Abstract
Objective
We tested the hypothesis that simulation of cardiac α1A-adrenergic receptors (α1A-AR) protects against the development of heart failure through induction of angiogenesis.
Approach and Results
4–6 weeks after permanent coronary artery occlusion (CAO), transgenic (TG) rats with cardiomyocyte-specific α1A-AR overexpression had less remodeling than their non-transgenic littermates (NTLs), with less fibrosis, hypertrophy and lung wt, and preserved left ventricular ejection fraction and wall stress, all p<0.05. Coronary blood flow, measured with microspheres, increased in the infarct zone in TG compared to NTLs (1.4±0.2 vs. 0.5±0.08ml/min/g) (p<0.05), which is consistent with angiogenesis, as reflected by a 20% increase in capillary density in the zone adjacent to the infarct. The question arose, how does TG overexpression of a gene in cardiomyocytes induce angiogenesis? We identified a paracrine mechanism, whereby VEGF-A mRNA and protein were increased in isolated TG cardiomyocytes, and also by NTL cardiomyocytes treated with an α1A-agonist, resulting in angiogenesis. Conditioned medium from cultured TG cardiomyocytes enhanced human umbilical vein endothelial cell (HUVEC) tubule formation, which was blocked by an anti-VEGF-A antibody. Moreover, improved cardiac function, blood flow and increased capillary density after chronic CAO in TG rats were blocked by either a MEK or a VEGF-A inhibitor.
Conclusions
Cardiomyocyte-specific overexpression of the α1A-AR resulted in enhanced MEK-dependent cardiomyocyte VEGF-A expression, which stimulates angiogenesis via a paracrine mechanism involving heterocellular cardiomyocyte/endothelial cell signalling, protecting against remodeling and heart failure following chronic CAO.
Keywords: Receptor, adrenergic, alpha, angiogenesis, myocardial infarction, heart failure
INTRODUCTION
Cardiac-specific α1A-adrenergic receptor (α1A-AR) stimulation has been proposed as a therapeutic strategy for heart failure1–5, as it increases myocardial contractility6, 7, and blockade of the α1-AR exacerbates heart failure2, 3. We investigated a different mechanism, namely that cardiomyocyte-specific overexpression of the α1A-AR induces angiogenesis through a paracrine mechanism, which could be therapeutically beneficial for heart failure, particularly that due to chronic myocardial infarction (MI). This is because, in the presence of permanent coronary artery occlusion (CAO), even cardioprotective interventions are destined to fail as they do not increase blood flow distal to the CAO, even to that observed in hibernating myocardium8, 9. This is required to limit ischemic damage to the myocardium. In fact, despite the hundreds, if not thousands, of studies identifying molecular pathways protecting the heart, there has been little clinical translation of these findings, and even approaches to enhance myocardial regeneration have met with uneven success10. In the permanent absence of blood flow to the ischemic heart, and in the absence of preformed collateral channels, almost any intervention is destined to fail. Although several TG models have shown cardioprotection in the setting of ischemia/reperfusion11–13, the key is that in reperfusion models blood flow is restored after a relatively short duration of ischemia, generally from 15 min to an hour.
For these reasons, we investigated here if α1A-AR overexpression also limits cardiac remodeling after chronic myocardial infarction resulting from permanent CAO. Specifically, we examined the effects of permanent CAO on the development of remodeling and heart failure in a rat model with 40-fold cardiomyocyte overexpression of the α1A-AR. Our hypothesis was that if we observed protection from remodelling after 4 weeks of permanent CAO in the TG rats, then there must have been some sustained blood flow to the ischemic myocardium, resulting in preservation of left ventricular (LV) ejection fraction and wall stress (See Fig. 1). Indeed, we did observe that the TG α1A-AR rat heart was protected from remodeling after permanent CAO, and since, unlike the dog and hamster14, the rat has few preformed collateral vessels, we also examined the extent to which protection against remodeling is mediated by angiogenesis. Accordingly, we measured coronary blood flow to the ischemic zone and quantified the percentage of viable myocardium within this zone, as well as the development of newly formed coronary vessels, and Ki67 positive myocytes. Since overexpression of the α1A-AR was restricted to cardiomyocytes, we then determined the mechanism underlying neo-angiogenesis in the TG hearts. Given that microarray studies, verified by qPCR, identified vascular endothelial growth factor (VEGF-A) as the sole angiogenesis gene upregulated in TG myocytes, we focused on this factor and also examined MEK/ERK signalling, which has been found to be activated with α1A-AR stimulation15–17 and to induce VEGF-A mediated angiogenesis18.
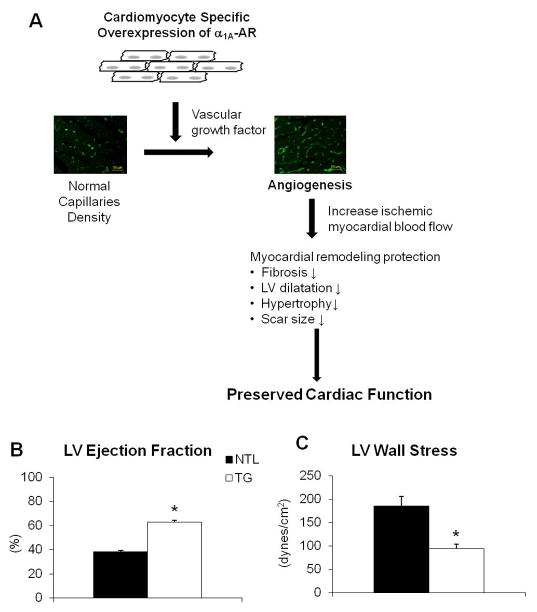
Fig. 1.
(A) Schematic mechanism for reduced remodeling after chronic myocardial infarction in α1A-AR TG rats. Angiogenesis induced through the MEK/VEGF pathway, provides blood flow to the ischemic zone, despite permanent coronary artery occlusion, to allow cardiomyocyte survival, reduce remodeling and preserve cardiac function, which was presented with preserved (B) LV ejection fraction and (C) wall stress. Results are expressed as the mean ± SEM. n=4–5/group; p<0.05 vs. NTLs.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement
RESULTS
Attenuated cardiac remodeling in transgenic rats after chronic MI
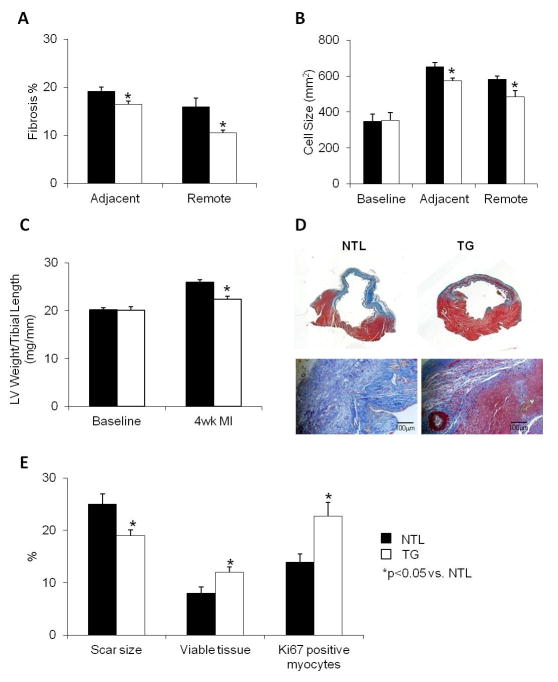
At 4–6 weeks post MI, compared to NTLs, TG rats showed significantly less fibrotic tissue deposition in the areas adjacent (25% less) and remote (33% less) to the ischemic zone (Fig. 2A). Compared to baseline the amount of cellular hypertrophy post MI was significantly less in the TG group in both the adjacent (573±14mm2) and remote zones (485±34mm2) vs. those seen in the NTLs (652±24mm2 in adjacent zone and 582±18mm2 in remote zone, p<0.05, Fig. 2B). Consistent with these findings, the ratio of LV weight to tibial length was reduced by 16% in TG rats compared to NTLs (Fig. 2C, p<0.05), the ratio of lung weight to tibial length was also lower in TGs (44±1.1mg/mm) than in NTLs (52±2.2mg/mm, p<0.05), which supports decreased post MI remodeling in the TG hearts. Scar size, measured by fibrotic tissue quantification within the ischemic zone and expressed as the percentage of the whole myocardial area, was markedly less in TG rats (19±1.1%) compared to NTLs (25±2.0%, p<0.05, Fig. 2E). This reduction in infarct size was accompanied by increased viable tissue within the ischemic zone in TGs (12±1.0% vs. 7.9±1.2% in NTLs, p<0.05, Fig. 2D, E), and was also accompanied by increased Ki67 positive myocytes in the ischemic zone (Fig. 2E). LV ejection fraction fell to 38% in NTL, but was significantly preserved at 63% in TG (Fig. 1B and Table 1). The low LV ejection fraction in NTLs 4–6 wks after CAO (39%) is at a level found in other studies of rats with heart failure, and in this study was supported by the increases in LV weight/tibial length and lung weight/tibial length in NTLs, indicative of heart failure. Mortality, by chi square analysis, with 4–6 wk CAO, was significantly reduced, p<0.05, in TG (18%) vs NTL (47%) rats.
Fig. 2.
Responses to 4–6 wks of permanent CAO in TG rats (open bars) and their NTLs (closed bars). (A) Fibrosis, quantified using picro-sirius red staining, was decreased in TG. (B) Myocyte size, determined using WGA fluorescent staining, was decreased in the zones bordering and remote to the ischemic area. (C) Left ventricular weight (LV) to tibial length (TL) ratio was decreased in TG rats. (D) Trichrome staining of LV rings (above) and transverse sections (below) of both NTL and TG hearts after chronic myocardial infarction demonstrates reduced scar size and fibrosis in the TG heart, as well as evidence of viable myocardium within the scar. (E) Scar size, presented as the percentage of scar size to LV surface area, was decreased in the TG vs NTL hearts, whereas the amount of viable myocardium within the ischemic zone was increased in TG vs. NTL hearts. This was accompanied by increased Ki67 positive myocytes in the ischemic zone in the TG vs NTL hearts. Results are expressed as the mean ± SEM. n=6–8/group; *p<0.05 vs. NTL.
Table 1.
Cardiac Function at Baseline and at 4–6 Weeks after MI
| Baseline | 4–6wk MI | |
|---|---|---|
| Heart Rate (beats/min) | ||
| NTL | 387±6.3 | 354±13 |
| TG | 358±11 | 329±7.6 |
| Mean Arterial Pressure (mmHg) | ||
| NTL | 112±14 | 113±12 |
| TG | 108±8.9 | 111±6.2 |
| LV Systolic Pressure (mmHg) | ||
| NTL | 143±3.9 | 143±3.9 |
| TG | 135±5.0 | 142±9.1 |
| LV Ejection Fraction (%) | ||
| NTL | 71±0.5 | 38±1.1 |
| TG | 87±1.2* | 63±1.8* |
| LV End-diastolic Diameter (mm) | ||
| NTL | 6.9±0.3 | 9.6±0.3 |
| TG | 6.7±0.3 | 7.8±0.1* |
| LV Systolic Wall Stress (dynes/cm2) | ||
| NTL | 36±6.3 | 185±21 |
| TG | 16±3.5* | 94±9.3* |
n = 4–5 at baseline, n = 8–9 at 4–6wk MI,
p<0.05 vs. NTL
Increased angiogenesis in transgenic rats with upregulation of VEGF-A
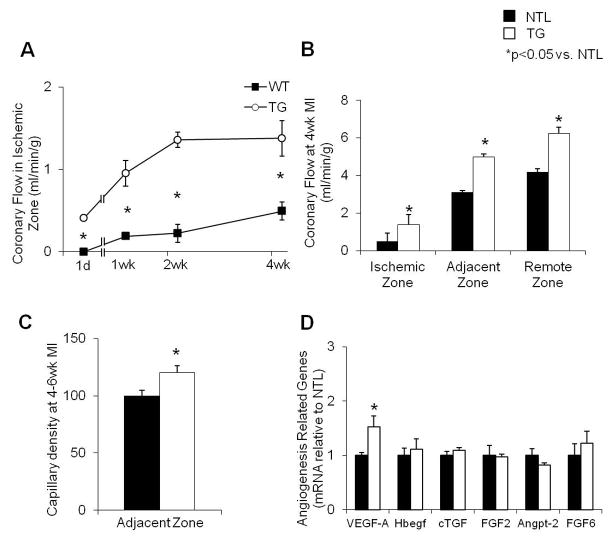
Myocardial blood flow was studied with microspheres injected at 1 day, 1 wk, 2 wk and 4 wk post-MI. Over the 4 wk period of permanent CAO, both groups showed a gradual recovery of blood flow within the ischemic zone. However, the rate of recovery was significantly faster and greater in TG rats as compared to their NTLs (Fig. 3A). At 4 wk post-MI, the blood flow in the central ischemic, adjacent and remote zones was consistently higher in TG group compared to NTL rats (p<0.05, Fig. 3B).
Fig. 3.
(A) Coronary blood flow in the ischemic zone at 1 day, 1 week, 2 weeks and 4 weeks after permanent CAO, showing that recovery of flow was significantly faster and of greater magnitude in TG vs. NTL rat hearts at the times indicated (n=4–6, p<0.05). (B) Coronary blood flow was increased in central ischemic, adjacent to ischemic and remote to ischemic zones of the heart. (C) Capillary density, normalized to NTLs, was increased in TG hearts at 4–6 wk post CAO in the zone adjacent to the scar. (D) Upregulation of angiogenic genes expressed in TG and NTL cardiomyocytes was determined by microarray analysis. The mRNA expression was further verified using qPCR. The significant increase in VEGF-A expression in TG hearts was verified. Results are expressed as the mean ± SEM. n=4–8; *p<0.05 vs. NTL. VEGF-A, vascular endothelial growth factor-A; Hbegf, Heparin-binding EGF-like growth factor; cTGF, connective tissue growth factor; FGF2, fibroblast growth factor-2; Angpt-2, angiopoietin 2; FGF6, fibroblast growth factor 6.
Capillary density was increased by 21% in the zone adjacent to the infarct in TGs compared to NTL at 4–6 weeks after MI, p<0.05, (Fig. 3C). This was consistent with the finding of more viable tissue within the ischemic zone in TGs (Fig. 2E), secondary to collateral blood flow through angiogenesis.
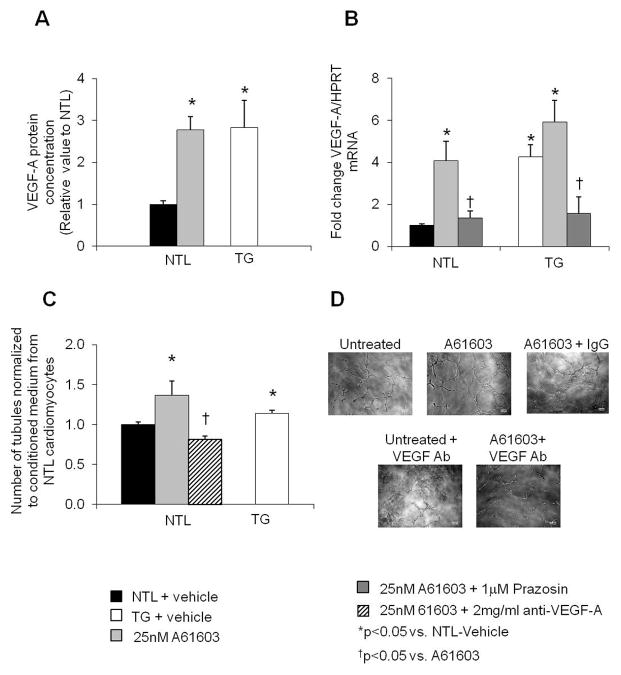
Determination of angiogenic genes using microarray analysis revealed 6 upregulated angiogenesis-related genes in cardiomyocytes of TG rats. Amongst the six genes, only VEGF-A mRNA was validated by qPCR to be significantly increased compared to NTLs (Fig. 3D). In addition, we found a 3-fold increases in VEGF-A protein level in TG mouse cardiomyocytes compared with NTLs (p<0.05) (Fig. 4A).
Fig. 4.
(A) Protein expression of VEGF-A was increased in cardiomyocytes isolated from TG vs. NTL mouse hearts, and increased to similar levels with A61603 treatment of NTL cardiomyocytes. (B) mRNA levels of VEGF-A are expressed as relative to the internal control, HPRT (hypoxanthine-guanine phosphoribosyltransferase). Compared to untreated NTL mouse cardiomyocytes, VEGF-A mRNA was increased in both untreated TG and 25nM A61603 treated cardiomyocytes in NTL. 1μM Prazosin blocked these increases in both NTL and TG VEGF-A mRNA levels. (C) HUVECs were cultured with conditioned medium collected from NTL, TG, A61603 treated NTL or anti-VEGF-A antibody treated NTL cardiomyocytes. Tubule formation, presented as fold change from unconditioned medium, was increased in HUVECs cultured with conditioned medium from TG or A61603-treated NTL cardiomyocytes, compared to medium from vehicle or 2μg/ml anti-VEGF-A antibody treated NTL cardiomyocytes. (D) Representative pictures of tubule formation in HUVECs treated with conditioned medium collected from vehicle or A61603 or VEGF antibody (Ab) treated NTL mouse myocytes. Results are expressed as the mean ± SEM. n=3–6; *p<0.05 vs. NTL.
Activation of the α1A-AR in cardiomyocyte induced angiogenesis via a paracrine mechanism
In isolated mouse cardiomyocytes, VEGF-A expression was found to be upregulated at both the protein and mRNA levels in TG mouse cardiomyocytes compared to those in NTLs. (Fig. 4A, B). VEGF-A levels also increased significantly after stimulation of NTL cardiomyocytes with α 1A-AR agonist, A61603 (25nM) (Fig. 4A, B). A61603 mediated upregulation of VEGF-A mRNA was abolished by pre-treatment of cardiomyocytes with the α1A-AR antagonist, prazosin (1μM) (Fig. 4B), indicating that the upregulation of VEGF-A is mediated by the α1A-AR.
To evaluate the underlying cellular mechanism of the α1A-AR-mediated angiogenic effect, we employed a Matrigel culture system using HUVECs. Tubule formation was increased after treating HUVECs with conditioned medium collected either from cultured TG cardiomyocytes, or from A61603-treated NTL cardiomyocytes, but was completely abolished with pre-treatment with an anti-VEGF-A antibody (Fig. 4C, 4D) but not by pre-treatment with control IgG (data not shown). This suggests that the growth factor, VEGF-A, is required for endothelial cell growth and organization into tubules, after its release from cardiomyocytes upon activation or with overexpression of the α1A-AR. Thus, the α1A-AR appears to program an angiogenic response within the myocardium that enhances endothelial cell organization through a paracrine mechanism involving heterocellular signalling.
Diminished myocardial remodeling in TG rats was abolished by inhibition of the MEK-VEGF-A pathway
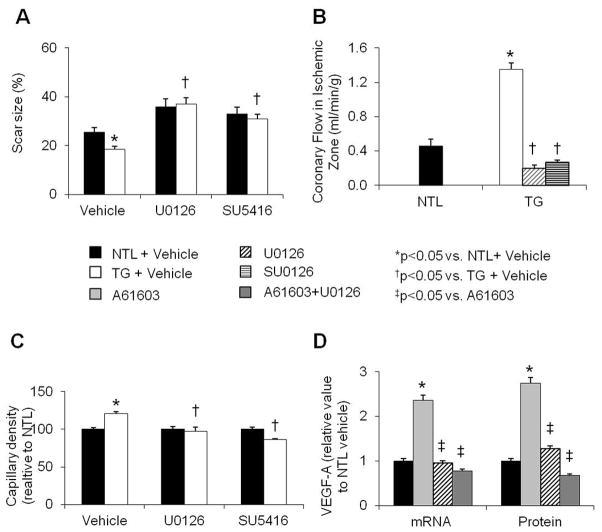
U0126, a MEK inhibitor, or SU5416, a VEGF receptor inhibitor, was administered throughout the 4 wk post MI period in both NTL and TG rats. Table 2 compares the effects of the MEK and VEGF receptor inhibitors in NTL and TG rats with that observed in NTL and TG rats given only vehicle. At 4 wk post MI, the preserved cardiac function and attenuated remodeling observed in vehicle-treated TG rats, as compared to their vehicle-treated NTLs, were abolished after treatment with either inhibitor. Thus, with inhibitor treatment, cardiac function, reflected by ejection fraction, LV +dP/dt, and LV wall stress were now similar in the TG animals and their NTLs (Table 2). Also, scar size was not significantly different between U0126- or SU5416-treated NTL and TG hearts at 4 wk post MI (Fig. 5A). Compared to vehicle treated TG hearts post MI, TG hearts treated with U0126 or SU5416 did not show an increase in myocardial blood flow, suggesting that treatment with either inhibitor caused regression of collateral vessels (Fig. 5B). Capillary density within the adjacent zone was no longer increased in TG rats treated with either inhibitor but was similar among the inhibitor-treated NTL and TG groups (Fig. 5C). In A61603 treated NTL mouse cardiomyocytes, pre-treatment with U0126 markedly reduced VEGF-A mRNA and protein levels (Fig. 5D).
Table 2.
Cardiac Function at 4 weeks after Myocardial Infarction Treated with Vehicle, U0126 or SU5416
| NTL | TG | |
|---|---|---|
| Heart Rate (beats/min) | ||
| Vehicle | 397±12 | 333±13* |
| U0126 | 409±24 | 350±11* |
| SU5416 | 425±31 | 397±18 |
| Mean Arterial Pressure (mmHg) | ||
| Vehicle | 89±5.5 | 100±3.1 |
| U0126 | 72±3.3 | 80±2.2* |
| SU5416 | 71±4.2 | 74±2.7 |
| LV Systolic Pressure (mmHg) | ||
| Vehicle | 102±5.6 | 112±3.2 |
| U0126 | 87±3.7 | 94±3.0 |
| SU5416 | 91±5.2 | 88±3.3 |
| LV Ejection Fraction (%) | ||
| Vehicle | 39±2.3 | 55±1.1* |
| U0126 | 37±0.9 | 40±1.8 |
| SU5416 | 33±2.1 | 27±1.0 |
| LV End-diastolic Diameter (mm) | ||
| Vehicle | 10±0.4 | 8.6±0.4* |
| U0126 | 11±0.2 | 11±0.5 |
| SU5416 | 9.7±0.3 | 9.5±0.6 |
| LV Systolic Wall Stress (dynes/cm2) | ||
| Vehicle | 193±22 | 137±11* |
| U0126 | 218±9.3 | 221±12 |
| SU5416 | 229±4.2 | 236±7.6 |
n = 4,
p<0.05 vs. NTL
Fig. 5.
Responses of NTL and TG rats to 4 wk of CAO and treatment with vehicle (10% DMSO), the MEK inhibitor, U0126 (400μg/kg/day), or the VEGF receptor inhibitor, SU5416 (25mg/kg/day). (A) Scar size was significantly smaller in the vehicle-treated TG vs. NTL hearts; a difference that was no longer observed after inhibitor treatment. (B) Coronary blood flow in the ischemic zone after 4 wk of CAO was significantly higher in TG vs. NTL hearts, and the increase in the TG hearts was abolished with either U0126 or SU5416 treatment for 4 wk. (C) Capillary density, normalized to NTLs, was increased in the adjacent zone of TG after 4 wk of CAO, and this increase was abolished with either U0126 or SU5416 treatment for 4 wk. (D) In NTL cardiomyocytes, A61603 increased VEGF-A mRNA and protein levels, and these increases were prevented when the α1A-AR agonist was combined with U0126. Results are expressed as the mean ± SEM. n=4–6; *p<0.05 vs. NTL + vehicle; †p<0.05 vs. TG + Vehicle; ‡p<0.05 vs. A61603 treated NTL cardiomyocytes.
DISCUSSION
In the present investigation, we demonstrated that overexpression of α1A-AR in cardiomyocytes protected the heart from the adverse effects of remodeling and heart failure that occurs after permanent CAO (Fig. 1). In the rat model of heart failure induced by permanent CAO, others have reported that LV ejection fraction falls to levels of 40–45% after heart failure develops19–21, and have utilized this model of heart failure to devise current heart failure therapy in patients. These values are similar to those we observed with CAO of NTLs in the present study, where LV ejection fraction fell to 38%, 4–6 wks after permanent CAO. Moreover, LV function was better preserved with less remodeling after CAO, in terms of fibrosis, myocyte hypertrophy, LV weight/tibial length and lung weight/tibial length and scar size, in the TG rats. Whereas cardiac α1A-AR stimulation has been proposed previously for the treatment of heart failure on the basis of its increased inotropic properties, and because α1A-AR blockade exerts an adverse effect in heart failure1–3, the results of the current investigation provide an additional novel mechanism that mediates α1A-AR protection against the remodeling and the development of heart failure that occurs after permanent CAO, i.e., α1A-AR-stimulated angiogenesis.
Since with complete CAO it is difficult for modulators of apoptosis or preconditioning to work without blood flow to keep the tissue alive, we concluded that the chronic protection must have ensued due to angiogenesis in the TG rats, which provided collateral blood flow to the central ischemic and adjacent zones after CAO, restored blood flow to the ischemic myocardium, resulting in less cell death with more viable tissue within the ischemic zone, and within the central infarct area. We documented the recovery of myocardial blood flow in the central ischemic zone and the zone adjacent to the infarct using the microsphere method, which is one of the only ways to measure regional myocardial blood flow after permanent CAO. It is important to appreciate that additional salvage of myocardium can come later in the process, due to the positive effects of remodeling, resulting in reduced LV wall stress and myocardial oxygen demand. This is the first report of the α1A-AR being linked to angiogenesis. Although norepinephrine was found to induce VEGF-A expression and angiogenesis22, the adrenergic receptor involved was not determined. There is evidence that the α-AR can stimulate angiogenesis24, however, our studies indicate for the first time, that in the heart, the α1A-AR/MEK/VEGF-A pathway appears to be the mediator of angiogenesis via heterocellular myocyte/endothelial cell signaling. Elucidation of this pathway was greatly aided by the availability of the TG rat model, which is more amenable to detailed physiological investigations than the mouse.
Involvement of the α1A-AR/MEK/VEGF-A pathway in angiogenesis is evident from the following observations. Firstly, we demonstrated that the TG rats with α1A-AR overexpression had preserved cardiac function with higher ejection fraction and lower LV wall stress than NTLs (Table 1). TG rats had similar LV systolic pressure with smaller LV chambers and significantly greater wall thickness at systole and their LV systolic wall stress was markedly lower than in the NTLs (Table 1). Reduced wall stress, per se, could have contributed to the cardioprotection, since it reduces myocardial oxygen requirements. However, as noted above, this by itself, in the absence of some preservation of blood supply, is not sufficient to protect the heart subjected to 4 weeks of permanent CAO. Secondly, using histological staining, scar size, measured as the percentage of the total myocardium, was markedly smaller in TG hearts and was accompanied by more viable tissue and capillaries within the scar and less fibrosis in ischemic and adjacent zones, suggesting that the mechanism of protection involved angiogenesis. The mechanisms mediating adjacent and remote remodeling are complex and likely different. The adjacent zone, which also suffers from some myocardial ischemia is influenced positively by angiogenesis and improved myocardial perfusion. This is not the case for the remote zone, which never experiences myocardial ischemia. The improvement in the remote zone is due more to the positive effects on remodeling and reduced LV wall stress and myocardial oxygen demand, which protects against further myocyte necrosis and secondary myocyte hypertrophy and fibrosis.
The conclusions from prior work on differences in regulation of alpha receptors in endothelial cells and cardiomyocytes are controversial23, 24. Some studies have implied that alpha receptors on endothelial cells may induce vasodilation23, 24, where others have suggested vasoconstriction25, 26. Studies have found different results on which subtypes of alpha receptors are predominant23, 24, and whether there are differences between regulation of RV vs. LV or normal vs. heart failure27. Whereas all of these points are interesting and important, they really do not bear on the major findings of the current paper.
We found that overexpressed alpha 1A receptors in myocytes induced angiogenesis both in vivo and in vitro and that the additional myocardial blood flow was necessary for improved function of the heart following chronic CAO. Previous studies have shown that alpha adrenergic receptor inhibition, rather than stimulation in brain and hindlimb endothelial cells induced angiogenesis28, which is opposite to our results in the heart. To our knowledge, there is no extant evidence showing that overexpression of any alpha receptor subtype in the heart can induce angiogenesis, and there are relatively few myocyte specific TG models for any gene that has been shown to protect against permanent CAO by inducing angiogenesis, unless that model also affected angiogenesis directly29, or through a pathway that induced angiogenesis, e.g., elaboration of an associated growth factor30.
We next determined the angiogenic factor that mediates the increase in blood flow and in capillary density in the α1A-AR TG rat model with permanent CAO. Microarray analysis revealed enhanced expression of 6 angiogenic growth factor genes in TG and NTL cardiomyocytes. However, only enhanced VEGF-A expression in TG cardiomyocytes could be verified by qPCR (Fig. 3C). VEGF-A promotes endothelial proliferation and migration resulting in tubule formation31–33, and as a proangiogenic factor, is known to be activated by hypoxia and has an important role in angiogenesis and in reducing hypoxic cellular damage32, 34.
Since the promoter used in the transgenic model was cardiac myocyte specific, there was no overexpression of α1A-AR in the vessels or endothelial cells, and therefore we postulated a paracrine mechanism, whereby angiogenesis was induced in the current myocyte-specific TG model through a crosstalk between the cardiomyocytes and endothelial cells, also known as heterocellular signalling, which has been described for the interaction between one cell type and another in the same organ35–38. Whereas heterocellular signalling has been described between several cell types35–39, the current investigation focused on cross-talk between cardiomyocytes overexpressing the α1A-AR and endothelial cells to induce angiogenesis. This paracrine mechanism appears to involve transmission of a signal from one cell type to an adjacent cell via the elaboration by the former of a secreted factor - in this case VEGF-A secretion from cardiomyocytes acting on endothelial cells and/or vascular smooth muscle cells, to induce angiogenesis as a result of their proliferation and migration29, 35, 37, 40, 41. To further confirm the link between α1A-AR stimulation and VEGF-A secretion, we examined the effects of α1A-AR agonist stimulation, using A6160342, directly on cultured NTL mouse cardiomyocytes. We elected to use cardiomyocytes isolated from mice instead of rats for the mechanistic studies because endogenous α1A-AR expression in mice is several fold less than rats, and is more akin to that in humans3. Treatment with A61603 enhanced VEGF-A expression at both the mRNA and protein levels; effects blocked by the non-specific α1-AR antagonist, prazosin (Fig. 4B). In addition, conditioned medium from cultured TG myocytes or from A61603 treated NTL myocytes induced vascular tubule formation by HUVECs (which do not express endogenous α1A-AR) significantly more than conditioned medium obtained from cultured untreated NTL cardiomyocytes. These findings support our concept of heterocellular signaling between cardiomyocytes and vascular cells. In keeping with this notion, enhanced myocardial VEGF-A production, observed in response to hypertrophic or ischemic signals, has been shown to activate endothelial cells and to induce angiogenesis40, 41.
The MEK/ERK pathway has been found to be upregulated in hearts with cardiomyocyte-specific α1A-AR overexpression15, 16, and is also activated with in vitro receptor stimulation of cardiomyocytes expressing native levels of the α1A-AR43. In the current study, inhibition of the MEK pathway with U0126 abolished the attenuated cardiac remodeling observed after MI in TG rats, by not only increasing LV wall stress and decreasing LV contractility, but also by decreasing capillary density. In agreement with our findings, previous studies have suggested that MEK/ERK signaling is essential for VEGF-regulated endothelial cell proliferation18, 44, 45. The MEK/ERK pathway has also been shown to mediate preconditioning in the CAO/reperfusion model11. However, as noted above, the molecular mechanism is not effective in the absence of blood flow.
One other point needs to be considered. Recently, Ye et al. reported that VEGF plays a role in cardiomyocyte differentiation from iPS cells46. Although there is substantial evidence that the increases in blood flow are sufficient to explain the preservation of myocytes, it is possible that an effect on cardiomyocyte differentiation contributes. We measured Ki67 positive myocytes and found that there was a significant increase in labeling in the TG vs NTL in the ischemic zone 47, suggesting the induction of new myocytes as well as preservation of older myocytes. It is difficult to distinguish which is the chicken vs. the egg, but it is conceivable that the increased blood flow due to angiogenesis permitted this to occur.
In summary, the results of this investigation suggest a novel therapeutic target for improved clinical outcomes by limiting post MI loss of myocardium and, hence, remodeling and heart failure as a result of α1A-AR-MEK/VEGF-A-mediated angiogenesis; an effect that also protects the function of marginally ischemic myocardium adjacent to the infarct. Protection against remodeling (less fibrosis and myocyte hypertrophy) also protects the non-ischemic zone after chronic CAO, a mechanism, unlike the adjacent zone, not dependent upon increased blood flow. Another novel finding was that overexpression of cardiomyocyte specific α1A-AR was able to induce angiogenesis, a mechanism that might be available for other myocyte proteins, and might promote a new line of research in the angiogenesis field. Although cardiac α1-AR stimulation has been proposed as a potential treatment for heart failure based on its ability to augment inotropy1, this is the first time that the α1A-AR has been linked to the enhanced expression of a potent proangiogenic factor, VEGF-A; a factor that also induces angiogenesis in response to ischemia. This mechanism improving perfusion to ischemic myocardium changes the paradigm explaining α1A-AR’s salutary action in heart failure, from simply increasing inotropy, which also increases myocardial oxygen consumption, to increasing inotropy while improving perfusion, which protects myocardial oxygen consumption.
Supplementary Material
SIGNIFICANCE.
This investigation demonstrates, at several levels, a novel mechanism for α1A-AR mediated protection from remodelling and heart failure following permanent CAO, i.e., angiogenesis. First, stimulation of the α1A-AR in cardiomyocytes has not been demonstrated previously to induce angiogenesis. Moreover, the fact that this occurs through heterocellular signalling from myocytes to coronary vessels will open new areas of investigation into the mechanisms of myo-vascular coupling, and potentially will lead to new therapeutic modalities. This is not only significant at the basic science level, but also from a clinical perspective, since cardiomyocyte α1A-AR stimulation has been proposed for the treatment of heart failure based on its action to increase myocardial contractility. Given the current findings, there is perhaps a more important reason to support cardiac α1A-AR stimulation for treatment of heart failure, particularly when it is of myocardial ischemic etiology, i.e., improvement of myocardial blood flow through angiogenesis.
Acknowledgments
We appreciate Ms. Grace Lee, Dr. Yimin Tian, and Dr. Chunbo Wang for technical support related to the histology and qPCR analyses.
FUNDING RESOURCES
Study supported by National Institute of Health grants 5R01HL119464, 3P01HL069020, 6T32HL069752, 6R01HL093481, 5R01HL106511, 1R01HL124282; University of New South Wales Goldstar Award RG14231, R.T. Hall Estate, National Health and Medical Research Council Program Grant 1074386, Australian Research Council Stem Cells Australia SR110001002.
ABBREVIATIONS
- α1A-AR
α1A-adrenergic receptor
- NTL
Non-transgenic littermates
- TG
Transgenic
- CAO
Coronary artery occlusion
- MI
myocardial infarction
- HUVEC
Human umbilical vein endothelial cell
Footnotes
DISCLOSURES: None
References
- 1.Jensen BC, O’Connell TD, Simpson PC. Alpha-1-adrenergic receptors: Targets for agonist drugs to treat heart failure. Journal of molecular and cellular cardiology. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen BC, O’Connell TD, Simpson PC. Alpha-1-adrenergic receptors in heart failure: The adaptive arm of the cardiac response to chronic catecholamine stimulation. Journal of cardiovascular pharmacology. 2014;63:291–301. doi: 10.1097/FJC.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connell TD, Jensen BC, Baker AJ, Simpson PC. Cardiac alpha1-adrenergic receptors: Novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacological reviews. 2014;66:308–333. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shannon R, Chaudhry M. Effect of alpha1-adrenergic receptors in cardiac pathophysiology. American heart journal. 2006;152:842–850. doi: 10.1016/j.ahj.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Landzberg JS, Parker JD, Gauthier DF, Colucci WS. Effects of myocardial alpha 1-adrenergic receptor stimulation and blockade on contractility in humans. Circulation. 1991;84:1608–1614. doi: 10.1161/01.cir.84.4.1608. [DOI] [PubMed] [Google Scholar]
- 6.Skomedal T, Borthne K, Aass H, Geiran O, Osnes JB. Comparison between alpha-1 adrenoceptor-mediated and beta adrenoceptor-mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. The Journal of pharmacology and experimental therapeutics. 1997;280:721–729. [PubMed] [Google Scholar]
- 7.Zakir RM, Folefack A, Saric M, Berkowitz RL. The use of midodrine in patients with advanced heart failure. Congest Heart Fail. 2009;15:108–111. doi: 10.1111/j.1751-7133.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen YT, Vatner SF. Mechanism of impaired myocardial function during progressive coronary stenosis in conscious pigs. Hibernation versus stunning? Circulation research. 1995;76:479–488. doi: 10.1161/01.res.76.3.479. [DOI] [PubMed] [Google Scholar]
- 9.Wijns W, Vatner SF, Camici PG. Hibernating myocardium. The New England journal of medicine. 1998;339:173–181. doi: 10.1056/NEJM199807163390307. [DOI] [PubMed] [Google Scholar]
- 10.Doppler SA, Deutsch MA, Lange R, Krane M. Cardiac regeneration: Current therapies-future concepts. Journal of thoracic disease. 2013;5:683–697. doi: 10.3978/j.issn.2072-1439.2013.08.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Park J, Ho D, Gao S, Yan L, Ge H, Iismaa S, Lin L, Tian B, Vatner DE, Graham RM, Vatner SF. Cardiomyocyte overexpression of the alpha1a-adrenergic receptor in the rat phenocopies second but not first window preconditioning. American journal of physiology. Heart and circulatory physiology. 2012;302:H1614–1624. doi: 10.1152/ajpheart.01072.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, Ginion A, Shah A, Pelat M, Bertrand L, Wagner T, Gaussin V, Vatner SF. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circulation research. 2006;98:280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- 13.Penna C, Brancaccio M, Tullio F, Rubinetto C, Perrelli MG, Angotti C, Pagliaro P, Tarone G. Overexpression of the muscle-specific protein, melusin, protects from cardiac ischemia/reperfusion injury. Basic research in cardiology. 2014;109:418. doi: 10.1007/s00395-014-0418-9. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: A critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovascular research. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, Colucci WS. Mek1/2-erk1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. Journal of molecular and cellular cardiology. 2001;33:779–787. doi: 10.1006/jmcc.2001.1348. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez MT, Sah VP, Zhao XL, Hunter JJ, Chien KR, Brown JH. The mekk-jnk pathway is stimulated by alpha1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. The Journal of biological chemistry. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 17.Sang RL, Johnson JF, Taves J, Nguyen C, Wallert MA, Provost JJ. Alpha(1)-adrenergic receptor stimulation of cell motility requires phospholipase d-mediated extracellular signal-regulated kinase activation. Chemical biology & drug design. 2007;69:240–250. doi: 10.1111/j.1747-0285.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 18.Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through mek/erk- and pi3k/akt-dependent signaling. Neuropharmacology. 2012;63:642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher PJ, Pfeffer JM, Pfeffer MA, Braunwald E. Left ventricular diastolic pressure-volume relations in rats with healed myocardial infarction. Effects on systolic function. Circulation research. 1981;49:618–626. doi: 10.1161/01.res.49.3.618. [DOI] [PubMed] [Google Scholar]
- 20.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S. Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;29:841–850. doi: 10.1159/000178526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circulation research. 1985;57:84–95. doi: 10.1161/01.res.57.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Kang JH, Jeong KJ, Lee J, Han JW, Choi WS, Kim YK, Kang J, Park CG, Lee HY. Norepinephrine induces vegf expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. International journal of cancer. Journal international du cancer. 2011;128:2306–2316. doi: 10.1002/ijc.25589. [DOI] [PubMed] [Google Scholar]
- 23.Mendez E, Calzada C, Ocharan E, Sierra A, Castillo C, Ramirez I, Meaney E, Meaney A, Asbun J, Miliar A, Herrera J, Ceballos G. Differential expression of alpha1-adrenergic receptor subtypes in coronary microvascular endothelial cells in culture. European journal of pharmacology. 2006;546:127–133. doi: 10.1016/j.ejphar.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 24.Jensen BC, Swigart PM, Montgomery MD, Simpson PC. Functional alpha-1b adrenergic receptors on human epicardial coronary artery endothelial cells. Naunyn-Schmiedeberg’s archives of pharmacology. 2010;382:475–482. doi: 10.1007/s00210-010-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guimaraes S, Moura D. Vascular adrenoceptors: An update. Pharmacological reviews. 2001;53:319–356. [PubMed] [Google Scholar]
- 26.Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The alpha(1d)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. The Journal of clinical investigation. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen BC, Swigart PM, De Marco T, Hoopes C, Simpson PC. {alpha}1-adrenergic receptor subtypes in nonfailing and failing human myocardium. Circulation. Heart failure. 2009;2:654–663. doi: 10.1161/CIRCHEARTFAILURE.108.846212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciccarelli M, Santulli G, Campanile A, Galasso G, Cervero P, Altobelli GG, Cimini V, Pastore L, Piscione F, Trimarco B, Iaccarino G. Endothelial alpha1-adrenoceptors regulate neo-angiogenesis. British journal of pharmacology. 2008;153:936–946. doi: 10.1038/sj.bjp.0707637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, Chang J, Fan GC. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of vegfr2. PloS one. 2012;7:e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korf-Klingebiel M, Kempf T, Schluter KD, Willenbockel C, Brod T, Heineke J, Schmidt VJ, Jantzen F, Brandes RP, Sugden PH, Drexler H, Molkentin JD, Wollert KC. Conditional transgenic expression of fibroblast growth factor 9 in the adult mouse heart reduces heart failure mortality after myocardial infarction. Circulation. 2011;123:504–514. doi: 10.1161/CIRCULATIONAHA.110.989665. [DOI] [PubMed] [Google Scholar]
- 31.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays vegf-dependent modulation of capillary structure and endothelial cell survival in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida S, Aihara K, Ikeda Y, Sumitomo-Ueda Y, Uemoto R, Ishikawa K, Ise T, Yagi S, Iwase T, Mouri Y, Sakari M, Matsumoto T, Takeyama K, Akaike M, Matsumoto M, Sata M, Walsh K, Kato S. Androgen receptor promotes sex-independent angiogenesis in response to ischemia and is required for activation of vascular endothelial growth factor receptor signaling. Circulation. 2013;128:60–71. doi: 10.1161/CIRCULATIONAHA.113.001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannarelli C, Alique M, Rodriguez DT, Yang DK, Jeong D, Calcagno C, Hutter R, Millon A, Kovacic JC, Weber T, Faries PL, Soff GA, Fayad ZA, Hajjar RJ, Fuster V, Badimon JJ. Alternatively spliced tissue factor promotes plaque angiogenesis through the activation of hypoxia-inducible factor-1alpha and vascular endothelial growth factor signaling. Circulation. 2014;130:1274–1286. doi: 10.1161/CIRCULATIONAHA.114.006614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. The New England journal of medicine. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 35.Heineke J, Auger-Messier M, Xu J, Oka T, Sargent MA, York A, Klevitsky R, Vaikunth S, Duncan SA, Aronow BJ, Robbins J, Crombleholme TM, Molkentin JD. Cardiomyocyte gata4 functions as a stress-responsive regulator of angiogenesis in the murine heart. The Journal of clinical investigation. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsch E, Nagai R, Thum T. Heterocellular signalling and crosstalk in the heart in ischaemia and heart failure. Cardiovascular research. 2014;102:191–193. doi: 10.1093/cvr/cvu073. [DOI] [PubMed] [Google Scholar]
- 37.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the akt/pkb signaling pathway. Genes & development. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 38.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between amp-activated protein kinase and akt signaling in endothelial cells. The Journal of biological chemistry. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, Gu Y, Nath AK, Huang Y, Hickey R, Dalton N, Peterson KL, Ross J, Jr, Chien KR, Ferrara N. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu CW, Zhang TP, Wang HX, Yang H, Li HH. Chip enhances angiogenesis and restores cardiac function after infarction in transgenic mice. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31:199–208. doi: 10.1159/000343361. [DOI] [PubMed] [Google Scholar]
- 42.Knepper SM, Buckner SA, Brune ME, DeBernardis JF, Meyer MD, Hancock AA. A-61603, a potent alpha 1-adrenergic receptor agonist, selective for the alpha 1a receptor subtype. The Journal of pharmacology and experimental therapeutics. 1995;274:97–103. [PubMed] [Google Scholar]
- 43.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O’Connell TD. An alpha1a-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 44.Nakatsu MN, Sainson RC, Perez-del-Pulgar S, Aoto JN, Aitkenhead M, Taylor KL, Carpenter PM, Hughes CC. Vegf(121) and vegf(165) regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Laboratory investigation; a journal of technical methods and pathology. 2003;83:1873–1885. doi: 10.1097/01.lab.0000107160.81875.33. [DOI] [PubMed] [Google Scholar]
- 45.Chang LH, Pan SL, Lai CY, Tsai AC, Teng CM. Activated par-2 regulates pancreatic cancer progression through ilk/hif-alpha-induced tgf-alpha expression and mek/vegf-a-mediated angiogenesis. The American journal of pathology. 2013;183:566–575. doi: 10.1016/j.ajpath.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 46.Ye L, Zhang S, Greder L, Dutton J, Keirstead SA, Lepley M, Zhang L, Kaufman D, Zhang J. Effective cardiac myocyte differentiation of human induced pluripotent stem cells requires vegf. PloS one. 2013;8:e53764. doi: 10.1371/journal.pone.0053764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park M, Vatner SF, Yan L, Gao S, Yoon S, Lee GJ, Xie LH, Kitsis RN, Vatner DE. Novel mechanisms for caspase inhibition protecting cardiac function with chronic pressure overload. Basic research in cardiology. 2013;108:324. doi: 10.1007/s00395-012-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.