Abstract
Social buffering, which is the attenuation of stress hormone release by a social partner, occurs in many species throughout the lifespan. Social buffering of the infant by the caregiver is particularly robust, and animal models using infant rodents are uncovering the mechanisms and neural circuitry supporting social buffering. At birth, the hypothalamic-pituitary-adrenal (HPA) stress system is functional but is suppressed via extended social buffering by the mother: the profound social buffering effects of the mother can last for one to two hours when pups are removed from the mother. At 10 days of age, pups begin to mount a stress response immediately when separated from the mother. The stimuli from the mother supporting social buffering are broad, for tactile stimulation, milk, and an anesthetized mother (no maternal behavior) all sufficiently support social buffering. The mother appears to produce social buffering by blocking norepinephrine (NE) release into the hypothalamic paraventricular nucleus (PVN), which blocks HPA activation. Since the infant amygdala relies on the presence of corticosterone (CORT), this suggests that social buffering of pups by the mother attenuates the neurobehavioral stress response in infancy and prevents pups from learning about threat within mother-infant interactions.
Keywords: social buffering, amygdala, paraventricular nucleus, corticosterone, norepinephrine
Social buffering refers to a phenomenon where a social partner can attenuate the release of stress hormones in response to a threatening or challenging situation. Social buffering occurs throughout the lifespan of social species, including in human, nonhuman primates, and rodents, and there are profound positive effects of social buffering on health and well-being (Ditzen & Heinrichs, 2014; Gee et al., 2014; Hennessy, Kaiser, & Sachser, 2009; Hostinar, Sullivan, & Gunnar, 2014; Uchino, Cacioppo, & Kiecolt-Glaser, 1996). The effective socially buffering partner changes with development. For example, in early life the caregiver is an effective partner for socially buffering the infant, while a friend or a rodent’s cage mate is a more appropriate source of social buffering in later life (Hostinar & Gunnar, 2013; Sandi & Haller, 2015). Using a rodent model, our goal is to describe the supporting neural mechanisms of social buffering and the profound effect of social buffering on behavior and learning during early life.
Characteristics of social buffering are remarkably similar across species and development (Gee et al., 2014; Kikusui, Winslow, & Mori, 2006). The HPA axis is functional at birth but goes through maturational changes over early life before acquiring a stable circadian rhythm and reliable response to stressful events (Klein & Romeo, 2013; Levine, 2001). In altricial species, such as the infant rat, prolonged separation of the infant from the caregiver appears stressful since the infant mounts a stress response, which is decreased by reuniting the infant with the caregiver or stimuli associated with the caregiver (Gunnar & Donzella, 2002; Levine, 2005; Sanchez, 2006). Stress hormones are also activated by traumatic events, and maternal presence (social buffering) attenuates this CORT release, although the infant still responds behaviorally to the pain or trauma producing the stress (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996).
Social buffering is complex and the degree of social buffering that occurs from one individual to another is influenced by the type of social relationship, previous experience with that individual, gender, and the developmental period (Hennessy et al., 2009). Learning and experience play a critical role in determining the socially buffering partner, with recent evidence suggesting that social buffering functions through a learned safety signal (Christianson et al., 2012; Moriceau, Roth, & Sullivan, 2010). The effectiveness of the caregiver to socially buffer its offspring decreases with maturation, and individuals from other relationships acquire the ability to socially buffer (Hostinar & Gunnar, 2013; Sandi & Haller, 2015). Finally, the quality of care received from the caregiver determines the effectiveness of social buffering (Nachmias et al., 1996; Sanchez, 2006). Together, this suggests that multiple social buffering circuits exist. However, identifying circuits that causally support social buffering, but are distinct from circuits supporting social behavior, stress, and learning is challenging, particularly due to the dramatic changes that all of these circuits make throughout development.
The Ontogeny of Rodent Social Buffering
Social buffering of the stress response is dependent on the infant’s ability to mount a stress response to a stressful situation. In the infant rat, and other altricial species, the HPA axis is functional, but not completely mature (Levine, 2001). While pups mount a stress response at birth, within a day, most stressors fail to elicit an increase in CORT (i.e. cold, endotoxin) and pups enter a developmental phase termed the Stress Hyporesponsive Period (SHRP) (Walker, Scribner, Cascio, & Dallman, 1991). This post birth suppression of the HPA axis appears to be controlled by strong, extended social buffering by social stimuli and behavior from the mother (Levine, 2000). Insights into the role of the mother as a powerful regulator of CORT came from maternal deprivation/separation research, which used a procedure where pups are removed from their mother for many hours, resulting in an increase in the pups’ blood CORT levels, as well as an increase of CRF mRNA expression in the hypothalamic PVN of the HPA axis (Plotsky et al., 2005). Also, the concept of the mother as a “hidden regulator” of infant neurobehavioral responses emerged when it was discovered that stimulation from the mother maintains pups’ physiological systems at homeostasis, with the intensity and patterning of different stimuli controlling different physiological systems (Hofer, 1973). For instance, the mother’s tactile stimulation of the pups maintains their levels of growth hormone, whereas, maternal odor maintains behavioral activity and arousal at optimal levels (Hofer, 1984; Kuhn, Butler, & Schanberg, 1978). Indeed, when the mother is removed and replaced by artificial stimulation of pups’ sensory systems, social buffering is reinstated. Specifically, mimicking maternal care (licking) with just tactile stimulation in the form of stroking, intubating pups to fill their stomach with milk, or presenting an anesthetized mother can reinstate low CORT levels in pups (van Oers, de Kloet, Whelan, & Levine, 1998). These results enabled the reconceptualization of the maternal separation paradigm as the removal of the maternal care that controls infant homeostasis. Furthermore, the discovery of the mother’s role in maintaining infant homeostasis clearly indicates the power of maternal control over infant CORT (i.e. social buffering) and reveals that social buffering is mediated through myriad sensory systems in pups.
The social buffering system seems to change around postnatal day (PN) 10, when pups begin to mount a stress response immediately following removal from the mother. That is, the mother’s ability to socially buffer her pups no longer extends into the period of pup separation from the mother. Specifically, when removed from the mother and immediately given a 0.5mA shock or exposure to a predator odor, an 8 day old pup does not mount a CORT increase, but an 11 day old pup does (Moriceau, Wilson, Levine, & Sullivan, 2006; Shionoya, Moriceau, Bradstock, & Sullivan, 2007; Stanton & Levine, 1990; Wiedenmayer, Magarinos, McEwen, & Barr, 2003). However, social buffering is reinstated in these older pups if the mother is present during the shock (Moriceau & Sullivan, 2006). The mother’s ability to socially buffer pups continues through weaning. As her ability to socially buffer begins to diminish with pup maturation, peers become potent sources of social buffering (Hennessy et al., 2009; Terranova, Cirulli, & Laviola, 1999). For example, the presence of familiar and unfamiliar conspecifics attenuates the CORT response to stressful conditions in developing squirrel monkeys (Hennessy, 1984, 1986; Vogt, Coe, & Levine, 1981). Social buffering by peers and cage-mates continues into adulthood (Kiyokawa, Kikusui, Takeuchi, & Mori, 2004).
Maternal social buffering and blockade of pup fear expression and learning
Social buffering of pups by the mother has profound effects on pup behavior, which is illustrated by the maternal control of infant amygdala-dependent threat (fear) learning. We have known for decades that infant rats show a sensitive period of attenuated aversion/threat learning (Camp & Rudy, 1988; Haroutunian & Campbell, 1979; Roth & Sullivan, 2005). Specifically, pairing an odor with either 0.5mA shocks or tail pinches causes a learned odor preference, rather than the aversion or threat (fear) learning that occurs in older pups and adults. The infant rat’s pain system is functional and hind paw threshold to shock does not appear to change during this period of development, thus odor preference learning is not due to differences in infant pain detection (Barr, 1995; Fitzgerald, 2005). We have shown that the functional emergence of the amygdala (basolateral complex) at PN 10 supports the onset of threat (fear) learning, which suggests that this learning requires a mature amygdala (Sullivan, Landers, Yeaman, & Wilson, 2000). Indeed, this threat (fear) learning is dependent upon the amygdala in both pups and adults (Fanselow & Gale, 2003; Johansen, Cain, Ostroff, & LeDoux, 2011; Maren, 2003; Moriceau et al., 2010).
However, with continued assessment of the development of amygdala-dependent learning, an understanding of the critical importance of CORT in this learning emerged. We have induced amygdala-dependent threat (fear) conditioning in pups as young as PN6 (still in the SHRP, when shock does not increase CORT) by artificially increasing amygdala or systemic CORT. Conversely, blocking systemic or amygdala CORT in older pups (between PN10-PN15) blocks threat (fear) learning (Moriceau & Sullivan, 2004, 2006; Moriceau et al., 2006; Roth & Sullivan, 2005; Shionoya et al., 2007; Shionoya et al., 2006; Wiedenmayer & Barr, 2001). Next, we took insight from the social buffering literature to produce naturalistic modifications of CORT via the mother during threat (fear) learning. Social buffering of pups via maternal presence during odor-shock conditioning blocks amygdala-dependent threat (fear) learning (Moriceau & Sullivan, 2006). While CORT can modify learning in adults, it modifies the strength of learning and is not required for amygdala plasticity (Corodimas, LeDoux, Gold, & Schulkin, 1994; Hui et al., 2004; Thompson, Erickson, Schulkin, & Rosen, 2004).
Presumably, the mother’s ability to block CORT, and thus block amygdala-dependent threat (fear) learning in early infancy, is to ensure pup attachment to the mother despite the quality of caregiving. In early infancy, the pup is solely dependent on the mother for survival, and must attach to her, even if she is abusive or neglectful. Even typical maternal behaviors sometimes involve brief painful stimuli, such as trampling or rough handling that may occur as the mom leaves and enters the nest (Hofer & Sullivan, 2001). Thus, social buffering may have evolved to prevent pups from avoiding or inhibiting responses toward the mother, despite the occurrence of pain. Furthermore, social buffering by the mother imparts an important context- dependency of threat (fear) learning. Following the emergence of threat (fear) learning at PN 10, pups only learn threat (fear) outside of the presence of the mother, thus the mother extends the sensitive period for attachment learning up until PN 15 (Upton & Sullivan, 2010). Overall, social buffering via the mother provides a mechanism that allows a pup to learn a preference for and attach to even the lowest quality caregiver (Perry & Sullivan, 2014). However, there are unfortunate consequences of poor caregiving on the pup’s development, including on the pup’s ability to be socially buffered by their mother (Raineki, Lucion, & Weinberg, 2014).
Early life adversity compromises maternal social buffering of pups
Infant rearing in adverse conditions compromises development of the HPA axis and increases CORT levels during early life, but also produces ubiquitous deleterious effects on neural development (Sanchez, 2006; Struber, Struber, & Roth, 2014). In humans and nonhuman primates, the quality of parental care is important for the effectiveness of social buffering, which indicates the strong role of learning in social buffering. For example, the primary caregiver is the most potent source of social buffering, regardless of biological relatedness of the father, mother, or sibling (Hennessy et al., 2009; Winslow, Noble, Lyons, Sterk, & Insel, 2003). Importantly, there is increasing evidence that adverse rearing conditions attenuate a caregiver’s ability to socially buffer their young, as well as other important social partners in humans (Nachmias et al., 1996; Wismer Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005). Experiencing high CORT and low social buffering from the caregiver as a result of early life adversity particularly impacts development of the amygdala, as has been demonstrated via animal models of attachment and threat (fear) learning (Perry & Sullivan, 2014; Rincon-Cortes & Sullivan, 2014). Specifically, early life adversity produces a precocious end to the SHRP as indicated by shock-induced CORT release and early emergence of amygdala-dependent threat (fear) learning (Moriceau, Shionoya, Jakubs, & Sullivan, 2009). Early life adversity also attenuates the ability of pups to be socially buffered by the mother (Raineki et al., 2014). An exploration of mechanisms suggests that early life adversity increases CORT and amygdala activity, but also produces increased corticotropin-releasing factor (CRF) release from the amygdala to the locus coeruleus (LC), which disrupts attachment learning to the mother (Moriceau et al., 2009).
Early life adversity also appears to produce precocious functional emergence of the prefrontal cortex (PFC), producing an early onset of adult-like fear retention and extinction (Cowan, Callaghan, & Richardson, 2013). While this may improve the individual’s ability to detect threat, the early onset of adult-like threat (fear) systems can lead to maladaptive behavior when threat is not immediate. For example, the persistent activation of threat (fear) systems, which can occur following early life adversity, can result in a maladaptive chronic state of fear. Such activation leads to hypervigilance and a focus on threat-associated cues, as well as increased anxiety and impulsivity. While this is often adaptive in the face of a threat, this state becomes maladaptive when the threat has passed (Perry & Pollard, 1998; Teicher et al., 2003). Furthermore, the overactivation of stress responses that occurs in this chronic state of fear leads to increased obesity, type II diabetes, hypertension, a multitude of psychiatric problems, and accelerated aging of brain structures (McEwen, 2003). Overall, early life adversity produces enduring changes in brain areas that regulate stress, cognition and emotion, with the alteration of these areas likely underlying the emergence of psychiatric disorders and other health complications (McEwen, 2003). Together, these results suggest that early life adversity produces early emergence of brain areas supporting threat (fear) learning, and diminishes the caregiver’s effectiveness at socially buffering pups, which together produces aberrant attachment and social behavior that will further compromise development.
The Hypothalamic-Pituitary-Adrenal Axis and Social Buffering in Pups
Defining the neural basis of social buffering relies heavily on the well-defined neural control of the HPA axis (Figure 1). As documented in the stress literature on rodents, and verified in humans and nonhuman primates, the HPA axis is typically initiated and attenuated at the level of the hypothalamus, via neurons in the medial parvocellular region of the paraventricular nuclei of the hypothalamus (PVN). When activated, the PVN secretes corticotrophin-releasing factor (CRF) and vasopressin (AVP), both of which travel to the anterior pituitary to independently and synergistically stimulate adrenocorticotropic hormone (ACTH) secretion into the blood stream. ACTH then activates the adrenal cortex of the adrenal gland for release of CORT into the blood stream. As we consider where along the HPA axis social stimuli might block this CORT release, we first consider direct PVN afferents, and then indirect PVN afferents (Rincon-Cortes & Sullivan, 2014; Sandi & Haller, 2015; Smith & Vale, 2006; Ziegler & Herman, 2002).
Figure 1.
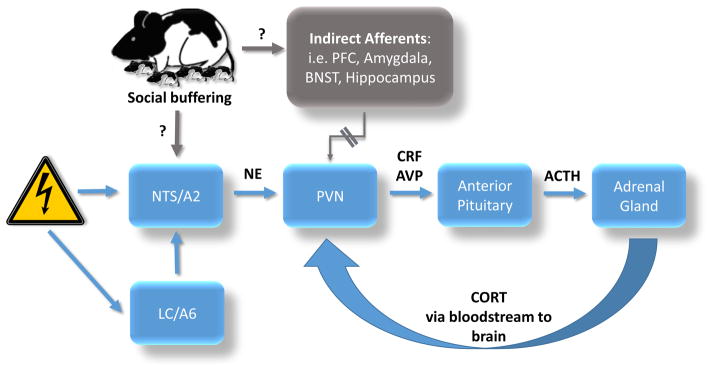
This is a schematic diagram of proposed circuits underlying social buffering. Stressors activate the nucleus of the solitary tract (NTS/A2) and locus coeruleus (LC/A6), which provide noradrenergic input to the hypothalamic paraventricular nucleus (PVN). Parvocellular neurosecretory cells of the PVN activate the hypothalamic pituitary adrenal (HPA) axis in response to stress. Parvocellular axons project to the median eminence to release peptides (corticotrophin-releasing factor (CRF) and vasopressin (AVP)) into hypothalamic-pituitary portal system blood vessels to the anterior pituitary, where adrenocorticotropic hormone (ACTH) is released to act upon the adrenal cortex to release corticosterone (CORT) into the bloodstream. The PVN receives diverse excitatory and inhibitory inputs, particularly from the brainstem, including the NTS/A2, which provides major noradrenergic input to the PVN, as well as the LC which provides major noradrenergic input indirectly to the PVN via the NTS. The excitatory transmitter norepinephrine (NE) input is a major activator of the HPA axis, and is known to be disrupted by social buffering. Social buffering may occur via circuits directly disrupting the major noradrenergic input to the PVN, thus leading to dampening of the HPA axis to reduce the saliency of stressors. However, evidence also exists suggesting that the effects of social buffering may be mediated by unique activation of brain regions that provide indirect afferents to the PVN, including areas of the prefrontal cortex (PFC), nuclei of the amygdala, the bed nucleus of the stria terminalis (BNST), and the hippocampus, among others.
Understanding the site of social buffering is complex because different stressors activate the HPA system differently, and different types of social buffering likely interact with the HPA system either directly at the level of the PVN or indirectly through one of the many PVN afferents. However, overall, the HPA system uses the parvocellular PVN CRF/AVP neurons as major integrators of this diverse excitatory and inhibitory input for the neuroendocrine control of the HPA axis (Sandi & Haller, 2015; Smith & Vale, 2006; Ziegler & Herman, 2002). Also to consider, CORT is a major subclass of steroid hormones and has very broad, complex roles in regulating metabolism, cardiovascular function, and immune system regulation and behavior, including social buffering. Together this provides an overwhelmingly complex system in which to attempt to identify mechanisms controlling social buffering (Beijers, Buitelaar, & de Weerth, 2014).
Innervation of the PVN hypophysiotropic neurons is diverse and derived from myriad brain regions, although the major afferent inputs are more limited (Smith & Vale, 2006; Ziegler & Herman, 2002). Cell groups include: 1) the brainstem, including the nucleus of the solitary tract (NTS-A2) and ventral medulla (C1), which relay visceral and stress information (via NE, epinephrine (E), and glucagon-like peptide 1(GLP-1)), 2) extra-PVN hypothalamic nuclei, which relay information from the endocrine system (via GABA), and 3) the lamina terminalis, which relays blood osmotic information (via glutamate (Glu) and angiotensin (Ang)). A host of other more minor inputs have been implicated in the activation of the HPA axis, which involves serotonin (5-HT), acetylcholine (Ach), and myriad neurohormones, directly altering HPA function (Makino, Hashimoto, & Gold, 2002; Ziegler & Herman, 2002). However, minor inputs can sometimes have profound effects on brain function. Lastly, hypothalamic-basal forebrain pathways can serve as local modulation of neuroendocrine messengers to interface and integrate information from higher brain areas.
Neurobiology of Social Buffering in Pups
Social buffering attenuates stress-induced activation of the brain. For example, being placed in a context associated with shock produces an increase in PVN c-Fos activity, but this is attenuated if the rat is accompanied by a conspecific partner (Kiyokawa et al., 2004). In pups, the enhancement of c-Fos and CRF-R2 expression in the ventromedial hypothalamus caused by maternal deprivation can be returned to control levels if maternal stimulation is mimicked (such as stroking the pup’s entire body with a brush) (Eghbal-Ahmadi, Avishai-Eliner, Hatalski, & Baram, 1999; van Oers et al., 1998).
It should be noted that when assessing the neurobiology of social buffering, the level of assessment utilized can produce varying interpretations of the mechanisms by which social buffering works (Figure 1). Some measures suggest that social buffering works simply via the attenuation of the response to stress, while other measures suggest that social buffering produces unique brain activation. For example, while shock produces an increase in PVN neural activity, maternal presence (anesthetized) during the shocks (social buffering) completely eliminates this response in the medial PVN (Maken, Weinberg, Cool, & Hennessy, 2010; Shionoya et al., 2007). However, microarray analyses of amygdala activity during shock with and without maternal presence shows unique neural responses, suggesting social buffering is working not simply via the elimination of stress responses, but likely by producing a unique neural signature (Barr et al., 2009).
Social buffering of the Stress Response via Modulation of Direct PVN Afferents
The paraventricular nucleus (PVN, PVA, or PVH) is a hypothalamic nucleus that serves as a major integrator of the brain’s stress system. Its activation is complex and extensive reviews of this topic already exist (Herman, Tasker, Ziegler, & Cullinan, 2002; Makino et al., 2002; Ziegler & Herman, 2002). Here we review only the major afferents to the PVN and their potential roles in mediating social buffering (Figure 1). Within the rodent animal literature, the search for the social buffering neural circuitry has primarily focused on the role of NE within the PVN. The rationale for this approach is based on the fact that NE has a major role in initiating HPA axis activation in the PVN (Plotsky, Cunningham, & Widmaier, 1989). Indeed, microinfusions of NE into the PVN activate CORT release (Leibowitz, Diaz, & Tempel, 1989; Pacak, Palkovits, Kopin, & Goldstein, 1995). Via direct afferent projections to the PVN, various stressful stimuli robustly increase NE release in the PVN, including shock used during threat (fear) conditioning (Otagiri, Wakabayashi, & Shibasaki, 2000; Pacak et al., 1992). Microdialysis of the PVN has verified stress-induced NE increase and microinfusions of NE receptor agonists into the PVN can activate the HPA axis, while infusions of NE receptor antagonists greatly attenuate this HPA activation in adults (Pacak, McCarty, Palkovits, Kopin, & Goldstein, 1995; Pacak, Palkovits et al., 1995; Plotsky, Otto, & Sutton, 1987; Shionoya et al., 2007; Szafarczyk, Malaval, Laurent, Gibaud, & Assenmacher, 1987).
The PVN’s major noradrenergic input come from direct projections from A1 and A2 noradrenergic cell groups, and additional minor direct input comes from the locus coeruleus (LC, also known as A6 noradrenergic cell group) (McKellar & Loewy, 1981; Pacak et al., 1992; Plotsky et al., 1989; Szafarczyk et al., 1987). Stress-dependent modulation of NE in the PVN is thought to primarily occur via modulation of A1 and A2 afferents, with possible contribution from the LC. However, while the LC is critically involved in arousal and stress, it appears to have a secondary role in HPA activation. LC lesions reduce stress-induced CORT secretion following restraint, but the connection of the LC to the PVN is primarily indirect via the nucleus tractus solitarius (NTS) of the brainstem (Cunningham & Sawchenko, 1988; Ziegler, Cass, & Herman, 1999). Interestingly, there appears to be direct input of pain information to the PVN that travels via noradrenergic neurons of the ventral medulla (C2), A2, and LC (Palkovits, Baffi, & Pacak, 1999). Overall, NE release within the PVN in response to stress is robust and represents one of the most significant PVN inputs, suggesting that the suppression of NE in the PVN is likely a neural mechanism underlying social buffering.
We assessed the role of PVN NE in the infant stress response to shock and social buffering by the mother. Using a threat (fear) conditioning paradigm, pups received odor-0.5mA shock pairings, either without the mother present where pups learn amygdala-dependent fear, or with the mother present which prevents CORT increase, blocks threat (fear) learning, and activates attachment learning (Moriceau & Sullivan, 2006; Shionoya et al., 2007). We explored whether maternal modulation of CORT altered PVN activity (autoradiography) and noradrenergic activity in the PVN (microdialysis), and finally asked if we could override maternal social buffering of pups and reinstate threat (fear) learning via microinfusions of NE α1 receptor agonists and antagonists into the PVN. As expected, following odor-0.5mA shock conditioning without maternal presence, pups learned an odor aversion and mounted a stress response, whereas maternal presence during odor-0.5mA shock conditioning permitted the odor preference learning and a blunted stress response. Furthermore, pups receiving shock without the maternal presence had significantly enhanced parvocellular PVN activity, while pups shocked in the presence of their mother looked similar to non-shocked control pups. This PVN activity was mirrored by PVN NE levels measured by microdialysis, with shock increasing PVN NE and maternal presence blocking NE release to a level similar to non-shocked controls. Finally, intra-PVN infusion of NE α1 antagonists blocked the aversion learning in absence of maternal presence, while infusion of NE α1 agonists prevented the effects of maternal presence and permitted pups to learn the odor threat conditioning (Shionoya et al., 2007).
A complementary social buffering system exists in the mother rat. The mother rat was long viewed as having a blunted stress response. However, in a set of experiments assessing the mother’s stress response with and without pups, it was noted that the presence of pups activates the mother’s stress response (Deschamps, Woodside, & Walker, 2003; Walker et al., 2004). That is, the mother has an SHRP in the absence of her pups and a robust stress response when in the presence of her pups. However, the mechanism for social buffering is the same in mother and infant, which is via modulation of NE in the PVN (Toufexis & Walker, 1996). The mother’s robust stress response in the presence of her pups presumably enables her to protect her pups, and underlies the maternal aggression phenomenon.
Social buffering of the Stress Response via Indirect PVN Afferents
Indirect afferent inputs to the PVN are extremely wide spread. Indirect inputs first receive information from sensory systems, which is typically then relayed and processed within other brain areas, such as the hippocampus, amygdala, and PFC. The indirect activation of the PVN is complex and extensive reviews of this topic already exist (Ferguson, Latchford, & Samson, 2008; Herman et al., 2003; Swanson & Sawchenko, 1980). Here we review only the major indirect afferents and their potential roles in mediating social buffering.
Sensory pathways are critical for the processing of sensory cues that mediate social buffering. Olfactory cues have been highlighted as particularly important in allowing social buffering in rodents, while humans and nonhuman primates appear to also rely on additional sensory systems (Kiyokawa, Wakabayashi, Takeuchi, & Mori, 2012; Parma, Bulgheroni, Tirindelli, & Castiello, 2014; Takahashi et al., 2013). Various brain areas known to indirectly modulate the HPA axis are also critical in permitting social buffering effects and have been clearly documented as vital for information processing and integration (Figure 1). Such areas include the amygdala, bed nucleus of the stria terminalis (BNST), PFC, and hippocampus, among others. For example, social buffering by a cage mate increases c-Fos expression in numerous brain areas, most of which have indirect connections with the PVN, including the PFC, the nucleus accumbens (NA), BNST, and medial, lateral, basal, cortical and central nuclei of the amygdala. These results suggest that indirect afferents to the PVN are part of the circuitry involved in inducing social buffering from a cage mate.
The amygdala in particular is important in modulating neuroendocrine functions, especially in response to threat, with each of the nuclei (medial, lateral, basolateral, central) performing specific functions (Fanselow & LeDoux, 1999; Ulrich-Lai & Herman, 2009). Thus, the amygdala is likely part of the circuitry underlying social buffering. Indeed, stressors activate the amygdala, and social buffering decreases amygdala activity related to threat and threat (fear) conditioning throughout the lifespan (Kiyokawa et al., 2012; Moriceau & Sullivan, 2006). The amygdala also has indirect connections to the PVN, with the central amygdala and basolateral complex projecting to the PVN via the NTS, BNST, and PFC (Schwaber, Kapp, Higgins, & Rapp, 1982). Furthermore, direct connections from the olfactory bulb to the amygdala are well documented, which could be important for allowing social buffering via olfactory cues (Prewitt & Herman, 1998; Schwaber et al., 1982). Social buffering by a familiar peer also reduces anxiety, and is dependent upon inhibitory interneurons within the basolateral complex of the amygdala, which are believed to be activated by the medial PFC (Truitt et al., 2007). Interestingly, different stressors appear to activate the PVN via divergent pathways. For example, physical stressors such as shock, increase NE within the PVN for HPA activation, while psychological stress may activate the PVN through the amygdala and potentially the BNST (Cunningham & Sawchenko, 1988; Herman, Cullinan, Ziegler, & Tasker, 2002; Prewitt & Herman, 1998; Roozendaal, Koolhaas, & Bohus, 1991). It should also be noted that although these findings implicate that the amygdala is involved in the neural circuits mediating social buffering, it is still unclear whether the amygdala plays a role in initiating social buffering, or shows altered neural activity simply as a result of low CORT levels induced by social buffering.
Work from the Hennessy lab, using guinea pigs, has provided one of the more comprehensive neural assessments of stress and social buffering (Hennessy et al., 2009; Hennessy et al., 2015). Specifically, separating guinea pig pups from their mother produces a stress response and a pharmacological inflammatory response, both of which can be buffered by maternal presence. A path from the medial amygdala through the BNST to PVN has been identified in driving HPA activation (Maken et al., 2010). However, reunion of the mother and pups following separation decreases neural activity of the PVN in pups, but does not reduce activity within the amygdala and BNST, suggesting suppression of the HPA axis during social buffering could be occurring via inhibition from another brain area (Maken et al., 2010). The Hennessy lab suggests that an inhibitory pathway from the prelimbic PFC may play a role in the initiation of social buffering via suppression of the HPA axis. Indeed, stimulation of the prelimbic PFC has been shown to inhibit the HPA stress response to forced restraint (Jones, Myers, & Herman, 2011). A recent study found activation of the prelimbic PFC to be associated with social buffering from an adult male, but not with maternal buffering, suggesting that prelimbic PFC may play a role in only certain types of social buffering (Hennessy et al., 2015).
In general, the PFC appears to be crucially involved in the modulation of the stress response and its attenuation by social buffering. The ventromedial PFC (vmPFC) has extensive connections with the amygdala and HPA axis, with dorsal regions inhibiting the HPA axis, and ventral regions exciting the HPA axis (Sullivan & Gratton, 1999). The anterior cingulate cortex (ACC) of the PFC is activated in response to social pain and rejection in adult humans (Eisenberger, Lieberman, & Williams, 2003). Furthermore, viewing pictures of one’s partner during a painful stimulation reduced pain-related activation of the ACC, and activated the vmPFC which is a neural region associated with safety signaling (Eisenberger et al., 2011). Similarly, maternal presence activates the PFC in children, and rodent studies in adults suggest there may be a causal link between PFC activation and social buffering (Gee et al., 2014). Specifically, lesioning prelimbic or anterior cingulate subareas of the PFC blocked social buffering effects of an adult partner (Herman, Ostrander, Mueller, & Figueiredo, 2005). While it is still unclear how the PFC is processing social information, the PFC has strong connections with the amygdala and BNST, which then connected to the PVN, possibly through the NTS (Figueiredo, Bodie, Tauchi, Dolgas, & Herman, 2003; Hennessy et al., 2009; Stefanacci & Amaral, 2002; Vertes, 2004).
An additional brain region that may be involved in social buffering is the NTS of the brain stem. The NTS appears to be a major relay station for the integration of stress and threat information, with afferents originating in the BNST and PFC (van der Kooy, Koda, McGinty, Gerfen, & Bloom, 1984; Zardetto-Smith, Moga, Magnuson, & Gray, 1988). The BNST afferents to PVN via the NTS are complex, with the anteroventral regions of the BNST controlling HPA excitation and the posterior regions controlling HPA inhibition (Choi et al., 2007). Lastly, the hippocampus is also a major limbic structure that has inhibitory control of the HPA axis, through projections to the PVN (Ulrich-Lai & Herman, 2009).
Putative biological mediators of social buffering also include hormones and neurotransmitters, such as oxytocin, dopamine, serotonin, and opioids, which has been largely reviewed (Hostinar et al., 2014). However, the review of these biological mediators span beyond the scope of this review.
Conclusion
Social buffering is a powerful, beneficial phenomenon that occurs throughout the lifespan in many species. Social buffering via a caregiver in early life is particularly robust, and impacts subsequent infant development. The quality of parental care determines the effectiveness of social buffering. Within a secure attachment, caregivers impart strong protection from the neurobiological consequences of stress to their infants, and in doing so prevent infant threat (fear) learning within the context of caregiver-infant interactions. Thus, through social buffering, high-quality parental care reduces infant distress, buffers their stress physiology, and regulates their learning about the environment.
Unfortunately, low-quality parental care is associated with poor social buffering of offspring, which causes early functional emergence of brain regions that allow threat (fear) learning. Therefore, removing powerful stress buffering, as occurs in the face of early life adversity, has profound consequences on the infant’s social, emotional, cognitive development.
Given the importance of social buffering on development, and the widely recognized benefits of social buffering on health and well being, understanding the neurobiology of social buffering is of vital importance. Further research is needed to fully understand the neural mechanisms of social buffering, which will inform interventions to provide social support to relieve stress and ameliorate the negative effects of early life adversity.
Acknowledgments
This work was supported by the NIH under Grants DC009910, MH091451, HD083217 to RMS.
Footnotes
Disclosure statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Barr GA. Ontogeny of nociception and antinociception. NIDA Res Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry. 2014;23(10):943–956. doi: 10.1007/s00787-014-0566-3. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27(8):2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. 27/8/2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Fernando AB, Kazama AM, Jovanovic T, Ostroff LE, Sangha S. Inhibition of fear by learned safety signals: a mini-symposium review. J Neurosci. 2012;32(41):14118–14124. doi: 10.1523/JNEUROSCI.3340-12.2012. 32/41/14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Ann N Y Acad Sci. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- Cowan CS, Callaghan BL, Richardson R. Acute early-life stress results in premature emergence of adult-like fear retention and extinction relapse in infant rats. Behav Neurosci. 2013;127(5):703–711. doi: 10.1037/a0034118. 2013-36104-010. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274(1):60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J Neuroendocrinol. 2003;15(5):486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32(1):149–162. doi: 10.3233/RNN-139008. M0228K0TM4T20210. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19(10):3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Master SL, Inagaki TK, Taylor SE, Shirinyan D, Lieberman MD. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proc Natl Acad Sci U S A. 2011;108(28):11721–11726. doi: 10.1073/pnas.1108239108. 1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–232. doi: 10.1016/s0896-6273(00)80775-8. S0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets. 2008;12(6):717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144(12):5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6(7):507–520. doi: 10.1038/nrn1701. nrn1701. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. 0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. S0306453001000452. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205(4409):927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Presence of companion moderates arousal of monkeys with restricted social experience. Physiol Behav. 1984;33(5):693–698. doi: 10.1016/0031-9384(84)90033-7. 0031-9384(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Effects of social partners on pituitary-adrenal activity during novelty exposure in adult female squirrel monkeys. Physiol Behav. 1986;38(6):803–807. doi: 10.1016/0031-9384(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. S0091-3022(09)00040-5. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml PA, Willen R, Watanasriyakul W, Johnson J, Garrett T. Selective social buffering of behavioral and endocrine responses and Fos induction in the prelimbic cortex of infants exposed to a novel environment. Dev Psychobiol. 2015;57(1):50–62. doi: 10.1002/dev.21256. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16(3):381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. S0091302203000293. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. S0278-5846(05)00269-1. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71(3):457–468. doi: 10.1016/s0091-3057(01)00681-5. S0091305701006815. [DOI] [PubMed] [Google Scholar]
- Hofer MA. The effects of brief maternal separations on behavior and heart rate of two week old rat pups. Physiol Behav. 1973;10(3):423–427. doi: 10.1016/0031-9384(73)90200-x. 0031-9384(73)90200-X. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: a psychobiologic perspective on bereavement. Psychosom Med. 1984;46(3):183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Sullivan RM. Towards a neurobiology of attachment. Cumberland, RI: MIT Press; 2001. [Google Scholar]
- Hostinar CE, Gunnar MR. Future directions in the study of social relationships as regulators of the HPA axis across development. J Clin Child Adolesc Psychol. 2013;42(4):564–575. doi: 10.1080/15374416.2013.804387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. 2013-13692-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81(1):67–74. doi: 10.1016/j.nlm.2003.09.002. S1074742703001126. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. S0092-8674(11)01207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104(2):266–271. doi: 10.1016/j.physbeh.2011.03.021. S0031-9384(11)00140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. 9152575N1012140R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behav Neurosci. 2004;118(4):798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y. The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2012;36(10):3429–3437. doi: 10.1111/j.1460-9568.2012.08257. [DOI] [PubMed] [Google Scholar]
- Klein ZA, Romeo RD. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Horm Behav. 2013;64(2):357–363. doi: 10.1016/j.yhbeh.2013.01.012. S0018-506X(13)00031-7. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Butler SR, Schanberg SM. Selective depression of serum growth hormone during maternal deprivation in rat pups. Science. 1978;201(4360):1034–1036. doi: 10.1126/science.684424. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Diaz S, Tempel D. Norepinephrine in the paraventricular nucleus stimulates corticosterone release. Brain Res. 1989;496(1–2):219–227. doi: 10.1016/0006-8993(89)91069-x. 0006-8993(89)91069-X. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405(1–3):149–160. doi: 10.1016/s0014-2999(00)00548-3. S0014299900005483. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73(3):255–260. doi: 10.1016/s0031-9384(01)00496-6. S0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–946. doi: 10.1016/j.psyneuen.2005.03.013. S0306-4530(05)00090-9. [DOI] [PubMed] [Google Scholar]
- Maken DS, Weinberg J, Cool DR, Hennessy MB. An investigation of the effects of maternal separation and novelty on central mechanisms mediating pituitary-adrenal activity in infant guinea pigs (Cavia porcellus) Behav Neurosci. 2010;124(6):800–809. doi: 10.1037/a0021465. 2010-22439-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73(1):147–158. doi: 10.1016/s0091-3057(02)00791-8. S0091305702007918. [DOI] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann N Y Acad Sci. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- McKellar S, Loewy AD. Organization of some brain stem afferents to the paraventricular nucleus of the hypothalamus in the rat. Brain Res. 1981;217(2):351–357. doi: 10.1016/0006-8993(81)90010-x. 0006-8993(81)90010-X. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Dev Psychobiol. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29(50):15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. 29/50/15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. Journal of Neuroscience. 2004;24(5):1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. Journal of Neuroscience. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Otagiri A, Wakabayashi I, Shibasaki T. Selective corticotropin-releasing factor type 1 receptor antagonist blocks conditioned fear-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. J Neuroendocrinol. 2000;12(10):1022–1026. doi: 10.1046/j.1365-2826.2000.00563.x. jne563. [DOI] [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: an in vivo microdialysis study. Brain Res. 1992;589(1):91–96. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688(1–2):242–246. doi: 10.1016/0006-8993(95)00566-9. 0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol. 1995;16(2):89–150. doi: 10.1006/frne.1995.1004. S0091-3022(85)71004-7. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Baffi JS, Pacak K. The role of ascending neuronal pathways in stress-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. J Neuroendocrinol. 1999;11(7):529–539. doi: 10.1046/j.1365-2826.1999.00365.x. jne365. [DOI] [PubMed] [Google Scholar]
- Parma V, Bulgheroni M, Tirindelli R, Castiello U. Facilitation of action planning in children with autism: the contribution of the maternal body odor. Brain Cogn. 2014;88:73–82. doi: 10.1016/j.bandc.2014.05.002. S0278-2626(14)00085-2. [DOI] [PubMed] [Google Scholar]
- Perry BD, Pollard R. Homeostasis, stress, trauma, and adaptation. A neurodevelopmental view of childhood trauma. Child Adolesc Psychiatr Clin N Am. 1998;7(1):33–51. viii. [PubMed] [Google Scholar]
- Perry R, Sullivan RM. Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev Psychobiol. 2014;56(8):1626–1634. doi: 10.1002/dev.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET, Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10(4):437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Otto S, Sutton S. Neurotransmitter modulation of corticotropin releasing factor secretion into the hypophysial-portal circulation. Life Sci. 1987;41(10):1311–1317. doi: 10.1016/0024-3205(87)90211-6. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. 1300769. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15(3):173–185. doi: 10.1016/s0891-0618(98)00045-3. S0891061898000453. [DOI] [PubMed] [Google Scholar]
- Raineki C, Lucion AB, Weinberg J. Neonatal handling: an overview of the positive and negative effects. Dev Psychobiol. 2014;56(8):1613–1625. doi: 10.1002/dev.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M, Sullivan RM. Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol (Lausanne) 2014;5:33. doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiol Behav. 1991;50(4):771–775. doi: 10.1016/0031-9384(91)90016-h. 0031-9384(91)90016-H. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biology Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. S0018-506X(06)00147-4. [DOI] [PubMed] [Google Scholar]
- Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. nrn3918. [DOI] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2(10):1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm Behav. 2007;52(3):391–400. doi: 10.1016/j.yhbeh.2007.06.004. S0018-506X(07)00138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Lunday L, Miner C, Roth TL, Sullivan RM. Development switch in neural circuitry underlying odor-malaise learning. Learn Mem. 2006;13(6):801–808. doi: 10.1101/lm.316006. lm.316006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev Psychobiol. 1990;23(5):411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451(4):301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Struber N, Struber D, Roth G. Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci Biobehav Rev. 2014;38:17–37. doi: 10.1016/j.neubiorev.2013.10.015. S0149-7634(13)00265-0. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19(7):2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31(6):410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Malaval F, Laurent A, Gibaud R, Assenmacher I. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinology. 1987;121(3):883–892. doi: 10.1210/endo-121-3-883. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. S0166-4328(12)00739-5. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. S0149763403000071. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology. 1999;24(6):639–656. doi: 10.1016/s0306-4530(99)00019-0. S0306453099000190. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav Brain Res. 2004;149(2):209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Res. 1996;737(1–2):71–77. doi: 10.1016/0006-8993(96)00627-0. 0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Sajdyk TJ, Dietrich AD, Oberlin B, McDougle CJ, Shekhar A. From anxiety to autism: spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology (Berl) 2007;191(1):107–118. doi: 10.1007/s00213-006-0674-y. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Dev Psychobiol. 2010;52(5):453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224(1):1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;18(23):10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt JL, Coe CL, Levine S. Behavioral and adrenocorticoid responsiveness of squirrel monkeys to a live snake: is flight necessarily stressful? Behav Neural Biol. 1981;32(4):391–405. doi: 10.1016/s0163-1047(81)90826-8. [DOI] [PubMed] [Google Scholar]
- Walker CD, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. J Psychiatry Neurosci. 2004;29(5):364–382. [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128(3):1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behavior Brain Research. 2001;126(1–2):147–157. doi: 10.1016/s0166-4328(01)00260-1. S0166432801002601. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr G. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Ann N Y Acad Sci. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.13001281300128. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102(47):17237–17240. doi: 10.1073/pnas.0504767102. 102/47/17237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Moga MM, Magnuson DJ, Gray TS. Lateral hypothalamic dynorphinergic efferents to the amygdala and brainstem in the rat. Peptides. 1988;9(5):1121–1127. doi: 10.1016/0196-9781(88)90099-x. 0196-9781(88)90099-X. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11(5):361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr Comp Biol. 2002;42(3):541–551. doi: 10.1093/icb/42.3.541. 42/3/541. [DOI] [PubMed] [Google Scholar]


