Abstract
Background
Up to 30% of patients with low-risk prostate cancer (PCa) are found to have features of aggressive disease at radical prostatectomy (RP). Several predictive nomograms and novel genomic markers have been developed to estimate the risk of adverse pathology in men eligible for active surveillance (AS). However, oncologic risk associated with these findings remains unknown.
Objective
To determine if the presence of adverse pathologic features at RP in patients eligible for AS is prognostic of poor oncologic outcome independent of pretreatment risk status.
Design, setting, and participants
A total of 2660 patients underwent immediate RP at our institution between 1998 and 2008. Patients were stratified as low, intermediate, or high risk according to the D’Amico clinical risk criteria.
Outcome measurements and statistical analysis
The rates of adverse pathology were reported, and the 5-yr risk of biochemical recurrence (BCR) was calculated in the presence of aggressive disease.
Results and limitations
The 5-yr risk of BCR in patients with extracapsular extension (n = 937) was 43% (95% confidence interval [CI], 40–46) overall but only 15% (95% CI, 11–22) for those who met the criteria for low risk (n = 181). For the 473 patients with pathologic Gleason score 4 + 3, the risk of recurrence at 5 yr was 41% (95% CI, 37–46) overall, 13% (95% CI, 5–27) for low-risk men (n = 1102), 41% (95% CI, 35–47) for intermediate-risk men (n = 1086), and 51% (95% CI, 43–60) for high-risk men (n = 472). Limitations include use of BCR as the study end point and surrogate for oncologic outcome in men who received curative treatment.
Conclusions
The presence of pathologically unfavorable disease in patients eligible for AS is not informative as to the safety of this treatment modality. We question the relevance of adverse pathology as the end point for predictive tools designed to guide treatment decisions in low-risk PCa.
Patient summary
The risk of biochemical recurrence associated with adverse pathologic findings at prostatectomy is reduced by approximately 50% in men with clinically low-risk prostate cancer.
Keywords: Prostatic neoplasm, Oncologic outcome, Biochemical risk, Prediction, Positive surgical margin
1. Introduction
Widespread use of prostate-specific antigen (PSA) as a primary screening tool for prostate cancer (PCa) has led to the increased detection of clinically insignificant tumors. It is estimated that up to 60% of men diagnosed by PSA screening have low-risk PCa [1], yet most of these men undergo invasive treatment involving surgery or radiotherapy [2, 3]. Such interventions carry a non-negligible risk of urinary and sexual dysfunction that can adversely affect a man’s quality of life.
As clinicians have grown aware of the hazards associated with overdiagnosis and overtreatment of PCa, active surveillance (AS) has emerged as a viable option for the conservative management of low-risk organ-confined disease. The D’Amico classification [4] is the most commonly used criterion for the definition of clinical risk that includes low-risk patients with Gleason scores ≤6, clinical stage T2a or lower, and PSA level ≤10 ng/ml. Such low-risk patients who are found to have low-volume disease on biopsy are offered AS, with close monitoring and the intent of curative treatment at signs of disease progression. Preliminary data from several prospective AS series have shown promising results, with very low rates of disease-specific mortality and moderate rates of intervention in the first few years of surveillance [5, 6]. However, definitive conclusions are premature because of the relatively short follow-up times available even in the longest series.
Some investigators have cautioned against overutilizing AS, citing a moderate incidence of pathologically unfavorable disease in patients with low-risk PCa who would have been eligible for AS. Reported rates of Gleason sum upgrading range from 20% to 54% and pathologic upstaging from 6% to 26%, depending on the stringency of the inclusion criteria applied [7–11]. These findings have raised concerns regarding the adequacy of current AS eligibility criteria to differentiate appropriately between candidates for conservative management and those who require definitive treatment.
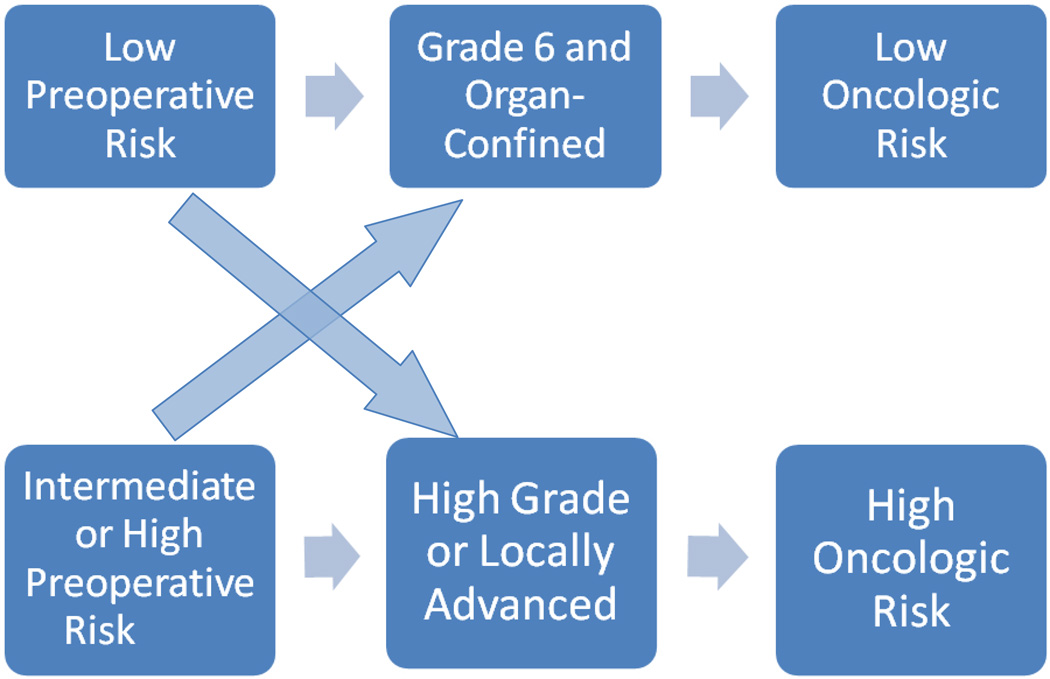
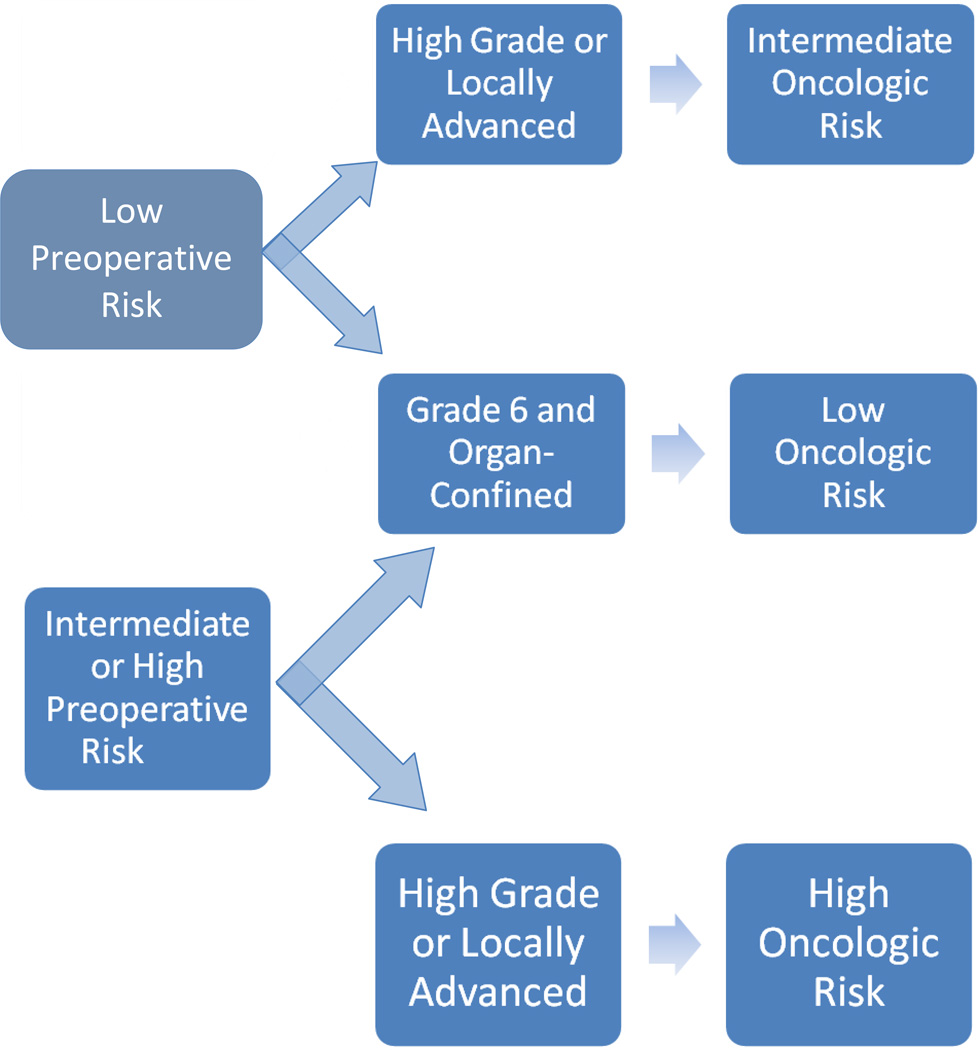
After examining the incidence of Gleason score upgrading in a low-risk patient population, Kulkarni et al [12] concluded that “caution should be exercised in recommending nonradical therapy to individuals with a high probability of undetected high-grade disease.” Similar conclusions were drawn by Isariyawongse et al [13], who found that the risk of upgrading increased with advanced age and advised that “caution should be exercised when recommending active surveillance in older men.” To this end, several nomograms have been constructed to predict the probability of pathologic upgrading in patients with low-risk PCa [14, 15]. Several novel genomic markers have been developed to predict the risk of disease recurrence and progression in patients who have undergone treatment for PCa [16, 17], as well as to better estimate the presence of pathologically unfavorable disease in men eligible for AS and to recommend immediate treatment for those with increased risk of upgrading or upstaging [18]. Such recommendations are based implicitly on the hypothesis that adverse pathology is prognostic of poor oncologic outcome in a manner relatively independent of pretreatment risk status (Fig. 1). An alternative hypothesis is that oncologic risk associated with adverse pathologic features is highly influenced by preoperative risk status (Fig. 2). To evaluate the second hypothesis, we analyzed data from patients with adverse pathologic features at radical prostatectomy (RP), examined the relationship between upstaging/upgrading with biochemical recurrence (BCR) (as a surrogate for oncologic outcome), and investigated the effect of preoperative risk on this relationship.
Fig. 1.
Hypothesis 1 implies that the presence of adverse pathologic features is prognostic of poor oncologic outcome relatively independent of pretreatment risk status.
Fig. 2.
Hypothesis 2 assumes oncologic risk associated with the presence of adverse pathologic features is influenced by pretreatment risk status.
2. Patients and methods
Following institutional review board approval, we performed a retrospective review of data collected from our PCa database on all patients undergoing immediate RP at Memorial Sloan Kettering Cancer Center (MSKCC) from 1998 to 2008 with complete clinical, pathologic, and follow-up data available (n = 3469). Patients were excluded if they had received neoadjuvant therapy prior to RP (n = 307) or received any adjuvant therapy secondary to adverse features at RP (n = 502).
Patients were stratified according to the D’Amico risk criteria for BCR based on clinical features: low risk (PSA ≤10 ng/ml, ≤cT2a, and biopsy Gleason ≤6), intermediate risk (PSA >10 and ≤20 ng/ml, cT2b, or biopsy Gleason 7), and high risk (PSA >20 ng/ml, lower than cT2b, or biopsy Gleason ≥8). We chose to use the D’Amico classification because it is the most commonly used criterion for the definition of clinical risk and used at many centers (including MSKCC) for inclusion of patients into AS protocols. The National Comprehensive Care Network recommends AS as an option for men with low-risk disease. We accept that there are a variety of different criteria for AS and many involve characteristics in addition to stage, grade, and PSA such as number of positive cores or percentage of core involvement. As is, our patient cohort constitutes a more diverse group that likely includes men with high-volume disease who at some centers would not be considered for or offered AS. We therefore repeated our analyses using more restrictive definitions of eligibility for AS.
The primary end point of the study was the effect of preoperative risk on BCR in men with adverse pathologic features at RP, defined as the presence of extracapsular extension (ECE), seminal vesicle invasion (SVI), lymph node invasion (LNI), or high-grade disease (Gleason sum >3 + 3). BCR was defined as a postoperative PSA elevation ≥0.2 ng/ml with a subsequent confirmatory value.
2.1. Statistical considerations
Univariable and multivariable Cox proportional hazards models were built with time to BCR as the outcome and preoperative risk as the covariate restricted to men with adverse pathologic features. The multivariable model was adjusted for pathologic Gleason scores and the presence of other adverse features (ECE, SVI, and LNI). BCR-free survival was estimated using the Kaplan-Meier method. The log-rank test was used to test differences between groups. All p values were two sided, with p < 0.05 considered a significant difference between groups. All statistical analysis was conducted using Stata v.12.0 (StataCorp, College Station, TX, USA).
3. Results
The final cohort comprised 2660 patients of which 1102 (41%) were classified as having low-risk disease, 1086 (41%) had intermediate-risk disease, and 472 (18%) had high-risk disease by D’Amico risk criteria. Median age for the entire cohort was 68 yr (interquartile range [IQR]: 63–73), and median PSA level was 5.3 ng/ml (IQR: 4–7.5). The median number of biopsy cores taken was eight (IQR: 4–12) with a median of two positive cores (IQR: 1–4). Median follow-up time for patients without BCR was 5.2 yr. Table 1 lists the clinicopathologic characteristics of our study population by risk category.
Table 1.
Clinicopathologic characteristics by risk group
| Low risk (n = 1102) |
Intermediate risk (n = 1086) |
High risk (n = 472) |
|
|---|---|---|---|
| Age at surgery, yr | 67 (63–72) | 68 (62–74) | 70 (65–75) |
| PSA level, ng/ml | 4.7 (3.5–6.3) | 5.6 (4.2–8.3) | 6.6 (4.8–11.9) |
| No. of total biopsy cores | 7.0 (3.0–12.0) | 8.0 (5.0–12.0) | 8.5 (6.0–12.0) |
| No. of positive cores | 2.0 (1.0–3.0) | 3.0 (2.0–5.0) | 4.0 (2.0–6.0) |
| Biopsy Gleason sum (%) | |||
| ≤6 | 1102 (100) | 165 (15) | 65 (14) |
| 7 | NA | 921 (85) | 144 (31) |
| ≥8 | NA | NA | 263 (56) |
| Pathologic Gleason sum (%) | |||
| ≤6 | 556 (50) | 127 (12) | 36 (7.6) |
| 7 | 538 (49) | 905 (83) | 254 (54) |
| ≥8 | 8 (0.7) | 54 (5.0) | 182 (39) |
| ECE (%) | 181 (16) | 444 (41) | 312 (66) |
| SVI (%) | 12 (1.1) | 90 (8.3) | 106 (22) |
| LNI (%) | 7 (0.7) | 73 (6.9) | 106 (23) |
| PSM (%) | 116 (11) | 184 (17) | 127 (27) |
ECE = extracapsular extension; LNI = lymph node invasion; NA, not applicable; PSA = prostate-specific antigen; PSM = positive surgical margins; SVI = seminal vesicle invasion.
All numbers are median (interquartile range) or frequencies (proportions).
Overall, 760 patients (29%) were upgraded at RP with 670 upgrades (88%) from Gleason sum ≤6 to Gleason sum 7. Of the patients whose tumors were upgraded to Gleason sum 7, 605 (90%) had primary pattern 3 (Gleason grades 3 + 4); 65 (10%) had primary pattern 4 (Gleason grades 4 + 3). Twelve patients were upgraded from Gleason sum ≤6 to Gleason sum ≥8; 78 were upgraded from Gleason sum 7 to ≥8. In the low-risk category, a total of 546 patients (50%) were upgraded at RP, with 99% of upgrades from Gleason sum 6 to 7 (n = 538). The rates of ECE, SVI, LNI, and positive surgical margins among low-risk patients were 16%, 1.1%, 0.7%, and 11%, respectively.
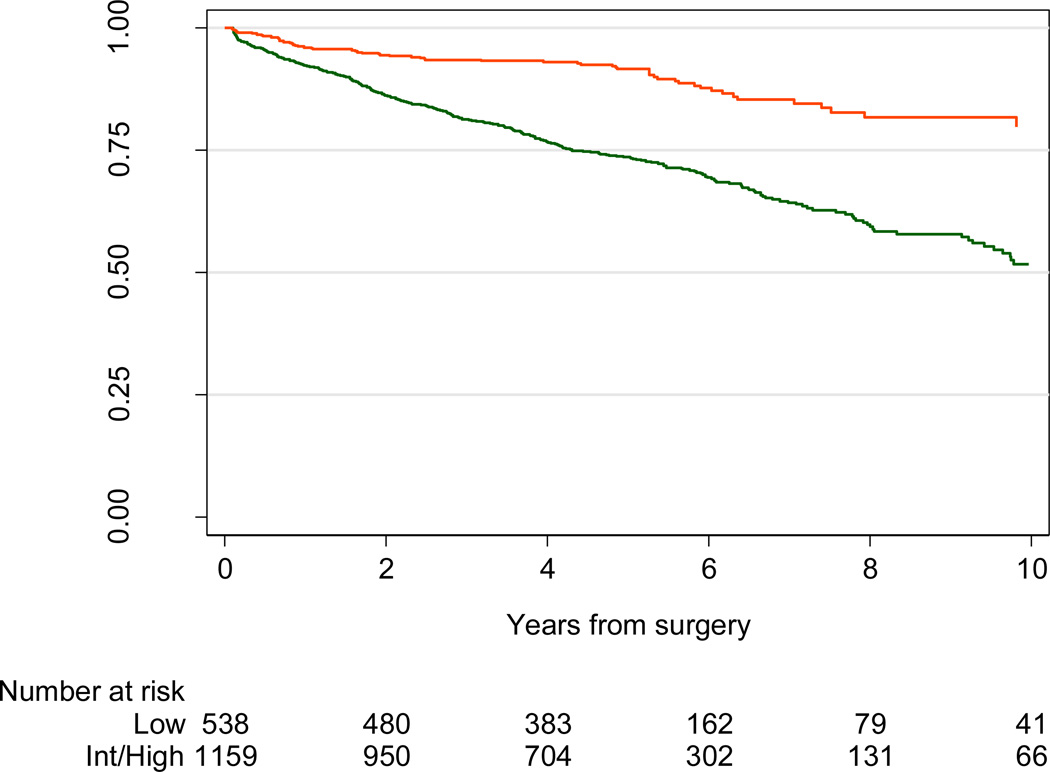
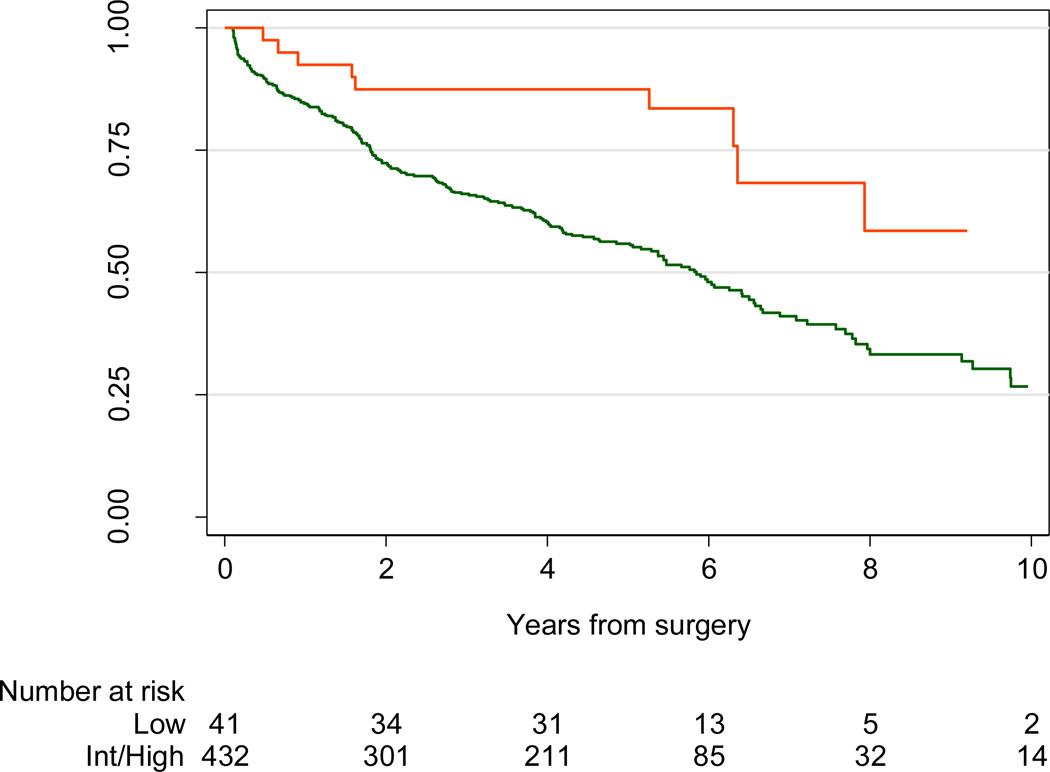
The 5-yr BCR risk in patients with ECE was 43% (95% confidence interval [CI], 40–46). However, risk of BCR varied dramatically depending on preoperative risk category: 15% (95% CI, 11–22) for low-risk patients, 39% (95% CI, 35–44) for intermediate-risk patients, and 65% (95% CI, 59–70) for high-risk patients with ECE. Similarly, although the overall 5-yr BCR risk for patients with pathologically high-grade disease was 21% (95% CI, 19–23), this risk was only 8% (95% CI, 6–11) for low-risk patients compared with 22% (95% CI, 20–25) for intermediate-risk and 40% (95% CI, 35–47) for high-risk men (Fig. 3; p < 0.005). More specifically, in the presence of pathologic Gleason 4 + 3 disease, the risk of BCR at 5 yr was 41% (95% CI, 37–46) overall, 12% (95% CI, 5–27) for preoperatively low-risk men, 41% (95% CI, 35–47) for intermediate-risk men, and 51% (95% CI, 43–60) for D’Amico high-risk men (Fig. 4; p < 0.005). By way of comparison, 5-yr BCR probability was close to 6% for patients who were preoperatively low risk and who did not have advanced stage or grade on pathology. Hence upgrading on surgical pathology raises 5-yr BCR probability for low-risk patients from 6% to 8%; upstaging increases risk from 6% to 15%.
Fig. 3.
Biochemical recurrence–free probability for all patients with pathologic Gleason sum 7 disease and low preoperative risk (red line) or intermediate or high risk (green line); p < 0.005 by log-rank test.
Int = intermediate.
Fig. 4.
Biochemical recurrence–free probability for all patients with pathologic Gleason 7, with predominant pattern 4 disease (Gleason grades 4 + 3) and low preoperative risk (red line) or intermediate or high risk (green line); p < 0.005 by log-rank test.
Int = intermediate.
Table 2 shows the results of our univariable and multivariable analysis of the association between BCR and preoperative risk in men with adverse pathology. The hazard ratio (HR) for low preoperative risk in predicting BCR for patients who have ECE on univariable analysis (HR: 0.29; 95% CI, 0.21–0.4; p < 0.0001), as well as multivariable analysis adjusted for pathologic Gleason scores and presence of other adverse features (HR: 0.51; 95% CI, 0.36–0.72; p = 0.0001), clearly illustrate that preoperative risk is highly prognostic. Similar findings were observed for men with high-grade disease at RP (univariate analysis: HR: 0.26; 95% CI, 0.20–0.34; p <0.0001; multivariable analysis: HR: 0.45; 95% CI, 0.35–0.60; p < 0.0001). The HRs for low-risk disease in patients who have SVI or LNI are <1 but did not reach statistical significance perhaps due to the low prevalence of SVI and LNI in low-risk men (1.1% and 0.7%, respectively). Low-risk patients with positive surgical margins were less likely to experience BCR than those with intermediate- or high-risk disease (HR: 0.38; 95% CI, 0.26–0.54; p < 0.0001); however, this association was not significant when adjusted for the effects of pathologic Gleason scores and adverse pathologic features (p = 0.2).
Table 2.
Univariable and multivariable analysis of the association between preoperative risk biochemical recurrence in patients with adverse pathology*
| Adverse feature (low-risk vs intermediate- and high-risk patients) |
Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| ECE | 0.29 (0.21–0.40) | <0.0001 | 0.51 (0.36–0.72) | 0.0001 |
| SVI | 0.70 (0.37–1.32) | 0.3 | 0.68 (0.35–1.31) | 0.2 |
| Pathologic Gleason ≥7 | 0.26(0.20–0.34) | <0.0001 | 0.45 (0.35–0.60) | <0.0001 |
| LNI | 0.53 (0.22–1.29) | 0.2 | 0.44 (0.18–1.08) | 0.073 |
| Positive surgical margins | 0.38 (0.26–0.54) | <0.0001 | 0.76 (0.50–1.14) | 0.2 |
CI = confidence interval; ECE = extracapsular extension; HR = hazard ratio; LNI = lymph node invasion; SVI = seminal vesical invasion.
For example, the HR of 0.29 for ECE means that, among men with ECE, the risk of recurrence was about two-thirds lower in men who had preoperative low-risk features in comparison with men with preoperative intermediate- or high-risk disease.
To account for changes in grading introduced by the 2005 International Society of Urologic Pathology modified Gleason scoring system [19], we repeated our analysis to include only patients diagnosed and treated after 2005. The overall rate of upgrading and of ECE, SVI, and LNI were virtually unchanged. The results of our univariable and multivariable Cox model did not change when applied to the patients operated on after 2005 (data not shown).
We also repeated our analyses using alternative definitions of low risk: either D’Amico criteria plus three or fewer positive cores or D’Amico plus three or fewer positive cores plus no more than 50% cancer in any core. Due to missing data on the number of cores, the analyses included 2221 and 1544 patients, respectively. The results were very similar to the main analysis. For D’Amico plus three or fewer positive cores, low preoperative risk was statistically associated with BCR on univariate analysis for all pathologic features (ECE, SVI, LNI, Gleason ≥7, and positive margins) with HRs ranging from 0.42 to 0.50. When low risk was additionally defined in terms of percentage of core affected, results were broadly similar, although CIs were wider due to a small cohort. Preoperative risk just missed statistical significance for LNI (HR: 0.36; 95% CI, 0.13–1.02; p = 0.054) and was nonsignificant for SVI (HR: 0.65; 95% CI, 0.26–1.62; p = 0.4). Hence our finding that preoperative low-risk status dramatically attenuates the negative prognostic impact of advanced pathology is robust to the definition of low risk used.
4. Discussion
Our findings confirm an association between the risk of BCR and adverse pathologic features; however, preoperative risk also plays an important role in this relationship. The absolute difference in 5-yr risk of BCR between low-risk patients with ECE and intermediate- and high-risk patients with ECE was 24% and 50%, respectively. A similar observation was made for patients with findings of high-grade disease in the low-risk category versus those in the intermediate- and high-risk groups, with an absolute difference of 14% and 32%, respectively. Among patients with pathologic Gleason sum 7 disease, with the predominant pattern 4 disease (Gleason grades 4 + 3), preoperatively low-risk patients had a third the risk of BCR at 5 yr compared with intermediate-risk patients (12% vs 41%) and a fourth the risk of high-risk patients (12% vs 51%). Based on these results, we suggest using a model in which high-grade or locally advanced disease confers a different probability of adverse oncologic outcome depending on pretreatment risk status (Fig. 2), rather than a model in which oncologic risk is determined by pathologic features independent of preoperative risk (Fig. 1).
Several investigators have extensively examined the rates of upgrading and upstaging at RC. For example, Kulkarni et al [12] found that 34% of patients thought to have low-risk disease were upgraded at RP and concluded that noninvasive treatment may not be suitable for those with risk of upgrading. Similarly, Ploussard et al [10] found that even with the use of the most stringent inclusion criteria on a 21-core biopsy protocol, the rates of upgrading were as high as 48%, and the rate of ECE was up to 11%. These findings are similar to our study. Although Ploussard et al did not address the prognostic value of these findings, they warned against the risk of finding pathologically unfavorable disease in patients eligible for AS and missing the opportunity for cure.
Klein et al [18] reported recently on the utility of novel genomic markers in the pretreatment setting of low-risk PCa. The authors validated a PCa risk score known as the Genomic Prostate Score (GPS) by testing biopsy specimens of PCa patients eligible for AS. GPS is calculated based on the expression of certain genes known to be independently predictive of disease recurrence and to have a high association with adverse pathology. According to the authors, “the biopsy-based 17-gene GPS improves prediction of the presence or absence of adverse pathology and may help men with PCa make more informed decisions between AS and immediate treatment.” Alternatively, of course, patients otherwise eligible for AS who are found to have GPS scores predictive of higher grade/stage disease should consider immediate intervention. Several studies have addressed how adverse pathologic feature influence the risk of BCR including randomized trials demonstrating the benefit of adjuvant radiotherapy in improving locoregional control and BCR-free survival in men with adverse pathologic features at RP [20, 21]. However, these trials included a heterogeneous cohort of patients without clear delineation of risk groups, and it is difficult to conclude which patients had the highest threat of recurrence and thus stood to gain the most from adjuvant intervention. Several retrospective reviews have attempted to answer this question. Swanson et al [22], for example, concluded that the risk of BCR in men with locally advanced disease varies widely depending on preoperative PSA value (<10 vs ≥10 ng/ml) and pathologic Gleason scores (<7 vs ≥7). Unlike our study, however, the authors did not analyze the effect of pretreatment risk on BCR; nor did they address the implications of Gleason sum upgrading at RP.
One key aspect of our study is that we used relatively liberal criteria for AS eligibility, analyzing patients defined at low risk by stage, grade, and PSA criteria, and omitting biopsy tumor characteristics such as number of positive cores or percentage of core involvement. Our patient population is therefore not representative of AS patients at many centers. However, we chose liberal inclusion criteria on the grounds that this would be a bias against our hypothesis that patients with low preoperative risk have low recurrence rates even if they are found to have locally advanced cancer on surgical pathology. If it is indeed the case that low-risk men with locally advanced cancer on final pathology have a low risk of BCR, then we would expect that very low-risk men who meet strict AS inclusion criteria and are found to have adverse pathologic features would be at even a lower risk of BCR.
Our study has important clinical implications. Although we demonstrated that adverse pathologic features at RP are associated with an increased risk of BCR, this risk is influenced substantially by pretreatment factors, and pathologic features in isolation cannot dictate treatment. In addition to counseling a patient who is considering AS about his chances of having pathologically unfavorable disease, we must also provide meaningful information regarding the oncologic risk associated with such findings in the specific context of preoperative risk. Furthermore, pretreatment risk status may also be used to guide a clinician in the selection of patients for post-RC adjuvant therapy, given the alarmingly increased rate of BCR in intermediate- and high-risk patients with locally advanced disease. Our findings may also be seen as providing general support for the notion that preoperative risk needs to be considered in addition to pathologic findings in predicting oncologic outcomes.
Some main limitations of our study warrant discussion. We acknowledge that BCR is not a perfect surrogate for oncologic outcome and that an estimation of disease-specific mortality is a more appropriate end point. With a relatively short follow-up time of 5.2 yr and a well-known protracted course of PCa biology, this end point could not be achieved in our study. However, the association between BCR and progression to metastatic disease and death is well documented in the literature, and in this respect, BCR as an end point fits our purpose sufficiently [23, 24]. Furthermore, all men in our series underwent RP, and it is far from certain how these men would have fared if they had not had definitive treatment. In addition, we report on a single institutional experience, and external validation of our findings is warranted to corroborate our results.
5. Conclusions
Our results indicate that the risk of BCR in men with adverse pathologic features is dramatically attenuated by low preoperative risk status that reduces the risk associated with findings such as ECE or high Gleason grade disease >50%. This suggests that preoperative risk is an important factor to consider when evaluating post-RP risk of BCR in the setting of adverse pathology. Our findings can be extrapolated to patients with low-risk PCa considering conservative management. For these patients who are otherwise eligible for AS, risk predictions of Gleason upgrading or locally advanced disease do not constitute a valid end point on which to base decisions for definitive treatment.
Take-home message.
We found that the risk of biochemical recurrence associated with adverse pathologic features at radical prostatectomy is reduced by approximately 50% in men with clinically low-risk prostate cancer.
Acknowledgments
Funding/Support and role of the sponsor: The Sidney Kimmel Center for Prostate and Urologic Cancers supported the study with funds provided by David H. Koch through the Prostate Cancer Foundation, T32 CA082088 from the National Cancer Institute, and by NIH/NCI Cancer Center Support Grant to MSKCC under award number P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Mariam Imnadze had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept, and design: Imnadze, Vickers.
Acquisition of data: Imnadze, Sjoberg, Vickers.
Analysis and interpretation of data: Imnadze, Sjoberg, Vickers.
Drafting of the manuscript: Imnadze, Vickers.
Critical revision of the manuscript for important intellectual content: Imnadze, Vickers.
Statistical analysis: Imnadze, Sjoberg.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Vickers.
Other (specify): None.
Financial disclosures: Mariam Imnadze certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 5.Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L. Active surveillance: the Canadian experience with an “inclusive approach”. J Natl Cancer Inst Monogr. 2012;2012:234–241. doi: 10.1093/jncimonographs/lgs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauval JB, Ploussard G, Soulie M, et al. Pathologic findings in radical prostatectomy specimens from patients eligible for active surveillance with highly selective criteria: a multicenter study. Urology. 2012;80:656–660. doi: 10.1016/j.urology.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 8.Conti SL, Dall’era M, Fradet V, Cowan JE, Simko J, Carroll PR. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–1633. doi: 10.1016/j.juro.2008.11.107. discussion 1633–4. [DOI] [PubMed] [Google Scholar]
- 9.Jeldres C, Suardi N, Walz J, et al. Validation of the contemporary Epstein criteria for insignificant prostate cancer in European men. Eur Urol. 2008;54:1306–1313. doi: 10.1016/j.eururo.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 10.Ploussard G, Salomon L, Xylinas E, et al. Pathological findings and prostate specific antigen outcomes after radical prostatectomy in men eligible for active surveillance--does the risk of misclassification vary according to biopsy criteria? J Urol. 2010;183:539–544. doi: 10.1016/j.juro.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Suardi N, Capitanio U, Chun FK, et al. Currently used criteria for active surveillance in men with low-risk prostate cancer: an analysis of pathologic features. Cancer. 2008;113:2068–2072. doi: 10.1002/cncr.23827. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni GS, Lockwood G, Evans A, et al. Clinical predictors of Gleason score upgrading: implications for patients considering watchful waiting, active surveillance, or brachytherapy. Cancer. 2007;109:2432–2438. doi: 10.1002/cncr.22712. [DOI] [PubMed] [Google Scholar]
- 13.Isariyawongse BK, Sun L, Banez LL, et al. Significant discrepancies between diagnostic and pathologic Gleason sums in prostate cancer: the predictive role of age and prostate-specific antigen. Urology. 2008;72:882–886. doi: 10.1016/j.urology.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Moussa AS, Kattan MW, Berglund R, Yu C, Fareed K, Jones JS. A nomogram for predicting upgrading in patients with low- and intermediate-grade prostate cancer in the era of extended prostate sampling. BJU Int. 2010;105:352–358. doi: 10.1111/j.1464-410X.2009.08778.x. [DOI] [PubMed] [Google Scholar]
- 15.Truong M, Slezak JA, Lin CP, et al. Development and multi-institutional validation of an upgrading risk tool for Gleason 6 prostate cancer. Cancer. 2013;119:3992–4002. doi: 10.1002/cncr.28303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Simko JP, Cowan JE, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31:1428–1434. doi: 10.1200/JCO.2012.46.4396. [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012;106:1095–1099. doi: 10.1038/bjc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66:550–560. doi: 10.1016/j.eururo.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 20.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 21.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 22.Swanson GP, Riggs M, Hermans M. Pathologic findings at radical prostatectomy: risk factors for failure and death. Urol Oncol. 2007;25:110–114. doi: 10.1016/j.urolonc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 24.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]