Abstract
Given the almost limitless variety of nanomaterials, it will be virtually impossible to assess the possible occupational health hazard of each nanomaterial individually. The development of science-based hazard and risk categories for nanomaterials is needed for decision-making about exposure control practices in the workplace. A possible strategy would be to select representative (benchmark) materials from various mode of action (MOA) classes, evaluate the hazard and develop risk estimates, and then apply a systematic comparison of new nanomaterials with the benchmark materials in the same MOA class. Poorly soluble particles are used here as an example to illustrate quantitative risk assessment methods for possible benchmark particles and occupational exposure control groups, given mode of action and relative toxicity. Linking such benchmark particles to specific exposure control bands would facilitate the translation of health hazard and quantitative risk information to the development of effective exposure control practices in the workplace. A key challenge is obtaining sufficient dose–response data, based on standard testing, to systematically evaluate the nanomaterials’ physical–chemical factors influencing their biological activity. Categorization processes involve both science-based analyses and default assumptions in the absence of substance-specific information. Utilizing data and information from related materials may facilitate initial determinations of exposure control systems for nanomaterials.
Keywords: Risk assessment, Occupational exposure limits, Comparative toxicity, Hazard groups, Exposure control groups, Health effects
Introduction
Due to the vast number of chemical and physical agents in the workplace without occupational exposure limits (OELs), there is a critical need to develop health-based criteria for the selection and evaluation of exposure controls. New substances such as nanomaterials typically have limited health hazard data from which to evaluate the workplace exposure control needs. Yet, nanomaterials in several major categories (e.g., carbon-based, metals, metal oxides) are currently in production and use, and wide variations exist in the properties of specific materials within these major categories (e.g., differences in shape, size, surface functionalization) which may affect biological activity of these materials, for example, if inhaled by a worker.
Trends in nanotechnology are moving from research and development toward manufacturing with a currently small, but growing, segment of the work-force (Invernizzi 2011). With the increased manufacturing of nanomaterials and nanomaterial-containing products comes the potential for occupational exposure to these materials during their production or use. Information is needed to make informed decisions about the level of exposure control needed to protect workers’ health. In general, the less that is known about a substance, the greater should be the precaution in selecting the level of exposure control (Schulte and Salamanca-Buentello 2007). In the absence of OELs for most nanomaterials, hazard and control banding approaches have been proposed, but these processes generally have not been validated concerning the level of health protection afforded by those systems.
The purpose of this paper is to describe the concepts and challenges of using benchmark particles in developing hazard- and risk-based categories for nanomaterials’ OELs. A benchmark particle is essentially a reference material which has been tested and evaluated according to standard criteria and to which new materials may reliably be compared. An example is provided in this paper of quantitative risk estimation and the comparative potency of various types of poorly soluble respirable particles (PSP), and one type of soluble particle, associated with lung cancer in rat chronic inhalation studies. These data were selected because PSP are an example of a possible mode of action (MOA) category due to their potential to cause chronic adverse lung effects related to their biopersistence in the lungs. The rat is a sensitive rodent species for adverse lung effects including persistent pulmonary inflammation and lung cancer associated with exposure to respirable PSP (Mauderly 1997; NIOSH 2011). Chronic health effects data are particularly needed in current hazard and control banding schemes.
With the growing variety of nanomaterials, there will be a greater need to identify the level of exposure control and containment needed for various types of nanomaterials. Developing a set of benchmark particles with a full quantitative risk assessment, and utilizing validated shorter-term studies to compare the nature and severity of response in standard assays, could increase the efficiency of OEL development and exposure controls for nanomaterials. Despite the large variation in nanomaterials, the exposure control options are much fewer, and may be defined within relatively few bands (e.g., order of magnitude) (Naumann et al. 1996; Ader et al. 2005; Hewett et al. 2006). Controlling exposures is the most important step in preventing occupational lung disease in workers.
An OEL strategy for nanomaterials
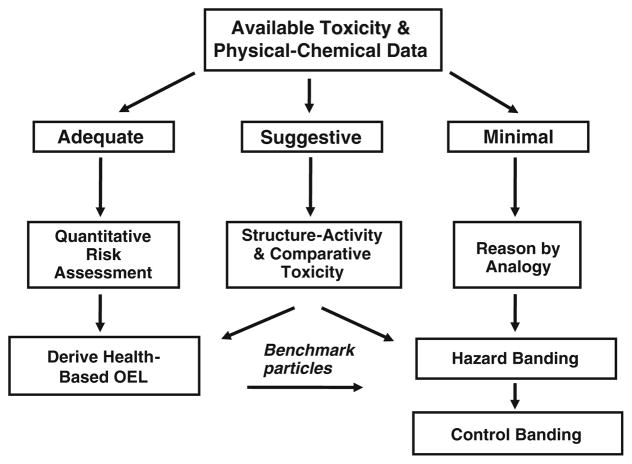
The process of evaluating health effects data for development of OELs can be viewed as three branches depending on the amount of available scientific evidence (Schulte et al. 2010): (1) Adequate (sufficient) dose–response data for quantitative risk assessment (QRA) and OEL development; (2) Suggestive (insufficient) data for QRA on the substance of interest, but adequate information on another substance with similar physical–chemical properties and likely biological mode of action; and (3) Minimal (limited) data on which to make a quantitative comparison so hazard and control bands are inferred by analogy to a similar type or class of materials (Fig. 1).
Fig. 1.
Possible strategy for developing exposure control limits and bands—incorporating risk-based estimates from comparative potency to benchmark particles (well-characterized substances with health-based OELs). Figure adapted from Schulte et al. (2010)
This strategy provides a systematic approach to developing health-based exposure controls for nanomaterials including those with suggestive or minimal data. The key to this approach is the identification of relevant benchmark (reference) particles, which can be defined as substances with adequate data on dose–response relationships and biological MOA for use in quantitative risk estimation and derivation of health-based OELs. Developing OELs for representative benchmark particles within each of these MOA categories (Table 1) would provide a basis for linking the health effects data to the exposure controls for nanomaterials with limited data. Using the example of control banding systems developed for pharmaceuticals and other types of dry powders, order of magnitude bins might be used as the first level of default categories (Fig. 2) with more narrow bands or specific OELs developed as data become available.
Table 1.
Possible group-specific categories and modes of action for nanoparticles related to hazard and risk assessment
| Group-specific considerations | Higher solubility particles | Poorly soluble, low toxicity (PSLT) particles | Poorly soluble, high toxicity particles | Fibrous particles |
|---|---|---|---|---|
| Example benchmark particles | Zinc oxide Copper oxide (I) |
Titanium dioxide Carbon black |
Crystalline silica Nickel oxide (III) |
Carbon nanotubes Carbon nanofibers |
| Adverse effects | Acute lung effects Systemic toxicity |
Lung inflammation and fibrosis; lung cancer (rats) | Chromiun oxide (III) Lung inflammation and fibrosis; lung cancer | Lung fibrosis, possible cancer, and mesothelioma |
| Mode of action | Toxic ions reach systemic tissues | Toxicity related to total deposited or retained particle dose in target respiratory tract region based on particle size | Same as PSLT; plus reactive surface (e.g., reactive oxygen species) | Durability/biopersistence Migration into alveolar walls and from lung tissue to the pleural Interference with normal cell division Genotoxicity |
| Dose metric related to adverse effects | Dissolution rate; amount absorbed into blood | Surface area Volume Mass or number, by particle size fraction |
Same dose metrics as PSLT; plus reactivity of particle surface | Number of fibers of certain dimensions Total surface area of fibers or nanotubes |
| Dose–response relationship | Slope and effect level may depend on dissolution | May be nonlinear at low doses | Steeper slope and lower effect level than PSLT | Linear dose–response for some endpoints |
Fig. 2.
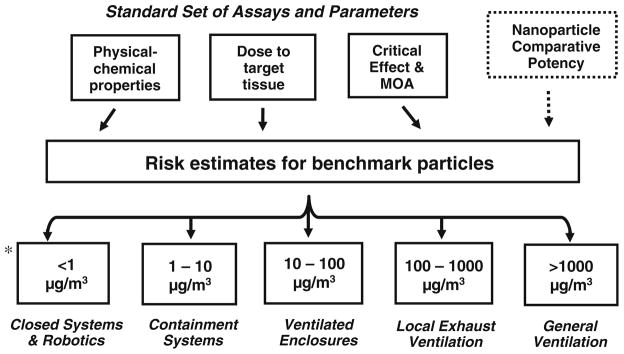
Integrating risk assessment with hazard and exposure control banding—an example of order of magnitude bins. * 8-h time-weighted average concentration. Exposure control limit bands and engineering control systems based on: Naumann et al. (1996); Ader et al. (2005); Zalk and Nelson (2008)
Categorical approaches
Several qualitative hazard-based categories and control banding schemes have been developed for hazardous substances in the workplace including nanomaterials. These include: relative hazard and risk ranking frameworks for nanomaterials (Linkov et al. 2007, 2009; Tervonen et al. 2009; Grieger et al. 2012), nanomaterial-specific control banding schemes (Zalk et al. 2009; ANSES 2010), and the United Nations’ globally harmonized system of classification and labelling of chemicals which was recently adopted in the U.S. (77 FR 17574, March 26, 2012). However, absolute risk estimates or risk-based OELs for reference or benchmark materials within these categories are needed to link the hazard and relative risk information to the level of exposure control needed to protect workers (e.g., at a minimum, order of magnitude bands, Naumann et al. 1996; Ader et al. 2005).
The concept of developing benchmark materials for hazard and risk assessment of nanomaterials, including by utilizing data from existing studies in humans and animals of exposure to inhaled particles and fibers, was proposed earlier (Kuempel et al. 2006, 2007). Since that time, additional toxicology data have become available for some nanomaterials and categorical approaches have become more widely recognized or adopted (BSI 2007; OECD 2007; NIOSH 2010a, b; Schulte et al. 2010)—providing greater opportunity and need to quantitatively evaluate these approaches. Yet, health effects data on most nanomaterials are still lacking. In the absence of nanomaterial-specific data, initial estimates of OELs for nanomaterials could be developed by adjusting the OELs for benchmark particles (e.g., larger particle size material of the same chemical composition) by the differences in the surface area, surface reactivity, and other factors that are associated with the adverse effect (Kuempel et al. 2007). However, OELs can vary widely with regard to the hazards and risks associated with exposure (e.g., due to differences in the derivation methods and technical feasibility of measuring and controlling exposure) and so do not provide a standard health basis for comparison. Thus, a quantitative comparison of nanoparticle toxicity to benchmark particles (including consideration of the role physical–chemical properties) was suggested as a preferable method if sufficient data were available (Kuempel et al. 2007). An example of a comparative toxicity approach (Schoeny and Margosches 1989) utilizes the “parallelogram” extrapolation method (Sobels 1993). These comparative toxicity analyses could be conducted in short-term assays for a set of nanomaterials in which the benchmark particles are the reference particles (e.g., positive and negative controls) to which the new materials would be compared (Fig. 3).
Fig. 3.
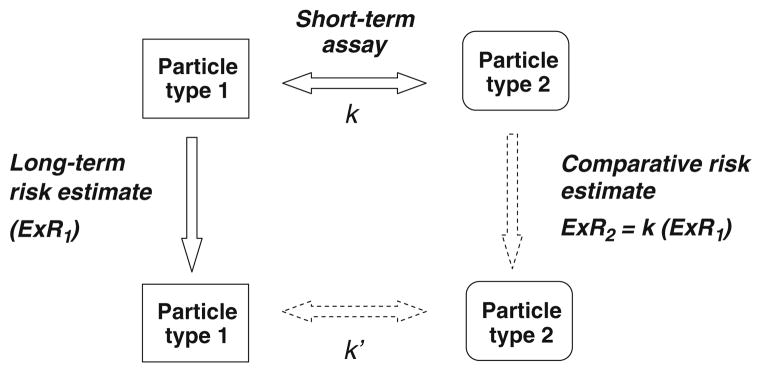
Comparative potency and parallelogram approaches to risk estimation for new materials. (Based on concepts discussed in: Schoeny and Margosches 1989; Sobels 1993 (and earlier papers); Sutter 1995). The relative potency (described by k) between particle type 1 and 2 in a short-term assay, along with the relationship between short-term and long-term toxicity and risk for particle type 1, is used to estimate the risk of adverse health effects from long-term exposure to particle type 2 (assuming the same relative potency, k’)
In an early example of categorical exposure limits, the British Standards Institute (BSI 2007) proposed setting “benchmark exposure limits” or “BELs” for nanomaterials based on analogy to substances in four main categories including (1) fibers; (2) carcinogenic, mutagenic, asthmatic, or reproductive toxicants; (3) insoluble; and (4) soluble substances (BSI 2007). BELs for nanomaterials were proposed at one-half to one-fifteenth of the OEL for the larger particles or fibers of similar chemical composition or structure (e.g., based on particle surface area of insoluble particles as in the 2005 NIOSH draft TiO2 recommended exposure limits (NIOSH 2011), or by means of uncertainty factor adjustments (BSI 2007)).
Within three broad categories (carcinogenic, high toxicity, low toxicity), Dolan et al. (2005) proposed a method to derive exposure limits (acceptable daily intake, ADI) for new substances with minimal data. The ADI for a new substance would be set at the 5th percentile of the ADIs for all nongenotoxic substances based on the assumption that a relatively unstudied compound would be unlikely to have a lower ADI once its true potency is determined (Dolan et al. 2005). A refinement of such methods, as discussed in this paper, would be to further develop the MOA categories and the hazard predictors (e.g., specific physical–chemical properties) in order to obtain better estimates of the actual hazard and risk of a nanomaterial.
Role of physical–chemical properties
The physical–chemical properties of particles and fibers, including nanomaterials, can influence the internal dose and the biological response to those materials through different modes of action (Table 1). Such properties include solubility, surface area, surface reactivity, size, and shape (Maynard and Kuempel 2005; Oberdörster et al. 2005a, b; Donaldson et al. 2010; Castranova 2000, 2011; Zhang et al. 2012). The size and shape of airborne structures influence their inhalability as well as deposition efficiency within the respiratory tract regions. As the airborne particle size decreases into the nanoparticle size range (<100 nm), the fraction of inhaled particles that deposit in the alveolar (gas-exchange) region of the lungs generally increases (up to ~50 %) (ICRP 1994; Maynard and Kuempel 2005). The dose metrics of mass, volume, number, or surface area of particles or fibers retained in the lungs have been associated with lung responses to inhaled particles or fibers in rats and mice (Morrow 1988; Oberdörster and Yu 1990; Muhle et al. 1991; Oberdörster et al. 1994; Tran et al. 2000; Elder et al. 2005; Nakanishi 2011; Pauluhn 2011; Murray et al. 2012). Working lifetime exposures to respirable particle mass and fiber number concentrations have been associated with nonmalignant and malignant lung diseases in workers (reviewed in: Kuempel and Maynard 2005; Oberdörster et al. 2005b; Rom and Markowitz 2006). In a recently published study, airborne exposure to the nanoscale diesel exhaust particulate (DEP) was associated with lung cancer in miners (Attfield et al. 2012). Cumulative exposure to the thinnest (<0.3 μm in diameter) and longest (>10 μm in length) structures of airborne chrysotile was the best predictor of asbestosis and lung cancer in textile workers (Stayner et al. 2008). Lung responses observed in both animals and humans, as reported in the studies cited above, include buildup and retention of particles or fibers in the lungs, pulmonary inflammation, fibrosis, and lung cancer.
The challenge remains to develop predictive models of the toxicity of nanoparticles based on their properties (e.g., quantitative structure activity relationships, QSAR). Thus, a basic set of data reported in all studies on particle characterization, dose metrics, and response measures would aid in the interpretation of findings across studies and facilitate the pooling of data. This would considerably increase the information base for comparing toxicity and estimating risk for individual nanomaterials or groups of nanomaterials. Benchmark particles would be included in the toxicity testing (e.g., as positive and negative control particles administered in the same test system as the nanomaterials) (Oberdörster et al. 2005a) along with a standard set of physical–chemical properties reported in each study (Oberdörster et al. 2005a; Warheit et al. 2007). Standardizing a minimum set of dose metrics and biological endpoints of relevance to humans would facilitate comparison of toxicity across nanomaterials for risk assessment (Table 2).
Table 2.
Basic parameters needed for risk assessment of inhaled particles
| Parameter | Purpose |
|---|---|
| Particle size, shape, density | Estimate inhalation and lung region-specific deposition fraction |
| Particle surface area, reactivity, solubility | Evaluate mode of action and local or systemic effects |
| Multiple exposure or dose groups | Describe dose–response relationship; estimate benchmark dose |
| Biological significance of response | Evaluate severity and relevance to humans |
| Body and lung weight; target lung region surface area and volume | Normalize dose from animals to humans |
Risk assessment framework
A hazard- and risk-based categorization approach to nanomaterials’ OELs, such as proposed in this paper, is consistent with the U.S. National Research Council (NRC) recommendations to increase the utility of risk assessment for risk management decision-making (NRC 2009). In its revised guidelines, the NRC (2009) recommended first to evaluate the options to reduce the hazard or exposure and then to determine what risk analyses are needed to decide among these options. Starting with the options for exposure control (e.g., order of magnitude bins and associated performance-based engineering control systems as previously proposed) (Fig. 2) provides a framework to link the hazard and risk information more directly to the exposure control options. Research priorities would include those studies that provide information to reduce uncertainty in decision-making concerning the exposure control options.
Example of quantitative risk assessment of poorly soluble particles
Methods
Standard quantitative risk assessment methods for inhaled particles including nanoparticles have been previously described (Kuempel et al. 2006). This general approach is applied here to evaluate the dose–response data from chronic inhalation studies in rats exposed to various types and sizes of airborne particles. Benchmark dose (BMD)1 methods (U.S. EPA 2010) were used to estimate human-equivalent working lifetime exposure concentrations associated with a 1/1000 (0.1 %) excess risk of lung cancer. This critical effect level was selected because the 1/1000 has been described a significant risk for a severe chronic health effect (leukemia in that case) (U.S. Supreme Court 1980). BMD methods have several advantages over other effect level estimates (e.g., no observed adverse effect level, NOAEL, or lowest observed adverse effect level, LOAEL) by providing a standardized, risk-based approach that uses all of the dose–response data and takes statistical account of the sample size and variability in the data.
The steps in this risk assessment approach include:
Identify the relevant animal model, dose metric, and disease response (in this example: rat, airborne particle exposure concentration or retained particle dose in the lungs, and lung cancer);
Model the animal dose–response relationship and estimate the critical effect level, BMD(L)2;
Extrapolate the animal critical effect level estimates to humans by adjusting for the factors that influence the deposited or retained lung dose in each species, assuming equal response at equivalent dose3;
Estimate the airborne exposure (8-h time-weighted average, TWA) that would result in the human-equivalent dose4;
The particles evaluated in this example are from chronic inhalation (2-year) bioassays of PSP in rats (NTP 1996–2000; Lee et al. 1985; Heinrich et al. 1995; Nikula et al. 1995). These studies were selected because they provide relevant toxicology data to identify chronic lung disease hazards and the doses associated with those effects. Data are presented for both fine and ultrafine (nanoscale) particles. The lung tumor responses include carcinoma and adenoma, but exclude squamous cell keratinizing cysts. No a priori differences in lung tumor responses by gender were assumed, and male and female rat dose–response data (if available in the same study) were first evaluated together. The multistage cancer model (polynomial degree 2) within the BMD software (U.S. EPA 2010) was selected in this analysis because it is a longstanding model in cancer risk assessment. When model fit to the data were not adequate (i.e., p < 0.05), data were evaluated separately by gender. In some cases, adequate dose–response was obtained for one gender only. When model fit to each was adequate, the lower BMD(L) estimates were selected for this analysis.
Results
Tables 3 and 4 provide estimates of the working lifetime exposure concentration associated with a 1/1,000 excess risk of lung cancer based on animal-to-human extrapolation of benchmark dose estimates from the NTP chronic inhalation studies in rats. Table 3 provides estimates based on the rat airborne exposure concentrations and Table 4 provides estimates for the subset of particles in Table 3 for which measured rat lung particle burden data were available at the end of the two-year exposure. Both maximum likelihood estimates (MLE) and 95 % lower confidence limit (LCL) estimates are provided. Within individual particle types, the MLE and LCL estimates are fairly similar, whereas there are clear differences in these estimates among particle types.
Table 3.
Excess risk estimates (0.1 %) of lung cancer associated with airborne particle exposure, based on exposure concentration in rat chronic inhalation studies and extrapolated to worker-equivalent concentration
| Particle type and sizea,b | Rat airborne exposure concentration (mg/m3)c
|
Worker-equivalent (8-h TWA) airborne concentration (mg/m3)d
|
||
|---|---|---|---|---|
| MLE | 95 % LCL | MLE | 95 % LCL | |
| TiO2 (fine) | 2.0 | 1.7 | 3.9 | 3.3 |
| MoO3 (fine) | 1.6 | 1.1 | 2.3 | 1.6 |
| CB (ultrafine) | 0.031 | 0.020 | 0.14 | 0.12 |
| DEP (ultrafine) | 0.031 | 0.020 | 0.13 | 0.086 |
| CoSO4 (fine) | 0.027 | 0.013 | 0.065 | 0.029 |
| NiO (fine) | 0.026 | 0.015 | 0.034 | 0.019 |
| Ni3S2 (fine) | 0.0081 | 0.0039 | 0.010 | 0.0050 |
| GaAs (fine) | 0.0041 | 0.0027 | 0.0065 | 0.0043 |
Data used in the analyses: TiO2 (fine) (Lee et al. 1985, male and female rats); CB (Nikula et al. 1995, female; Heinrich et al. 1995, female); DEP (Nikula et al. 1995, female); MoO3 (male and female), GaAs (female), CoSO4 (male), NiO (female), and Ni3S2 (female) (NTP 1996–2000)
MLE Maximum likelihood estimate; 95 % LCL 95 % Lower confidence limit; DEP Diesel exhaust particulate; CB Carbon black; TWA Time-weighted average
All are respirable, nonfibrous, poorly soluble particles (e.g.,<0.1 g/100 ml in water for NiO, Ni3S2, GaAs; 0.5 g/100 ml for MoO3) (Melnick et al. 2003), except CoSO4 which is soluble. Size category refers to primary particle (ultrafine <100 nm)
MLE or 95 % LCL estimate based on multistage model (polynomial degree 2) estimate of 10 % excess risk with linear extrapolation to 0.1 % excess risk
Adjusted for rat and human differences in air inhaled per day (~0.24–0.36 m3/24-h based on rat body weight (EPA 1987) and 9.6 m3/8-h day in workers (ICRP 1994)), exposure duration (6–18 h/d rat and 8-h/dayhumans), alveolar deposition fraction (estimated in MPPD 2.0 (CIIT and RIVM 2006) based on airborne particle size reported in animal studies), and alveolar lung surface area (102 m2 human/0.4 m2 rat)
Table 4.
Excess risk estimates (0.1 %) of lung cancer associated with airborne particle exposure based on particle lung burden after chronic inhalation exposure in rats and extrapolated to worker-equivalent concentration
| Particle type and sizea | Rat lung burden (mg/g lung)b
|
Worker-equivalent (8-h TWA) airborne concentration (mg/m3)c
|
||
|---|---|---|---|---|
| MLE | 95 % LCL | MLE | 95 % LCL | |
| TiO2 (fine) | 1.9 | 1.6 | 1.6 | 1.3 |
| DEP (ultrafine) | 0.42 | 0.37 | 0.26 | 0.23 |
| TiO2 (ultrafine) | 0.20 | 0.17 | 0.16 | 0.14 |
| CB (ultrafine) | 0.16 | 0.11 | 0.15 | 0.10 |
MLE Maximum likelihood estimate; 95 % LCL: 95 % lower confidence limit; DEP Diesel exhaust particulate; CB Carbon black. TWA Time-weighted average
Data used in the analyses TiO2 (fine) (Lee et al. 1985, male and female); DEP (Nikula et al. 1995, male and female; Heinrich et al. 1995, female); CB (Nikula et al. 1995, female; Heinrich et al. 1995, female); TiO2 (ultrafine) Heinrich et al. 1995, female
All are nonfibrous, poorly soluble particles. Size category refers to primary particle
MLE or 95 % LCL estimates based on multistage model (polynomial degree 2) estimate of 10 % excess risk with linear extrapolation to 0.1 % excess risk; except, TiO2 estimates were based on model fit to the particle surface area lung dose of fine and ultrafine TiO2 data combined due to insufficient dose groups to model ultrafine TiO2 alone (equivalent mass dose was estimated by means of specific surface area, 48 m2/g ultrafine TiO2 and 4.99 m2/g fine TiO2) (NIOSH 2011)
Working lifetime exposure concentration associated with human-equivalent lung burden was estimated from the MPPD human lung dosimetry model (CIIT and RIVM 2006) by means of the Yeh and Schum deposition model; reference worker breathing parameters equivalent to 9.6 m3/8-h day (ICRP 1994; NIOSH 2011) for 2250 week (5 days/week, 50 week/year, for 45 year; airborne particle size and density as reported in animal studies; oronasal normal augmenter; and inhalability adjustment)
The working lifetime exposure concentration (8-h TWA) associated with 0.1 % excess risk of lung cancer based on rat chronic inhalation exposure data differs by approximately three orders of magnitude across the various particle types and sizes (Table 3). The lowest human-equivalent airborne concentrations are estimated for nickel (NiO and Ni2S3),5 cobalt, and gallium arsenide, which are all fine-sized particles. Slightly higher human-equivalent airborne concentrations are estimated for the ultrafine particles (carbon black, CB, and DEP), while the highest airborne concentrations are estimated for fine-sized oxides of molybdenum and titanium. The working lifetime 8-h TWA concentration estimates are within a factor of two or three for those particles with both exposure concentration and 2-year lung burden data (i.e., fine- sized TiO2 and, DEP, ultrafine CB) (Tables 3, 4).
By means of the 95 % LCL estimates of working lifetime exposure concentrations associated with 0.1 % excess risk of lung cancer, the various particles in these analyses (Tables 3, 4) can be ranked by potency as follows:
Low [>1 mg/m3 bin] (1,000–4,000 μg/m3): fine TiO2 and MoO3 (fine-sized particles)
Moderate [~0.1–1 mg/m3 bins] (~90–250 μg/m3): CB, DEP, and ultrafine TiO2 (all are ultrafine particles)
High [0.01–0.1 mg/m3 bin] (20–30 μg/m3): NiO and CoSO4 (fine particles) (CoSO4 is soluble)
Very high [0.001–0.01 mg/m3 bin] (4–5 μg/m3): Ni3S2 and GaAs (fine particles)
Both order of magnitude bins (e.g., Fig. 2) and the more specific exposure concentration estimates Tables 3 and 4 are shown for these particles. The more potent and hazardous particles are those with the lower 8-h TWA concentration estimates associated 1/1,000 excess risk of lung cancer over a 45 year working lifetime. Thus, these ultrafine (nanostructured) particles would not be the lowest health-based OEL group among these various types of respirable particles in this analysis.
Discussion of QRA example
These results show that even by means of standard risk assessment methods and rodent bioassay data, the risk-based estimates can vary depending on the models and methods used. In this example, estimates are generally within a factor of two–three based on either exposure concentration or retained lung burden (Tables 3, 4). The lung dose estimates (Table 4) would be expected to be more biologically based by accounting for the long-term clearance and retention of particles in the lungs.
Differences in the male and female rat dose–response relationships were observed for some particles. For CB and DEP, the tumor response in female rats (Nikula et al. 1995; Heinrich et al. 1995) (shown in Table 3) was higher than that in male rats (Nikula et al. 1995) based on the airborne exposure data (which resulted in ~5× higher estimates than those in Table 3). Based on the lung burden data for DEP (Table 4), the dose–response relationship was similar in male and female rats, which provided marginally adequate fit (p = 0.07) by combining these data (Nikula et al. 1995; Heinrich et al. 1995). Similar MLE (95 % LCL) estimates of 0.24 (0.22) to those in Table 4 were obtained for the female data only (Nikula et al. 1995; Heinrich et al. 1995) with better goodness of fit (p = 0.6). For NiO, the male rat exposure concentration data were also adequately fit by the multistage model (p = 0.1) and resulted in ~2× higher BMD(L) estimates compared to the female rats (p = 0.2). These data suggest that even without inclusion of the squamous cell keratinizing cystic tumors, there is some tendency for the female rats to be more sensitive to developing PSP exposure-related lung tumors. It may also be that some of the studies did not fully differentiate between the squamous cell carcinoma and the squamous cell keratinizing cystic tumors, which have been observed in higher proportions of female rats after chronic exposure to PSP (e.g., TiO2) (NIOSH 2011).
Some of these 8-h TWA concentration estimates are similar to current OELs, and others differ considerably. For example, the 95 % LCL estimates associated with the 1/1,000 excess risk of lung cancer from working lifetime exposure to DEP (0.09–0.23 mg/m3, 8-h TWA) (Tables 3, 4) are similar to the current permissible exposure limit (PEL) for DEP in mines (0.16 mg/m3) (CFR 2001). However, the human databased lung cancer risk estimates associated with DEP exposure are generally higher than those based on the rat data, suggesting that the rat model may underpredict the human working lifetime lung cancer risk from DEP (Kuempel et al. 2009).
In contrast, the worker-equivalent airborne concentration estimates for ultrafine CB (i.e., 0.10–0.12 mg/m3, 95 % LCL estimates, Tables 3 and 4) are considerably lower than the current NIOSH recommended exposure limit (REL) and the Occupational Safety and Health Administration (OSHA) PEL (3.5 mg/m3; in addition, the NIOSH REL includes 0.1 PAHs/m3) (NIOSH 2005). The estimates for ultrafine and fine TiO2 (Tables 3, 4) are similar to the NIOSH RELs (0.3 and 2.4 mg/m3, respectively) (NIOSH 2011), although different dose–response models were used to derive these estimates (i.e., a multistage model with linear low-dose extrapolation in this example for consistency across particles evaluated vs. a weighted average of three nonlinear models in NIOSH 2011). An earlier analysis of a subset of these data showed that the lung dosimetry model selection and the interspecies dose normalization assumptions also influence the quantitative risk estimates (Kuempel et al. 2006), although the relative risk rankings of particles were consistent.
The derived OELs may also depend on the response endpoint. For example, the American Conference of Governmental Hygienists’ (ACGIH) threshold limit value (TLV) for GaAs of 0.3 μg/m3 (ACGIH 2008) is based on pulmonary inflammation, which is lower than the 8-h TWA concentration of 4.3 μg/m3 (95 % LCL) estimated in this example to be associated with a 0.1 % excess risk of lung cancer (Table 3). [NIOSH and OSHA do not list OELs for GaAs]. In general, an earlier stage, more sensitive response (i.e., develops at lower exposures) would be expected to result in lower OELs than those based on later-stage, more severe responses.
Some comparative potency information for non-cancer lung responses (including pulmonary fibrosis) is provided in Table 5 for single- or multi-walled carbon nanotubes (SWCNT, MWCNT) and possible benchmark particles including ultrafine carbon black, crystalline silica, and asbestos. CNT was estimated to be from 1.5 to 10× more potent than ultrafine CB (Table 5). The factor of 10× is from a comparison of NOAELs, which may depend on the dose spacing across the studies versus the within-study comparisons for the other potency factor estimates. Since human studies have been published on pulmonary fibrosis and/or lung cancer responses from occupational exposure to these possible benchmark particles, these studies provide data to evaluate the concordance of animal- and human-based risk estimates, which can help to reduce the uncertainty in inter-species extrapolation (Kuempel et al. 2009).
Table 5.
Comparative potency of carbon nanotubes to other types of particles or fibers associated with noncancer lung responses following short-term or subchronic exposure in rats or mice
| Comparisona | Relative potency factorb | Reference |
|---|---|---|
| SWCNT/SiO2 | 2.5 | Shvedova et al. (2005) |
| SWCNT/CB-UF | 2.5 | Shvedova et al. (2005) |
| MWCNT/chrysotile asbestos | ~1 | Muller et al. (2005) |
| MWCNT/CB-UF | 1.5 | Muller et al. (2005) |
| MWCNT/CB-UF | 10c |
Pauluhn (2010)
Elder et al. (2005) |
SWCNT Single-walled carbon nanotubes; MWCNT Multi-walled carbon nanotubes; SiO2 Respirable crystalline silica; CB Carbon black; UF Ultrafine
Comparisons based on same dose and duration: Shvedova: alveolar interstitial connective tissue thickness (measure of pulmonary fibrosis); dose: 40 μg per mouse, 28 days (SWCNT and CB-UF), or 60 days (SWCNT and SiO2) after pharyngeal aspiration; Muller: amount of type 1 soluble collagen (measure of pulmonary fibrosis); dose: 2 mg per rat, 60 day after intratracheal instillation
Potency is the inverse of the critical effect levels; that is, the lower the dose associated with a critical effect, the greater the potency. Thus, potency factor is calculated: (dose associated with CNT effect/dose associated with comparison effect)−1
No observed adverse effect levels (NOAELs) in rats after subchronic (13-week) to CNT or CB, i.e., [0.1 mg/m3 (Pauluhn 2010); 1 mg/m3 (Elder et al. 2005] −1
Discussion and next steps
To deal with the large number of nanomaterials without OELs, categorization approaches have been proposed based on similar physical–chemical properties, biological mode of action, and comparative potency analyses (Kuempel et al. 2006, 2007; BSI 2007; OECD 2007). Benefits of a categorization approach to developing OELs include: more efficient use of data, reduced costs and animal use, increased sample size, greater robustness of results, and increased biological plausibility for other materials in the same mode of action category (OECD 2007).
Benchmark particles are needed to link the hazard data to the level of exposure control needed to protect workers’ health as well as to provide a standard basis for developing OELs across substances (e.g., chronic inhalation hazards). The OEL for the benchmark particle would provide information about the health risk of a nanomaterial in the same mode of action category, either estimated directly or adjusted by the physical and chemical properties that modify the potency (e.g., increased particle surface area, surface reactivity, or solubility). Characterizing the distribution of effect levels is an important component of developing MOA categories and could also be used to estimate an OEL for a new member to the group (e.g., Dolan et al. 2005). If the variability in potency within an MOA category is great (e.g., overlaps the exposure control options that would impact decision-making), this may indicate a biological or practical need to split the category into subcategories. Crystalline silica is an example of a substance that has qualitatively the same mode of action as other poorly soluble particles (i.e., generation of reactive oxygen species resulting in chronic pulmonary inflammation), but it is much more potent and causes pulmonary inflammation at a much lower dose than lower toxicity particles (e.g., titanium dioxide) (Maynard and Kuempel 2005; NIOSH 2011). Thus, crystalline silica may be a benchmark particle for the MOA category of poorly soluble particle with highly reactive surfaces. On the other hand, ultrafine TiO2, DEP, and CB are all PSP that give similar risk estimates for lung cancer in the rat model; that is, the working lifetime exposure concentrations associated with the 1/1,000 excess risk of lung cancer for these substances all fall within a fairly narrow range (~0.1–0.2 mg/m3 based on model estimates in Table 4). To be most health protective, OELs would be based on earlier stage, lower severity effects (e.g., inflammation) if the earlier effect is on the causal pathway between exposure and a chronic adverse health effect. In that case, prevention of the earlier effect would also be expected to reduce the probability of a more severe chronic effect.
Categorical OELs could be based on qualitative and/or quantitative comparisons with benchmark particles. Some of the MOAs and dose metrics for inhaled particles and fibers are likely to apply to nanomaterials. For example, the total surface area dose has been associated with adverse lung responses for various types and sizes of poorly soluble particles (Oberdörster and Yu 1990; Oberdörster et al. 1994, 2005b; Driscoll 1996; Tran et al. 2000) as well as various types of carbon nanotubes (Nakanishi 2011). In other cases, the reactivity of the surface (e.g., generation of reactive oxygen species) needs to be considered (Duffin et al. 2007; Rushton et al. 2010). Particle mass or volume dose has also been associated with the rat lung responses to poorly soluble low toxicity particles (Morrow 1988; Muhle et al. 1991) including MWCNT (Pauluhn 2011). The number concentration of specific sized structures has been associated with the inflammatory effects of nanotubes and fibers (Donaldson et al. 2010). Finally, the solubility of nanoparticles may be increased compared to the same mass of larger particles due to the increased available surface of nanoparticles. Even poorly soluble particles may be sufficiently soluble in the acidic fluid inside lung alveolar macrophages to trigger a biological effect as has been shown for immune responses associated with chronic beryllium disease (Stefaniak et al. 2011). The range of possible dose metrics illustrates that toxicity studies need to provide sufficient particle characterization to convert among the various dose metrics, which would facilitate hypothesis testing and identification of the most predictive dose metric.
Not all poorly soluble inhaled particles may cause lung cancer in rats or mice through an inflammation mode of action, but they may be genotoxic by other mechanisms including direct DNA damage (Melnick et al. 2003). Some nanomaterials have been shown to disrupt normal cell processes including cell division (mitosis) resulting in genotoxicity including aneuploidy (abnormal chromosome number) (Sargent et al. 2009 2011). Some CNT can also cause rapid development of pulmonary fibrosis by a different mechanism (i.e., acting as a lung basement membrane, encouraging lung fibroblast growth) (Wang et al. 2010). This suggests that standard toxicity tests based on inflammation may not detect the fibrotic hazard of CNTs. Alternative modes of action and relevant assays should also be evaluated to identify any critical effects beyond the lungs.
The array of candidate benchmark particles discussed in this paper could be expanded as additional dose–response data become available particularly in short-term or subchronic studies. For example, the OECD (2010a, b) list of nanomaterials currently undergoing standardized toxicity testing could be evaluated by means of comparative potency analyses when relevant benchmark particles are used as controls. As more systematic data are available, further development of QSAR-based predictive models may also be feasible to improve the throughput for hazard and risk estimation and exposure control decisions.
As a next step, we are compiling data of possible benchmark particles within these four (or more) MOA categories (Table 1). The analysis steps will include identification of the health endpoint(s) of concern, selection of relevant toxicology assay data, and evaluation of dose–response relationships and effect levels for a range of nanoparticles and benchmark particles within MOA categories. A framework such as this provides a basis to develop an initial matrix of nanomaterial hazard- and risk-based exposure control bins, and to guide the testing needs to evaluate whether the initial estimates are reasonable.
As illustrated in this paper, even a relatively simple case involving “gold standard” animal bioassay data (chronic inhalation exposure of various types of fine and ultrafine particles) raises a number of questions concerning the implementation of standard response endpoints and risk assessment methods. One criterion for meaningful differences in the hazard/risk groups may be the extent to which different options are available for exposure control to those levels. Hazard-and risk-based categorical OELs are consistent with the concept of exposure control banding approaches and are useful in risk management decision-making (Fig. 2).
Benchmark particles could provide a quantitative link to the current hazard and control banding schemes that have qualitative descriptors of severity and likelihood of adverse effects (e.g., low to high severity, and unlikely to probable) (Maynard 2007; Schulte et al. 2008; Zalk et al. 2009; ANSES 2010). Exposure control decisions are typically based on exposure frequency, amount used, and dustiness of material as well as the hazardous properties of the material. Benchmark particles could also provide risk estimates for calibration and validation of other nanomaterials’ risk analysis frameworks such as those based on multi-criteria decision analysis (MCDA) methods (Linkov et al. 2007, 2009; Grieger et al. 2012).
In any occupational exposure control strategy, including control banding, worker health protection is the primary concern. Thus, the upper limit of the bands should not be interpreted as the maximum permissible average exposure. Rather, exposures should be well controlled within that band based on the demonstrated performance of the applicable engineering control systems and statistical confidence in the sampling results including accounting for variability in exposures. The target for designing a control system should be at the low end of the band, recognizing that there may be excursions, so that exposures are controlled within the band. If appropriate controls are installed and used properly, then exposure monitoring could be performed periodically to verify that the system is functioning as designed (including using surrogate substances to check for release if a nanomaterial-specific method is not available). The technical feasibility of measuring or controlling exposures is a continuing challenge. Jones and Nicas (2006) reported that the margins of safety (MOS) (i.e., ratios of worker exposures/animal effect levels) were too low (<1–100) using the recommended exposure controls of COSSH Essentials and ILO control banding toolkits, whereas higher MOS factors would be more health protective given the uncertainty in exposure control estimates based on animal data. Benchmark particles could also be used for such evaluations.
In summary, a hazard- and risk-based categorical approach would have several benefits including:
Employing standard tools and methods to develop initial OELs and exposure control bands (and to reassess as new data become available);
Identifying the minimum data standards for harmonization across studies;
Allowing for development of a database with standard parameters for use in pooled or comparative analyses;
Providing a framework for testing and refining hypotheses;
Facilitating the systematic evaluation to select—or design—safer nanomaterials.
A number of challenges also exist in developing a categorical approach to evaluating the health hazards and risks across the various nanomaterials. In particular, further development of predictive models is needed, including comparison of short-term to long-term in vivo responses, and multivariate models with parameters for the various physical–chemical properties. Some advances have been made in developing models and methods for in vitro to in vivo comparison of toxicity and potency across a range of particle types and sizes including nanoparticles (Donaldson et al. 2008; Rushton et al. 2010). Standard sets of particle descriptors, dose metrics, and response parameters (Tables 1 and 2) are also needed in order to compare mode of action and dose–response relationships across studies.
Conclusions
Given the many different types of nanoparticles, comprehensive data for quantitative risk assessment on each specific type of nanoparticles are not likely to be feasible. Toxicological studies suggest that current OELs developed for larger respirable particles may not be adequate to protect workers exposed to some nanoparticles over a working lifetime. As illustrated here, existing scientific literature on ultrafine and fine particles can be used to estimate workplace exposure concentrations and provide a set of possible benchmark or reference particles. Linkages between short-term and chronic responses and in vitro to short-term in vivo responses are needed to increase testing efficiency of comparative toxicity evaluations. Benchmark particles may also be used to calibrate and validate the various hazard and control banding schemes.
Acknowledgments
We would like to thank Mr. Randall Smith for helpful discussions concerning statistical aspects of this paper.
Footnotes
A benchmark dose (BMD) is the dose associated with a specified increase (e.g., 10 %) in the probability of a given response known as the benchmark response (BMR) (Crump 1984). The BMD is a maximum likelihood estimate, and the BMDL is the 95 % LCL of the BMD.
A critical effect level of 0.1 % excess risk of lung cancer is estimated in this example by linear extrapolation of the 10 % BMD and BMDL estimates. The BMD(L) estimates are based on lung burden at the end of the two-yr exposure if those data were available or on airborne exposure concentration otherwise.
For those particles with 2-yr rat lung burden data, the rat critical lung dose (as particle mass or surface area dose per g lung) was converted to mg/lung to use as the target lung dose in a human lung dosimetry model (assuming average worker lung weight of 1000 g) (ICRP 1975) (CIIT and RIVM 2006). For those particles without 2-yr rat lung burden data, the deposited daily dose (mg/d) was calculated by accounting for the species differences in ventilation rates and alveolar deposition fractions (Kuempel et al. 2006).
Human-equivalent 45-yr working lifetime concentrations were estimated in a human lung dosimetry model (CIIT and RIVM 2006) for those particles with rat lung burden data. For those particles without rat lung burden data, the human 8-hr TWA concentrations were estimated by adjusting for the species differences in the alveolar surface area (102 m2 human/0.4 m2 rat), particle size-specific deposition fraction, and ventilation (assuming reference worker rate of 9.6 m3/8-hr d) (ICRP 1994) (Kuempel et al. 2006).
The greater tumor potency of Ni3S2 compared to NiO may be due to oxidative DNA damage (8-OH-dG), which was observed in cultured cells treated with Ni3S2, but not in cells treated with NiO or NiSO4 (Kawanishi et al. 2002).
This article is part of the Topical Collection on Nanotechnology, Occupational and Environmental Health
This article is based on a presentation at the 5th Int’l NanOEH, Boston, MA, August 9–12, 2011.
Disclaimer The findings and conclusion in this paper are those of the authors and do not necessarily represent the view of the National Institute for Occupational Safety and Health.
Contributor Information
E. D. Kuempel, Email: ekuempel@cdc.gov, Education and Information Division, Nanotechnology Research Center (NTRC), National Institute for Occupational Safety and Health (NIOSH), Cincinnati, OH, USA
V. Castranova, Health Effects Laboratory Division and NTRC, NIOSH, Morgantown, WV, USA
C. L. Geraci, Education and Information Division, NTRC, NIOSH, Cincinnati, OH, USA
P. A. Schulte, Education and Information Division, NTRC, NIOSH, Cincinnati, OH, USA
References
- ACGIH. Threshold limit values for chemical substances and physical agents and biological exposure indices. American Conference of Governmental Industrial Hygienists; Cincinnati: 2008. Gallium arsenide; p. 12. [Google Scholar]
- Ader AW, Farris JP, Ku RH. Occupational health categorization and compound handling practice systems: roots, application and future. Chem Health Safety. 2005 Jul-Aug;:20–24. [Google Scholar]
- ANSES. Development of a specific control banding tool for nanomaterials. Agence nationale de sécurité sanitarie. Maisons-Alfort Cedex 2010 [Google Scholar]
- Attfield MD, Schleiff PL, Lubin JH, Blair A, Stewart PA, Vermeulen R, Coble JB, Silverman DT. The diesel exhaust in miners study: a cohort mortality study with emphasis on lung cancer. J Natl Cancer Inst. 2012;104(11):869–883. doi: 10.1093/jnci/djs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BSI. Nanotechnologies, Part 2. PD 6699-2:2007: guide to safe handling and disposal of manufactured nanomaterials. British Standards Institution; London: 2007. [Google Scholar]
- Castranova V. From coal mine dust to quartz: mechanisms of pulmonary pathogenicity. Inhal Toxicol. 2000;3:7–14. doi: 10.1080/08958378.2000.11463226. [DOI] [PubMed] [Google Scholar]
- Castranova V. Overview of current toxicological knowledge of engineered nanoparticles. JOEM. 2011;53(6 Suppl):S14–S17. doi: 10.1097/JOM.0b013e31821b1e5a. [DOI] [PubMed] [Google Scholar]
- CFR. Code of federal regulations: 30 CFR Section 57.5060. US Government Printing Office, Office of the Federal Register; Washington, DC: 2001. Limit on exposure to diesel particulate matter, Mine Safety and Health Administration. [Google Scholar]
- CIIT, RIVM. Multiple-path particle dosimetry (MPPD V 2.0): a model for human and rat airway particle dosimetry. Research Triangle Park, NC, USA: Centers for Health Research (CIIT) and the Netherlands: National Institute for Public Health and the Environment (RIVM); 2006. [Google Scholar]
- Crump KS. A new method for determining allowable daily intakes. Fund Appl Toxicol. 1984;4:854–871. doi: 10.1016/0272-0590(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Dolan DG, Naumann BD, Sargent EV, Maier A, Dourson M. Application of the threshold of toxicological concern concept to pharmaceutical manufacturing operations. Regul Toxicol Pharmacol. 2005;43:1–9. doi: 10.1016/j.yrtph.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Borm PJ, Oberdörster G, Pinkerton KE, Stone V, Tran CL. Concordance between in vitro and in vivo dosimetry in the proinflammatory effects of low-toxicity, low-solubility particles: the key role of the proximal alveolar region. Inhal Toxicol. 2008;20:53–62. doi: 10.1080/08958370701758742. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;22(7):5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll KE. Role of inflammation in the development of rat lung tumors in response to chronic particle exposure. In: Mauderly JL, McCunney RJ, editors. Particle overload in the rat lung and lung cancer: implications for human risk assessment. Taylor & Francis; Philadelphia: 1996. pp. 139–152. [Google Scholar]
- Duffin R, Tran L, Brown D, Stone V, Donaldson K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal Toxicol. 2007;19(10):849–856. doi: 10.1080/08958370701479323. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Finkelstein JN, Driscoll KE, Harkema J, Oberdörster G. Effects of subchronically inhaled carbon black in three species. I. Retention kinetics, lung inflammation, and histopathology. Toxicol Sci. 2005;88(2):614–629. doi: 10.1093/toxsci/kfi327. [DOI] [PubMed] [Google Scholar]
- Grieger KD, Linkov I, Hansen SF, Baun A. Environmental risk analysis for nanomaterials: review and evaluation of frameworks. Nanotoxicol. 2012;6(2):196–212. doi: 10.3109/17435390.2011.569095. [DOI] [PubMed] [Google Scholar]
- Heinrich U, Fuhst R, Rittinghausen S, Creutzenberg O, Bellmann B, Koch W, Levsen K. Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust, carbon black, and titanium dioxide. Inhal Toxicol. 1995;7:533–556. [Google Scholar]
- Hewett P, Logan P, Mulhausen J, Ramachandran G, Banerjee S. Rating exposure control using Bayesian decision analysis. J Occup Environ Hyg. 2006;3:568–581. doi: 10.1080/15459620600914641. [DOI] [PubMed] [Google Scholar]
- ICRP. Report of the task group on reference man: a report prepared by a task group of committee 2. International Commission on Radiological Protection; Pergamon; Elmsford: 1975. [Google Scholar]
- ICRP. International commission on radiological. Elsevier; Oxford: 1994. Human respiratory tract model for radiological protection. protection publication no. 66. [PubMed] [Google Scholar]
- Invernizzi N. Nanotechnology between the lab and the shop floor: what are the effects on labor? J Nanopart Res. 2011 doi: 10.1007/s11051-011-03033-z. [DOI] [Google Scholar]
- Jones RM, Nicas M. Margins of safety provided by COSHH essentials and the ILO chemcial control toolkit. Ann Occup Hyg. 2006;50(2):149–156. doi: 10.1093/annhyg/mei054. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Tran CL, Castranova V, Bailer AJ. Lung dosimetry and risk assessment of nanoparticles: evaluating and extending current models in rats and humans. Inhal Toxicol. 2006;18(10):717–724. doi: 10.1080/08958370600747887. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Geraci CL, Schulte PA. Risk assessment approaches and research needs for nanoparticles: an examination of data and information from current studies. In: Simeonova P, Opopol N, Luster M, editors. Nanotechnology: toxicological issues and environmental safety; Proceedings of the NATO Advanced Research Workshop on Nanotechnology: Toxicological Issues and Environmental Safey; Varna, Bulgaria. August 12–17, 2006; New York: Springer; 2007. pp. 119–145. [Google Scholar]
- Kuempel ED, Smith RJ, Dankovic DA, Stayner LT. Rat-and human-based risk estimates of lung cancer from occupational exposure to poorly-soluble particles: a quantitative evaluation. J Phys Conf Series. 2009;151:012011. [Google Scholar]
- Lee KP, Trochimowicz HJ, Reinhardt CF. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for 2 years. Toxicol Appl Pharmacol. 1985;79:179–192. doi: 10.1016/0041-008x(85)90339-4. [DOI] [PubMed] [Google Scholar]
- Linkov I, Satterstrom FK, Steevens J, Ferguson E, Pleus RC. Multi-criteria decision analysis and environmental risk assessment for nanomaterials. J Nanopart Res. 2007;9(4):543–554. [Google Scholar]
- Linkov I, Steevens J, Adlakha-Hutcheon F, Bennett E, Chappell M, Colvin V, Davis M, Davis T, Elder A, Hansen SF, Hakkinen PB, Hussain SM, Karkan D, Korenstein R, Lynch I, Metcalfe C, Ramadan AB, Satterstrom FK. Emerging methods and tools for environmental risk assessment, decision-making, and policy for nanomaterials: summary of NATO advanced research workshop. J Nanopart Res. 2009;11:513–527. doi: 10.1007/s11051-008-9514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauderly JL. Relevance of particle-induced rat lung tumors for assessing lung carcinogenic hazard and human lung cancer risk. Environ Health Perspect. 1997;105(Suppl 5):1337–1346. doi: 10.1289/ehp.97105s51337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard AD. Nanotechnology: the next big thing, or much ado about nothing? Ann Occup Hyg. 2007;51(1):1–12. doi: 10.1093/annhyg/mel071. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Kuempel E. Airborne nanostructured particles and occupational health. J Nanoparticle Res. 2005;7(6):587–614. [Google Scholar]
- Melnick RL, Bucher JR, Roycroft JH, Hailey JR, Huff J. Carcinogenic and toxic effects of inhaled, nonfibrous, poorly soluble particulates in rats and mice contradict threshold lung cancer hypotheses that are dependent on chronic pulmonary inflammation. Eur J Oncol. 2003;8(3):177–186. [Google Scholar]
- Morrow PE. Possible mechanisms to explain dust overloading of the lungs. Fund Appl Toxicol. 1988;10(3):369–384. doi: 10.1016/0272-0590(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Muhle H, Bellmann B, Creutzenberg O, Dasenbrock C, Ernst H, Kilpper R, MacKenzie JC, Morrow P, Mohr U, Takenaka S, Mermelstein R. Pulmonary response to toner upon chronic inhalation exposure in rats. Fund Appl Toxicol. 1991;17:280–299. doi: 10.1016/0272-0590(91)90219-t. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Murray AR, Kisin ER, Tkach AV, Yanamala N, Mercer R, Young S-H, Fadeel B, Kagan VE, Shvedova AA. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Particle Fibre Toxicol. 2012;9:10. doi: 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi J. New Energy and Industrial Technology Development Organization (NEDO) project (P06041) “Research and Development of Nanoparticles Characterization Methods”. National Institute of Advanced industrial Science and Technology (AIST); 2011. Risk Assessment of Manufactured Nanomaterials: Carbon Nanotubes (CNT). Final report issued on August 12, 2011. Available at http://www.aistriss.jp/main/?ml_lang=en. [Google Scholar]
- Naumann BD, Sargent EV, Starkman BS, Fraser WJ, Becker GT, Kirk GD. Performance-based exposure control limits for pharmaceutical active ingredients. Am Ind Hyg Assoc J. 1996;57:33–42. doi: 10.1080/15428119691015197. [DOI] [PubMed] [Google Scholar]
- Nikula KJ, Snipes MB, Barr EB, Griffith WC, Henderson RF, Mauderly JL. Comparative pulmonary toxicities and carcinogenicities of chronically inhaled diesel exhaust and carbon black in F344 rats. Fundam Appl Toxicol. 1995;25:80–94. doi: 10.1093/toxsci/25.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. NIOSH pocket guide to chemical hazards and other databases. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati: 2005. DHHS (NIOSH) Publication No. 2005–149. [Google Scholar]
- NIOSH. Strategic plan for NIOSH nanotechnology research and guidance filling the knowledge gaps. U.S. Department of Health and Human Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati: 2010a. DHHS (NIOSH) Publication No. 2010–105. [Google Scholar]
- NIOSH. Draft for public comment. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati: 2010b. Current intelligence bulletin: occupational exposure to carbon nanotubes and nanofibers. NIOSH Docket Number: NIOSH 161-A. [Google Scholar]
- NIOSH. Current intelligence bulletin 63: occupational exposure to titanium dioxide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati: 2011. NIOSH (DHHS) Publication No. 2011–160. [Google Scholar]
- NRC. Committee on improving risk analysis approaches used by the U.S. EPA, Board on Environmental Studies and Toxicology, Division on Earth and Life Studies, National Research Council of the National Academies. 2009. Science and decisions: advancing risk assessment. [Google Scholar]
- NTP (1996–2000) National Toxicology Program, Technical Report Series: Toxicology and carcinogenesis in F344/N rats and B6C3F1 mice (inhalation studies). US Department of Health and Human Services, National Institutes of Health (NIH), Research Triangle Park, NC. Reports referenced include: Cobalt sulfate heptahydrate (NIH 1998, Pub. No. 98–3961, NTP TR 471); gallium arsenide (NIH 2000, Pub. No. 00-3951, NTP TR 492); nickel oxide (NIH 1996, Pub. No. 96-3367, NTP TR 451); nickel subsulfide (NIH 1996, Pub. No. 96-3369, NTP TR 453); and molybdenum trioxide (NIH 1997, Pub. No. 97-3378, NTP TR 462)
- Oberdörster G, Yu CP. The carcinogenic potential of inhaled diesel exhaust: a particle effect? J Aerosol Sci. 1990;21(Suppl 1):S397–S401. [Google Scholar]
- Oberdörster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(Suppl 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Report of the international life sciences institute research foundation/risk science institute nano-material toxicity screening working group. Part Fibre Toxicol. 2005a;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nano-toxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005b;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. Guidance on grouping of chemicals. Series on testing and Assessment, No. 80. ENV/JM/MONO(2007)28. Organization for Economic Cooperation and Development, Environmental Health and Safety Publications; 2007. [Google Scholar]
- OECD. List of manufactured nanomaterials and list of endpoints for phase one of the sponsorship programme for the testing of manufactured nanomaterials: revision. No. 27. Organization for Economic Cooperation and Development, Series on the Safety of Manufactured Nanomaterials; 2010a. ENV/JM/MONO(2010)46. [Google Scholar]
- OECD. Guidance manual for the testing of manufactured nanomaterials: OECD’s sponsorship programme. 2010b. first revision. ENV/JM/MONO(2009)20/REV. [Google Scholar]
- Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol Sci. 2010;113(1):226–242. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Poorly soluble particulates: searching for a unifying denominator of nanoparticles and fine particles for DNEL estimation. Toxicology. 2011;279(1–3):176–188. doi: 10.1016/j.tox.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Rom WN, Markowitz S. Environmental and occupational medicine. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, Oberdorster G. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J Toxicol Environ Health A. 2010;73:445–461. doi: 10.1080/15287390903489422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML, Lowry DT, Murray AR, Kisin ER, Friend S, McKinstry KT, Battelli L, Reynolds SH. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen. 2009;50(8):708–717. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Hubbs AF, Young SH, Kashon ML, Dinu CZ, Salisbury JL, Benkovic SA, Lowry DT, Murray AR, Kisin ER, Siegrist KJ, Battelli L, Mastovich J, Sturgeon JL, Bunker KL, Shvedova AA, Reynolds SH. Single-walled carbon nanotube-induced mitotic disruption. Mutat Res. 2011b;745(1–2):28–37. doi: 10.1016/j.mrgentox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeny RS, Margosches E. Evaluating comparative potencies: developing approaches to risk assessment of chemical mixtures. Toxicol Indust Health. 1989;5(5):825–837. doi: 10.1177/074823378900500518. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Salamanca-Buentello F. Ethical and scientific issues of nanotechnology in the workplace. Environ Health Perspect. 2007;115(1):5–12. doi: 10.1289/ehp.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte P, Geraci C, Zumwalde R, Hoover M, Kuempel E. Occupational risk management of engineered nanoparticles. JOEH. 2008;5:239–249. doi: 10.1080/15459620801907840. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Murashov V, Zumwalde R, Kuempel ED, Geraci CL. Occupational exposure limits for nanomaterials: state of the art. J Nanopart Res. 2010;12:1971–1987. [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Sobels FH. Approaches to assessing genetic risks from exposure to chemicals. Environ Health Perspect. 1993;101(Suppl 3):327–332. doi: 10.1289/ehp.93101s3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayner L, Kuempel E, Gilbert S, Hein M, Dement J. An epidemiological study of the role of chrysotile asbestos fibre dimensions in determining respiratory disease risk in exposed workers. Occup Environ Med. 2008;65(9):613–619. doi: 10.1136/oem.2007.035584. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, Virji MA, Day GA. Dissolution of beryllium in artificial lung alveolar macrophage phagolysosomal fluid. Chemosphere. 2011;83(8):1181–1187. doi: 10.1016/j.chemosphere.2010.12.088. [DOI] [PubMed] [Google Scholar]
- Sutter JR. Molecular and cellular approaches to extrapolation for risk assessment. Environ Health Perspect. 1995;103:386–389. doi: 10.1289/ehp.95103386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervonen T, Linkov I, Figueira FR, Steevens J, Chappell M, Merad M. Risk-based classification system of nanomaterials. J Nanopart Res. 2009;11:757–766. [Google Scholar]
- Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K. Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance. Inhal Toxicol. 2000;12(12):1113–1126. doi: 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Environmental criteria and assessment office, office of health and environmental assessment, office of research and development. U.S. Environmental Protection Agency; Cincinnati: 1987. Aug, Recommendations for and documentation of biological values for use in risk assessment. [Google Scholar]
- U.S. EPA. Benchmark dose software, version 2.1.2. U.S. Environmental Protection Agency, National Center for Environmental Assessment; Washington: 2010. [Google Scholar]
- U.S. Supreme Court. Industrial Union Department, AFL-CIO v. American Petroleum Institute et al., Case Nos. 78–911, 78–1036. Supreme Court Reporter. 1980;100:2844–2905. [Google Scholar]
- Wang L, Mercer RR, Rojanasakul Y, Qiu A, Lu Y, Scabilloni JF, Wu N, Castranova V. Direct fibrogenic effects of dispersed single-walled carbon nanotubes on human lung fibroblasts. J Toxicol Environ Health Part A. 2010;73(5):410–422. doi: 10.1080/15287390903486550. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Zalk DM, Nelson DI. History and evolution of control banding: review. J Occup Environ Hyg. 2008;5:330–346. doi: 10.1080/15459620801997916. [DOI] [PubMed] [Google Scholar]
- Zalk DM, Paik SY, Swuste P. Evaluating the control banding nanotool: a qualitative risk assessment method for controlling nanoparticle exposures. J Nanopart Res. 2009;11:1685–1704. [Google Scholar]
- Zhang H, Ji Z, Xia T, Meng H, Low-Kam C, Liu R, Pokhrel S, Lin S, Wang X, Liao YP, Wang M, Li L, Rallo R, Damoiseaux R, Telesca D, Mädler L, Cohen Y, Zink JI, Nel AE. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano. 2012;6(5):4349–4368. doi: 10.1021/nn3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]