Abstract
Multiple Sclerosis is a neurologic disease caused by immune cell infiltration into the central nervous system, resulting in grey and white matter inflammation, progressive demyelination and neuronal loss. Astrocytes, the most abundant cell population in the CNS, have been considered inert scaffold- or housekeeping cells for many years. However, recently it has become clear that this cell population actively modulates the immune response in the CNS at multiple levels. While being exposed to a plethora of cytokines during ongoing autoimmune inflammation, astrocytes modulate local CNS inflammation by secreting cytokines and chemokines, among other factors. This review article gives an overview of the most recent understanding about cytokine networks operational in astrocytes during autoimmune neuroinflammation and highlights potential targets for immunomodulatory therapies for Multiple Sclerosis.
Keywords: Multiple Sclerosis, astrocyte, cytokine, chemokine, blood brain barrier
Introduction
Astrocytes are the most abundant cell population in the human central nervous system (CNS) and account for one third of the cells in the murine CNS. Yet, defining their particular function has been a difficult undertaking. So far, morphologic criteria (star-shaped, protoplasmic vs fibroblastic), the expression of glial fibrillary acidic protein (GFAP) and other markers like Aldh1l1 are used to define this population, yet are neither in- nor exclusive. Astrocytes are a heterogenous population not only with respect to their morphological characteristics. It has recently become clear that the morphological variety of astrocytes is reflected by distinct functions. Depending on their location in the CNS (e. g. spinal cord vs frontal white matter vs cortical grey matter) and their association to adjacent brain structures, astrocyte’s activities span from providing structural support, forming extracellular matrix, maintaining stable extracellular ion and neurotransmitter conditions and forming and regulating the blood brain barrier to modulating synaptic function in “tripartite synapses” between neurons and astrocytes. Thus, addressing astrocytes as one unique population may not reflect their multifaceted role in the healthy CNS.
Another degree of complexity is added when analyzing the function of astrocytes during Multiple Sclerosis and its murine model experimental autoimmune encephalomyelitis (EAE). It has become clear that all of the functions outlined above are modulated during ongoing inflammation by interactions between astrocytes and infiltrating immune cells. However, even before the breakdown of the blood brain barrier, cytokines produced in the peripheral immune compartment get access to the CNS. Astrocytes, being part of the blood brain barrier, are among the first to encounter these cytokines and react by producing inflammatory mediators such as chemokines and cytokines of their own without the need of direct cellular contact.
Based on these observations, this review will focus on the regulation of astrocyte activity during Multiple Sclerosis and its animal model Experimental autoimmune encephalomyelitis, EAE. We will also highlight potential links to novel therapeutic strategies derived from these observations and conclude with open questions worth of further study.
Role of selected pro- and anti-inflammatory cytokines
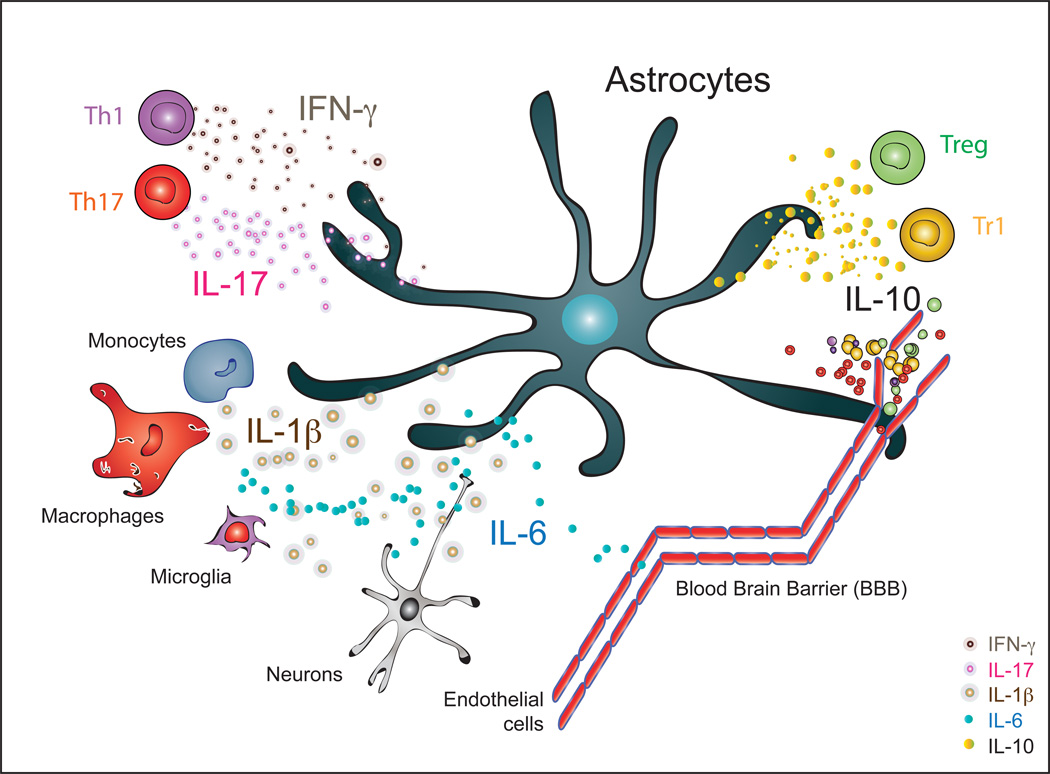
In this first section we will review the most recent advancements and experimental work of select pro- and anti-inflammatory cytokines on astrocyte biology during inflammatory diseases of the CNS. Figure 1 summarizes cellular sources of the mentioned cytokines.
Figure 1. Cellular sources of cytokines that impact astrocyte biology during autoimmune CNS inflammation.
Signature cytokines of CNS infiltrating T cell subsets including Th1, Th17, Treg and Tr1 cells influence astrocyte biology and direct their behavior. Moreover, monocytes, macrophages as well as resident microglia secret IL-1β and IL-6, among others, and influence the local cytokine environment, blood brain barrier integrity and astrocyte and neuronal function.
A. Interferon-γ
Interferon-γ is produced by members of both the adaptive and innate immune system and is the signature cytokine of pathogenic Th1 cells. Its role during autoimmune inflammation is context-specific and depends on multiple factors such as disease stage, cytokine environment and availability as well as activation status of antigen presenting cells. One of the strongest cellular effects of Interferon-γ is the upregulation of MHC-II on antigen presenting cells to induce their ability to present antigen and prime CD4 T cells(1). Astrocytes, as opposed to microglia and oligodendrocytes, express high levels of IFNGR1(2) in vivo and upregulate MHC-II expression upon stimulation with Interferon-γ in vitro (3), emphasizing their potential function as local antigen presenting cells in the CNS. In this context, early in vitro investigations demonstrated that expression of low levels of MHC-II on non-activated astrocytes results in a decrease of T cell proliferation (4) and induces hyporesponsiveness of T and B cells (5), potentially via secretion of the downmodulatory cytokine IL-27(6,7). In contrast, when activated with Interferon-γ, the ability of astrocytes to produce IL-27 is impaired resulting in increased proliferation and pathogenic potential of MOG-specific T cells ex vivo(6). The ability of astrocytes to prime naïve CD4 T cells, however, is still controversial and might also be dependent on the expression of co-stimulatory molecules, which are only in part expressed on astrocytes (8,9). Taken together, these observations, which were mainly obtained in vitro, point towards a proinflammatory net effect of Interferon-γ on astrocytes.
In vivo studies using mice with astrocyte specific dominant negative Interferon-γ receptor expression (H-2b GFAP/IFN-γR1-IC) exhibit more severe EAE and especially fail to recover during later stages of the disease. Mechanistically, an increase in demyelination and axonal loss in KO mice, mediated by enhanced production of proinflammatory mediators (CCL2, CCL5, CXCL10, IL-6, iNos, IL-1, TNF) in combination with elevated expression of MHC-II on microglia were considered responsible for the failure of recovery in these KO mice. In contrast to these observations in vitro, IL-27 was induced by Interferon-γ in vivo mediating increased availability of inflammation-resolving IL-10 (10).
The above cited study suggested a role for Interferon-γ mediated signaling on astrocytes specifically during late stages of the disease, when down-modulatory and reparative mechanisms are mostly dependent on astrocytes driving remodeling of the injured tissue and depend on the availability of astrocytic Interferon-γ signaling. However, potential sequestration in overall bioavailable Interferon-γ due to binding to the dominant negative receptor may have influenced the results of this study. Further investigation using inducible KO models during different stages of the disease are thus needed to validate these findings and further investigate the role of Interferon-γ on astrocytes. To date, Interferon-γ signaling in astrocytes seems to have distinct functions depending on induction, peak or recovery phase of CNS inflammation. This is exemplified by the dichotomy of the presumably pro-inflammatory effects of MHC-II upregulation on astrocytes during early disease stages as opposed to its effect in late disease stages, where it initiates and perpetuates reparative mechanisms.
B. IL-17A
The founding family member of the IL-17 family of cytokines IL-17A has profound effects on different resident cell types in the CNS during inflammatory, ischemic and degenerative diseases (reviewed in (11)) and its impact on astrocytes has been under investigation ever since the discovery of IL-17A in 1993(12). Both murine (13) and human (14) astrocytes express IL-17RA, and its activation upon binding of IL-17A is translated into recruitment of Act1 and ultimately activation of NF-κB(11). In vitro, stimulation of murine and human astrocytes with IL-17A leads to the induction of a proinflammatory state characterized by the production of various cytokines and chemokines (IL-6, TNFα, CCL2, CCL3, CCL20, CXCL1, CXCL2, CXCL9, CXCL10, CXCL11) and these transcripts were also detected in astrocytes during EAE (11,14–18). In addition, blockade of IL-17A by the monoclonal antibody Secukinumab reduces human astrocyte activation as indicated by IL-6 production(14).
Deficiency of IL-17RA in astrocytes has been experimentally mimicked by strategies targeting Act1 directly downstream of IL-17RA both by permanent astrocyte-conditional knockout as well as lentiviral knockdown at different stages of EAE with comparable results. In mice lacking Act1 in neuroectoderm-derived cells (NestinCre Act1fl/fl, affecting neurons, astrocytes and oligodendrocytes) EAE severity was markedly reduced with astrocytes being the main IL-17 responsive and disease mediating population (19). In line with these findings, targeting Act1 in astrocytes by lentiviral knockdown (shAct1) during induction, onset and peak of EAE ameliorated disease while reducing infiltrating inflammatory cells and percentage of Th17 cells in the CNS. This effect was attributed to a reduction in Th17 related mediators in total spinal cord RNA preparations (IL-17A/F, IL-23, IGF1, FGF2, MMP3, CXCL1, CXCL2, CXCL20) (20). This finding could be reproduced to some extent using an astrocyte-conditional knockout strategy (GFAPCre Act1fl/fl), while targeting Ng2+ glial cells (oligodendrocyte precursor cells, OPCs) proved superior in terms of disease mitigation(21). However, it has to be stated that the effects observed when targeting Act1 do not necessarily reflect IL-17RA signaling only, Act1 integrates additional input signals (e. g. CD40L/BAFF signaling) and is thus not private to IL-17 signaling(22,23).
Molecules further downstream in IL-17RA signaling, such as NF-κB and its negative regulator ubiquitin-modifying enzyme A20 have also shown astrocyte-intrinsic effects on CNS inflammation (11,24). Taken together, astrocyte-intrinsic IL-17A mediated signaling seems to promote CNS inflammation. However, recent conflicting data concerning the effects of IL-17A on neuronal stem cells and oligodendrocyte precursor cells(25) highlight the need for further investigations before targeting this immune axis in cell-type specific therapies for Multiple Sclerosis.
C. IL-1β
IL-1β, a proinflammatory member of the IL-1 family of cytokines released not only by macrophages, monocytes, microglia and brain endothelial cells(26), has been shown to affect astrocytes during inflammatory CNS diseases. In in vitro studies, IL-1β was demonstrated to increase blood brain-barrier (BBB) permeability in an astrocyte-intrinsic sonic-hedgehog protein dependent manner to induce chemotactic CCL2, CCL20, and CXCL2 in astrocytes exacerbating BBB disruption and ultimately promoting influx of inflammatory cells (27,28). IL-1β has also been shown to activate Hif-1α, resulting in angioneogenesis and increased BBB permeability(29).
Astrocytic metabolism has also been demonstrated to be influenced by IL-1β: IL-1β upregulates purinergic receptor P2X7 in human astrocytes in vitro to react to changing concentrations of extracellular ADP and ATP (30). Moreover, both in vitro and in vivo IL-1β has been demonstrated to promote expression of astrocytic heme oxygenase-1 expression and the deposition of mitochondrial iron (31). These signals have been demonstrated to induce a reactive astrocyte state that favors astrogliosis and chronic inflammation (32). On the subcellular level, IL-1β result in the NF-kB dependent production of pro-inflammatory cytokines such as CCL2, CCL20 and CXCL2, among others (33).
Astrocytes also participate in the production of IL-1β in response to pro-inflammatory stimuli and thus form part of a positive feed-forward loop that maintains ongoing inflammation and tissue destruction. In these lines, therapeutic concepts such as the blockade of S1P receptors by FTY720 (34), inhibition of COX2 signaling by analogues of celecoxib (35), increase of cytosolic cGMP by sildenafil (36), as well as other immunomodulatory drugs like resveratrol (37), mitoxantron (38) and dimethylumarate (39) have been demonstrated to inhibit IL-1β production in astrocytes during EAE, the cuprizone induced model of demyelination in vivo as well as LPS induced astrocyte activation in vitro.
Thus, the existing knowledge about the action of IL-1β on astrocytes supports the concept of an overall pro-inflammatory role by the induction of a chemoattractant gradient, disruption of the blood brain barrier as well as the impairment of cellular metabolism and the induction of chronic gliosis. Further mechanistic studies using targeted deletion of IL-1R1 in astrocytes, given its importance not only in Th17 differentiation (40,41), or with the IL-1β neutralizing antibody Canakinumab (42) may provide a deeper understanding of the role of IL-1β signaling in astrocytes and might point to novel cell-type specific strategies with a potential role in treating late stage disease.
D. IL-6
While astrocytic production of IL-6 has been used as a surrogate parameter to identify a bona fide pro-inflammatory phenotype in a multitude of experimental studies (24,43,44), surprisingly limited knowledge exists about the functional role of astrocyte-derived IL-6 during EAE and Multiple Sclerosis. While global IL-6 deficient mice are resistant to EAE most likely due to defects in the interplay between innate and adaptive immunity during the priming phase causing failure to raise pathogenic T cell responses (45), recent studies made use of transgenic mice with GFAP-driven expression of IL-6 on a globally IL-6 deficient background (GFAP-IL-6-IL-6 KO mice). These mice showed restored susceptibility to EAE with an atypical, ataxic phenotype which was driven by inflammation, demyelination and astrogliosis sparing the spinal cord while mostly targeting the cerebellum, thus recapitulating a phenotype previously seen in GFAP-IL-6 overexpressing mice on a IL-6 sufficient background (46,47). These observations have profound implications and raise questions as to the importance of IL-6 produced in the periphery as well as the existence of cytokine spill from the CNS across the (intact?) blood brain barrier during the priming phase of immune cells in the peripheral immune compartment. However, it has to be mentioned that in the model system used in these experiments, IL-6 is overexpressed under the control of GFAP promoter “unphysiologically”, and without the need for its induction by other inflammatory stimuli (48). Moreover, GFAP expressing cells have also been described in other organs including the gut, kidney, testes, skin, pancreas, and liver, among others,(49) raising the possibility of IL-6 produced in the peripheral immune compartment confounding the described effects.
In contrast to these studies underlining the generally assumed pro-inflammatory role of astrocyte derived IL-6, astrocyte conditional gp130 deficient mice, which are unable to respond to the IL-6 family of cytokines (including IL-6, IL-11, IL-27, LIF, oncostatin M, ciliary neurotrophic factor (CNTF), B cell-stimulating factor 3 (BSF3), and cardiotrophin (CT)-1) exhibit more severe EAE caused by increases in numbers and activation status of proinflammatory T cells, elevated apoptosis of astrocytes and increased demyelination(50). In this setting, gp130 signaling on astrocytes seems to be overall beneficial. This would argue against a positive autocrine pro-inflammatory feed-forward loop of astrocyte derived IL-6 on astrocytes themselves. However, mutual compensatory mechanisms may be operational in this broad signaling deficient system, which thus allows only for the judgment of the net effect of this cytokine family, but not for definite statements as to the contribution of a single cytokine, e.g. IL-6. Collectively, however, these studies suggest a pro-inflammatory role of astrocyte-derived IL-6. However, further studies are needed to decipher the role of astrocyte derived IL-6 during CNS autoimmune inflammation and possible autocrine effects of astrocyte-derived IL-6.
E. Role of astrocyte derived Nitric oxide
Nitric oxide (NO), a free radical promoting mainly deleterious effects on resident cells in the CNS, is detected in elevated levels in inflammatory lesions and blood, urine and cerebrospinal fluid (CSF) of MS patients (51). Its production during ongoing inflammation has been attributed to the expression of the inducible form of the nitric oxide synthase (iNOS) in macrophages, endo-and epithelial cells, neurons, microglia as well as astrocytes by inflammatory stimuli like IL-1β, IL-17 and TNF (52,53). NO ultimately promotes inflammation and impairs reparative mechanisms by oligodendrocytes and their precursors(54). Mechanistically, NO has been shown to inhibit the production of the blood brain-barrier integrity promoting chemokine CXCL12 in astrocytes through suppression of p38 MAPK (55). Therapeutically, it has been shown that IFN-β, one of the main therapeutics of MS in the peripheral immune compartment, decreases NO production by a human astrocytoma cell line in vitro upon stimulation with proinflammatory cytokines (56). Also, both human and murine astrocytes express Sphingosine 1 Phosphate receptors (S1P) and the immunmodulatory drug fingolimod reduces NO levels both in vivo and in vitro(4). However, fingolimod failed to modulate primary progressive MS in the INFORMS study (Novartis, Miller DH et al. AAN 2013; P07.116), suggesting that its effects on CNSresident cells might be cell type and disease-stage specific (58,59).
Moreover, few mechanistic studies exist addressing the effect of NO on astrocytes themselves. As mentioned above, since iNos is expressed in most of the resident and infiltrating cell types during EAE and MS, reducing oxidative stress in general as opposed to astrocyte-derived NO only might provide the more valuable and promising therapeutic strategy.
F. Effect of anti-inflammatory IL-10 on astrocytes
IL-10 is a well-studied anti-inflammatory cytokine. The most important cellular sources in MS and EAE for IL-10 constitute certain CD4+ T helper cell subsets, namely Forkhead box protein 3 (FoxP3)- expressing natural Treg cells (Tregs) and IL-10 producing T regulatory type 1 (Tr1) cells (60). IL-10 has been shown to exert a multitude of mostly down-modulatory functions upon binding to its receptor resulting in the termination of ongoing immune responses(61,62). Most of these immune mechanisms have been examined in hematopoietic and antigen presenting cells such as T, B and dendritic cells(63).
Only recently the down-modulatory effects of IL-10 on astrocytes are starting to be appreciated. In vitro, rat astrocytes pre-activated with IL-17 and IFN-γ show increased expression of the chemokine CXCL12, which regulates blood brain barrier integrity in a down-modulatory fashion (see below), upon exposure to IL-10 (64). Moreover, activation of the cellular stress response, as determined by upregulation of heat shock protein 60 (Hsp60), has been demonstrated in human astrocytes upon IL-10 encounter (44). Further mechanistic studies carried out in the Theiler’s virus demyelination model (TMEV) have demonstrated that TMEV infected murine astrocytes respond to IL-10 treatment with upregulation of IκBα mRNA impairing NF-κB translocation to the nucleus. This, in turn, abrogates NF-κB induced expression of proinflammatory iNos as well as COX-2 (65,66). Taken together, these studies demonstrate that IL-10 induces anti-inflammatory programs in astrocytes that down-modulate ongoing inflammation. Therapeutic approaches have already been taken to exploit this observation: IL-10 expression was induced in astrocytes during EAE upon their interaction with Glatirameracetate-specific T cells (67). Adult neuronal stem cells genetically modified by a lentiviral vector to secrete IL-10 mitigate inflammation after intraventricular or intravenous injection during EAE, promote remyelination and oligodendrocyte differentiation of neuronal stem cells, and, ultimately reduce astrogliosis causing improved outcome after an inflammatory attack (68).
Astrocytes have also been shown to produce IL-10: human astrocyte cell lines stimulated with IFN-γ1a or combinations of proinflammatory cytokines (LPS/IFN-γ) plus IFN-γ1a produce IL-10, consistent with an anti-inflammatory program as reflected by reduced levels of iNos (69). TLR3 stimulation induces anti-inflammatory cytokines including IL-10 in adult human astrocytes and reduce the production of astrogliosis in terms of “dysfunctional scars” hindering neuronal regeneration (70). This observation was recapitulated in treatment experiments applying LPS to neonatal rats, which decreased pro-inflammatory responses in EAE at later age, as measured by increased IL-10 in the spinal cord (71). However, conflicting results for TLR4 stimulation have been recently published for the treatment of pregnant mice with LPS, which show declined IL-10 production from mixed glial cultures from newborn mice restimulated with LPS(43).
In human MS tissue, IL-10 (but not IL-10R) expression has been demonstrated histologically in chronic active MS-lesions and the hypercellular rim of chronic active MS-lesions in astrocytes(72–74), which has risen the question of whether this expression of IL-10 might contribute to the perseveration of chronic inflammation and the formation of chronic active MS lesions. However, given the previously cited in vitro experiments, IL-10 production in chronic active lesions might also reflect (futile) counter regulation in response to chronic inflammation in these areas.
Taken together, further studies should address open questions as to the effects of IL-10 during autoimmune inflammation in animal experiments in vivo, the effects of TLR stimulation on astrocytic IL-10 production during different stages of ontogenesis and the net effect of astrocyte derived IL-10 during different stages of Multiple Sclerosis. To date, no astrocyte conditional IL-10 or IL-10R knockouts have been generated to address these questions.
Chemokines secreted by astrocytes
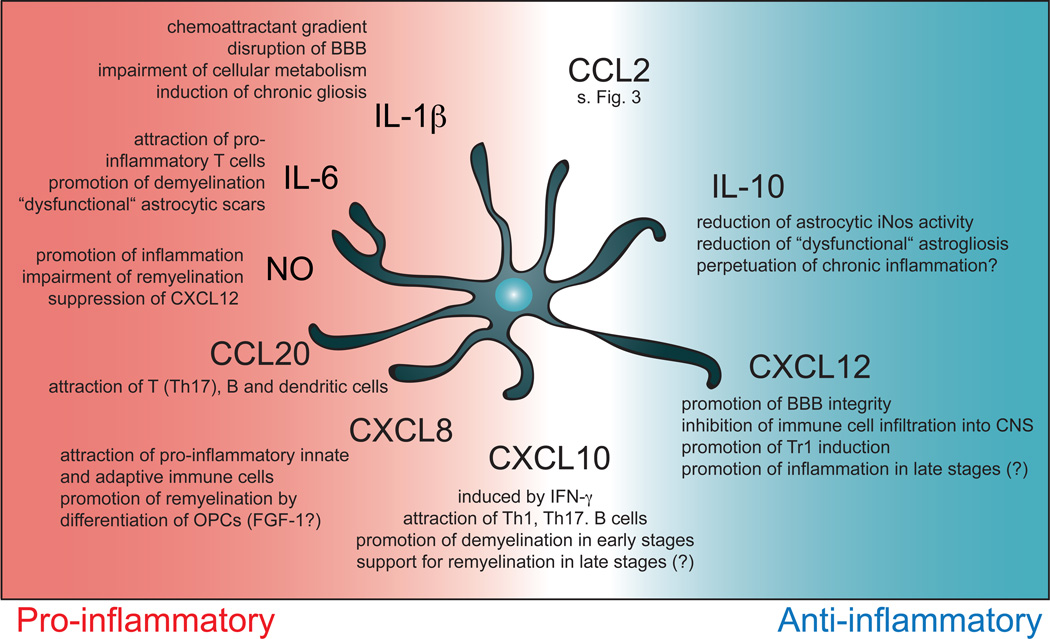
Chemokines modulate ongoing immune responses by recruiting distinct sets of immune cells to the site of inflammation. In Multiple Sclerosis and its animal model EAE, various chemokines have been identified as major players in the control of the immune response. In particular, astrocytes are an important source of chemokines, an important factor considering their strategic position at the interface between brain parenchyma and blood vessels at the blood brain barrier, where they form not only a selective barrier for the entry of immune cells into the CNS but are also capable of secreting chemoattractant mediators into the blood circulation (75). However, the current knowledge about the role of distinct astrocytic chemokines is limited. We will focus on the most recent studies of astrocytic chemokines involved in MS and EAE. An overview of the mentioned chemokines as well as select cytokines is depicted in Figure 2.
Figure 2. Cytokines and chemokines produced by astrocytes regulate CNS inflammation at multiple levels.
Astrocytes create a chemoattractant gradients that guide immune cell infiltration into the CNS, shape the local cytokine milieu to influence recruited and resident cells in the CSN such monocytes and oligodendrocytes and ultimately determine the outcome after local inflammation.
G. CCL2
CCL2 was initially purified in 1989 from the supernatant of the human astroglioma cell line U-105 MG as monomeric polypeptide with a molecular weight of approximately 13 kDa. Its predominant biological activity was determined as the chemoattraction of monocytes, but not neutrophils; hence it was termed “Monocyte chemoattractant protein-1” (MCP-1) and thought to be related to neoplastic diseases (76,77). Since then, extensive research has been undertaken to determine the role of CCL2 and its receptor CCR2 in infectious, inflammatory and neoplastic diseases(78). Not only monocytes, but also macrophages, basophils, mast cells, T cells and microglia express CCR2 and are guided by a CCL2 gradient into different immunological niches.
In the context of autoimmune CNS inflammation, CCL2 remains the best-studied chemokine in Multiple Sclerosis and its animal model to date. Its cellular sources in the CNS have been determined by time course studies during EAE in different rodent strains (79,80) as well as elegant approaches such as Laser Capture Microdissection from different cell types in the inflamed CNS: Microvasculature endothelial cells and, to an even higher extent, astrocytes have been determined as the main resident cell sources of CCL2 in the CNS during EAE (81).
In vitro, combinations of proinflammatory stimuli such as LPS, IL-1β and TNF-α, potentiated by IFN-γ, but also glycolipids (82) induce the expression of CCL2 in murine astrocytes and promote chemotaxis of microglial cells and macrophages(83). However, in vivo IFN-γ signaling seems to be dispensable for the induction of CCL2, since IFNGR−/− mice do not show alterations in the expression levels of CCL2 in the CNS(84). Mechanistically, astrocytic CCL2 expression is controlled by binding of NF-κB to the CCL2 promoter and enhancer (85) as well as activation of stat1(24). Both of these transcription factors are modulated at multiple levels to induce ccl2 transcription, as exemplified by the activation of stat1 and NF-Kb by ubiquitin-modifying protein A20 in astrocytes causing increased chemokine production in vitro and more severe EAE in vivo (24).
So far, three experimental studies have addressed the role of CCL2 production in astrocytes during EAE making use of astrocyte conditional CCL2 knock out mice (GFAPCre CCL2fl/fl). All of them showed decreased disease severity, most pronounced during late diseases stages. As expected based on CCL2’s mode of action as a chemoattractant, numbers of infiltrating macrophages and T cells were found to be reduced. Moreover, the activation status of macrophages, microglia and astrocytes was shifted towards anti-inflammatory phenotypes, which was paralleled by reduced activation of astrocytes and microglia(86). While most of these studies did not detect a change in T cell phenotype(87), CD4 lymphocytes seemed to be confined to CNS perivascular spaces with an impaired ability to migrate through the glia limitans (88). Paul et al compared astrocytic vs endothelial CCL2 deficiency and found that expression of CCL2 in astrocytes regulates overall blood brain barrier integrity by destabilizing endothelial tight junctions and ultimately enables leukocyte penetration into the CNS. In contrast, endothelial CCL2 gives the stimulus for the exit of leukocytes out of the blood vessel (87). This mechanistic view explains their observations, in which endothelial CCL2 deficient mice showed delayed disease onset with comparable severity, while astrocyte conditional CCL2 deficiency causes a similar onset but reduced disease severity. Finally, GFAPCre CCL2fl/fl exhibit reduced long-term axonal loss and demyelination(88). Interestingly, this seems not only to be the result of a decrease and mitigation of the pro-inflammatory infiltrate following a chemotactic stimulus, but is also caused by CNS-intrinsic CCL2 actions.
In the murine model of TNF-α induced demyelination (TID that recapitulates some aspects of demyelination in Multiple sclerosis, CCL2 was up-regulated in astrocytes. Moreover, astrocytes themselves expressed CCR2 both in vivo and in vitro, migrated towards a CCL2 chemotactic gradient underlining the functionality of their CCR2 and showed decreased apoptosis upon CCL2 stimulation via induction of NF-κB by the PI3-kinase and Akt pathways (89). Of note, in experiments using CCR2 deficient astrocytes, those effects were not entirely abolished pointing towards the existence of an alternative receptor for CCL2 on astrocytes. Already in 2003, this alternative receptor for CCL2 and other chemokines, named orphan mouse chemokine receptor L-CCR, was shown to be expressed on infiltrating macrophages, but not T cells, during EAE; moreover, L-CCR was also demonstrated to be expressed on astrocytes and microglia (90) and to be induced by LPS signaling in vivo and in vitro (91,92). Taken together (see Figure 3), astrocytes not only produce CCL2 upon pro-inflammatory stimuli to promote BBB disruption, guide inflammatory cells into the CNS, shape their pro-inflammatory attributes and ultimately promote axonal loss and neurodegeneration, but they also respond to CCL2 themselves by two discrete receptors promoting their survival and chemotaxis. In this partly autocrine positive feed forward loop, astrocytic CCL2 plays a detrimental role and is thus worth being considered for therapeutic approaches.
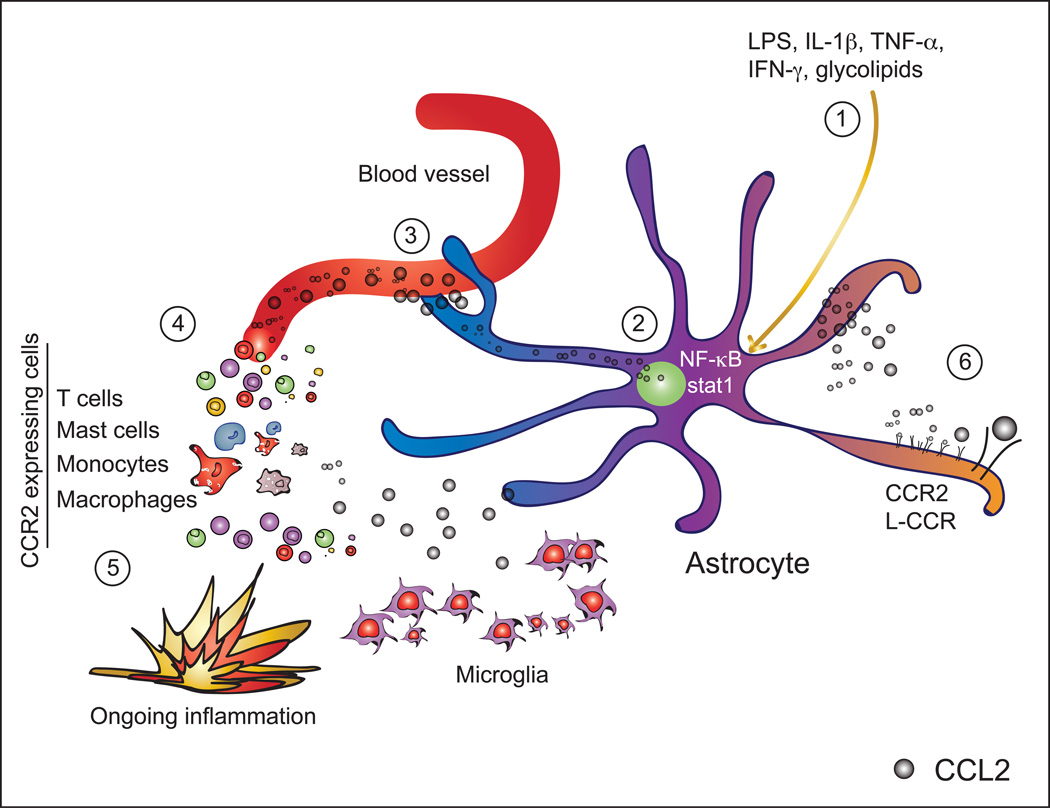
Figure 3. Biology of CCL2 production by astrocytes.
Pro-inflammatory stimuli like LPS, IL-1β, TNF-α, IFN-γ or glycolipids (1), activate NF-κB and stat1 signaling in astrocytes. These transcription factors bind to the CCL2 promoter and its enhancer (2). Favored by their intimate location at the blood brain barrier, astrocytes secrete CCL2 into the circulation (3) creating a chemotactic gradient for cells expressing CCR2 such as T cells, mast cells, monocytes or macrophages (4), which are guided to the site of inflammation (5). Also, autocrine effects of CCL2 upon binding of its receptors CCR2 and L-CCR, both expressed on astrocytes, lead to astrocytic migration towards a CCL2 gradient as well as reduced apoptosis (6).
Bindarit, a small synthetic indazolic derivative that preferentially inhibits transcription of the monocyte chemoattractant subfamily of CC chemokines (CCL2, CCL7, CCL8), has high BBB permeability and was shown to block the synthesis of CCL2 in astrocytes, microglia, and endothelial cells in vitro. Bindarit decreased CNS availability of CCL2 and ameliorated EAE with decreased incidence, onset and severity in a preventive regimen (93). Similarly, treatment with Fasudil, a Rho-kinase inhibitor, improved EAE clinical course by reducing proinflammatory cytokine production from astrocytes, including CCL2 (94). Last, activation of Estrogen receptor α (ERα) by estrogen receptor modulators ameliorates EAE by decreasing astrocytic CCL2 and CCL7 (85,95).
However, some of these therapeutic approaches are confined by side-effects of the drug or lack prove of efficiency in the human system. All in all, more selective and fine-tuned approaches will be needed targeting CCL2 expression in distinct subsets of CNS intrinsic cells, e. g. by specific antagonization or knock-down strategies, in order to dampen autoimmune responses in the CNS without compromising or jeopardizing local immune surveillance.
H. CCl20
CCL20, previously termed Macrophage inflammatory protein-3α (MIP-3α), is a CC motif chemokine functioning as a chemoattractant for cells expressing its receptor CCR6 including certain fractions of Th17 cells and CD4+ T cells. It was first described in 1998 in EAE in vivo as well as in ischemic and MS brain tissue and was shown to be induced in vitro by TNF-α in astrocytes, microglia and macrophage cultures (96) (97). Since then, astrocytes have been demonstrated to be the main source of CCL20 production in response to pro-inflammatory stimuli such as TNF-α, IL-6, IL-17 (mainly in combination with IL-6), IL-1β, IL-9 as well as TLR3 and 4 ligands poly(I:C) and LPS in the CNS (14,17,33,98–100). Interestingly, IL-1β and TNF-α seem to be the most potent inducers of CCL20, while IFN-γ has no effect on its expression(28). Following astrocyte activation by these proinflammatory stimuli, the transcription of CCL20 is induced by phosphorylation of NF-κB and activation of stat3(17). This pathway can be modulated by estrogen receptor signaling, since the selective estrogen receptor modulator Raloxifene decreases astrocytic CCL20 production in vitro and during EAE(33), most likely by inhibiting NF-κB and stat3 mediated signaling. The net effect of CCL20 during EAE is the attraction of T and B cells as well as immature dendritic cells, creating a pro-inflammatory milieu characterized mostly by Th17 cytokines, which further promotes the attraction of immune cells in a positive feed forward loop (101). So far, no astrocyte specific genetic knockout study exists; however, modification of this signaling pathway, as demonstrated for estrogen receptor modulators, might provide a promising strategy worth of further investigation in EAE and MS.
I. Cxcl8 (IL-8)
CXCL8, also known as IL-8 or neutrophil chemotactic factor, is well characterized for its role in viral (102) and protozoic (103) infections to attract neutrophils and, to a lesser extent than CCL2, monocytes (104); extensive studies have focused on its effects during inflammatory diseases like psoriasis or pulmonary conditions like COPD as well as its role in cancer promotion, metastasis and angiogenesis. In these conditions its role as chemotactic agent for neutrophils, as well as its role in angiogenesis are well characterized.
In the context of CNS autoimmune inflammation, Cxcl8 expression in endothelial cells was found to promote Th1 recruitment (105). In addition, CXCL8 is a member of the inflammatory “secretome” of astrocytes induced by TNF-α, IL-1β and IFN-γ in vitro (106–108) (104,109) as well as TLR3 signaling (110). These observations suggest a role for CXCL8 in attracting inflammatory cells of both the innate and adaptive immune system, which promote inflammation and ultimately detrimental effects.
Challenging this conclusion, however, beneficial effects of CXCL8 on remyelination after an autoimmune inflammatory attack have been recently reported. A study by Mohan et al (111) suggest that CXCL8 and LIF are involved in the protective effects of FGF1: in a comparative screening of remyelinated and unmyelinated chronic MS lesions, the authors identified FGF1 as an putative inductor of remyelination. This hypothesis was further tested in vitro, when exposure of myelinating and organotypic cerebellar cultures showed enhanced myelination upon exposure to FGF1. This effect was dependent on the presence of astrocytes. Finally, exposure of astrocyte cultures to FGF-1 led to the induction of LIF and CXCL8, both of which were thus suggested to be involved in the recruitment and differentiation of oligodendrocyte precursor cells (OPCs) and oligodendrocytes to promote remyelination(112). Taking these observations together, CXCL8 is induced in astrocytes by FGF-1 and might induce chemotaxis of OPCs and thus indirectly promote remyelination. These observations make astrocytic CXCL8 an interesting candidate for the promotion of remyelination and recovery after an inflammatory event even in later stages of the disease. However, further experimental studies are needed to corroborate and functionally validate these findings.
J. Cxcl10
CXCL10 belongs to the CXC family of cytokines with a molecular weight of round 8.7 kDa. It has been described as an “inflammatory” chemokine mediating pro-inflammatory immune cell tissue infiltration and is best studied in the context of chronic viral infections such as Hepatitis C(113). As documented in several cell types including keratinocytes, hepatocytes, pancreatic acinar cells, as well as kidney mesangial cells(114), expression of CXCL10 in astrocytes and microglia during EAE depends on the availability of immune cell derived Interferon-γ This has been demonstrated by its absence in Ifngr−/− and Ifng−/− mice (84,115) and its inducibility by adenovirus encoded Interferon-γ (AdIFNg) (116), underlining the convenience of its previous name Interferon-γ inducible protein 10 (IP-10) (79,117). Moreover, other proinflammatory stimuli (IL-1β, TNF-α) (118) as well as TLR3/4 signaling in mouse astrocytes (119) (120) induce CXCL10 production by astrocytes.
In MS and EAE, expression of CXCL10 has been demonstrated in astrocytes surrounding inflammatory lesions (121), where it attracts CXCR3+ Th1 cells and antibody secreting B cells during acute inflammation (122). A recent study using an astrocyte conditional knockout of CXCL10 proved astrocytes to be the main producers of CXCL10 in the CNS and detected diminished disease severity with delayed onset, which was attributed to reduction in CD4 T cell accumulation in perivascular spaces of the spinal cord, a decrease in the ratio of Th1 to Th17 cells as well as reduced acute spinal cord demyelination. However, progressive spinal cord axonal loss at later stages was not affected in these mice, altogether pointing towards a proinflammatory role of astrocytic CXCL10 in acute inflammation with little effect on chronic inflammatory or reparative processes such as remyelination (123). Of note, in EAE studies using globally deficient mice for either CXCL10 or its receptor CXCR3, which is expressed mostly on Th1 cells and shared with the chemokines CXCL9 and CXCL11, contradictory findings exist and favor anti-inflammatory effects of CXCL10 in EAE due to compartmentalization effects between perivascular space and parenchyma (8,9). However, in these studies specific conclusions as to the role of astrocyte derived CXCL10 are hampered by the use of systems globally deficient for CXCL10 or its (shared) receptor.
In the cuprizone model of demyelination astrocyte produced CXCL10 guide microglia to sites of inflammatory damage for the removal of myelin debris, enabling remyelination (126). These findings have been recently challenged by new studies in which early microglia activation by CXCL10 (not CCL2 or CCL3) has detrimental effects on oligodendrocyte survival, remyelination and axonal damage (127). In line with these observations, in vitro studies using human OPCs showed impaired differentiation when co-cultured with pro-inflammatory T cells or macrophages, in part by indirect mechanisms mediated astrocytic CXCL10(128).
The differences observed in these studies may reflect the use of different experimental models, readouts and time points. Given the high expression of the receptor for CXCL10, CXCR3, on T cells in the CSF of MS patients, its elevation in the CSF of patients with active MS(129) as well as evidence for a role of CXCL10 in the promotion of demyelination and axonal damage during later disease stages(130), further experimentation combining different experimental approaches with astrocyte intrinsic deficiency of CXCL10 will be needed to clarify the value of targeting CXCL10 in the treatment of acute and late stages of Multiple Sclerosis.
K. CXCL12
CXCL12, also termed stromal cell derived factor 1α, is known as a key inhibitor of leukocyte entry into the CNS(131–133). While most of the studies available focus on the expression of CXCL12 in endothelial cells of the luminal part of the blood brain barrier, several studies suggest a protective role for CXCL12 in astrocytes as well. First, IL-10 (62) has been shown to stimulate the expression of CXCL12 in rat astrocytes (64) and astrocytic expression of CXCL12 induced by IL-10 is potentiated by TNF-α (134). Second, neurotoxic NO inhibits CXCL12 expression in vitro and iNos expression negatively correlates with the level of CXCL12 transcripts in astrocytes. In vivo, suppression of NO production recovers CXCL12 expression in the CNS (55,135). Third, astrocyte derived CXCL12 redirects the polarization of Th1 cells into CD25-Foxp3-IL-10+ antigen-specific regulatory T cells restraining the autoimmune inflammatory process in an IL-10 dependent manner suggesting a positive feed-forward loop involving IL-10 and CXCL12(136). Taken together, these studies point towards a protective role of CXCL12 generated from astrocytes by mechanisms involving shifts in T-cell polarization, dampening of pro-inflammatory mediators and inhibition of immune cell entry into the CNS.
Conversely, earlier studies demonstrated that astrocytes themselves respond to CXCL12 by the release of potentially neurotoxic glutamate (137). Moreover, Calderon et al (138) reported that CXCL12 is expressed to a low extent by a small number of astrocytes in normal brain tissue and shows its highest expression in astrocytes in active MS-lesions, followed by decreased expression in chronic lesions particularly in hypertrophic astrocytes near the lesion edge. In this study, pro-inflammatory IL-1β and MBP induced CXCL12 expression in astrocytes by signaling pathways involving ERK and Pi3-K, based on which the authors concluded that CXCL12 in astrocytes may initiate or augment the inflammatory response during MS(138). However, these observations might reflect the expression of CXCL12 as a down-modulatory mechanism triggered by inflammation. Additional experiments should be carried out to determine whether CXCL12 induction represents a suitable tool to down-modulate CNS inflammation. Taken together, experimental models are consistent in the idea that CCL2 is essential for the recruitment of inflammatory cells into the CNS while CXCL10 and CXCL12 might have a more context dependent function and can actually inhibit inflammatory infiltrates in the CNS.
Conclusion
Astrocyte derived cytokines and chemokines play important roles in the recruitment of immune cells into the CNS, shape the quality of the ongoing inflammation and are involved in late stage disease mechanisms such as chronic demyelination, axonal loss as well as reparative functions. As outlined in this article, some of these mediators promise therapeutic potential for the treatment of different stages of Multiple Sclerosis. Future pharmacologic studies need to address the accessibility of these mediators for blocking or activation strategies in order to shape the quantity and quality of the immune response. A deeper understanding of cytokines and chemokines active in Multiple Sclerosis might therefore pave the way to novel therapies targeting specific aspects of astrocyte biology for MS and other neurologic diseases.
Acknowledgments
Research in the Quintana laboratory is supported by the National Institutes of Health, the National Multiple Sclerosis Society, the International Progressive MS Alliance and the American Cancer Society. V. R. received support from Mallinckrodt Pharmaceuticals and German Research Foundation (DFG, RO4866-1/1).
Literature
- 1.Muhlethaler-Mottet A, Di Berardino W, Otten LA, Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 2.Hashioka S, Klegeris A, Schwab C, Yu S, McGeer PL. Differential expression of interferon-gamma receptor on human glial cells in vivo and in vitro. J Neuroimmunol. 2010;225:91–99. doi: 10.1016/j.jneuroim.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Nikcevich KM, Gordon KB, Tan L, Hurst SD, Kroepfl JF, Gardinier M, Barrett TA, Miller SD. IFN-gamma-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997;158:614–621. [PubMed] [Google Scholar]
- 4.Sun D, Coleclough C, Whitaker JN. Nonactivated astrocytes downregulate T cell receptor expression and reduce antigen-specific proliferation and cytokine production of myelin basic protein (MBP)-reactive T cells. J Neuroimmunol. 1997;78:69–78. doi: 10.1016/s0165-5728(97)00083-0. [DOI] [PubMed] [Google Scholar]
- 5.Gold R, Schmied M, Tontsch U, Hartung HP, Wekerle H, Toyka KV, Lassmann H. Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death. A model for T-cell apoptosis in vivo. Brain. 1996;119(Pt 2):651–659. doi: 10.1093/brain/119.2.651. [DOI] [PubMed] [Google Scholar]
- 6.Yang JF, Tao HQ, Liu YM, Zhan XX, Liu Y, Wang XY, Wang JH, Mu LL, Yang LL, Gao ZM, et al. Characterization of the interaction between astrocytes and encephalitogenic lymphocytes during the development of experimental autoimmune encephalitomyelitis (EAE) in mice. Clin Exp Immunol. 2012;170:254–265. doi: 10.1111/j.1365-2249.2012.04661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald DC, Zhang G-X, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJM, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 8.Aloisi F, Ria F, Columba-Cabezas S, Hess H, Penna G, Adorini L. Relative efficiency of microglia, astrocytes, dendritic cells and B cells in naive CD4+ T cell priming and Th1/Th2 cell restimulation. Eur J Immunol. 1999;29:2705–2714. doi: 10.1002/(SICI)1521-4141(199909)29:09<2705::AID-IMMU2705>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Sedgwick JD, Mössner R, Schwender S, Meulen ter V. Major histocompatibility complex-expressing nonhematopoietic astroglial cells prime only CD8+ T lymphocytes: astroglial cells as perpetuators but not initiators of CD4+ T cell responses in the central nervous system. J Exp Med. 1991;173:1235–1246. doi: 10.1084/jem.173.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindinger C, Bergmann CC, Hinton DR, Phares TW, Parra GI, Hussain S, Savarin C, Atkinson RD, Stohlman SA. IFN-γ signaling to astrocytes protects from autoimmune mediated neurological disability. PLoS ONE. 2012;7:e42088. doi: 10.1371/journal.pone.0042088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015:1–13. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- 12.Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 13.Sarma Das J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J, Fitzgerald DC, Shindler KS, Rostami A. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2009;6:14. doi: 10.1186/1742-2094-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elain G, Jeanneau K, Rutkowska A, Mir AK, Dev KK. The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A–induced levels of IL6 in human astrocytes. Glia. 2014;62:725–735. doi: 10.1002/glia.22637. [DOI] [PubMed] [Google Scholar]
- 15.Yi H, Bai Y, Zhu X, Lin L, Zhao L, Wu X, Buch S, Wang L, Chao J, Yao H. IL-17A induces MIP-1α expression in primary astrocytes via Src/MAPK/PI3K/NF-kB pathways: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2014;9:629–641. doi: 10.1007/s11481-014-9553-1. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y, Jin J, Chang M, Nakaya M, Hu H, Zou Q, Zhou X, Brittain GC, Cheng X, Sun S-C. TPL2 mediates autoimmune inflammation through activation of the TAK1 axis of IL-17 signaling. J Exp Med. 2014;211:1689–1702. doi: 10.1084/jem.20132640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meares GP, Ma X, Qin H, Benveniste EN. Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia. 2012;60:771–781. doi: 10.1002/glia.22307. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Guo J, Liu J, Wang Z, Liang D. Toll-like receptor signaling directly increases functional IL-17RA expression in neuroglial cells. Clin Immunol. 2014;154:127–140. doi: 10.1016/j.clim.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Y, Ding X, Li K, Ciric B, Wu S, Xu H, Gran B, Rostami A, Zhang G-X. CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes. Mol Ther. 2012;20:1338–1348. doi: 10.1038/mt.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci. 2013;16:1401–1408. doi: 10.1038/nn.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X. Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine. 2008;41:105–113. doi: 10.1016/j.cyto.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Wu L, Zepp J, Li X. Function of Act1 in IL-17 family signaling and autoimmunity. Adv Exp Med Biol. 2012;946:223–235. doi: 10.1007/978-1-4614-0106-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Deckert M, Xuan NT, Nishanth G, Just S, Waisman A, Naumann M, Schlüter D. Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-κB- and STAT 1-dependent chemokine production in astrocytes. Acta Neuropathol. 2013;126:711–724. doi: 10.1007/s00401-013-1183-9. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Li K, Zhu L, Kan Q, Yan Y, Kumar P, Xu H, Rostami A, Zhang G-X. Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC Immunol. 2013;14:20. doi: 10.1186/1471-2172-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidary M, Rakhshi N, Pahlevan Kakhki M, Behmanesh M, Sanati MH, Sanadgol N, Kamaladini H, Nikravesh A. The analysis of correlation between IL-1B gene expression and genotyping in multiple sclerosis patients. J Neurol Sci. 2014;343:41–45. doi: 10.1016/j.jns.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A. Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS ONE. 2014;9:e110024. doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambrosini E, Columba-Cabezas S, Serafini B, Muscella A, Aloisi F. Astrocytes are the major intracerebral source of macrophage inflammatory protein-3alpha/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia. 2003;41:290–300. doi: 10.1002/glia.10193. [DOI] [PubMed] [Google Scholar]
- 29.Argaw AT, Zhang Y, Snyder BJ, Zhao M-L, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 30.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2×7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehindate K, Sahlas DJ, Frankel D, Mawal Y, Liberman A, Corcos J, Dion S, Schipper HM. Proinflammatory cytokines promote glial heme oxygenase-1 expression and mitochondrial iron deposition: implications for multiple sclerosis. J Neurochem. 2001;77:1386–1395. doi: 10.1046/j.1471-4159.2001.00354.x. [DOI] [PubMed] [Google Scholar]
- 32.John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J Neurosci. 2004;24:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Xu W, Chen Y, Qiu W, Shu Y, Wu A, Dai Y, Bao J, Lu Z, Hu X. Raloxifene suppresses experimental autoimmune encephalomyelitis and NF-κB-dependent CCL20 expression in reactive astrocytes. PLoS ONE. 2014;9:e94320. doi: 10.1371/journal.pone.0094320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Leong SY, Moore CS, Cui Q-L, Gris P, Bernier L-P, Johnson TA, Séguéla P, Kennedy TE, Bar-Or A, et al. Dual effects of daily FTY720 on human astrocytes in vitro: relevance for neuroinflammation. J Neuroinflammation. 2013;10:41. doi: 10.1186/1742-2094-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Penta A, Chiba A, Alloza I, Wyssenbach A, Yamamura T, Villoslada P, Miyake S, Vandenbroeck K. A trifluoromethyl analogue of celecoxib exerts beneficial effects in neuroinflammation. PLoS ONE. 2013;8:e83119. doi: 10.1371/journal.pone.0083119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunes AK de S, Rapôso C, Luna RL de A, Cruz-Höfling MAD, Peixoto CA. Sildenafil (Viagra®) down regulates cytokines and prevents demyelination in a cuprizone-induced MS mouse model. Cytokine. 2012;60:540–551. doi: 10.1016/j.cyto.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Wight RD, Tull CA, Deel MW, Stroope BL, Eubanks AG, Chavis JA, Drew PD, Hensley LL. Resveratrol effects on astrocyte function: relevance to neurodegenerative diseases. Biochem Biophys Res Commun. 2012;426:112–115. doi: 10.1016/j.bbrc.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burns SA, Lee Archer R, Chavis JA, Tull CA, Hensley LL, Drew PD. Mitoxantrone repression of astrocyte activation: relevance to multiple sclerosis. Brain Res. 2012;1473:236–241. doi: 10.1016/j.brainres.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation. 2010;7:30. doi: 10.1186/1742-2094-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 41.Fukaya T, Someya K, Hibino S, Okada M, Yamane H, Taniguchi K, Yoshimura A. Loss of Sprouty4 in T cells ameliorates experimental autoimmune encephalomyelitis in mice by negatively regulating IL-1β receptor expression. Biochem Biophys Res Commun. 2014;447:471–478. doi: 10.1016/j.bbrc.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch JD, Gnanasakthy A, Lale R, Choi K, Sarkin AJ. Efficacy of Canakinumab vs. triamcinolone acetonide according to multiple gouty arthritis-related health outcomes measures. Int J Clin Pract. 2014;68:1503–1507. doi: 10.1111/ijcp.12521. [DOI] [PubMed] [Google Scholar]
- 43.Zager A, Peron JP, Mennecier G, Rodrigues SC, Aloia TP, Palermo-Neto J. Maternal immune activation in late gestation increases neuroinflammation and aggravates experimental autoimmune encephalomyelitis in the offspring. Brain Behav Immun. 2015;43:159–171. doi: 10.1016/j.bbi.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Bajramović JJ, Bsibsi M, Geutskens SB, Hassankhan R, Verhulst KC, Stege GJ, de Groot CJ, van Noort JM. Differential expression of stress proteins in human adult astrocytes in response to cytokines. J Neuroimmunol. 2000;106:14–22. doi: 10.1016/s0165-5728(99)00260-x. [DOI] [PubMed] [Google Scholar]
- 45.Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–2187. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 46.Giralt M, Ramos R, Quintana A, Ferrer B, Erta M, Castro-Freire M, Comes G, Sanz E, Unzeta M, Pifarré P, et al. Induction of atypical EAE mediated by transgenic production of IL-6 in astrocytes in the absence of systemic IL-6. Glia. 2013;61:587–600. doi: 10.1002/glia.22457. [DOI] [PubMed] [Google Scholar]
- 47.Quintana A, Müller M, Frausto RF, Ramos R, Getts DR, Sanz E, Hofer MJ, Krauthausen M, King NJC, Hidalgo J, et al. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. The Journal of Immunology. 2009;183:2079–2088. doi: 10.4049/jimmunol.0900242. [DOI] [PubMed] [Google Scholar]
- 48.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Haroon F, Drögemüller K, Händel U, Brunn A, Reinhold D, Nishanth G, Mueller W, Trautwein C, Ernst M, Deckert M, et al. Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. The Journal of Immunology. 2011;186:6521–6531. doi: 10.4049/jimmunol.1001135. [DOI] [PubMed] [Google Scholar]
- 51.Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 52.Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill KE, Zollinger LV, Watt HE, Carlson NG, Rose JW. Inducible nitric oxide synthase in chronic active multiple sclerosis plaques: distribution, cellular expression and association with myelin damage. J Neuroimmunol. 2004;151:171–179. doi: 10.1016/j.jneuroim.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Brenner T, Brocke S, Szafer F, Sobel RA, Parkinson JF, Perez DH, Steinman L. Inhibition of nitric oxide synthase for treatment of experimental autoimmune encephalomyelitis. J Immunol. 1997;158:2940–2946. [PubMed] [Google Scholar]
- 55.Petković F, Blaževski J, Momčilović M, Mostarica Stojkovic M, Miljković D. Nitric oxide inhibits CXCL12 expression in neuroinflammation. Immunol Cell Biol. 2013;91:427–434. doi: 10.1038/icb.2013.23. [DOI] [PubMed] [Google Scholar]
- 56.Guthikonda P, Baker J, Mattson DH. Interferon-beta-1-b (IFN-B) decreases induced nitric oxide (NO) production by a human astrocytoma cell line. J Neuroimmunol. 1998;82:133–139. doi: 10.1016/s0165-5728(97)00172-0. [DOI] [PubMed] [Google Scholar]
- 57.Colombo E, Di Dario M, Capitolo E, Chaabane L, Newcombe J, Martino G, Farina C. Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol. 2014;76:325–337. doi: 10.1002/ana.24217. [DOI] [PubMed] [Google Scholar]
- 58.Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89:225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Al-Izki S, Pryce G, Jackson SJ, Giovannoni G, Baker D. Immunosuppression with FTY720 is insufficient to prevent secondary progressive neurodegeneration in experimental autoimmune encephalomyelitis. Mult Scler. 2011;17:939–948. doi: 10.1177/1352458511400476. [DOI] [PubMed] [Google Scholar]
- 60.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231–244. doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bettini M, Vignali DAA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heinemann C, Heink S, Petermann F, Vasanthakumar A, Rothhammer V, Doorduijn E, Mitsdoerffer M, Sie C, Prazeres da Costa O, Buch T, et al. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nat Commun. 2014;5:3770. doi: 10.1038/ncomms4770. [DOI] [PubMed] [Google Scholar]
- 63.Beebe AM, Cua DJ, de Waal Malefyt R. The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS) Cytokine Growth Factor Rev. 2002;13:403–412. doi: 10.1016/s1359-6101(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 64.Blaževski J, Petković F, Momčilović M, Jevtić B, Miljković D, Mostarica Stojkovic M. High interleukin-10 expression within the central nervous system may be important for initiation of recovery of Dark Agouti rats from experimental autoimmune encephalomyelitis. Immunobiology. 2013;218:1192–1199. doi: 10.1016/j.imbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Molina-Holgado E, Arévalo-Martín A, Castrillo A, Boscá L, Vela JM, Guaza C. Interleukin-4 and interleukin-10 modulate nuclear factor kappaB activity and nitric oxide synthase-2 expression in Theiler’s virus-infected brain astrocytes. J Neurochem. 2002;81:1242–1252. doi: 10.1046/j.1471-4159.2002.00925.x. [DOI] [PubMed] [Google Scholar]
- 66.Molina-Holgado E, Arévalo-Martín A, Ortiz S, Vela JM, Guaza C. Theiler’s virus infection induces the expression of cyclooxygenase-2 in murine astrocytes: inhibition by the anti-inflammatory cytokines interleukin-4 and interleukin-10. Neurosci Lett. 2002;324:237–241. doi: 10.1016/s0304-3940(02)00209-4. [DOI] [PubMed] [Google Scholar]
- 67.Aharoni R, Kayhan B, Eilam R, Sela M, Arnon R. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proc Natl Acad Sci USA. 2003;100:14157–14162. doi: 10.1073/pnas.2336171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H, Zhao Z, Li Y, Ciric B, Curtis M, et al. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009;119:3678–3691. doi: 10.1172/JCI37914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohsenzadegan M, Fayazi MR, Abdolmaleki M, Bakhshayesh M, Seif F, Mousavizadeh K. Direct immunomodulatory influence of IFN-β on human astrocytoma cells. Immunopharmacol Immunotoxicol. 2015;37:214–219. doi: 10.3109/08923973.2015.1014559. [DOI] [PubMed] [Google Scholar]
- 70.Bsibsi M, Persoon-Deen C, Verwer RWH, Meeuwsen S, Ravid R, van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 71.Li X-L, Lv J, Xi N-N, Wang T, Shang X-F, Xu H-Q, Han Z, O’Byrne KT, Li X-F, Zheng R-Y. Neonatal endotoxin exposure suppresses experimental autoimmune encephalomyelitis through regulating the immune cells responsivity in the central nervous system of adult rats. Biochem Biophys Res Commun. 2010;398:302–308. doi: 10.1016/j.bbrc.2010.06.086. [DOI] [PubMed] [Google Scholar]
- 72.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 73.Cannella B, Raine CS. Multiple sclerosis: cytokine receptors on oligodendrocytes predict innate regulation. Ann Neurol. 2004;55:46–57. doi: 10.1002/ana.10764. [DOI] [PubMed] [Google Scholar]
- 74.Hulshof S, Montagne L, De Groot CJA, Van Der Valk P. Cellular localization and expression patterns of interleukin-10, interleukin-4, and their receptors in multiple sclerosis lesions. Glia. 2002;38:24–35. doi: 10.1002/glia.10050. [DOI] [PubMed] [Google Scholar]
- 75.Cheng W, Chen G. Chemokines and chemokine receptors in multiple sclerosis. Mediators Inflamm. 2014;2014:659206–8. doi: 10.1155/2014/659206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 78.O Connor T, Borsig L, Heikenwalder M. CCL2-CCR2 signaling in disease pathogenesis. Endocr Metab Immune Disord Drug Targets. 2015 doi: 10.2174/1871530315666150316120920. [DOI] [PubMed] [Google Scholar]
- 79.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 80.Adamus G, Machnicki M, Amundson D, Adlard K, Offner H. Similar pattern of MCP-1 expression in spinal cords and eyes of Lewis rats with experimental autoimmune encephalomyelitis associated anterior uveitis. J Neurosci Res. 1997;50:531–538. doi: 10.1002/(SICI)1097-4547(19971115)50:4<531::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 81.Shrestha B, Ge S, Pachter JS. Resolution of central nervous system astrocytic and endothelial sources of CCL2 gene expression during evolving neuroinflammation. Fluids Barriers CNS. 2014;11:6. doi: 10.1186/2045-8118-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayo L, Trauger SA, Blain M, Nadeau M, Patel BN, Alvarez JI, Mascanfroni ID, Yeste A, Kivisakk P, Kallas K, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014 doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME. Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. J Neuroimmunol. 1995;60:143–150. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 84.Glabinski AR, Krakowski M, Han Y, Owens T, Ransohoff RM. Chemokine expression in GKO mice (lacking interferon-gamma) with experimental autoimmune encephalomyelitis. J Neurovirol. 1999;5:95–101. doi: 10.3109/13550289909029750. [DOI] [PubMed] [Google Scholar]
- 85.Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proceedings of the National Academy of Sciences. 2010;107:8416–8421. doi: 10.1073/pnas.0910627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, Voskuhl RR. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;274:53–61. doi: 10.1016/j.jneuroim.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paul D, Ge S, Lemire Y, Jellison ER, Serwanski DR, Ruddle NH, Pachter JS. Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J Neuroinflammation. 2014;11:10. doi: 10.1186/1742-2094-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, Pleasure D. Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J Neurosci. 2014;34:8175–8185. doi: 10.1523/JNEUROSCI.1137-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quinones MP, Kalkonde Y, Estrada CA, Jimenez F, Ramirez R, Mahimainathan L, Mummidi S, Choudhury GG, Martinez H, Adams L, et al. Role of astrocytes and chemokine systems in acute TNFalpha induced demyelinating syndrome: CCR2-dependent signals promote astrocyte activation and survival via NF-kappaB and Akt. Mol Cell Neurosci. 2008;37:96–109. doi: 10.1016/j.mcn.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brouwer N, Zuurman MW, Wei T, Ransohoff RM, Boddeke HWGM, Biber K. Induction of glial L-CCR mRNA expression in spinal cord and brain in experimental autoimmune encephalomyelitis. Glia. 2004;46:84–94. doi: 10.1002/glia.10352. [DOI] [PubMed] [Google Scholar]
- 91.Zuurman MW, Heeroma J, Brouwer N, Boddeke HWGM, Biber K. LPS-induced expression of a novel chemokine receptor (L-CCR) in mouse glial cells in vitro and in vivo. Glia. 2003;41:327–336. doi: 10.1002/glia.10156. [DOI] [PubMed] [Google Scholar]
- 92.Biber K, Zuurman MW, Homan H, Boddeke HWGM. Expression of L-CCR in HEK 293 cells reveals functional responses to CCL2, CCL5, CCL7, and CCL8. J Leukoc Biol. 2003;74:243–251. doi: 10.1189/jlb.0802415. [DOI] [PubMed] [Google Scholar]
- 93.Ge S, Shrestha B, Paul D, Keating C, Cone R, Guglielmotti A, Pachter JS. The CCL2 synthesis inhibitor bindarit targets cells of the neurovascular unit, and suppresses experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9:171. doi: 10.1186/1742-2094-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo M-F, Meng J, Li Y-H, Yu J-Z, Liu C-Y, Feng L, Yang W-F, Li J-L, Feng Q-J, Xiao B-G, et al. The inhibition of Rho kinase blocks cell migration and accumulation possibly by challenging inflammatory cytokines and chemokines on astrocytes. J Neurol Sci. 2014;343:69–75. doi: 10.1016/j.jns.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 95.Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CRL, Stapornkul B, Itoh N, Sofroniew MV, Voskuhl RR. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons. J Neurosci. 2013;33:10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Utans-Schneitz U, Lorez H, Klinkert WE, da Silva J, Lesslauer W. A novel rat CC chemokine, identified by targeted differential display, is upregulated in brain inflammation. J Neuroimmunol. 1998;92:179–190. doi: 10.1016/s0165-5728(98)00204-5. [DOI] [PubMed] [Google Scholar]
- 97.Varona R, Zaballos A, Gutiérrez J, Martín P, Roncal F, Albar JP, Ardavín C, Márquez G. Molecular cloning, functional characterization and mRNA expression analysis of the murine chemokine receptor CCR6 and its specific ligand MIP-3alpha. FEBS Lett. 1998;440:188–194. doi: 10.1016/s0014-5793(98)01450-1. [DOI] [PubMed] [Google Scholar]
- 98.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 99.Zhou Y, Sonobe Y, Akahori T, Jin S, Kawanokuchi J, Noda M, Iwakura Y, Mizuno T, Suzumura A. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. The Journal of Immunology. 2011;186:4415–4421. doi: 10.4049/jimmunol.1003307. [DOI] [PubMed] [Google Scholar]
- 100.Liu X, Tian Y, Lu N, Gin T, Cheng CHK, Chan MTV. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. PLoS ONE. 2013;8:e75804. doi: 10.1371/journal.pone.0075804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reboldi A, Reboldi A, Coisne C, Coisne C, Baumjohann D, Baumjohann D, Benvenuto F, Benvenuto F, Bottinelli D, Bottinelli D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 102.van Heteren JT, Rozenberg F, Aronica E, Troost D, Lebon P, Kuijpers TW. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutières syndrome. Glia. 2008;56:568–578. doi: 10.1002/glia.20639. [DOI] [PubMed] [Google Scholar]
- 103.Uddin J, Garcia HH, Gilman RH, Gonzalez AE, Friedland JS. Monocyte-astrocyte networks and the regulation of chemokine secretion in neurocysticercosis. J Immunol. 2005;175:3273–3281. doi: 10.4049/jimmunol.175.5.3273. [DOI] [PubMed] [Google Scholar]
- 104.Zheng JC, Huang Y, Tang K, Cui M, Niemann D, Lopez A, Morgello S, Chen S. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-alpha: involvement of mitogen-activated protein kinases and protein kinase R. J Neuroimmunol. 2008;200:100–110. doi: 10.1016/j.jneuroim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Subileau EA, Rezaie P, Davies HA, Colyer FM, Greenwood J, Male DK, Romero IA. Expression of chemokines and their receptors by human brain endothelium: implications for multiple sclerosis. J Neuropathol Exp Neurol. 2009;68:227–240. doi: 10.1097/NEN.0b013e318197eca7. [DOI] [PubMed] [Google Scholar]
- 106.Choi SS, Lee HJ, Lim I, Satoh J-I, Kim SU. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS ONE. 2014;9:e92325. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- 108.Jing T, Wu L, Borgmann K, Surendran S, Ghorpade A, Liu J, Xiong H. Soluble factors from IL-1β-stimulated astrocytes activate NR1a/NR2B receptors: implications for HIV-1-induced neurodegeneration. Biochem Biophys Res Commun. 2010;402:241–246. doi: 10.1016/j.bbrc.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- 110.So EY, Kang MH, Kim BS. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler’s murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia. 2006;53:858–867. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- 111.Mohan H, Friese A, Albrecht S, Krumbholz M, Elliott CL, Arthur A, Menon R, Farina C, Junker A, Stadelmann C, et al. Transcript profiling of different types of multiple sclerosis lesions yields FGF1 as a promoter of remyelination. Acta Neuropathol Commun. 2014;2:178. doi: 10.1186/s40478-014-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelland EE, Gilmore W, Weiner LP, Lund BT. The dual role of CXCL8 in human CNS stem cell function: multipotent neural stem cell death and oligodendrocyte progenitor cell chemotaxis. Glia. 2011;59:1864–1878. doi: 10.1002/glia.21230. [DOI] [PubMed] [Google Scholar]
- 113.Khalifa KA, Radwan WM, Attallah KM, Hosney H, Eed EM. Pretreatment serum interferon gamma inducible protein-10 as biomarker of fibrosis and predictor of virological response in genotype 4 hepatitis C virus infection. Acta Gastroenterol Belg. 2014;77:401–407. [PubMed] [Google Scholar]
- 114.Vazirinejad R, Ahmadi Z, Kazemi Arababadi M, Hassanshahi G, Kennedy D. The biological functions, structure and sources of CXCL10 and its outstanding part in the pathophysiology of multiple sclerosis. Neuroimmunomodulation. 2014;21:322–330. doi: 10.1159/000357780. [DOI] [PubMed] [Google Scholar]
- 115.Carter SL, Müller M, Manders PM, Campbell IL. Induction of the genes for Cxcl9 and Cxcl10 is dependent on IFN-gamma but shows differential cellular expression in experimental autoimmune encephalomyelitis and by astrocytes and microglia in vitro. Glia. 2007;55:1728–1739. doi: 10.1002/glia.20587. [DOI] [PubMed] [Google Scholar]
- 116.Millward JM, Caruso M, Campbell IL, Gauldie J, Owens T. IFN-gamma-induced chemokines synergize with pertussis toxin to promote T cell entry to the central nervous system. J Immunol. 2007;178:8175–8182. doi: 10.4049/jimmunol.178.12.8175. [DOI] [PubMed] [Google Scholar]
- 117.Glabinski AR, Tani M, Tuohy VK, Tuthill RJ, Ransohoff RM. Central nervous system chemokine mRNA accumulation follows initial leukocyte entry at the onset of acute murine experimental autoimmune encephalomyelitis. Brain Behav Immun. 1995;9:315–330. doi: 10.1006/brbi.1995.1030. [DOI] [PubMed] [Google Scholar]
- 118.Rubio N, Arevalo M-A, Cerciat M, Sanz-Rodriguez F, Unkila M, Garcia-Segura LM. Theiler’s virus infection provokes the overexpression of genes coding for the chemokine Ip10 (CXCL10) in SJL/J murine astrocytes, which can be inhibited by modulators of estrogen receptors. J Neurovirol. 2014;20:485–495. doi: 10.1007/s13365-014-0273-3. [DOI] [PubMed] [Google Scholar]
- 119.Imaizumi T, Numata A, Yano C, Yoshida H, Meng P, Hayakari R, Xing F, Wang L, Matsumiya T, Tanji K, et al. ISG54 and ISG56 are induced by TLR3 signaling in U373MG human astrocytoma cells: possible involvement in CXCL10 expression. Neurosci Res. 2014;84:34–42. doi: 10.1016/j.neures.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 120.Imaizumi T, Murakami K, Ohta K, Seki H, Matsumiya T, Meng P, Hayakari R, Xing F, Aizawa-Yashiro T, Tatsuta T, et al. MDA5 and ISG56 mediate CXCL10 expression induced by toll-like receptor 4 activation in U373MG human astrocytoma cells. Neurosci Res. 2013;76:195–206. doi: 10.1016/j.neures.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 121.Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 122.Phares TW, Stohlman SA, Hinton DR, Bergmann CC. Astrocyte-derived CXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. J Virol. 2013;87:3382–3392. doi: 10.1128/JVI.03307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mills Ko E, Ma JH, Guo F, Miers L, Lee E, Bannerman P, Burns T, Ko D, Sohn J, Soulika AM, et al. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J Neuroinflammation. 2014;11:105. doi: 10.1186/1742-2094-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Müller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJC, et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- 125.Lalor SJ, Segal BM. Th1-mediated experimental autoimmune encephalomyelitis is CXCR3 independent. Eur J Immunol. 2013;43:2866–2874. doi: 10.1002/eji.201343499. [DOI] [PubMed] [Google Scholar]
- 126.Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M, Baumgärtner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain. 2013;136:147–167. doi: 10.1093/brain/aws262. [DOI] [PubMed] [Google Scholar]
- 127.Clarner T, Janssen K, Nellessen L, Stangel M, Skripuletz T, Krauspe B, Hess F-M, Denecke B, Beutner C, Linnartz-Gerlach B, et al. CXCL10 Triggers Early Microglial Activation in the Cuprizone Model. The Journal of Immunology. 2015:1401459. doi: 10.4049/jimmunol.1401459. [DOI] [PubMed] [Google Scholar]
- 128.Moore CS, Cui Q-L, Warsi NM, Durafourt BA, Zorko N, Owen DR, Antel JP, Bar-Or A. Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. The Journal of Immunology. 2015;194:761–772. doi: 10.4049/jimmunol.1401156. [DOI] [PubMed] [Google Scholar]
- 129.Sørensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sørensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, Lassmann H, Olsen DB, Strieter RM, Ransohoff RM, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 131.Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Momčilović M, Mostarica Stojkovic M, Miljković D. CXCL12 in control of neuroinflammation. Immunol Res. 2012;52:53–63. doi: 10.1007/s12026-012-8282-x. [DOI] [PubMed] [Google Scholar]
- 133.McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- 134.Blaževski J, Petković F, Momčilović M, Jevtić B, Stojković MM, Miljković D. Tumor necrosis factor stimulates expression of CXCL12 in astrocytes. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 135.Timotijević G, Petković F, Blaževski J, Momčilović M, Mostarica Stojkovic M, Miljković D. CXCL12-γ expression is inhibited in neuroinflammation. Brain Res. 2013;1519:120–126. doi: 10.1016/j.brainres.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 136.Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–2655. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 138.Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]