Abstract
Recognition of ischemic heart disease (IHD) is often delayed or deferred in women. Thus, many at risk for adverse outcomes are not provided specific diagnostic, preventive, and/or treatment strategies. This lack of recognition is related to sex-specific IHD pathophysiology that differs from traditional models using data from men with flow-limiting coronary artery disease (CAD) obstructions. Symptomatic women are less likely to have obstructive CAD than men with similar symptoms, and tend to have coronary microvascular dysfunction, plaque erosion, and thrombus formation. Emerging data document that more extensive, nonobstructive CAD involvement, hypertension, and diabetes are associated with major adverse events similar to those with obstructive CAD. A central emerging paradigm is the concept of nonobstructive CAD as a cause of IHD and related adverse outcomes among women. This position paper summarizes currently available knowledge and gaps in that knowledge, and recommends management options that could be useful until additional evidence emerges.
Keywords: Adverse outcomes, Ischemia, Sex-specific pathophysiology
Introduction
Recognition of ischemic heart disease (IHD) is often delayed or deferred in women. Consequently, many at risk for related adverse outcomes are not provided specific diagnostic, preventive, and/or treatment strategies. In part, this lack of recognition is related to sex-specific cardiovascular disease (CVD) pathophysiology in women that differs from the traditional male-pattern model. The latter model is based largely upon studies where the majority of subjects were men with flow-limiting atherosclerotic coronary artery disease (CAD). The current state centers on the emerging paradigm of nonobstructive CAD relationships to myocardial ischemia and related adverse outcomes among women. Women are less likely to have flow-limiting obstructive CAD compared with men presenting with similar symptoms (1). This nonobstructive CAD pattern and the tendency among women to have plaque erosion with subsequent thrombus formation, along with CMD, are not well recognized. Importantly, data are emerging to show that more extensive nonobstructive CAD involvement is associated with a rate of major adverse cardiovascular events (MACE) that may approximate that of obstructive CAD (2). However, there are many limitations to our understanding of nonobstructive CAD, a consequence of numerous gaps in current knowledge.
This position paper summarizes the available knowledge and important gaps in knowledge, and recommends management options that could be useful for the clinician until additional evidence becomes available. We expect this report to raise awareness of clinical presentations, adverse outcomes, diagnostic strategies, and therapeutic options, and to help guide efforts to further improve outcomes among patients with acute and chronic ischemia syndromes (e.g., IHD) and nonobstructive CAD, who are predominantly women.
The Problem of Nonobstructive CAD: Definition, Prevalence, and Pathophysiological Implications for Management
Nonobstructive CAD may be considered in patients with symptoms/signs of IHD where atherosclerotic epicardial CAD does not limit coronary blood flow, but other processes may adversely influence myocardial supply/demand relationship. Nonobstructive CAD is highly prevalent in women, including those presenting with typical symptoms of IHD (e.g., angina).
Historical Considerations and Terminology
Although it has long been recognized that selected conditions other than obstructive CAD may cause ischemia and related symptoms and signs, the prevailing opinion was that these situations were relatively infrequent and had no clinical implications beyond those associated with the selected condition (e.g., severe aortic valve stenosis, hypertrophic cardiomyopathy, pulmonary hypertension). However, several factors have contributed to a change in that position.
For example, approximately 20% to 30% of angina patients with technically successful coronary revascularization, by either coronary bypass graft or percutaneous coronary intervention (PCI), have persistent signs and/or symptoms of IHD (3,4). Explanations for ischemia among these patients include incomplete revascularization, unrecognized remaining obstructive disease, coronary spasm, and/or coronary microvascular dysfunction (CMD). Next, a large cohort of patients with chronic angina and objective evidence of ischemia at stress testing have no demonstrable obstructive CAD by angiography (5,6). This was initially explained as false positive findings for ischemia, despite the documentation of ischemia by methods ranging from the electrocardiogram (6), positron emission tomographic (PET) imaging (7), contrast cardiac magnetic resonance imaging (cMRI) (8), and cardiomyocyte metabolism (9-11). Then, ischemia with nonobstructive CAD was viewed as a benign form because these patients generally had normal left ventricular (LV) systolic function and good short-term outcomes. However, patchy areas of ischemia in the subendocardium and/or midwall of the LV are often not associated with major reductions in systolic function (7). Additionally, issues such as survival bias, high rates of variability in quality and/or interpretation of angiograms related to lack of core labs, and incomplete follow-up limit much of this past outcomes literature. Indeed, many well-designed, more recent cohorts document a heightened rate of adverse outcomes among patients with symptoms and signs of ischemia and no obstructive CAD versus similar patients without symptoms and signs of ischemia (1,12-25). Importantly, multiple cohorts link other mechanisms for ischemia, such as coronary endothelial and microvascular dysfunction, and risk for adverse outcomes among symptomatic patients with nonobstructive CAD (2,19,26-28).
Definitions for nonobstructive CAD vary in the literature, in part from variable methods used to interpret coronary angiograms (individual operator or group consensus readings using simple visual estimation, differing methods to quantify narrowings, dedicated core lab, and so on). Experience from the Women's Ischemic Syndrome Evaluation (WISE) angiographic core lab, using standardized qualitative and quantitative methods, indicates that essentially any observed luminal irregularity, measured quantitatively, yields at least a 20% diameter reduction versus the most completely normal-appearing reference segment in the same part of the coronary artery under evaluation (23). In addition, as a result of vessel tapering, it is common to obtain narrowing ranging from 0% to 19% when measuring such “normal” segments. Thus, it follows that a patient with no apparent CAD or normal-appearing coronary arteries may be defined as having normal-appearing coronary arteries and, when measured, no stenosis ≥20% diameter narrowing in any epicardial coronary artery. Nonobstructive CAD may be defined as at least 1 stenosis ≥20 but <50%, whereas obstructive (single-, double-, or triple-vessel) CAD may be defined as at least 1 stenosis ≥50%.
Recently, the Veterans Administration Cart National Registry (16) defined nonobstructive CAD as any stenosis ≥20%, but <70% narrowing, in any epicardial artery or ≥20%, but <50%, in the left main artery. Normal coronary anatomy was defined as <20% stenosis in all coronary arteries, consistent with the definition for normal used in the WISE.
Pathophysiology
From the pathophysiology standpoint, a number of different terms have been used to describe these patients: nonobstructive CAD; IHD patients without obstructive CAD; open artery IHD; myocardial infarction (MI) with no coronary artery obstruction; CMD; microvascular angina; and cardiac syndrome X. The latter term has been unfortunate, as there is no consensus in the literature on its definition, and there is now sufficient knowledge to sunset this terminology (28,29).
Coronary intravascular ultrasound (IVUS) and, to some extent, cardiac computed tomography angiography (CTA) studies indicate that essentially all patients (within the limitations of sampling by these techniques) with suspected IHD reported to date with “normal-appearing coronary arteries by angiography” have some evidence for atherosclerosis (plaque). Thus, it seems most appropriate to endorse the descriptive term nonobstructive CAD in the absence of another cause for the syndrome.
Another concern is exclusion of concealed obstructive CAD due to diffuse epicardial coronary artery narrowing. The only study (19) addressing this in a prospective, systematic approach found that 5% of cases (7 of 139) had a fractional flow reserve ≤0.80 among patients otherwise thought to have normal or nonobstructive CAD by quantitative angiography. Interestingly, most of the cases (4 of 7) had other, coexisting reasons for ischemia (myocardial bridging and/or severe endothelial dysfunction), as all 7 had some evidence for endothelial dysfunction. So, it seems reasonable to conclude that diffuse or concealed obstructive CAD alone rarely explains this syndrome of symptoms/signs of ischemia.
Approximately 60% to 70% of women and 30% of men undergoing coronary angiography to further evaluate suspected clinically stable IHD have nonobstructive CAD (1). Thus, this nonobstructive pattern is common, but more highly prevalent among women. This is despite the fact that symptomatic women are generally 10 to 15 years older than symptomatic men when they present, and often have greater density (number) and magnitude of risk factors (hypertension, diabetes, smoking, dyslipidemia). In the presence of nonobstructive CAD, microvascular and/or endothelial dysfunction, and many other processes (e.g., epicardial and microvascular spasm, myocardial bridging, conduit vessel stiffening) may contribute to myocardial ischemia (30)(Table 1). These features appear to be much more frequent in women than in men. The presence of coronary microvascular and/or endothelial coronary dysfunction predicts adverse outcomes (26,31), although specific mechanisms responsible for these mortality/morbidity outcomes are not fully understood. Our limited understanding of these nonobstructive disease patterns is particularly relevant for young women, who have an unfavorable prognosis compared with men of the same age (32,33). Clearly, nonobstructive CAD requires better recognition and investigation if we are to develop effective prevention, diagnosis, and treatment approaches for this population, which includes large numbers of women.
Table 1. Proposed Mechanisms for Stable Ischemic Heart Disease Syndromes.
| Type | Location of Defect | Potential Mechanisms |
|---|---|---|
| Vascular | ||
| Coronary macrovessels | Flow-limiting stenosis (e.g., atherosclerosis) Nonflow-limiting stenosis (e.g., atherosclerosis) Endothelial dysfunction (e.g., athero RFs, viruses) VSM dysfunction/spasm (e.g., athero RFs, ANS, drugs, viruses) Thrombotic (e.g., hypercoagulation, enhanced platelet act, plaque rupture/erosion/fissuring) Embolic (e.g., AF, prosthetic valve, LV thrombus, SBE) Inflammation (atherosclerosis, transplant, col dis [e.g., SLE, PAN, RA]) Congenital (muscle bridge, aberrant origin) Dissection (e.g., pregnancy, chest trauma, Marfan) Misc. |
|
| Coronary microvessels | Microvascular dysfunction (VSM dysfunction/spasm (e.g., athero RFs, ANS, viruses, drugs) Endothelial dysfunction (e.g., athero RFs, viruses) Endothelial cell-x cell “crosstalk” (e.g., EC-VSM, mononuclear cell, cardiomyocye) Microparticle occlusion (e.g., atheroma, cells, platelet microaggregation, cholesterol) Thrombotic (e.g., hypercoagulable state, platelet activation, plaque rupture/erosion) Microembolic (e.g., atheroma, AF, prosthetic valve, SBE) Inflammation (athero, transplant, col dis [e.g., SLE, PAN, RA]) Capillary insufficiency (e.g., LVH) Misc. |
|
| Other vessels | ||
| Capacitance | Increased Ao-F stiffness (e.g., aging, calcification, hypertension, CRI) | |
| Nonvascular | Cardiomyocyte | |
| Transcellular | Oxygen transport (reduced diffusion [e.g., infiltrate, amyloid) Energy substrate (e.g., depleted FFA, glucose) ? |
|
| Intracellular | Oxygen transport (e.g., defective myoglobin) Energy substrate (e.g., depleted FFA, glucose) ? |
|
| Mitochondria | Mitochondrial dysfunction/adaptation (ischemic injury/protection, HF, DM, aging) ? |
|
| Adventitia/Matrix | Stroma-connective tissue proliferation Adipocytes-estrogens (from androgens), leptins, and so on. Leukocytes-cytokines, angiotensin II, and so on. Mast cells, histamine, serotonin, proteoglycans, serine proteases, eicosanoids, and so on. Sympathetic nerve activation Vasa vasorum-capillary leak ? |
|
| Other | CNS, bone marrow-derived cells (e.g., CD34/CD133), T cells, among others. Adipose-derived cells, among others. ? |
AF = atrial fibrillation; ANS = autonomic nervous system; Ao-F = ; athero RF = atherosclerosis risk factors; CNS = central nervous system; CRI = chronic renal insufficiency; DM = diabetes mellitus; FFA = free fatty-acid; HF = heart failure; LV = left ventricular; LVH = left ventricular hypertrophy; PAN = polyarteritis nodosa; RA = rheumatoid arthritis; SBE = subacute bacterial endocarditis; SLE = systemic lupus erythematosus; VSM = vasomotor; ? = unknown.
Clinical Management Implications
Numerous guideline-recommended strategies target prevention of atherosclerosis progression in obstructive CAD to improve outcomes, and promising innovative therapies that target obstructive CAD are under development. Although most nonobstructive CAD patients likely have coronary atherosclerosis, no guideline-recommended therapy is available (except for symptom relief and CVD risk factor management) for the large proportion of patients with signs and symptoms of IHD and nonobstructive CAD and none appear on the horizon. As a result, these patients are often dismissed from specialty care and even general care, and the majority are women. It is noteworthy that guideline-directed care for patients with nonobstructive CAD was not included in the recent stable IHD practice guideline that focused on obstructive CAD as the pathophysiology of ischemia (34). The evidence reviewed here can inform the clinical community and support a focused guideline update for clinicians facing dilemmas regarding diagnosis and management of patients (particularly women) with nonobstructive CAD.
To recapitulate, patients with nonobstructive CAD encompass all of the acute and chronic IHD syndromes. This disease pattern is associated with heightened risk for adverse outcomes, yet current guidelines do not inform clinicians regarding assessment and management of these patients. The foregoing has generated several important questions.
Is Coronary Atherosclerosis Present?
Without an obvious focal epicardial stenosis, remodeling renders the angiogram insensitive to the presence of atherosclerosis and invalidates use of a so-called “normal reference segment” to estimate stenosis severity. Studies using IVUS (19,35,36) or CTA (13) have documented that coronary artery remodeling makes it very challenging to determine whether or not atherosclerosis is present from the “lumenogram” presented by selective coronary angiography. Some have proposed developing a method for quantifying angiographic estimates of coronary artery segmental size and shape (e.g., tapering) compared with sex- and segment-specific, population-derived, normal values (37). Registry data report that positive or expansive remodeling is associated with an elevated risk of acute coronary syndrome (ACS) (38-41). In the absence of obstructive stenosis, coexisting low-attenuation plaque on CTA or echolucent plaque on IVUS are also noted, which further increase ACS risk (42).
Coronary artery IVUS confirmed that >80% of the women evaluated in a WISE substudy had evidence for atherosclerotic plaque (36,43). Lee et al. (19) found IVUS evidence for atherosclerosis in all 139 patients with angina in the absence of obstructive CAD, of whom 107 (77%) were women.
Thus, atherosclerosis is present in most of the cases included in reports to date, but this may be influenced by selection bias related to atherosclerosis risk factor threshold contributing to referral for invasive studies.
Is Myocardial Ischemia Really Present?
Findings of normal global LV systolic function and good short-term clinical outcomes led to speculation that ischemia might not be present. Yet, there is uniformity of data supporting risk stratification across conventional stress imaging procedures, concluding that identification of ischemia in women (and men) is associated with elevated risk for cardiac events (34). Specifically, in cohorts of women with prevalent nonobstructive CAD, as the extent and severity of inducible ischemia increases, so do IHD-related event rates (17,21,24). Furthermore, because most clinical methods to assess ischemia rely on detecting relatively large regional differences in LV perfusion and/or wall motion in epicardial coronary territories, it became apparent that most patients without obstructive CAD do not have major global perfusion differences. Instead, with pharmacological vasodilator stress, perfusion of the subendocardium and/or midventricular wall fails to increase appropriately (7,8,44). The most useful methods for these cases are those that measure coronary blood flow reserve (directly with Doppler or PET) and/or myocardial metabolism (31P magnetic resonance spectroscopy and cMRI with gadolinium).
Is Coronary Microvascular Dysfunction Present?
Without a more proximal flow-limiting stenosis, coronary resistance vessels (e.g., arterioles <100 μm diameter), the microcirculation predominantly modulates myocardial perfusion. Considerable data document that CMD contributes to myocardial perfusion abnormalities in regions supplied by vessels without epicardial stenosis (45-48) in patients with risk factors and/or angina, but without epicardial stenosis (49-52). In the Multi-Ethnic Study of Atherosclerosis (MESA), both myocardial flow (cMRI) during adenosine-induced hyperemia and flow reserve were inversely associated with risk factor burden (53). CMD has been documented among symptomatic women without flow-limiting coronary stenosis in the WISE (26,54) by directly-measured (Doppler flow wire) coronary flow, by cMRI (44), and by PET (20). These studies have linked CMD and atherosclerosis risk factors with adverse outcomes over follow-up. CMD has also been replicated in another female cohort (55), providing additional support for its link with several risk factors. TIMI frame counts and some noninvasive measures to assess contrast flow as an index of myocardial blood flow have also been used (56-58).
Even among individuals with obstructive CAD, noninvasive imaging has documented abnormal perfusion in myocardial regions supplied by vessels without apparent obstructive CAD (48). Histology of nonischemic LV myocardium, remote from vessels with obstructive CAD, shows that women have less interstitial fibrosis, but similar perivascular fibrosis versus men (59). The arteriolar wall area/circumference ratio, a measure of arteriolar wall thickness, was almost 50% greater in these women versus men. Cardiomyocyte width, capillary length density, diffusion radius, and cardiomyocyte width/body surface area ratio were similar for men and women, but women had greater diffusion radius/body surface area ratio and diffusion radius/cardiomyocyte width ratio, with lower plasma vascular endothelial growth factor (VEGF) receptor-1 levels and VEGF receptor-1/VEGF-A ratios. These findings imply that women have greater arteriolar wall thickness and diffusion radius relative to body surface area and to cardiomyocyte width than men, which may predispose them to ischemia. Studies of larger numbers of women with less extensive CAD are required to confirm these findings and elucidate mechanisms of underlying CMD.
Syndromes Associated With Nonobstructive CAD and Adverse Outcomes
Chronic Stable Angina
So-called “normal coronary arteries” and “no obstructive CAD” by selective angiography may be reported in as many as 60% to 70% of symptomatic women referred to angiography for evaluation of chronic stable symptoms (principally angina) (1). Much of this information originated from the WISE, a prospective cohort study of 936 women presenting to coronary angiography to further evaluate symptoms (chest pain) and/or signs of IHD. One a priori objective was to determine the frequency and impact of ischemia in the absence of significant coronary stenosis. All underwent a baseline physical examination with collection of demographic, medical history, and symptom data using standardized questionnaires. Angiograms were quantitatively and qualitatively evaluated for presence and extent of CAD by the core laboratory (masked to all other data). Among the 883 completing the angiogram and available for follow-up, no obstructive CAD was found in 547 (62%, mean age 56 years), defined as no stenosis ≥50% in any artery (60). Outcome data were collected by a scripted interview 6 weeks after initial assessment and annually thereafter. The initial follow-up analysis was done at 5.2 years, mean (18). Death certificates, clinical data, and hospital summaries were reviewed by the Events Committee masked to angiographic data, to determine the cause of death (cardiac vs. noncardiac). Another analysis, which also included a National Death Registry search, extended the follow-up to 10 years (23). The same Events Committee, masked to angiographic information, also reviewed these additional deaths to determine the cause of death.
The WISE data indicated that about two-thirds of the women referred for angiography to further evaluate symptoms/signs of IHD had nonobstructive CAD. At 5 years, the nonobstructive CAD cohort had a 2.5% yearly risk of MACE (first occurrence of death [all-cause], nonfatal MI, nonfatal stroke, or heart failure hospitalization) that was 3-fold higher than the case-matched asymptomatic reference cohort (18). At 10 years, cardiovascular death or MI had occurred in 6.7% of women with “no obstructive CAD” (e.g., ≤20% diameter reduction) versus 12.8% of those with nonobstructive CAD (e.g., >20% but <50% narrowing) (23). Limitations of these findings were largely related to the design: absence of men, small sample size, and selection bias by the respective centers.
However, multiple larger studies, free of selection bias, from the United States, Canada, and Europe, have replicated the high prevalence of nonobstructive CAD among angina patients, the adverse prognosis in women, and extended these findings to men (1,22,25). Some have found that the outcomes associated with nonobstructive CAD are worse for women.
One study included all patients ≥20 years with stable angina undergoing coronary angiography (n = 13,695) in British Columbia, Canada, from July 1999 to December 2002 (22). Using the WISE angiographic definitions, outcomes were assessed to 3 years follow-up. Among women with stable angina, 42%, versus 14% of men, had no obstructive CAD. Women with no obstructive CAD were ∼3 times more likely than men to experience a MACE (same definition as WISE) within the first year of angiography.
A larger registry study of all patients with suspected stable angina in Eastern Denmark having a “first” coronary angiography between 1998 and 2009 (1) identified 11,223 patients and 5,705 participants from the Copenhagen City Heart Study for reference. Within the symptomatic population (4,711 women and 6,512 men), significantly more women (65%) than men (32%) had no obstructive CAD (<50% stenosis). Interestingly, this fraction progressively increased over the 10-year study period. Although event rates were higher among women, in models adjusted for age, body mass index, diabetes, smoking, and lipid-lowering or antihypertensive medication use, risks associated with no obstructive CAD were similar in women and men. In a pooled analysis (women plus men), the risks for MACE (as cardiovascular death, MI, stroke, or heart failure), and all-cause death increased with increasing degrees of nonobstructive CAD: adjusted HRs were 1.52 (95% CI: 1.27 to 1.83) for patients with normal coronary arteries and 1.85 (95% CI: 1.51 to 2.28) for those with diffuse nonobstructive CAD versus the reference population. For all-cause mortality, normal coronary arteries and diffuse nonobstructive CAD were associated with HRs of 1.29 (95% CI: 1.07 to 1.56) and 1.52 (95% CI: 1.24 to 1.88), respectively. It is noteworthy that these stable angina patients with nonobstructive CAD also had higher rates of cardiovascular hospitalization versus reference individuals, after adjustment for cardiac risk factors and exclusion of cardiovascular comorbidities.
Among all veterans undergoing elective angiography from 2007 to 2012, ∼75% of 3,181 women had no obstructive CAD (e.g., either “normal” or “nonobstructive CAD”) and among 36,590 men only ∼44% had these findings (16). But, as noted previously, “nonobstructive CAD” was defined as any stenosis ≥20%, but <70% narrowing, in any epicardial artery or ≥20%, but <50%, in the left main artery. Relative to patients with no apparent CAD, those with 1-vessel nonobstructive CAD had a hazard ratio (HR) of 2 for MI at 1 year, which increased to 4.6 for 2-vessel nonobstructive CAD.
In community-based, predominantly symptomatic (angina) patients (n = 23,854) without known CAD, the CONFIRM registry found that about a third had nonobstructive CAD by CTA (14), which occurred more frequently in symptomatic women versus men. All-cause mortality risk associated with nonobstructive CAD was similar among women and men (HR: 1.67 and 1.52, respectively), but these risks were similar or higher compared to those with obstructive 1-vessel CAD (HR: 1.17). Importantly, when stratified by age, older (≥65 years) women with nonobstructive CAD experienced a more than 5-fold higher all-cause mortality risk (adjusted HR: 8.08) versus younger women (HR: 1.59) that was greater than the risk observed for older men (HR: 7.79).
Referral for another coronary angiogram is costly, is associated with additional risk, and adversely impacts quality of life. In the WISE cohort of women having an angiogram to evaluate stable angina symptoms, those with nonobstructive CAD had rates of repeat angiography of 3.7%, 12.3%, and 15.7% at 1, 3, and 5 years, respectively (60). These findings have been confirmed and extended to men (61). Over 7.8 years, 23% of women and 30% of men had at least 1 additional angiogram. In both women and men, those with no obstructive CAD had 3- to 5-fold higher rates of repeat angiography per 1,000 years at risk versus asymptomatic reference individuals. Overall, the risk for repeat angiography was >2-fold higher in patients with “angiographically normal” coronary arteries and ∼6-fold higher for those with diffuse nonobstructive CAD.
Our studies comparing WISE participants with nonobstructive CAD and a reference group of asymptomatic, apparently healthy women matched for age, height, and body mass index identified significant differences related to indexes of increased arterial stiffness, reflected by aortic pulse wave velocity, augmentation index, systolic blood pressure, and pulse pressure. These observations suggest that these factors may contribute to the development of nonobstructive CAD, but additional studies are clearly warranted (62).
In summary, about 30% to 60% of patients with stable angina undergoing selective coronary angiography have no obstructive CAD. This frequency appears higher in women (40% to 60%) versus men. Using CTA, this frequency of nonobstructive CAD is about one-third. It is also clear that those with nonobstructive CAD have considerably greater risk burdens in terms of CVD hospitalization, disability, repeat angiography, and MACE versus reference individuals. It is unclear if these adverse outcomes associated with nonobstructive CAD are significantly higher among women with stable angina versus men. However, contrary to common perception, excluding obstructive CAD by angiography in stable angina patients does not assure a benign cardiovascular prognosis.
Acute Coronary Syndromes
Women with ACS are less likely than men to have obstructive CAD, suggesting that different ACS mechanisms operate among women compared with men (63). More than one-third of women with MI and nonobstructive CAD have plaque rupture or ulceration when examined with IVUS (64,65). Within 180 days of an ACS presentation, women with normal-appearing coronary arteries by angiography are 4 times more likely than men to be readmitted for ACS/chest pain (66,67). Over 1 to 5 years, they have a 40% risk of rehospitalization for chest pain and a 30% rate of repeat coronary angiography. Details of patient characteristics and outcomes are provided in 2 large data sets summarized in the subsequent discussion.
Relative to non-ST-segment elevation (NSTE) ACS, data from 37,101 patients, 3,555 with no obstructive CAD, were reported in a patient-level meta-analysis of 8 randomized trials (68). Overall, ∼10% had nonobstructive CAD; they were younger (60 vs. 66 years), more frequently women (56% vs. 29% men), and fewer had diabetes (15% vs. 26%), prior MI (15% vs. 32%), or PCI (10% vs. 20%) compared with those with obstructive CAD. Patients with nonobstructive CAD were treated less often with guideline-recommended drugs before angiography and this difference increased after angiography. Death or MI at 30 days was less frequent among patients with nonobstructive (2.2%) versus obstructive CAD (13.3%, OR; 0.15; 95% CI: 0.11 TO 0.20); 6-month mortality was also lower (0.19 [95% CI: 0.14 to 0.25] and OR: 0.37 [95% CI: 0.28 to 0.49], respectively). In the nonobstructive CAD group, patients with 30-day death/MI had higher GRACE risk scores and elevated cardiac markers at presentation versus those without these events. These data are limited by wide variability across trials in fractions of patients undergoing angiography (52% to 97%) and with findings of nonobstructive CAD (6.9% to 13%), and adverse outcomes that generally related to refractory ischemia or urgent revascularization. These data are also limited by bias related to different entry criteria and treatments mandated by each trial.
Such limitations are not present in the population-based CRUSADE registry of 51,608 NSTE ACS patients (69). Overall, no obstructive CAD was found in 4,903 (9.5%, 60% women), but was twice as likely among women (15.1% vs. 6.8% of men). Women were older (63 vs. 53 years of age), and more likely to have hypertension (65% vs. 52%), diabetes (19% vs. 16%), dyslipidemia (35% vs. 31%), heart failure (11% vs. 8.7%), or stroke (6.4% vs. 3.6%), and less likely to be smokers (23% vs. 36%) vs. men. There were no significant differences in medications given in the initial 24 h by sex. Although women were as likely as men (89 vs. 87%) to have troponin elevation, they were less likely to undergo early angiography. At discharge, women were more likely to receive calcium antagonists and either angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers. Similar percentages of women and men died or had reinfarction or stroke. The strongest predictors of nonobstructive CAD were female sex and younger age (70).
From these datasets, it is appropriate to conclude (Table 2) that ∼10% of NSTE ACS patients have nonobstructive CAD and death or MI occurred in ∼2% of them by 30 days. Sex differences in characteristics and outcomes were similar to those found with obstructive CAD. Although women with NSTE ACS are about twice as likely to have nonobstructive CAD than men, those with nonobstructive CAD have lower event rates than patients with obstructive CAD. But their death and MI rates are not negligible. Furthermore, ACS patients like those selected for these analyses (e.g., reaching hospital alive and undergoing early angiography) usually have lower event rates than those seen in the general, all-inclusive ACS population. Women were less likely than men to undergo angiography within 48 h of admission and to receive guideline recommended therapies, despite similar proportions with troponin elevation, suggesting opportunities for improved management. Therefore, the presence of nonobstructive CAD alone does not justify dismissing opportunities for secondary prevention.
Table 2. Tabulation of Findings in NSTE ACS Patients With Nonobstructive CAD.
|
ACS = acute coronary syndrome; CAD = coronary artery disease; MI = myocardial infarction; NSTE = non-ST-segment elevation
Relative to ST-segment elevation myocardial infarction (STEMI), at the time of hospitalization it is known that women are generally older than men and less frequently have chest pain/discomfort, which, in addition to social factors, contributes to delayed treatment and delayed symptom-to-balloon time versus men (71). Optimal recognition and timely management of STEMI is important, especially reducing delay in seeking care and physician decision making. Although presence of chest pain/discomfort is the hallmark of acute MI, it is important to account for age when considering sex differences in presentation and mortality. Relationships between sex and symptom presentation and hospital mortality, before and after accounting for age, were examined in registry patients (481,581 women and 661,932 men) with MI (71). The proportion presenting without pain in the chest, arm, neck or jaw was higher for women than men (42.0% vs. 30.7%): this sex-related difference was larger in younger (<45 years) versus older patients.
Among patients hospitalized with MI, women are more likely to present without chest pain and this is linked with higher mortality versus men of the same age. However, sex differences in clinical presentation without chest pain and in mortality are attenuated with increasing age.
The effect of sex on incidence of acute MI without obstructive CAD was assessed among 95,849 patients undergoing angiography in the Swedish Coronary Angiography and Angioplasty Registry (72). Analyses in 2,268 STEMI and 10,904 non-ST-segment elevation myocardial infarction (NSTEMI) patients without obstructive CAD (<50% stenosis) revealed the presence of nonobstructive CAD in 7% of STEMI (6% men, 10% women) and 17% of NSTEMI patients (11% men, 28% women). During 2.6 years follow-up, 8% of STEMI and 5% of NSTEMI patients died. Sex-associated differences in risk were observed in NSTEMI patients, with HRs for mortality (HR: 0.90, 95% CI: 0.50 to 0.73) and heart failure (HR: 0.61, 95% CI: 0.52 to 0.72) lower in women than in men. Women also had less revascularization. They concluded that nonobstructive CAD was more common in NSTEMI versus STEMI patients, as well as in women versus men. Mortality in patients with nonobstructive CAD was higher after STEMI than NSTEMI. These differences in outcomes support the suggestion that there are important sex-related differences in underlying pathogenesis of MI without obstructive CAD.
Although there has been controversy concerning the incidence of nonobstructive disease leading to STEMI when on the basis of angiograms, recent IVUS studies (PROSPECT [Providing Regional Observations to Study Predictors of Events in the Coronary Tree] and VIVA [virtual histology IVUS (VH-IVUS) in Vulnerable Atherosclerosis]) have shown responsible lesions are usually only mild (on the basis of adjacent reference segment lumens), but severe by IVUS on the basis of large atheroma volume with severe cross-sectional area narrowing (73). Mechanistically, other studies have noted that the greater the atheroma burden, the greater the burden of lipid and necrotic core, the thinner the fibrous cap, the more severe the inflammation, the more deranged the vaso vasorum, and the more abnormal the stress-strain relationships (23,27). That nonobstructive lesions can lead to STEMI has also been confirmed after thrombus aspiration (74).
Details on the extent and composition of atherosclerosis contributing to ACS (697 patients, 24% women) were provided in the multicenter PROSPECT Study (75). Three-vessel multimodality intracoronary imaging (quantitative coronary angiography, grayscale, and radiofrequency IVUS) was performed after culprit lesion(s) treatment. Women were older and had more comorbid disease than men. By angiography, women had a similar number of culprit lesions, but fewer nonculprit lesions and fewer vessels with nonculprit lesions than men. By IVUS, women had fewer nonculprit lesions, but similar plaque burden per lesion, and female sex was not predictive of severe (>70%) plaque burden. Plaque rupture was significantly less frequent among women (6.6% vs. 16.3% in men), even after adjusting for comorbidities, and their total necrotic core volume was also less. Frequencies of pathological intimal thickening, thin-cap fibroatheromas (TCFA), and thick-cap fibroatheromas were similar in women and men. Rates of MACE attributed to culprit and nonculprit lesions during follow-up were not significantly different between women and men, although women were rehospitalized more frequently due to culprit lesion–related angina. For men, nonculprit lesion minimal lumen area ≤4.0 mm2, plaque burden ≥70%, and TCFA predicted nonculprit MACE at 3 years, but for women, only TCFA and plaque burden were predictive. These data lead to the conclusion that among ACS patients, women have more comorbid risk factors, but less extensive CAD by angiographic and IVUS measures than men. Furthermore, women have less plaque rupture, less necrotic core and calcium, and similar plaque burden, but smaller coronary lumens, and TCFA may be a stronger marker of plaque vulnerability versus men.
A more thorough understanding is needed of the complex interplay among procoagulant, antiplatelet, and fibrinolytic properties of normal and diseased endothelium to provide additional insight into mechanisms and directions for management of ACS associated with sudden coronary thrombosis in the absence of obstructive coronary atherosclerosis.
Cardiac Sudden Death
Interestingly, an alternative explanation for the relative lack of severely obstructive CAD among women versus men presenting with ACS could be that women with obstructive CAD are more likely to die before reaching the hospital versus women with nonobstructive CAD.
Overall, women have a lower incidence of cardiac sudden death than men, even when adjusted for predisposing conditions such as CHD, MI, and HF. Additionally, their percentage of cardiac sudden deaths due to obstructive CAD is lower: CAD is found in ∼half of women versus 80% to 90% of men (76). But details relative to nonobstructive CAD by angiography are unclear.
Pathological findings have been described in many fatal cases with nonobstructive CAD (64,77-79). The most complete evaluations of sex differences in the extent and severity of coronary and myocardial findings in fatal IHD are from autopsy reports on people aged 21 to 54 years (78,79). According to the medical examiner, obstructive CAD (≥75% cross-sectional area stenosis in an epicardial vessel or ≥50% left main) was significantly less likely among women (63% vs. 77% of men). Yet, pathologic evidence of MI was present in almost half of the cases, 17% with nonobstructive CAD. The frequency of MI did not vary by sex overall or among those without significant CAD (∼23%) versus those with obstructive CAD. Thus, among younger adults determined at autopsy to have died of IHD, fewer women have obstructive CAD, consistent with angiographic data from other IHD syndromes. Furthermore, pathological evidence of MI exists in many without obstructive CAD.
These findings do not support the notion that the relative lack of severely obstructive CAD among women versus men with ACS is related to higher pre-hospital death risk among women with obstructive CAD. Yet, the mechanisms for these deaths are unknown.
Predictors of Adverse Outcomes With Nonobstructive CAD: Role of Hypertension, Diabetes, and Related Insulin-Resistant States
Predictors of adverse outcomes among individuals with nonobstructive CAD appear similar to those documented among the population with obstructive CAD, with some exceptions. The strong association with LV systolic function observed in those with obstructive CAD is not present, as LV systolic function is usually preserved among those with nonobstructive CAD. Measures of the extent and severity of CAD also appear important in the nonobstructive cohort but, as discussed later, are not well developed. Patient characteristics, including hypertension, diabetes, and smoking, have been identified in the WISE (23) and other nonobstructive CAD cohorts as important. Because of the high and increasing prevalence of hypertension, diabetes, and metabolic syndrome among women, and their known associations with microvascular disease, these areas will be addressed in more detail.
In the large prospective CONFIRM Registry, hypertension was present in the majority with nonobstructive, as well as obstructive, CAD (14). Furthermore, hypertension (HR: 1.93), diabetes (HR: 2.13) and smoking (HR: 1.47) were all significantly associated with increased risk. The multivariable adjusted risk for all-cause mortality, stratified by sex, found nonobstructive CAD was associated with a 67% excess risk among women with versus without CAD. These findings are consistent with hypertension being the major risk factor for atherosclerosis that underlies most nonobstructive and obstructive CAD. Hypertensive postmenopausal women have abnormal endothelium-dependent vascular function, and hypertension is a known cause of microvascular complications in the heart, brain, eye, and kidney. Antihypertensive treatment improves endothelial and other microvascular functions, which identifies patients who possibly have a more favorable prognosis (80). Effective control of hypertension in women is proven to decrease CVD risks, but it is unclear if the benefit is the same among women with nonobstructive CAD versus obstructive CAD.
Elevated glucose (type 2 diabetes and pre-diabetes) is highly prevalent among women and is associated with insulin resistance. Insulin resistance is strongly linked with both microvascular and macrovascular disorders, resulting in organ and tissue damage. Numerous studies document that these disorders convey higher risk for CVD morbidity and mortality in women versus men (81-83). Even women with type 1 diabetes have 40% excess risk of fatal and nonfatal CVD events versus men with type 1 diabetes (84). Accordingly, nonobstructive CAD is more prevalent among those with diabetes versus those without, and coronary microvascular abnormalities are highly prevalent and progress with worsening glucose intolerance. Vascular damage associated with insulin resistance has long been recognized (85-90).
Coronary endothelial dysfunction occurs with hypertension and diabetes and is associated with increased risk for CVD events. Stress myocardial blood flow by PET has characterized CMD in various states of insulin resistance. Compared with insulin-sensitive individuals, endothelium-dependent coronary dilation is progressively diminished in insulin-resistant (-56%), impaired glucose-tolerant (-85%), and normotensive (-91%), as well as hypertensive diabetic subjects (-120%). Thus, progressive worsening of CMD occurs with increasing severity of insulin-resistance and carbohydrate intolerance.
These observations are important because CMD is an independent predictor for mortality among patients with diabetes, providing incremental risk stratification (91). Patients with diabetes and normal CFR have low annual CVD mortality, similar to patients without diabetes or obstructive CAD who had normal myocardial perfusion with stress. But patients with diabetes and impaired CFR have CVD mortality similar to that of patients with obstructive CAD, but no diabetes.
Early detection of CMD among women without obstructive CAD and insulin resistance is particularly important given their increased prevalence of hypertension and the worsening worldwide epidemic of diabetes. In addition, CMD in hypertension and diabetes can be normalized with blood pressure control and insulin, as well as with many insulin-sparing drugs.
Proposed Mechanistic Classification of IHD Syndromes With Nonobstructive CAD
In addition to CMD, many other potential etiologies exist beyond the traditional “flow-limiting stenosis” (Table 1). Each of these mechanisms may operate alone, but they more frequently operate in concert (19). Nevertheless, the specific mechanisms operating in any given patient are likely to remain elusive unless the diagnostic approach moves beyond the coronary angiogram currently done as usual care for evaluation of IHD. Several reports, including one limited to women (36), document that the additional testing required can safely be conducted in experienced hands.
Knowledge Gaps
Although the need for cardiovascular research focusing on women has recently been emphasized by the American College of Cardiology, American Heart Association, European Society of Cardiology, and other organizations, none have specifically addressed the issue of IHD with nonobstructive CAD. Clearly, there are many important gaps in our existing knowledge. Information about the following is essential:
1. Identify specific mechanism(s)
Whether the underlying pathophysiology of IHD without documentation of obstructive CAD is different in women and men, or is different from those with obstructive CAD, requires further investigation. The current focus is on nonobstructive CAD associated with limitations in flow reserve at the coronary microvascular level or CMD. However, evolving data suggest that CMD also occurs among patients with obstructive CAD and carries a particularly poor prognosis (92). Does CMD contribute to the development or characteristics of upstream plaque in large coronary arteries? If so, does this contribute to plaque vulnerability to erosion or rupture? Is microvascular flow limited due to microvascular spasm (93)? If so, is this spasm due to endothelial dysfunction (e.g., loss of nitrous oxide [insufficient production/excessive inactivation or both]), intrinsic heightened vascular smooth muscle activation state, sympathetic nervous system activation, platelet microaggregates with direct plugging or release of vasoactive substances, and so on? What about the complex interplay among white blood cells and the procoagulant, antiplatelet, and fibrinolytic properties of a diseased coronary endothelium? Many other possibilities exist (see Table 1) alone or in combination. Finally, why does CMD seem to be more prevalent in women?
2. Define optimal diagnostic approaches
It is unclear how to best identify nonobstructive CAD. Additional information on its clinical predictors is needed. The incidence of nonobstructive CAD in women with complications of pregnancy could be important. The need for lower radiation doses is obvious and the effective dose with CTA overall is ≈10 mSv, but is <2 to 5 mSv with current dose-reduction techniques (94). Data comparing different imaging modalities for identification of nonobstructive plaque and ischemia in patients with nonobstructive CAD are lacking. This is clearly an important knowledge gap for future study. Most of the evidence for nonobstructive CAD with CMD as a mechanism for ischemia was obtained using directly measured coronary flow, either by catheter (Doppler-flow or thermal dilution to calculate microvascular resistance) or PET. Although the latter provides flow/g of LV muscle, both methods are costly, have radiation and other hazards, and are not applicable to large studies, particularly where repeated measurements are needed. The WISE has advanced adenosine-stimulated gadolinium perfusion cMRI, which can be quantified using available techniques, has no radiation hazard, and can be repeated. The challenge is its lack of widespread availability.
Biomarkers, such as circulating endothelial and bone marrow-derived cells and ischemia metabolites, are under current evaluation. The study of noncoronary microvascular beds thus far appears to have limited applicability to the coronary circulation, although the retina holds promise.
It should also be noted that the previously mentioned studies all required either selective coronary angiography or CTA as an imaging method to exclude obstructive CAD and quantify functional changes of the large coronary vessels. To this end, new functional imaging modalities that do not require radiation are clearly needed.
3. Discover novel treatment strategies and their follow-up
Most treatment strategies and current IHD guidelines center on identification of high risk, which is code for finding flow-limiting stenosis, identifying candidates for revascularization, and then deciding the most appropriate revascularization strategy among percutaneous and surgical approaches. This is followed by lifetime modification of life-style and medical management directed at prevention of atherosclerosis progression. It would be truly useful to discover novel treatment strategies for women that do not begin with finding flow-limiting stenosis, searching for candidates for revascularization, and end with lifetime medical treatments and repeated hospitalizations and/or costly testing. Also important is the need to follow atherosclerosis progression in patients treated for nonobstructive disease. To this end, evolving lower-dose CTA and cMRI techniques may offer promise, but this is an important knowledge gap.
Clinical Trials to Provide Evidence-Based Guideline Development
Prior, short-term, single-agent studies of symptomatic subjects with ischemia and nonobstructive CAD testing antiatherosclerotic and/or anti-ischemic therapies suggest benefit in patients with nonobstructive CAD. Briefly, statins and ACE inhibitors counteract oxidative stress and improve endothelial function (95,96), and CMD (97) may benefit (96,98). Beneficial effects of statins on CMD are also documented (99), and many trials in patients with obstructive CAD show prevention of atherosclerosis progression in nonobstructed segments. Drug combinations (e.g., statins with ACE inhibitors) may potentially amplify benefits (96). Calcium antagonists fail to ameliorate CMD in these patients (100), but may prevent spasm. Conversely, beta-blockers appear effective for management of angina (101), and superior to calcium antagonists (101,102). Few controlled studies have been done with nitrates. Exercise training beneficially modulates adrenergic and nitric oxide pathways (103). Imipramine improves angina, possibly through visceral analgesic, anticholinergic, and alpha-antagonist effects (104). L-arginine improved angina and vascular function (105), but was adverse in an obstructive CAD trial (106). Postmenopausal hormone therapy improved emotional well-being, but had no effect on angina or exercise tolerance (107). Several studies reported symptom and ischemia improvement with less coronary vascular dysfunction (97,108). However, appropriately-powered outcome trials testing various strategies have not been performed in such patients. Existing guidelines focus on reassurance and symptom management (109,110). This is inappropriate because of the elevated MACE rate (1,18,22,23,25,26), symptom recurrence, and health resource consumption comparable to that of obstructive CAD (60). Pragmatic trials testing real-world strategies of antiatherosclerotic and anti-ischemic therapies are needed to advise guidelines for this growing population with nonobstructive CAD.
Even without definitive information about mechanisms, it may be possible to use the limited information currently available to test new strategies to improve outcomes. To accelerate development of new diagnostic and therapeutic regimens, an integrated approach to phase II and III clinical trials that incorporates multiple efficacy variables, including angiography, biomarkers of microvascular dysfunction, and other factors should be considered.
Summary and Conclusions (Central Illustration)
Central Illustration. Nonobstructive CAD in Women: Sex-Specific CAD and Need for Ischemic Cardiac Disease Definition Changes.
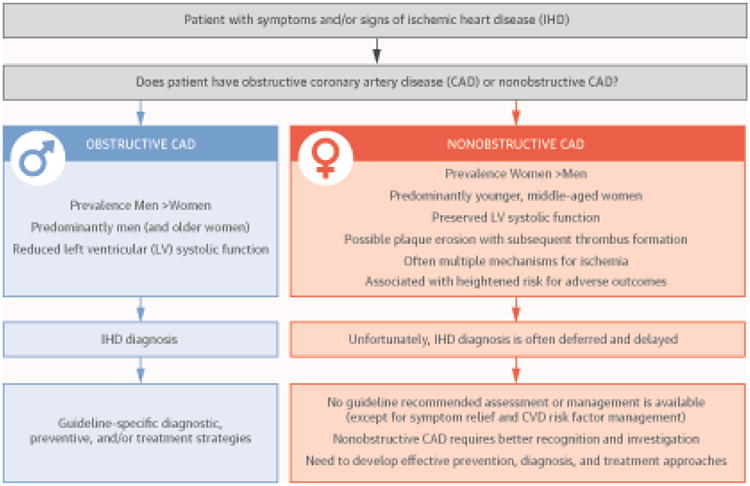
Among patients presenting with symptoms/signs suspect for IHD, the presence of obstructive CAD (e.g., identifying a flow-limiting lesion, FFR <0.80) is highly prevalent in men (and older women), and often associated with reduced LV systolic function. Diagnosis and risk stratification are prompt because guideline-specific diagnostic, preventive, and/or treatment strategies are available.
In contrast, nonobstructive CAD (e.g., FFR ≥80) is highly prevalent among women (mostly younger and middle-aged women) with preserved LV systolic function. Additionally, pharmacological testing with acetylcholine and adenosine distinguishes those with macrovascular or microvascular spasm, endothelial dysfunction, and/or coronary microvascular dysfunction (CMD). These latter findings are associated with increased risk of adverse outcomes that include heart failure with preserved systolic function (HFpEF), acute coronary syndromes, and cardiovascular-related hospitalizations, as well as repeated testing, Unfortunately, no guideline-recommended assessment or management is available, except for symptom relief and CVD risk factor management.
CAD = coronary artery disease; CVD = cardiovascular disease; IHD = ischemic heart disease; LV = left ventricular.
Nonobstructive CAD is relatively common in women in acute or chronic coronary syndromes. Reassurance of an excellent prognosis is inappropriate among symptomatic patients with no or minimal epicardial coronary artery obstruction (>0%, but <50% stenosis). Suboptimal clinical outcomes of patients with symptoms and/or signs of ischemia and nonobstructive CAD must be better understood so that a near-normal or “normal” angiogram does not drive diagnostic and therapeutic complacency (30,92). Importantly, given their impaired prognosis, a search for cause(s) of ischemia must be much more comprehensive than simply a diagnostic angiogram. Additional testing must be considered to attempt to identify some of the processes reviewed earlier (endothelial and/or microvascular dysfunction, coronary spasm, angiographically nonevident plaques causing diffuse narrowing, etc.) followed by additional research to fully understand the pathophysiology, treatments, and outcomes for this condition.
This information should foster: 1) development of more precise tools to better risk-stratify patients with nonobstructive CAD; 2) prospective trials to assess benefits of intensive medical therapy directed at ischemia and atherosclerosis progression in these patients; and 3) discovery of novel management strategies for these patients, most of whom are women. In the future, if we aim at preventing these events, the paradigm of risk stratification for prevention must move from identification of obstructive atherosclerosis to an earlier stage that includes nonobstructive coronary disease.
Acknowledgments
Financial Support: This work was supported by contracts N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164 from the National Heart, Lung and Blood Institutes; grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging; GCRC grant MO1-RR00425 from the National Center for Research Resources; the National Center for Advancing Translational Sciences Grants UL1TR000124 and UL1TR000064; and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ; The Women's Guild of Cedars-Sinai Medical Center, Los Angeles; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA; QMED, Inc., Laurence Harbor, NJ; the Edythe L. Broad and the Constance Austin Women's Heart Research Fellowships; Cedars-Sinai Medical Center, Los Angeles; the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles; The Society for Women's Health Research (SWHR), Washington, D.C.; The Linda Joy Pollin Women's Heart Health Program; and the Erika Glazer Women's Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California.
Abbreviations
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CMD
coronary microvascular dysfunction
- HR
hazard ratio
- IHD
ischemic heart disease
- IVUS
intravascular ultrasound
- LV
left ventricular
- MI
myocardial infarction
- NSTE
non-ST-segment elevation
- STEMI
ST-segment elevation myocardial infarction
Footnotes
Disclosures: All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 2.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–91. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 3.Cohen DJ, Van Hout B, Serruys PW, et al. Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) Investigators. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med. 2011;364:1016–26. doi: 10.1056/NEJMoa1001508. [DOI] [PubMed] [Google Scholar]
- 4.Serruys PW, Unger F, Sousa JE, et al. Arterial Revascularization Therapies Study Group. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–24. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- 5.Redwood DR, Epstein SE. Uses and limitations of stress testing in the evaluation of ischemic heart disease. Circulation. 1972;46:1115–31. doi: 10.1161/01.cir.46.6.1115. [DOI] [PubMed] [Google Scholar]
- 6.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967;276:1063–6. doi: 10.1056/NEJM196705112761904. [DOI] [PubMed] [Google Scholar]
- 7.Galassi AR, Crea F, Araujo LI, et al. Comparison of regional myocardial blood flow in syndrome X and one-vessel coronary artery disease. Am J Cardiol. 1993;72:134–9. doi: 10.1016/0002-9149(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 8.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 9.Bemiller CR, Pepine CJ, Rogers AK. Long-term observations in patients with angina and normal coronary arteriograms. Circulation. 1973;47:36–43. doi: 10.1161/01.cir.47.1.36. [DOI] [PubMed] [Google Scholar]
- 10.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 11.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 12.Leipsic J, Taylor CM, Gransar H, et al. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology. 2014;273:393–400. doi: 10.1148/radiol.14140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58:510–9. doi: 10.1016/j.jacc.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 14.Min JK, Dunning A, Lin FY, et al. CONFIRM Investgators. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 15.Shah S, Bellam N, Leipsic J, et al. CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry) Investigators. Prognostic significance of calcified plaque among symptomatic patients with nonobstructive coronary artery disease. J Nucl Cardiol. 2014;21:453–66. doi: 10.1007/s12350-014-9865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis MB, Maddox TM, Langner P, et al. Characteristics and outcomes of women veterans undergoing cardiac catheterization in the Veterans Affairs Healthcare System: insights from the VA CART Program. Circ Cardiovasc Qual Outcomes. 2015;8:S39–47. doi: 10.1161/CIRCOUTCOMES.114.001613. [DOI] [PubMed] [Google Scholar]
- 17.Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol Img. 2010;3:1030–6. doi: 10.1016/j.jcmg.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–60. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pazhenkottil AP, Nkoulou RN, Ghadri JR, et al. Prognostic value of cardiac hybrid imaging integrating single-photon emission computed tomography with coronary computed tomography angiography. Eur Heart J. 2011;32:1465–71. doi: 10.1093/eurheartj/ehr047. [DOI] [PubMed] [Google Scholar]
- 22.Sedlak TL, Lee M, Izadnegahdar M, et al. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44. doi: 10.1016/j.ahj.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–41. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Werkhoven JM, Schuijf JD, Gaemperli O, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol. 2009;53:623–32. doi: 10.1016/j.jacc.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Maddox TM, Stanislawski M, Grunwald G, et al. Non-obstructive coronary artery disease is not benign: Insights from the VA CART program on the association between non-obstructive disease and cardiac events. Circ Cardiovasc Qual Outcomes. 2014;7:A24. abstr. [Google Scholar]
- 26.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Col Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 28.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–11. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermeltfoort IA, Raijmakers PG, Riphagen II, et al. Definitions and incidence of cardiac syndrome X: review and analysis of clinical data. Clin Res Cardiol. 2010;99:475–81. doi: 10.1007/s00392-010-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepine CJ. Multiple causes for ischemia without obstructive coronary artery disease: not a short list. Circulation. 2015;131:1044–6. doi: 10.1161/CIRCULATIONAHA.115.015553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Britten MB, Zeiher AM, Schächinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259–64. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 32.Dreyer RP, Smolderen KG, Strait KM, et al. Gender differences in pre-event health status of young patients with acute myocardial infarction: A VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2015 Feb 3; doi: 10.1177/2048872615568967. [E-pub ahead of print], http://dx.doi.org/10.1177/2048872615568967. [DOI] [PMC free article] [PubMed]
- 33.Gupta A, Wang Y, Spertus JA, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–45. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Khalig A, Johnson BD, Anderson RD, et al. Relationships between components of metabolic syndrome and coronary intravascular ultrasound atherosclerosis measures in women without obstructive coronary artery disease: the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Cardiovasc Endocrinol. 2015 doi: 10.1097/XCE.0000000000000049. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancini GB, Ryomoto A, Kamimura C, et al. Redefining the normal angiogram using population-derived ranges for coronary size and shape: validation using intravascular ultrasound and applications in diverse patient cohorts. Int J Cardiovasc Imaging. 2007;23:441–53. doi: 10.1007/s10554-006-9199-z. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Nishikawa H, Mukai S, et al. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 2001;37:63–9. doi: 10.1016/s0735-1097(00)01097-4. [DOI] [PubMed] [Google Scholar]
- 39.Pasterkamp G, Schoneveld AH, van der Wal AC, et al. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J Am Coll Cardiol. 1998;32:655–62. doi: 10.1016/s0735-1097(98)00304-0. [DOI] [PubMed] [Google Scholar]
- 40.Schoenhagen P, Ziada KM, Kapadia SR, et al. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000;101:598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 41.Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–43. doi: 10.1161/hc0802.104327. [DOI] [PubMed] [Google Scholar]
- 42.Schmid M, Pflederer T, Jang IK, et al. Relationship between degree of remodeling and CT attenuation of plaque in coronary atherosclerotic lesions: an in-vivo analysis by multi-detector computed tomography. Atherosclerosis. 2008;197:457–64. doi: 10.1016/j.atherosclerosis.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Banks K, Lo M, Khera A. Angina in women without obstructive coronary artery disease. Curr Cardiol Rev. 2010;6:71–81. doi: 10.2174/157340310790231608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction A National Heart, Lung, and Blood Institute-Sponsored Study From the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8:e002481. doi: 10.1161/CIRCIMAGING.114.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heistad DD, Armstrong ML, Marcus ML, et al. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984;54:711–8. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- 46.Sambuceti G, Marzullo P, Giorgetti A, et al. Global alteration in perfusion response to increasing oxygen consumption in patients with single-vessel coronary artery disease. Circulation. 1994;90:1696–705. doi: 10.1161/01.cir.90.4.1696. [DOI] [PubMed] [Google Scholar]
- 47.Sellke FW, Armstrong ML, Harrison DG. Endothelium-dependent vascular relaxation is abnormal in the coronary microcirculation of atherosclerotic primates. Circulation. 1990;81:1586–93. doi: 10.1161/01.cir.81.5.1586. [DOI] [PubMed] [Google Scholar]
- 48.Uren NG, Marraccini P, Gistri R, et al. Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal arteries in patients with coronary artery disease. J Am Coll Cardiol. 1993;22:650–8. doi: 10.1016/0735-1097(93)90172-w. [DOI] [PubMed] [Google Scholar]
- 49.Gimelli A, Schneider-Eicke J, Neglia D, et al. Homogeneously reduced versus regionally impaired myocardial blood flow in hypertensive patients: two different patterns of myocardial perfusion associated with degree of hypertrophy. J Am Coll Cardiol. 1998;31:366–73. doi: 10.1016/s0735-1097(97)00503-2. [DOI] [PubMed] [Google Scholar]
- 50.Pitkänen OP, Nuutila P, Raitakari OT, et al. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47:248–54. doi: 10.2337/diab.47.2.248. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama I, Momomura S, Ohtake T, et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472–7. doi: 10.1016/s0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama I, Ohtake T, Momomura S, et al. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation. 1996;94:3232–8. doi: 10.1161/01.cir.94.12.3232. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Jerosch-Herold M, Jacobs DR, Jr, et al. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:565–72. doi: 10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 54.Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–75. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal M, Shufelt C, Mehta PK, et al. Cardiac risk factors and myocardial perfusion reserve in women with microvascular coronary dysfunction. Cardiovasc Diagn Ther. 2013;3:146–52. doi: 10.3978/j.issn.2223-3652.2013.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caiati C, Montaldo C, Zedda N, et al. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol. 1999;34:1193–200. doi: 10.1016/s0735-1097(99)00342-3. [DOI] [PubMed] [Google Scholar]
- 57.Petersen JW, Johnson BD, Kip KE, et al. TIMI frame count and adverse events in women with no obstructive coronary disease: a pilot study from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) PLoS One. 2014;9:e96630. doi: 10.1371/journal.pone.0096630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun H, Fukumoto Y, Ito A, et al. Coronary microvascular dysfunction in patients with microvascular angina: analysis by TIMI frame count. J Cardiovasc Pharmacol. 2005;46:622–6. doi: 10.1097/01.fjc.0000181291.96086.ae. [DOI] [PubMed] [Google Scholar]
- 59.Campbell DJ, Somaratne JB, Jenkins AJ, et al. Differences in myocardial structure and coronary microvasculature between men and women with coronary artery disease. Hypertension. 2011;57:186–92. doi: 10.1161/HYPERTENSIONAHA.110.165043. [DOI] [PubMed] [Google Scholar]
- 60.Shaw LJ, Merz CN, Pepine CJ, et al. Women's Ischemia Syndrome Evaluation (WISE) Investigators. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 61.Jespersen L, Abildstrom SZ, Hvelplund A, et al. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PLoS One. 2014;9:e93170. doi: 10.1371/journal.pone.0093170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nichols WW, Denardo SJ, Johnson BD, et al. Increased wave reflection and ejection duration in women with chest pain and nonobstructive coronary artery disease: ancillary study from the Women's Ischemia Syndrome Evaluation. J Hypertens. 2013;31:1447–54. doi: 10.1097/HJH.0b013e3283611bac. discussion 1454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw LJ, Shaw RE, Merz CN, et al. American College of Cardiology–National Cardiovascular Data Registry Investigators. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 64.Burke AP, Virmani R, Galis Z, et al. 34th Bethesda Conference: Task force #2--What is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41:1874–86. doi: 10.1016/s0735-1097(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Humphries KH, Pu A, Gao M, et al. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155:375–81. doi: 10.1016/j.ahj.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 67.Kothawade K, Bairey Merz CN. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr Probl Cardiol. 2011;36:291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Ferrari GM, Fox KA, White JA, et al. Outcomes among non-ST-segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37,101 patients. Eur Heart J Acute Cardiovasc Care. 2014;3:37–45. doi: 10.1177/2048872613489315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gehrie ER, Reynolds HR, Chen AY, et al. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. 2009;158:688–94. doi: 10.1016/j.ahj.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Patel MR, Chen AY, Peterson ED, et al. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152:641–7. doi: 10.1016/j.ahj.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 71.Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–22. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnston N, Jönelid B, Christersson C, et al. Effect of gender on patients with ST-elevation and non-ST-elevation myocardial infarction without obstructive coronary artery disease. Am J Cardiol. 2015;115:1661–6. doi: 10.1016/j.amjcard.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Stone GW, Narula J. The myth of the mild vulnerable plaques. J Am Coll Cardiol Img. 2013;6:1124–6. doi: 10.1016/j.jcmg.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 74.De Araújo Gonçalves P, Brito J, Sousa PJ, et al. Nonobstructive coronary disease leading to STEMI: assessment of residual stenosis after thrombus aspiration. Coron Artery Dis. 2013;24:154–9. doi: 10.1097/MCA.0b013e32835c46bd. [DOI] [PubMed] [Google Scholar]
- 75.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. J Am Coll Cardiol Img. 2012;5:S62–72. doi: 10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–906. doi: 10.1161/CIRCRESAHA.116.304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burke AP, Kolodgie FD, Farb A, et al. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 78.Johnson BD, Eteiba W, Sharaf B, et al. Sudden cardiac death and severity of obstructive coronary artery disease A report from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2009;120:S394. abstr. [Google Scholar]
- 79.Smilowitz NR, Sampson BA, Abrecht CR, et al. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J. 2011;161:681–8. doi: 10.1016/j.ahj.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 80.Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–10. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 81.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Juutilainen A, Kortelainen S, Lehto S, et al. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27:2898–904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 83.Natarajan S, Liao Y, Cao G, et al. Sex differences in risk for coronary heart disease mortality associated with diabetes and established coronary heart disease. Arch Intern Med. 2003;163:1735–40. doi: 10.1001/archinte.163.14.1735. [DOI] [PubMed] [Google Scholar]
- 84.Huxley RR, Peters SA, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206. doi: 10.1016/S2213-8587(14)70248-7. [DOI] [PubMed] [Google Scholar]
- 85.Di Carli MF, Charytan D, McMahon GT, et al. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med. 2011;52:1369–77. doi: 10.2967/jnumed.110.082883. [DOI] [PubMed] [Google Scholar]
- 86.Iozzo P, Chareonthaitawee P, Rimoldi O, et al. Mismatch between insulin-mediated glucose uptake and blood flow in the heart of patients with Type II diabetes. Diabetologia. 2002;45:1404–9. doi: 10.1007/s00125-002-0917-3. [DOI] [PubMed] [Google Scholar]
- 87.Nahser PJ, Jr, Brown RE, Oskarsson H, et al. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–40. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- 88.Prior JO, Quiñones MJ, Hernandez-Pampaloni M, et al. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111:2291–8. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 89.Quiñones MJ, Hernandez-Pampaloni M, Schelbert H, et al. Coronary vasomotor abnormalities in insulin-resistant individuals. Ann Intern Med. 2004;140:700–8. doi: 10.7326/0003-4819-140-9-200405040-00009. [DOI] [PubMed] [Google Scholar]
- 90.Schindler TH, Cardenas J, Prior JO, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–95. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 91.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van de Hoef TP, van Lavieren MA, Damman P, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–11. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 93.Ong P, Athanasiadis A, Borgulya G, et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59:655–62. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 94.Mieres JH, Gulati M, Bairey Merz N, et al. American Heart Association Cardiac Imaging Committee of the Council on Clinical Cardiology and the Cardiovascular Imaging and Intervention Committee of the Council on Cardiovascular Radiology and Intervention. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130:350–79. doi: 10.1161/CIR.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 95.Kayikcioglu M, Payzin S, Yavuzgil O, et al. Benefits of statin treatment in cardiac syndrome-X1. Eur Heart J. 2003;24:1999–2005. doi: 10.1016/s0195-668x(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 96.Pizzi C, Manfrini O, Fontana F, et al. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–8. doi: 10.1161/01.CIR.0000100722.34034.E4. [DOI] [PubMed] [Google Scholar]
- 97.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE) Am Heart J. 2011;162:678–84. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen JW, Hsu NW, Wu TC, et al. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–82. doi: 10.1016/s0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 99.Hamasaki S, Higano ST, Suwaidi JA, et al. Cholesterol-lowering treatment is associated with improvement in coronary vascular remodeling and endothelial function in patients with normal or mildly diseased coronary arteries. Arterioscler Thromb Vasc Biol. 2000;20:737–43. doi: 10.1161/01.atv.20.3.737. [DOI] [PubMed] [Google Scholar]
- 100.Sütsch G, Oechslin E, Mayer I, et al. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol. 1995;52:135–43. doi: 10.1016/0167-5273(95)02458-9. [DOI] [PubMed] [Google Scholar]
- 101.Lanza GA, Colonna G, Pasceri V, et al. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–6. A8. doi: 10.1016/s0002-9149(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 102.Bugiardini R, Borghi A, Biagetti L, et al. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. 1989;63:286–90. doi: 10.1016/0002-9149(89)90332-9. [DOI] [PubMed] [Google Scholar]
- 103.Eriksson BE, Tyni-Lennè R, Svedenhag J, et al. Physical training in Syndrome X: physical training counteracts deconditioning and pain in Syndrome X. J Am Coll Cardiol. 2000;36:1619–25. doi: 10.1016/s0735-1097(00)00931-1. [DOI] [PubMed] [Google Scholar]
- 104.Cannon RO, III, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–7. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 105.Lerman A, Burnett JC, Jr, Higano ST, et al. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–8. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 106.Sun T, Zhou WB, Luo XP, et al. Oral L-arginine supplementation in acute myocardial infarction therapy: a meta-analysis of randomized controlled trials. Clin Cardiol. 2009;32:649–52. doi: 10.1002/clc.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adamson DL, Webb CM, Collins P. Esterified estrogens combined with methyltestosterone improve emotional well-being in postmenopausal women with chest pain and normal coronary angiograms. Menopause. 2001;8:233–8. doi: 10.1097/00042192-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 108.Guarini G, Huqi A, Morrone D, et al. Pharmacological approaches to coronary microvascular dysfunction. Pharmacol Ther. 2014;144:283–302. doi: 10.1016/j.pharmthera.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 109.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 110.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]


