Abstract
Oxytocin modulates many aspects of social cognition and behaviors, including maternal nurturing, social recognition and bonding. Natural variation in oxytocin receptor (OXTR) density in the nucleus accumbens (NAcc) is associated with variation in alloparental behavior, and artificially enhancing OXTR expression in the NAcc enhances alloparental behavior and pair bonding in socially monogamous prairie voles. Furthermore, infusion of an OXTR antagonist into the nucleus accumbens (NAcc) inhibits alloparental behavior and partner preference formation. However, antagonists can promiscuously interact with other neuropeptide receptors. To directly examine the role of OXTR signaling in social bonding, we used RNA interference to selectively knockdown, but not eliminate, OXTR in the NAcc of female prairie voles and examined the impact on social behaviors. Using an adeno-associated viral vector expressing a short hairpin RNA (shRNA) targeting Oxtr mRNA, we reduced accumbal OXTR density in female prairie voles from juvenile age through adulthood. Females receiving the shRNA vector displayed a significant reduction in alloparental behavior and disrupted partner preference formation. These are the first direct demonstrations that OXTR plays a critical role in alloparental behavior and adult social attachment, and suggest that natural variation in OXTR expression in this region alone can create variation in social behavior.
Keywords: pair bonding, alloparental care, striatum
INTRODUCTION
Oxytocin (OT) is a hypothalamic neuropeptide first identified for its role in coordinating the onset of labor and the milk ejection reflex during nursing (Burbach et al., 2006). Centrally released OT also facilitates the induction of maternal nurturing and the mother-infant bond in rats and sheep (Ross and Young, 2009; Rilling and Young, 2014). OT is thought to modulate a host of social behaviors by increasing the salience of social stimuli and by enhancing the rewarding aspects of social interactions (Young, 2015). In both male and female mice, OT facilitates individual recognition based on social olfactory cues (Ferguson et al., 2001; Choleris et al., 2007; Choleris et al., 2009). OT knockout mice fail to recognize other mice to which they have been exposed, but display normal olfactory habituation to non-social stimuli (Ferguson et al., 2000). The social recognition deficits can be rescued with an infusion of OT into the medial amygdala before, but not after the initial social exposure (Ferguson, Aldag et al. 2001). These data suggest that OT enhances the neural processing of social olfactory cues and promotes social learning.
Monogamous prairie voles have become an important animal model for investigating the role of OT in modulating social cognition and behavior (Carter et al., 1992; Young and Wang, 2004; McGraw and Young, 2010). Correlational studies of natural variation in oxytocin receptor (OXTR) expression and pharmacological manipulations suggest that OT enhances alloparental behavior and pair bonding, as determined by the partner preference test, by acting on OXTR in the nucleus accumbens (NAcc). The highly social prairie vole has high densities of OXTR in the NAcc compared to relatively asocial non-monogamous vole species (Insel and Shapiro, 1992; Young and Wang, 2004). Infusing an OXTR antagonist into the NAcc of female prairie voles reduces alloparental behavior and prevents mating-induced partner preference formation (Young, 2001; Young and Wang, 2004; Olazábal and Young, 2006a). Dopamine D2 and mu opioid signaling in the striatum are also critical for partner preference formation in prairie voles (Young and Wang, 2004; Burkett et al., 2011; Resendez et al., 2013). The interaction of these systems and the role of OT in the processing of social stimuli suggest that OXTR signaling in the NAcc facilitates pair bonding by linking the neural encoding of the olfactory cues of the partner with the reinforcing aspects of affiliative interactions and mating (Johnson and Young, 2015). Thus partner preference formation may be useful for identifying therapies to increase social cognitive function in psychiatric disorders (Modi and Young, 2011; Modi and Young, 2012).
There is remarkable individual variation in the density of OXTR in the NAcc and variation in NAcc OXTR density is associated with variation in social behaviors in female prairie voles. Adult females with high densities of OXTR binding in the NAcc display more spontaneous alloparental nurturing responses and are less likely to ignore or attack pups compared to those with low densities of OXTR, consistent with the hypothesis that OT modulates social motivation (Olazábal and Young, 2006a). Furthermore, in 20-day old juvenile females, there is a positive correlation between NAcc OXTR density and duration of alloparental responsiveness (Olazábal and Young, 2006b). Enhancing OXTR expression in the NAcc of adult females using viral vector mediated gene transfer facilitates partner preference formation, but has no effect on alloparental behavior (Ross et al., 2009). However, when the same viral vector was administered in the NAcc of juveniles at weaning (21 days of age) to enhance OXTR expression, the prepubertally treated female prairie voles displayed a robust increase in both alloparental behavior and partner preference behavior as adults (Keebaugh and Young, 2011). Early-life social isolations, in a model of neglect, disrupt the ability of female prairie voles to form pair bonds as adults, but only in those with low densities of OXTR in the NAcc (Barrett et al., Under Revision). These studies suggest that OXTR signaling in the NAcc can have both activational and organizational effects on adult social behaviors.
In humans, single nucleotide polymorphisms (SNPs) in the OXTR locus have been associated with variation in social behaviors, including maternal sensitivity and physiological responses to infants (Bakermans-Kranenburg and van Ijzendoorn, 2008) (Riem et al., 2010; Rilling and Young, 2014), face recognition memory (Skuse et al., 2014), emotion recognition (Rodrigues et al., 2009) and pair-bonding and relationship quality (Walum et al., 2012). Furthermore, intranasal administration of OT enhances emotion recognition and behaviors associated with monogamy (Scheele et al., 2012; Scheele et al., 2013) and enhance parenting behaviors, including positive affect, social gaze and vocal synchrony in humans (Weisman et al., 2012; Rilling and Young, 2014).
Several candidate gene association studies have reported links between SNP’s in OXTR and either Autism Spectrum Disorder (ASD) diagnosis, or ASD sub-domains such as social interactions and communication (Wu et al., 2005; Ylisaukko-oja et al., 2006; Jacob et al., 2007; Yrigollen et al., 2008; Liu et al., 2010; Campbell et al., 2011; Walum et al., 2012; LoParo and Waldman, 2014), while others have failed to find an association with the diagnosis of ASD (Parker et al., 2014; Skuse et al., 2014). All of the SNPs associated with ASD or social behaviors lie within non-coding regions of OXTR, suggesting that the impact on behavior is mediated by variation in OXTR expression, not structure. Finally, CpG islands in the first intron of the OXTR gene are hypermethylated in the cortex of subjects with ASD, and in a limited sample size, OXTR mRNA expression in the cortex of subjects with ASD is reduced compared to healthy controls (Gregory et al., 2009).
While the natural variation in OXTR density and pharmacological studies are consistent with a critical role for NAcc OXTR signaling in alloparental behavior and pair bonding, they are not direct evidence that variation in endogenous OXTR signaling is essential or contributes to variation in these behaviors among individuals. The OXTR antagonists used previously can interfere with other neuropeptide receptor systems, including the vasopressin 1a receptor (Manning et al., 2008). For example, infusion of an OXTR antagonist into the NAcc could interfere with vasopressin 1a receptor signaling in the adjacent ventral pallidum. In order to better understand how developmental variation in OXTR signaling may contribute to variation in social behavior we use RNA interference (RNAi) to selectively compromise OXTR expression in the NAcc of female prairie voles. Specifically, we developed adeno-associated viral vectors expressing short hairpin RNAs to selectively degrade Oxtr mRNA in infected cells. This approach allows us to achieve targeted OXTR reduction, but not elimination, which may better reflect both natural variation in prairie voles as well as humans. We then tested the effect of reduced OXTR expression on alloparental behavior and partner preference formation, a laboratory index of pair bonding.
METHODS AND MATERIALS
Animals and Animal Care
Animals were laboratory-bred prairie voles, originally derived from an Illinois field-captured population. The animals were maintained on a 14:10 light:dark cycle with lights on at 7:00AM. Housing consisted of ventilated Plexiglass cages (36 x18 x19 cm) lined with bed-o-cob bedding (Maumee, OH). Access to food (Purina high-fiber rabbit chow) and water was available ad libitum in a room maintained at 22°C. At 21 days of age, animals were weaned into same-sex groups (2–3 voles/cage). At the time of weaning, experimental females were injected with either scrambled control virus (N= 18), or shRNA-Oxtr virus (N=21) into the NAcc. Littermates were assigned to different treatment groups to control for variability within litters and within cages. No more than 2 littermates were assigned to a single group. Subjects remained undisturbed until young adulthood, postnatal day (PND) 35, at which time they were tested for alloparental behavior. As adults, animals were tested for the development of a partner preference. Stimulus pups for the alloparental behavior tests were 2–5 days of age and of mixed sex, and stimulus males for the partner preference test were sexually experienced adults. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Emory University Institutional Animal Care and Use Committee.
shRNA Viral Vector Production
Short hairpin RNA sequences (shRNAs) targeting the prairie vole Oxtr mRNA, as well as a scrambled control sequence, were developed using Invitrogen’s BLOCK-iT RNAi Designer software (Invitrogen, Carlsbad, CA). In order to minimize off-target effects, a BLAST search against other Microtus sequences was systematically performed to ensure a single mRNA sequence was targeted. More recently the prairie vole genome was published (taxid: 79684; http://www.ncbi.nlm.nih.gov/genome/?term=microtus+ochrogaster) and we confirmed post hoc that the shRNA sequences did not target any gene other than Oxtr. Three shRNA sequences targeting Oxtr mRNA were tested in in vivo assays using HEK 293 cells transfected with plasmid encoding the prairie vole OTR-GFP fusion protein as previously described for the vasopressin receptor by our lab (Barrett et al., 2013). The most efficient shRNA targeted a region just over 600 bp into the Oxtr gene (sense target sequence: TGGATCACGCTTGCCGTCTACATTG; scrambled sequence: CTGATATCGGTACGCGTCTTACCTC). The shRNA’s spliced downstream of the U6 promotor using the pENTR/U6 plasmid (Invitrogen, Gateway Cloning Technology) were cloned into adeno-associated viral vectors (AAV) as described recently (Barrett et al., 2013). AAV was produced by the Emory NINDS Viral Vector Core Facility (http://neurology.emory.edu/ENNCF/viral_vector/index.html). Both scrambled (Oxtr control) and OTR shRNA (shRNA-pvOxtr) vectors also express green fluorescent protein for visualization of the transfected neurons.
Viral Vector Infusion
AAV infusions were performed on 21-day-old females under isoflurane anesthesia in a Kopf stereotax fitted with an Ultra Micro Pump II (World Precision Instruments, Sarasota, FL) and a 26 gauge 5 ml Hamilton syringe (Hamilton as described previously (Keebaugh and Young, 2011). Females were injected bilaterally into the nucleus accumbens (NAcc; anterioposterior +1.7mm, mediolateral ±0.9mm, dorsoventral −0.45mm) with 2µl per side of either shRNA-pvOxtr (N=21) or the scrambled Oxtr control vector (N=18). Virus was infused at a rate of 5nl/s and the syringe was left in place for 5 minutes following the injection to minimize diffusion up the needle track. Following AAV injection, animals were group housed until behavioral testing in young adulthood.
Alloparental Behavior
At 35 days of age, female prairie voles were tested for alloparental behavior to assess their willingness to care for novel, unrelated stimulus pups as previously described (Olazábal and Young, 2006a; Olazábal and Young, 2006b). The latency and frequency to approach or attack the pups, as well as the duration of licking, carrying, huddling and retrieving the pups was recorded and scored by an observer blind to the experimental condition using Stopwatch (http://www.cbn-atl.org/research/stopwatch.shtml) as previously described (Ahern et al., 2009). Females were considered “alloparental” if they spent >30s huddling over the pups and >5s licking the pups without attacking as previously described (Olazábal and Young, 2005). A latency of 900s was assigned to animals that did not approach the pups for the purpose of statistical analysis. Latency values were not included in the analysis for females that attacked pups.
Partner Preference Testing
At approximately 70 days of age, gonadally-intact experimental females were placed in a clean cage (28 × 17 × 12 cm) at 9:00 AM with an age-matched sexually-experienced stimulus male for 24 hours. Females were not estrogen primed and therefore not immediately sexually receptive. In prairie vole females, however, a 24 hour cohabitation even in the absence of mating typically results in partner preference formation (Williams et al., 1992). Following the cohabitation period, females were examined vaginally to determine if mating had likely occurred. Females with vaginal redness and vaginal opening were considered to have mated. This method has been previously validated to accurately reflect mating (Ahern and Young, 2009). All animals displayed evidence of mating based on vaginal examination (shRNA-pvOxtr N=21, Oxtr scrambled control N=18), and were therefore included in the partner preference testing. All animals then underwent a 3 h partner preference test as described previously (Williams et al., 1992). During the partner preference test, the male partner is tethered in one chamber, and a second novel sexually experienced male (stranger) is tethered in another chamber of a three-chambered testing arena. The experimental female is placed in the middle chamber and is free to roam throughout the arena for three hours. Time spent in immobile contact (huddling) with the partner and the stranger was recorded and analyzed using an automated system (Social Scan 2.0, Clever Sys Inc., Reston, VA) as previously described (Ahern et al., 2009). Distance traveled was also quantified as an index of motor activity. Animals were considered to have formed a partner preference if they spent twice as much time in immobile contact with the partner versus the stranger.
Brain Collection and Processing
Following the behavioral testing, experimental females were euthanized with isofluorane inhalation and rapidly decapitated. Brains were removed from the skull, frozen on powdered dry ice, and stored at −80°C until sectioning. Using a cryostat, the brains were sectioned through the NAcc in 6 series at 20µM onto Fisher Frost plus slides (12-550-15, Fisher Scientific). Slides were stored at −80°C until analysis for OXTR knockdown using autoradiography and injection site accuracy using GFP fluorescence.
GFP Visualization
Sections were removed from −80°C storage, allowed to air dry and cover slipped using Krystalon (EMD Chemicals). Green Fluorescence Protein (GFP) expression was visualized by fluorescent microscopy at the injection site. Animals with injection sites outside of the NAcc were excluded from all analyses (shRNA-pvOxtr N=2/21 misses with final N=19; scrambled Oxtr control N=3/18 misses with final N=15).
Receptor Binding Autoradiography
Receptor binding for OXTR was used to assess whether group differences in expression were present as previously described (Ahern and Young, 2009). Autoradiograms were quantified as previously described (Barrett et al., 2013). Optical density readings were measured and converted to decompositions per minute (d.p.m./milligram tissue equivalent) based on I125 autoradiographic standards (ARI 0133A, American Radiolabeled Chemicals) using an automated computer-based image analysis system and AIS™ software version 6.0 (Imaging Research Inc., Onterio, Canada).
Optical density measures of OXTR binding for the caudate putamen (CP), nucleus accumbens (NAcc), and prefrontal cortex (PFC) was measured using four brain sections for each region and averaged across sections. Background readings were taken from an adjacent area with no OXTR binding and averaged. It should be noted that each reading was made on the same four brain sections across all regions. Specific binding was calculated by subtracting the average non-specific background binding for each brain from the readings for all brain regions taken from that specific animal.
Statistical Analysis
OXTR binding in the CP, NAcc and PFC was analyzed by Student’s t-test with a bonnferoni correction (α=0.05/3=0.016). Alloparenting behaviors were analyzed using a One-way ANOVA. In addition, subjects were categorized as either being alloparental or non-alloparental as described above. Fisher's exact test was used to compare the proportion of alloparental females between groups and the proportion of females that attacked pups. Data from the partner preference tests were analyzed using two-way ANOVAs in which stimulus animal (partner or stranger) and treatment (shRNA-pvOxtr and Oxtr scrambled control) were factors. When a main effect of stimulus animal was found Student's t-test were used to compare time in contact with the partner and stranger within each treatment group. Data collected for locomotor activity during the partner preference test was analyzed using a one-way ANOVA with treatment as the independent factor.
RESULTS
Receptor Binding Autoradiography
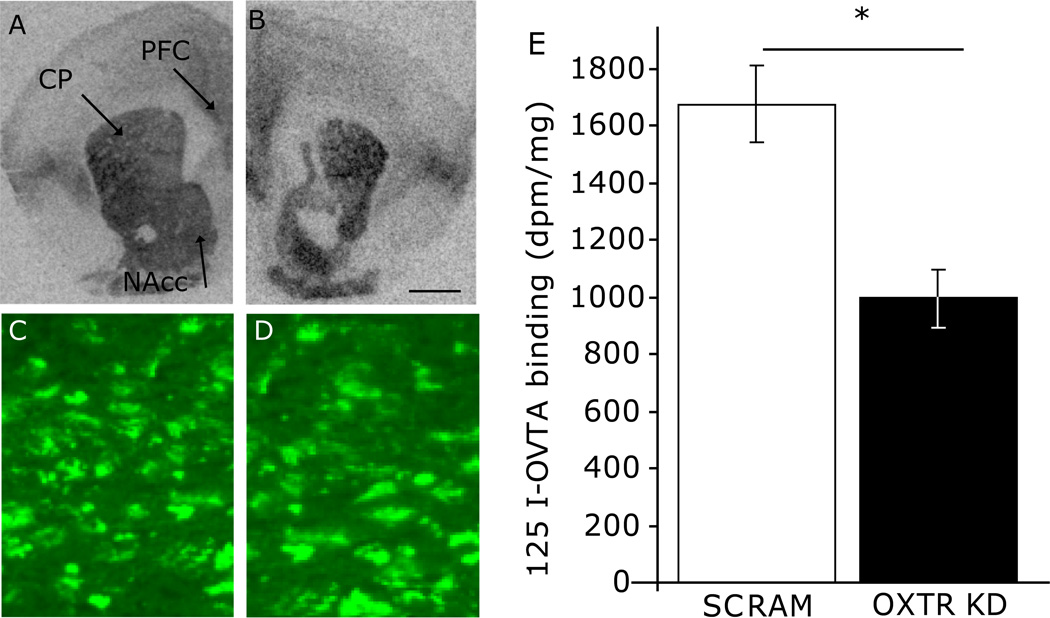
shRNA-pvOxtr females displayed a 45% reduction in OXTR binding in the NAcc compared to Oxtr scrambled control females (p=0.005, df=32, Student’s t-test bonferroni correction alpha=0.016; Figure 1A,B,E). OXTR density was not reduced in the caudate putamen (CP; control 1109 ± 134 dmp/mg, shRNA-pvOxtr 1289 ± 121 dmp/mg; p=0.64, df=32, Student’s t-test) or pre-limbic cortex (PLC; control, 1891 ± 99 dmp/mg, shRNA-pvOxtr 1907 ± 56 dmp/mg; p=0.73, df=32, Student’s t-test). The reduction in binding in the NAcc was not due to tissue damage, as this region robustly expressed the GFP (Figure 1C,D).
Figure 1.
In vivo Knockdown of prairie vole OXTR expression. Twenty-one day old females were injected with an AAV virus expressing a shRNA targeting Oxtr mRNA (shRNA-pvOxtr) or targeting a scrambled sequence (scrambled Oxtr control). Receptor autoradiography showing OXTR density in the nucleus accumbens (NAcc), caudate putamen (CP) and prefrontal cortex (PFC) of females injected with (A) scrambled Oxtr control or (B) shRNA-pvOxtr. Expression of the shRNA expressing virus did not damage neurons as both the scrambled Oxtr control (C) and shRNA-pvOxtr (D) treated females expressed the green fluorescent protein. (E) shRNA-pvOxtr females expressed approximately 45% less OXTR binding than scrambled Oxtr control females. Asterisk represents a p-value less than 0.05. Data are represented as mean ± SEM.
Scale bar, 1 mm.
Alloparental Behavior
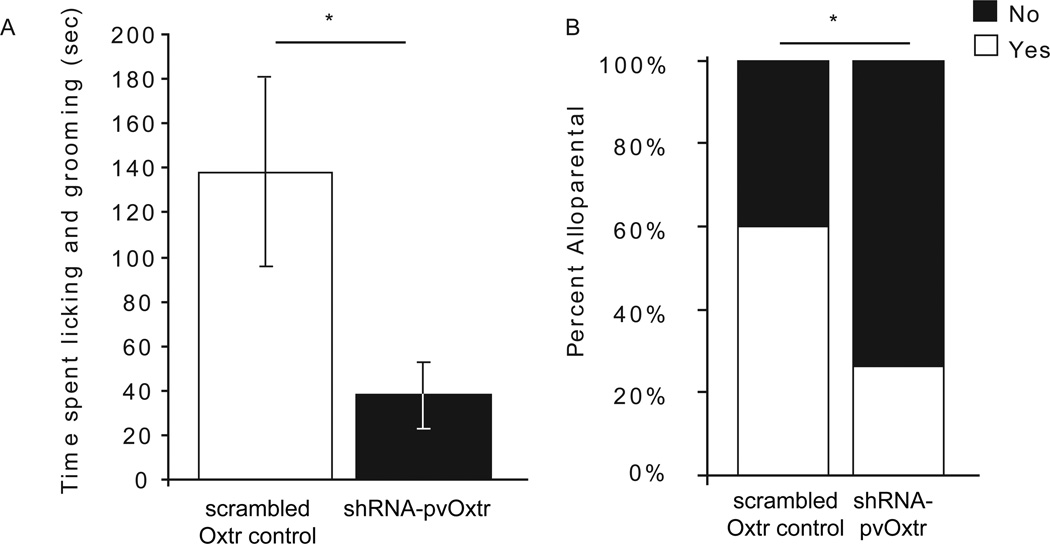
There was a significant treatment effect of time spent licking and grooming the pups (F1,32=5.791, p=0.022, one-way ANOVA) where shRNA-pvOxtr females spent less time licking and grooming pups than females injected with the Oxtr scrambled control virus (Figure 2A). No significant differences were noted in the time spent hovering or retrieving pups. The number of shRNA-pvOxtr females that met the categorical criteria for displaying alloparental behavior (N=5/19) was significantly less than the control females (N=9/15; p=0.0397, Fisher's exact test, Figure 2B). Six shRNA-pvOxtr females and one Oxtr scrambled control female attacked pups, and were therefore categorized as non-alloparental and removed from the analyses of individual behaviors. No significant difference in the number of attacks between groups (p=0.1039, Fisher's exact test) was noted.
Figure 2.
Graph showing the effect of OXTR knockdown on alloparental behavior. (A) Scrambled Oxtr control females spend more time licking and grooming pups than shRNA-pvOxtr females. (B) Scrambled Oxtr control females showed normal variation in propensity to engage in alloparental behavior, with about 60% showing spontaneous nurturing care. However, shRNA-pvOxtr females displayed inhibited alloparental care, indicating that decreased OXTR expression in the NAcc is directly related to decrease alloparental behavior. Asterisk represents a p-value less than 0.05. Data are represented as mean ± SEM.
Partner Preference Testing
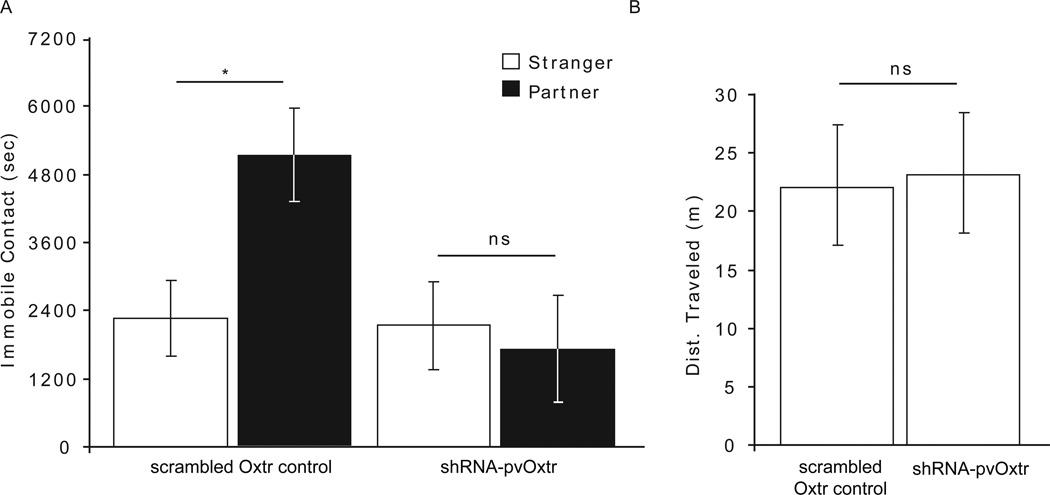
Following 24 hours of cohabitation, there were significant main effects of treatment (F1,56=9.778, p=0.003, two-way ANOVA) and stimulus animal (F1,50=7.424, p=0.009, two-way ANOVA). Moreover, there was a significant interaction effect (F1,50=15.101, p<0.001, two-way ANOVA). To determine which treatment group spent more time in immobile contact with either the partner or stranger, a Student's t-test was performed. Oxtr scrambled control females spent significantly more time in immobile contact with their partners compared with strangers (p=0.009, df=32, Student's t-test). shRNA-pvOxtr females did not display this preference (p=0.190, df=32, Student's t-test) (Figure 3A). Locomotor activity did not differ between treatment groups following a 24-hour cohabitation (F1,56=0.448, p=0.510; Figure 3B).
Figure 3.
Partner preference behavior in shRNA-pvOxtr and scrambled Oxtr control females at 70 days old treated at 21 days old. (A) After 24 hours cohabitation period and mating, only the scrambled Oxtr control females displayed a significant preference for the partner over the stranger. (B) After 24 hours cohabitation period and mating, there were no differences in locomotor behavior. Asterisk represents a p-value less than 0.05. Data are represented as mean ± SEM.
DISCUSSION
Species differences in OXTR density in the NAcc have been associated with species differences in affiliative behavior, including pair bonding and alloparental behavior (Olazábal and Young, 2006b). In the current study, we used RNA interference beginning at PND 21 to decrease OXTR density in the NAcc of prepubertal female prairie voles and tested for alloparental care and partner preference formation at PND 35 and 70. Our results demonstrate that a 45% knockdown in accumbal OXTR expression during development leads to deficits in social behavior and supports our hypothesis that natural variation in endogenous OXTR signaling directly contributes to natural intraspecies variation in these behaviors. As expected from previous pharmacological blockade studies, suppressing OXTR expression in the NAcc reduced alloparental behavior and disrupted partner preference formation in female prairie voles (Young et al., 2001; Olazábal and Young, 2006b). Previous studies have shown that the effects of OXTR signaling on these behaviors are dissociable, and therefore likely involve different cognitive processes (Ross et al., 2009; Keebaugh and Young, 2011). The disruption in alloparental behavior likely reflects impaired social motivation due to a reduction in the reinforcing nature of pup cues, which appears to be modulated by OXTR signaling in the NAcc through development (Keebaugh and Young, 2011). In contrast, the disruption in partner preference formation may reflect a deficit in the ability to learn the association between social olfactory cues and the reinforcing aspects of mating, as well as reduced social motivation to preferentially associate with the partner (Ross et al., 2009). It is important to note that while we ensured mating during the 24 hr cohabitation, more careful quantitative analyses including affiliation and movement during the cohabitation period was not done. Therefore, decreased accumbal OTR may have quantitatively changed interaction during the cohabitation period, thereby contributing to the lack partner preference formation.
The role of OXTR signaling in the NAcc in regulating alloparental motivation and partner preference formation has previously been suggested using OXTR antagonists to disrupt signaling and viral vector overexpression to enhance signaling (Ross et al., 2009). However, antagonists and viral vectors often diffuse to off-target brain regions near the site of infusion. OXTR antagonists used in previous studies also have antagonistic effects on the vasopressin V1a receptor, which are abundant in forebrain regions adjacent to the accumbens, such as the ventral pallidum and lateral septum. There is no clear delineation between OTR in the NAcc and V1a receptors expressed in neuronal processes expressing extending from the ventral pallidum (Lim et al., 2004). V1a receptors are critical to pair bond formation in males (Winslow et al., 1993; Lim and Young, 2004; Donaldson et al., 2010) and mediate several aspects of female social behaviors, including maternal care and aggression, and anxiety (Bosch et al., 2005), which may confound interpretation of pharmacological studies. In addition to binding to related receptor subtypes, OXTR antagonists only produce acute effects limiting the feasibility of assessing multiple behavioral phenotypes or the effects of chronic decreases in OXTR signaling. Overexpression studies using general promoters such as the CMV promoter result in ectopic expression in cells that may not typically express OXTR, and therefore are limited in utility for inferring the role of endogenous OXTR signaling (Ross et al., 2009). However, shRNA knockdown is ideal for investigating the role of endogenous ligand signaling as it degrades only the endogenously expressed Oxtr mRNA. It has the further advantage over antagonists in that it produces site-specific long-term disrupted signaling which allows animals to be run over multiple behavioral tests. This viral vector mediated shRNA knockdown is the only approach to produce a long-term region-specific reduction in OXTR signaling, comparable to the degree of naturalistic variation observed in the wild. Therefore we believe it is currently the best method to determine the causal effects of diversity in neuropeptide receptor expression on behavioral outcomes in an ethologically relevant manner.
Individual variation in OXTR expression in the NAcc has been correlated to individual variation in spontaneous nurturing and social attachment behaviors (Olazábal and Young, 2006b; Olazábal and Young, 2006a; Ophir et al., 2012). The present study supports the hypothesis that developmental variation in OXTR expression results in variation in adult social behavior. While there is evidence that individual differences in accumbal OXTR expression are influenced by early life environment (Bales et al., 2011; Cao et al., 2014), we have data suggesting that a single nucleotide polymorphism in the prairie vole Oxtr gene predicts OXTR binding density in the NAcc of adults (King and Young, Society for Neuroscience Abstract 2014). Furthermore, studies in humans suggest that polymorphisms in the OXTR gene are associated with altered social behavior including facial recognition, as well as confer an increased risk for ASD (Lerer et al., 2008; Wermter et al., 2010; Skuse et al., 2014).
Natural variation in Oxtr expression may interact with experiences over development to shape an individual’s brain and behavior. Human studies have demonstrated that polymorphisms in the OXTR gene interact with early life adversity to predict quality of mothering and postpartum mood (Mileva-Seitz et al., 2013), in addition to adult emotion regulation and attachment style (Bradley et al., 2011). Experimental studies have demonstrated that OT circuits are sensitive to early social context, and early-life manipulation of these systems have long-term effects on later life social attachment behaviors (Ahern and Young, 2009; Ahern et al., 2011; Barrett et al., 2014). More recently, individual variation in NAcc OXTR density has been shown to interact with early-life social experiences to influence the ability to form partner preferences in adult prairie voles (Barrett et al., Under Revision).
The present study is the first direct demonstration that the OXTR plays a critical role in alloparental behavior and adult social attachment, and suggest that natural variation in OXTR expression in this region alone can create variation in social behavior. Moreover, this work further supports the use of the prairie vole as an experimental animal model to explore the interaction between genetics, brain receptor density and the environment on complex social behaviors not exhibited in other laboratory rodents.
ACKNOWLEDGEMENTS
We would like to thank Lorra Mathews and Pravina Fernandez for animal care, the Emory Cloning Core facility for assistance in cloning shRNA’s into the AVV plasmid and the Emory Neuroscience NINDS Core Facilities for production of AAV virus.
Grant support: This work was funded by a McKnight Foundation Technological Innovations in Neuroscience grant, NSF 10S-1035975, and R01MH096983 to LJY, NIH P51OD011132 to YNPRC, and NINDS P30NS055077 to the Emory Neuroscience NINDS Core Facility.
Footnotes
FINANCIAL DISCLOSURES
None
REFERENCES
- Ahern TH, Hammock EA, Young LJ. Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster) Dev Psychobiol. 2011;53(2):118–131. doi: 10.1002/dev.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009;182(2):180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. Are behavioral effects of early experience mediated by oxytocin? Front Psychiatry. 2011;2:24. doi: 10.3389/fpsyt.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry. doi: 10.1038/tp.2015.73. (Under Revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63(3):518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Modi ME, Zhang BC, Walum H, Inoue K, Young LJ. Neonatal melanocortin receptor agonist treatment reduces play fighting and promotes adult attachment in prairie voles in a sex-dependent manner. Neuropharmacology. 2014;85C:357–366. doi: 10.1016/j.neuropharm.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder EB, Jovanovic T, Crain D, et al. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Dev Psychopathol. 2011;23(2):439–452. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach P, Young LJ, Russell J. Knobil an Neill’s Physiology of Reproduction. J. D. Neill, Elsevier; 2006. Oxytocin: Synthesis, Secretion and Reproductive Functions; pp. 3055–3127. [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of mu-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36(11):2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Datta D, Jones ST, Batey Lee E, Sutcliffe JS, Hammock EA, et al. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J Neurodev Disord. 2011 doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Wu R, Tai F, Zhang X, Yu P, An X, et al. Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen receptor alpha mRNA expression in the MeA and NAcc, and serum oxytocin in mandarin voles. Horm Behav. 2014;65(1):57–65. doi: 10.1016/j.yhbeh.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Carter CS, Williams JR, Witt DM, Insel TR. Oxytocin and social bonding. Ann N Y Acad Sci. 1992;652:204–211. doi: 10.1111/j.1749-6632.1992.tb34356.x. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci U S A. 2007;104(11):4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124(1):159–163. doi: 10.1037/a0018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25(3):284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417(1):6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. The Neural Mechanisms of Pair Bonding and Social Attachment. Current Opinion in Behavioral Sciences. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60(5):498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13(10):980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468(4):555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55(3):137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, et al. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One. 2013;8(4):e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Young LJ. D-Cycloserine Facilitates Socially Reinforced Learning in an Animal Model Relevant to Autism Spectrum Disorders. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61(3):340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ. Variability in "spontaneous" maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster) Dev Psychobiol. 2005;47(2):166–178. doi: 10.1002/dev.20077. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate "spontaneous" maternal behavior in adult female prairie voles. Neuroscience. 2006a;141(2):559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006b;49(5):681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav. 2012;61(3):445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, et al. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci U S A. 2014;111(33):12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, et al. mu-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci. 2013;33(21):9140–9149. doi: 10.1523/JNEUROSCI.4123-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Pieper S, Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci U S A. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29(5):1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Gunturkun O, Deutschlander S, Maier W, Kendrick KM, et al. Oxytocin modulates social distance between males and females. J Neurosci. 2012;32(46):16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 2013;110(50):20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, et al. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci U S A. 2014;111(5):1987–1992. doi: 10.1073/pnas.1302985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, et al. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatry. 2012;71(5):419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biol Psychiatry. 2012;72(12):982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Wermter AK, Kamp-Becker I, Hesse P, Schulte-Korne G, Strauch K, Remschmidt H. Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):629–639. doi: 10.1002/ajmg.b.31032. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58(1):74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Alarcon M, Cantor RM, Auranen M, Vanhala R, Kempas E, et al. Search for autism loci by combined analysis of Autism Genetic Resource Exchange and Finnish families. Ann Neurol. 2006;59(1):145–155. doi: 10.1002/ana.20722. [DOI] [PubMed] [Google Scholar]
- Young LJ. Oxytocin and vasopressin as candidate genes for psychiatric disorders: lessons from animal models. Am J Med Genet. 2001;105(1):53–54. [PubMed] [Google Scholar]
- Young LJ. Oxytocin, social cognition and psychiatry. Neuropsychopharmacology. 2015;40(1):243–244. doi: 10.1038/npp.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Yrigollen CM, Han SS, Kochetkova A, Babitz T, Chang JT, Volkmar FR, et al. Genes controlling affiliative behavior as candidate genes for autism. Biol Psychiatry. 2008;63(10):911–916. doi: 10.1016/j.biopsych.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]