR siRNA cells was significantly abolished compared with control siRNA-untreated cells (Fig. 5F), indicating a VDR-dependent effects of 1,25(OH)2D3 on LXRα and its target genes. We further examined the role of VDR in the 1,25(OH)2D3-induced cholesterol efflux using liquid scintillation counter. The ability of 1,25(OH)2D3-induced cholesterol efflux in VDR siRNA cells was markedly decreased compared to control siRNA-treated cells (Fig. 5G).
DISCUSSION
Although clinical studies suggest that vitamin D deficiency is related to a higher risk for cardiovascular disease, the exact role of vitamin D in the progression of cardiovascular diseases has not been well defined [23]. Because a higher plasma vitamin D concentration is usually associated with a healthier lifestyle, it is unclear whether vitamin D status is causally related to disease or is merely a marker of health [8,10]. In this study, we fed hypercholesterolemic swine with different vitamin D diet resulting in different plasma 25(OH)D levels that provide the direct evidence for the effect of vitamin D status on the progress of the disease. We found that vitamin D deficiency promoted the progress of atherosclerosis and decreased plasma HDL-C level, a crucial cardiovascular protective factor, under hypercholesterolemic condition. Supplementation of vitamin D3 in the diet (3,000 IU per day × 24 weeks) significantly prevented the development of atherosclerosis and increased the level of HDL-C, suggesting the beneficial effect of vitamin D3 supplementation in modifying lipid profile and decreasing cardiovascular disease. In a randomized clinical study including 57 postmenopausal vitamin D deficient women, Catalano et al. found that the supplementation with 25(OH)D3 (calcifediol) for 24 weeks significantly increased HDL-C level even after adjustment for age, baseline BMI, 25(OH)D3 and lipid levels[24]. In another randomized double-blind placebo-controlled clinical trial including 70 participants with type 2 diabetes it was found that the supplementation of calcitriol (1,25(OH)2D3, 0.5 μg per day) for 12 weeks increased the HDL-C level compared to the placebo group[25]. However, Ponda et al. have reported no improvement in the lipid profile following short-term supplementation of vitamin D3 (50,000 IU of vitamin D3 weekly for 8 Weeks) in vitamin D-deficient adults with elevated risk for cardiovascular disease, suggesting that the dose and duration of treatment determine the outcomes and this remains to be elucidated.
Oxysterols are the endogenous ligands for the LXRs, ligand-activated transcription factors that can regulate a crucial checkpoint in cholesterol homeostasis [26]. The sterol 27-hydroxylase (CYP27A1) is a mitochondrial cytochrome P450 and is involved in the synthesis of oxysterols, 27-hydoxycholesterol (HOC), 3ß-hydoxy-5-cholestenoic acid and 25(OH)D3, which play a crucial role in cholesterol, bile acids and vitamin D metabolism[27]. It was reported previously that vitamin D3 and its biologically active form, 1,25(OH)2D3, can regulate expression of various cytochromes P450 (CYP) [28–30]. Utilizing a microarray method, we found that vitamin D-deficiency induces a response for the expression of the mRNA of genes associated with oxidative stress, lipid metabolism, and inflammation pathways in the liver. Vitamin D-supplementation increased liver mRNA levels of oxidation-reduction and oxysterol-related CYP genes, including CYP1A1, CYP1A2, CYP2J34, and CYP27A1. In addition, we found that vitamin D upregulates the expression of LXRs and ABCA1 in liver in a dose-dependent manner.
ABCA1 is a lipid transport protein transcriptionally regulated by nuclear receptors LXRα, which facilitates cellular cholesterol efflux to lipid-poor apoAI and plays a key role in the formation and function of HDL [31]. In mice, specific lack of ABCA1 in hepatocytes almost totally reduces the circulating HDL-C level [32]. Mutations of ABCA1 in human underlie the HDL deficiency syndrome, Tangier disease, suggesting that primarily hepatic ABCA1 determines the generation of nascent HDL particles [31,32]. To clarify whether the regulated effect of vitamin D on the HDL metabolism is related to the ABCA1 expression, we investigated the effect of 1,25(OH)2D3 on the expression of ABCA1 and nascent HDL formation in hepatocytes, and found 1,25(OH)2D3 promoted cellular cholesterol efflux to lipid-poor apoAI, a major component of nascent HDL via upregulating ABCA1 expression. In several cholesterol-loaded cells, including hepatocytes and macrophages, LXR-α/ABCA1 pathway has been found to be upregulated to promote the cholesterol efflux via a 27-HOC dependent manner [33,34]. In this study, we have shown for the first time that 1,25(OH)2D3 facilitates the upregulation of LXR-α/ABCA1 in cholesterol-loaded hepatocytes and macrophages via promoting the expression of CYP27A1. This effect could be supported by a recent clinical data, showing a significant positive correlation between the levels of 27-OHC and 25(OH)D3 under hypercholesterolemic condition [35]. Interestingly, the LXR target genes such as ABCG5/8, SREBP-1c, and SR-B1 were not found to be regulated by 1,25(OH)2D3 in hepG2 cells (Figure II in the online-only Data Supplement). The conserved consensus cis-acting element DR4-dependent transcription is necessary for LXR-α-induced ABCA1 expression in HepG2 cells [36]. The activation of VDR by 1,25(OH)2D3 has been found to recruit co-activators at DR4-type response element that leads to the expression of target genes [37,38]. These results suggest that the recruitment of co-activators at the response element induced by 1,25(OH)2D3 may be playing a critical role in supporting the transactivation of LXR-α/ABCA1 pathway, though the exact mechanisms remain to be further examined.
A number of studies have shown that 1,25(OH)2D3 can regulate the differentiation, maturation and function of macrophages[7,39,40], which prompted us to investigate whether the anti-atherogenic effects of vitamin D results from the direct effect of vitamin D on the arterial wall. In this study, we found VDR and CYP27B1, a key enzyme to control the synthesis of 1,25(OH)2D3, are mainly expressed at the M2 macrophage foam cell-accumulated cores of plaques in the coronary artery. Vitamin D regulates the macrophage M1/M2 phenotype in diabetic nephropathy rats36. In vitro, 1,25(OH)2D3 has also been found to switch high glucose-induced M1/M2 polarization to suppress cholesteryl ester formation and to enhance cholesterol efflux in M2 macrophages [41,42]. In atherosclerotic lesion, we found that VDR is primarily co-expressed with ABCA1/ABCG1 at M2 macrophage-derived foam cell. In vitro, 1,25(OH)2D3 has been found to promote the cholesterol efflux and polarize macrophages to pro-inflammatory M2-phenotype. In macrophages, ABCA1 and ABCG1, another ABC family of transporter, play critical role in mobilizing cholesterol out of macrophages and onto extracellular HDL [43]. In addition to its lipid transport function, ABCA1 and ABCG1 have been found to play a crucial role in the regulation of cytokine-triggered macrophage polarization programs and inflammatory response [44,45]. Several studies have shown that the cholesterol export activity of ABCA1/ABCG1 could account for its potent anti-inflammatory properties [46,47]. Knockdown of ABCA1 in macrophage results in an increase of cell membrane cholesterol and lipid raft content, which facilitate toll-like receptor 4 - MyD88-mediated pro-inflammatory response [48]. In this study, we found 1,25(OH)2D3 markedly induced the expression of ABCA1 and ABCG1 in macrophages, suggesting that enhanced cholesterol efflux may play a crucial role in anti-inflammatory effect of vitamin D.
Previously, JNK/c-jun pathway has been reported to upregulate the expression of CYP27A1 in hepatocytes [20]. 1,25(OH)2D3 activates the JNK/c-jun pathway in various cell lines[21,22,49]. Consistently, our study demonstrated that 1,25(OH)2D3 upregulates the phosphorylation of JNK in macrophages. Furthermore, inhibitor of JNK activation blocks 1,25(OH)2D3-induced upregulation of CYP27A1 and 27HOC content, suggesting that JNK/c-Jun activation plays an important role in anti-atherogenic activity of vitamin D. Both VDR and LXRα are crucial lipid-activated nuclear receptors that shape macrophage function [50]. Earlier studies have shown that VDR is regulated by LXRα through its ability to modulate sterol regulatory element binding protein present in the promoter region of VDR gene [51]. In our study, we found that LXRα pathway is also regulated by VDR through its ability to modulate CYP27A1 and 27HOC (Fig. 6), suggesting the existence of crosstalk between LXR and VDR that stimulates the intracellular pathway to exert the anti-atherogenic effect.
Figure 6. Schematic representation of vitamin D regulation of nascent HDL formation and macrophage polarization via promoting cholesterol efflux in hepatocytes and macrophage-derived foam cells.
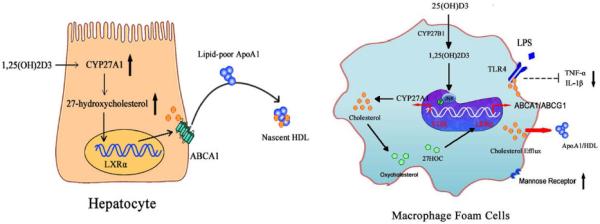
In hepatocytes (Left), the 1,25(OH)2D3 increases CYP27A1 expression as well as the production of the endogenous LXR ligand, 27-hydroxycholesterol(27HOC), which stimulated ABCA1-mediated cholesterol efflux and nascent HDL formation. In macrophages-derived foam cells (Right), 1,25(OH)2D3 markedly induced CYP27A1 expression and increased 27HOC levels, which induced LXRα, ABCA1 and ABCG1 expression, stimulated intercellular cholesterol efflux, reduced the cholesterol accumulation and attenuated LPS induced M1-polarization via a VDR-dependent JNK/SAPK signal manner.
In conclusion, the findings in this study revealed a novel mechanistic link between vitamin D deficiency and cardiovascular risk. Vitamin D supplementation significantly increased the levels of plasma HDL, and the expression of 27HOC/LXRα pathway, which contributes to alleviate cholesterol toxicity and macrophage pro-inflammatory polarization, resulting in protection against the development of atherosclerosis.
Supplementary Material
Significance.
Epidemiological studies suggest that vitamin D deficiency is related to a pro-atherogenic lipid profile and inflammation. However, the exact role of vitamin D in the progress of atherosclerosis has not been fully elucidated. Here, we found that vitamin D deficiency accelerated the development of atherosclerosis in hypercholesterolemic swine, which is very similar to the lipid metabolism and the progress of atherosclerosis in human. Vitamin D deficiency results in impaired cholesterol efflux in the liver and artery that lead to decreased high-density lipoprotein (HDL) level, cholesterol accumulation and M1 macrophage polarization in vascular wall. The 1,25(OH)2D3 markedly induced CYP27A1 expression and increased 27HOC levels, which induced cholesterol efflux. These results suggest that vitamin D supplementation might serve as a novel therapeutic modality to increase HDL level and decrease severity of atherosclerosis in patients with cardiovascular disease risk.
Acknowledgement
None
Sources of Funding: This work was supported by research grants HL112597, HL116042 and HL120659 from the National Institutes of Health, USA to DK Agrawal. Kai Yin is supported, in part, by the research grants 81100213 and 81470569 from National Natural Science Foundation of China (NSFC).
The content of this research article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and NSFC.
Abbreviations
- ABCA1
ATP binding cassette transporter A1
- ABCG1
ATP binding cassette transporter G1
- CYP
cytochrome P450
- HDL
high density lipoprotein
- HOC
27-hydroxy cholesterol
- LDL
low density lipoprotein
- LXR
liver-X-receptor
- MetS
metabolic syndrome
- VD
vitamin D
- VD-Def
vitamin D deficient
- VD-Suf
vitamin D sufficient
- VDR
vitamin D receptor
Footnotes
Disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
REFERENCES
- 1.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sene A, Khan AA, Cox D, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17:549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das R, Ganapathy S, Mahabeleshwar GH, Drumm C, Febbraio M, Jain MK, Plow EF. Macrophage gene expression and foam cell formation are regulated by plasminogen. Circulation. 2013;127:1209–1218. doi: 10.1161/CIRCULATIONAHA.112.001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153–166. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- 5.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1120–1126. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG. Role of vitamin D in atherosclerosis. Circulation. 2013;128:2517–2531. doi: 10.1161/CIRCULATIONAHA.113.002654. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 10.Ponda MP, Huang X, Odeh MA, Breslow JL, Kaufman HW. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation. 2012;126:270–277. doi: 10.1161/CIRCULATIONAHA.111.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 12.Vitezova A, Zillikens C, van Herpt T, Sijbrands E, Hofman A, Uitterlinden A, Franco O, Kiefte-de Jong J. Vitamin D status and metabolic syndrome in the elderly: the Rotterdam Study. Eur J Endocrinol. 2015;172:327–335. doi: 10.1530/EJE-14-0580. [DOI] [PubMed] [Google Scholar]
- 13.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short-term effects of vitamin D repletion on cholesterol: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2012;32:2510–2515. doi: 10.1161/ATVBAHA.112.254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staprans I, Rapp JH, Pan XM, Hardman DA, Feingold KR. Oxidized lipids in the diet accelerate the development of fatty streaks in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1996;16:33–38. doi: 10.1161/01.atv.16.4.533. [DOI] [PubMed] [Google Scholar]
- 16.Adachi J1, Kudo R, Asano M, Ueno Y, Hunter R, Rajendram R, Martin C, Preedy VR. Skeletal muscle and liver oxysterols during fasting and alcohol exposure. Metabolism. 2006;55:119–127. doi: 10.1016/j.metabol.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.da Rocha RF, De Bastiani MA, Klamt F. Bioinformatics approach to evaluate differential gene expression of M1/M2 macrophage phenotypes and antioxidant genes in atherosclerosis. Cell Biochem Biophys. 2014;70:831–839. doi: 10.1007/s12013-014-9987-3. [DOI] [PubMed] [Google Scholar]
- 18.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, Dong F, Zaid M, Kumar A, Zha X. ABCA1 protein enhances Toll-like receptor 4 (TLR4)-stimulated interleukin-10 (IL-10) secretion through protein kinase A (PKA) activation. J Biol Chem. 2012;287:40502–40512. doi: 10.1074/jbc.M112.413245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norlin M, Pettersson H, Tang W, Wikvall K. Androgen receptor-mediated regulation of the anti-atherogenic enzyme CYP27A1 involves the JNK/c-jun pathway. Arch Biochem Biophys. 2011;506:236–241. doi: 10.1016/j.abb.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Wang X, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D3. J Cell Biochem. 2003;89:1087–1101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- 22.Sundaram K, Sambandam Y, Tsuruga E, Wagner CL, Reddy SV. 1alpha,25-dihydroxyvitamin D3 modulates CYP2R1 gene expression in human oral squamous cell carcinoma tumor cells. Horm Cancer. 2014;5:90–97. doi: 10.1007/s12672-014-0170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangge H, Weghuber D, Prassl R, Haara A, Schnedl W, Postolache TT, Fuchs D. The Role of Vitamin D in Atherosclerosis Inflammation Revisited: More a Bystander than a Player? Curr Vasc Pharmacol. 2015;13:392–398. doi: 10.2174/1570161111666131209125454. [DOI] [PubMed] [Google Scholar]
- 24.Catalano A, Morabito N, Basile G, Cucinotta D, Lasco A. Calcifediol improves lipid profile in osteopenic atorvastatin treated postmenopausal women. Eur J Clin Invest. 2015;45(2):144–149. doi: 10.1111/eci.12390. [DOI] [PubMed] [Google Scholar]
- 25.Eftekhari MH, Akbarzadeh M, Dabbaghmanesh MH, Hassanzadeh J. The effect of calcitriol on lipid profile and oxidative stress in hyperlipidemic patients with type 2 diabetes mellitus. ARYA Atheroscler. 2014;10:82–88. [PMC free article] [PubMed] [Google Scholar]
- 26.Hafner M, Rezen T, Rozman D. Regulation of hepatic cytochromes p450 by lipids and cholesterol. Curr Drug Metab. 2011;12:173–185. doi: 10.2174/138920011795016890. [DOI] [PubMed] [Google Scholar]
- 27.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsunawa M, Akagi D, Uno S, Endo-Umeda K, Yamada S, Ikeda K, Makishima M. Vitamin D receptor activation enhances benzo[a]pyrene metabolism via CYP1A1 expression in macrophages. Drug Metab Dispos. 2012;40:2059–2066. doi: 10.1124/dmd.112.046839. [DOI] [PubMed] [Google Scholar]
- 30.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814:186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Van Eck M. ATP-binding cassette transporter A1: key player in cardiovascular and metabolic disease at local and systemic level. Curr Opin Lipidol. 2014;25:297–303. doi: 10.1097/MOL.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 32.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szanto A, Benko S, Szatmari I, Balint BL, Furtos I, Ruhl R, Molnar S, Csiba L, Garuti R, Calandra S, Larsson H, Diczfalusy U, Nagy L. Transcriptional regulation of human CYP27 integrates retinoid, peroxisome proliferator-activated receptor, and liver X receptor signaling in macrophages. Mol Cell Biol. 2004;24:8154–8166. doi: 10.1128/MCB.24.18.8154-8166.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 35.Nagasaka H, Okano Y, Kimura A, et al. Oxysterol changes along with cholesterol and vitamin D changes in adult phenylketonuric patients diagnosed by newborn mass-screening. Clin Chim Acta. 2013;416:54–59. doi: 10.1016/j.cca.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhang LH, Kamanna VS, Ganji SH, Xiong XM, Kashyap ML. Niacin increases HDL biogenesis by enhancing DR4-dependent transcription of ABCA1 and lipidation of apolipoprotein A-I in HepG2 cells. J Lipid Res. 2012;53:941–950. doi: 10.1194/jlr.M020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deb DK1, Wang Y, Zhang Z, Nie H, Huang X, Yuan Z, Chen Y, Zhao Q, Li YC. Molecular mechanism underlying 1,25-dihydroxyvitamin D regulation of nephrin gene expression. J Biol Chem. 2011;286:32011–32017. doi: 10.1074/jbc.M111.269118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277:25125–25132. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217:1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XL, Guo YF, Song ZX, Zhou M. Vitamin D Prevents Podocyte Injury via Regulation of Macrophage M1/M2 Phenotype in Diabetic Nephropathy Rats. Endocrinology. 2014;155:4939–4950. doi: 10.1210/en.2014-1020. [DOI] [PubMed] [Google Scholar]
- 42.Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol. 2013;136:309–312. doi: 10.1016/j.jsbmb.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Activation of liver X receptor decreases atherosclerosis in Ldlr(−)/(−) mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler Thromb Vasc Biol. 2014;34:279–284. doi: 10.1161/ATVBAHA.113.302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin K, Liao DF, Tang CK. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol Med. 2010;16:438–449. doi: 10.2119/molmed.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pradel LC, Mitchell AJ, Zarubica A, Dufort L, Chasson L, Naquet P, Broccardo C, Chimini G. ATP-binding cassette transporter hallmarks tissue macrophages and modulates cytokine-triggered polarization programs. Eur J Immunol. 2009;39:2270–2280. doi: 10.1002/eji.200838867. [DOI] [PubMed] [Google Scholar]
- 46.Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–170. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 47.Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buitrago CG, Ronda AC, de Boland AR, Boland R. MAP kinases p38 and JNK are activated by the steroid hormone 1alpha,25(OH)2-vitamin D3 in the C2C12 muscle cell line. J Cell Biochem. 2006;97:698–708. doi: 10.1002/jcb.20639. [DOI] [PubMed] [Google Scholar]
- 50.Kiss M, Czimmerer Z, Nagy L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology. J Allergy Clin Immunol. 2013;132(2):264–286. doi: 10.1016/j.jaci.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 51.Dave VP, Kaul D, Sharma M. Crosstalk between RXR, LXR and VDR within blood mononuclear cellular model. Indian J Exp Biol. 2012;50:35–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.