Abstract
Purpose of review
Cancer-related muscle loss, or cachexia, is the cause of death for approximately 2 million people worldwide and severely reduces quality of life. The degree of cachexia is inversely correlated with survival time; however, the exact mechanisms behind cancer-induced muscle wasting remain under investigation.
Recent findings
Cytokines such as TNF-α trigger degradatory pathways through NF-κB signaling that activate the ubiquitin-proteasome system and muscle proteolysis. Androgen treatment has been shown to reduce inflammatory cytokines and even stimulate anti-inflammatory cytokine production. Amino acid supplementation has been shown to induce muscle protein synthesis in ovarian cancer patients.
Summary
Targeted anabolic therapies aimed at preventing or reversing cancer cachexia might involve the combined use of androgens and amino acids working concurrently to enhance muscle protein synthesis and reduce muscle protein breakdown. Additional focused clinical studies are needed to identify muscle-specific targets or biomarkers for defined therapeutic approaches to slow or prevent cancer cachexia. In this paper we review the pathogenesis of cancer-related muscle wasting and discuss potential interventions at reversing or preventing cancer-related muscle loss.
Keywords: MAFbx (muscle atrophy F box), androgens, amino acids, MuRF-1 (Muscle-specific RING-Finger-1), cachexia
Introduction
It is estimated that approximately 2 million people die annually worldwide due to the consequences of cancer-related cachexia (1–3). Cachexia is responsible for the death of about 20% of cancer patients (2, 4) with muscle wasting as the most important phenotypic feature. It is the principal cause of functional impairment, fatigue and respiratory complications. Loss of skeletal muscle proteins and adipose tissue reaches 75% and 85% respectively when the patient has lost 30% body weight; and without therapeutic intervention, often leads to death (5). Cancer-related muscle loss results in asthenia and functional impairments similar to that seen with age-related sarcopenia (6) and there are common metabolic abnormalities to both cancer cachexia and sarcopenia; such as altered hormone levels (7, 8), elevated cytokines (2, 7–9), increased insulin resistance (7), increased muscle proteolysis (8, 10, 11), elevated synthesis of acute phase proteins (7) and altered nutrient utilization (8). Yet despite these commonalities, the rapid loss of muscle mass associated with cachexia, in contrast to the progressive declines seen with sarcopenia, more negatively impacts the quality of life and well-being of cancer patients.
In the case of cancer, a significant nitrogen flux can occur from the skeletal muscle to the liver (12). This decreases the precursor supply of key branched-chain amino acids (BCAAs) in the plasma necessary to stimulate muscle protein synthesis. The liberated amino acids are used for both acute phase protein (APP) synthesis and gluconeogenesis (13, 14), rather than muscle protein synthesis. The APP response is activated by inflammatory cytokines such as IL-6, IL-8 and TNF-α (15, 16). A recent study has shown that cancer cachexia results in an increase in TNF-α receptor gene expression in both skeletal muscle and adipose tissue (7). Further, systemic inflammation has been shown to correlate with increased expression of skeletal muscle ubiquitin in cancer patients demonstrating increased levels of IL-6, sTNF-R and C-reactive protein (CRP) (17). In this paper we review the pathogenesis of cancer-related muscle wasting and discuss potential interventions at reversing or preventing cancer-related muscle loss.
Overview of Cachexia-Related Muscle Protein Degradation
The most important proteolytic pathway in cancer cachexia is considered to be ubiquitin-dependent proteolysis of the skeletal muscle (5, 18). It is now well established that the ubiquitin-proteasome system is of primary importance during protein degradation that occurs in muscle as it atrophies (5, 18). The ubiquitin-proteasome system involves two successive steps. First, the target protein is polyubiquitinated and then degraded by the 26S proteasome (10). Polyubiquitination involves the sequential action of the ubiquitin-activating enzyme (E1), ubiquitin conjugating enzyme (E2) and ubiquitin-protein ligase (E3), which is the key enzyme in the process. E1 has low expression in skeletal muscle and its mRNA level is not regulated in catabolic states (10). Conversely, E2s are expressed in multiple mammalian cells, however only a few are over-expressed in certain instances of muscle wasting (10). Typically, one E2 interacts with one or a limited number of E3 species, which recognize specific protein substrates. E3 forms by far the largest family of ubiquitination enzymes; however, only a limited number of E3s have been identified to be up-regulated in muscle wasting (10).(19)
Two muscle-specific E3s, atrogin-1/MAFbx (muscle atrophy F-box protein) and MuRF-1 (muscle-specific RING-Finger-1), seem to be over-expressed in several catabolic conditions (20–22). Atrogin-1/MAFbx and MuRF-1 interact with the α-actinin-2-calcineurin A complex in cardiomyocytes (10, 20), and with various proteins in skeletal muscle cells (10). The increased transcription of atrogin-1/MAFbx seems to be under the control of the forkhead transcription factor, FoxO (forkhead box O) (23, 24), while MuRF-1 transcription is driven by the activation of NF-κB (25).
Pathogenesis of Muscle Wasting in Cancer Cachexia
The interplay between proteolytic, degradative pathways and anabolic, synthetic pathways has been well summarized (26) and can be upset by events such as aging and various disease states (Figure 1). Muscle atrophy occurs when the balance shifts to an increase in muscle protein breakdown, a decrease in muscle protein synthesis, or both. Cancer cachexia likely involves both sides of the metabolic equation. This switch is regulated at a genetic level by activation of transcription factors such as NF-κB (9, 25). Activation of NF-κB by cytokines (8, 9, 27) and proteolysis-inducing factor (PIF) (8) is an important first step in the intracellular signaling pathway leading to activation of NF-κB mediated muscle protein breakdown (8, 27) as well as apoptosis (8, 27).
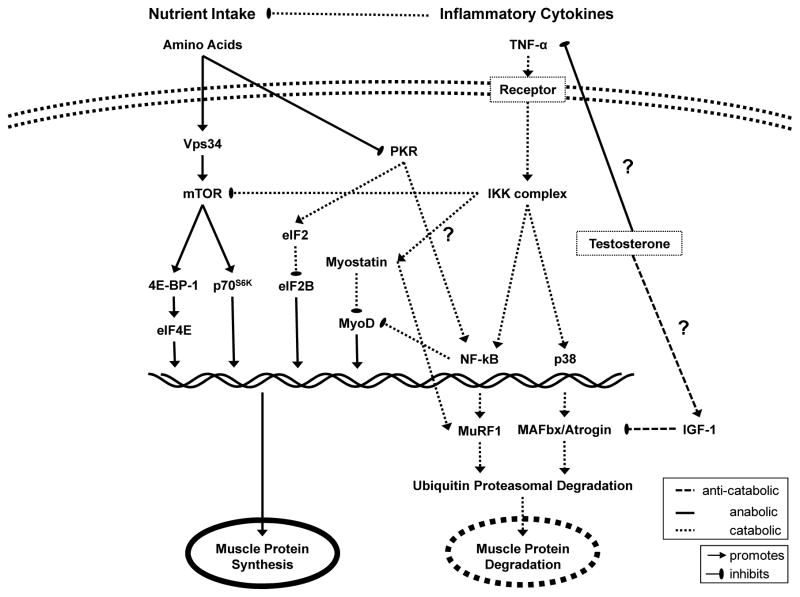
Figure 1. Signaling pathways likely involved in cancer cachexia and proposed effects of amino acid and testosterone treatment on muscle protein synthesis.
A large contributing factor to cancer cachexia is the presence of inflammatory cytokines such as TNF-α which act to both suppress appetite and stimulate the proteasomal degredation pathway through activation of NF-κB. Testosterone may work to directly decrease the inflammatory cytokine burden, downstream to suppress upregulation of MuRF-1 and MAFbx or a combination of both pathways. Testosterone treatment, in conjunction with oral amino acid supplements, may then help tip the balance away from muscle degradation and improve quality of life in patients suffering from cancer cachexia.
While the exact molecular mechanism remains elusive, it has been demonstrated that NF-κB-dependent muscle wasting is activated by late-stage cancer or chronic inflammation (28). Further, NF-κB stimulates the proteasomal machinery by activating muscle specific E3 ligase MuRF-1 (29). While one of the main activators of NF-κB is the cytokine TNF-α (9), recent in vivo data has also shown NF-κB is capable of inducing muscle protein breakdown via a noncytokine-mediated pathway in cachectic conditions (8).
Cytokines, Inflammation and Cancer
A central mechanism of action in cachexia has been postulated for many cytokines. In particular, IL-1 (30) and TNF (30) have been linked to cancer-related anorexia (30, 31), perhaps by increasing corticotropin-releasing hormone (CRH) levels, a neurotransmitter that suppresses food intake. However, cytokines can induce both systemic and local inflammation which can globally affect the patient’s metabolism and locally activate muscle protein breakdown.
Cytokines by themselves are capable of inducing weight loss. TNF-α has long been associated with muscle pathology and was originally designated ‘cachectin’ in recognition of its catabolic action. In humans, muscle catabolism has been attributed to TNF-α in inflammatory diseases that include cancer, congestive heart failure, AIDS, and chronic obstructive pulmonary disease (COPD). To date, evidence of increased TNF-α in plasma of cancer patients is controversial perhaps due to different sensitivities of the assay methods, short half-life of TNF in vivo, or localized paracrine production of TNF.
The mechanism of TNF-α effects in vivo remains largely enigmatic, although it has long been recognized that TNF-α may stimulate catabolism via indirect mechanisms (Figure 1). TNF-α alters circulating levels of hormones that regulate muscle growth and affects tissue sensitivity to such factors. TNF-α also stimulates production of catabolic cytokines and induces anorexia. Any of these effects could indirectly promote muscle wasting. Mechanisms by which TNF-α might directly stimulate catabolism are less clear. One potential mechanism is by inhibiting myoblast differentiation that could limit the regenerative response of satellite cells to muscle injury (32). A second mechanism could be a direct catabolic effect on differentiated muscle. Accelerated protein loss can be induced using TNF-α levels that do not stimulate cell death, by either apoptosis or necrosis, and are within the range measured clinically (32).
At least three major pathways mediate the cellular response to TNF-α with activation of NF-κB, a primary mediator of transcriptional control, being the major candidate for catabolic signaling. TNF-α stimulates the activation and nuclear translocation of NF-κB in skeletal muscle cells. This is a rapid, dose-dependent response that involves phosphorylation and proteasomal degradation of the NF-κB-inhibitory protein, Iκ-Bα (33). NF-κB activity in the cell nucleus peaks within 30 minutes of TNF-α exposure and then rapidly decays. However, this transient stimulus is sufficient to alter gene expression and cause prolonged changes in muscle protein levels. NF-κB signaling is essential for TNF-α-induced catabolism in differentiated muscle cells (32).
In addition to TNF-α, IL-6 is the main mediator in the hepatic acute phase response in cancer cachexia. IL-6 inhibits hepatic albumin production and correlates positively with serum levels of CRP in pancreatic cancer patients. Increased levels of IL-6 are associated with large tumor size, significantly greater weight loss and overall poor prognosis (34). In vitro administration of anti-IL-6 antibodies has been successful in attenuating the cachectic and the acute phase response (6, 35). Therefore, IL-6 is considered a prime regulator of the acute phase response in cachectic patients.
Anabolic Role of Androgens
The metabolic significance of human skeletal muscle is often overlooked, despite that it comprises ~40–50% of total body weight, and is one of the most metabolically active tissues in the body. Besides its obvious importance for human locomotion, the metabolic flexibility of muscle as well as its substrate storage capacity makes it an ideal hormonal target. Skeletal muscle serves as an abundant repository of protein and free amino acids. The homeostatic balance of muscle is affected by fasting (36, 37), feeding (36, 38, 39), exercise (36, 40), aging (36, 41) and disease (36, 42, 43). Hormones such as testosterone, growth hormone (GH), insulin, insulin-like growth factor-1 (IGF-1) and glucocorticoids have profound influences on human skeletal muscle and are important regulators of this remodeling process (36). How a specific hormone exerts its anabolic action on muscle depends on factors such as age, sex, duration of exposure and amount administered. Ultimately, hormones are responsible for modulating the switch toward positive or negative muscle protein balance, which can significantly alter one’s health status.
Our understanding of the in vivo actions of hormones both at the whole-body level and the level of the muscle has greatly advanced owing to the use of isotopic tracer technologies (36). One short term study in healthy males showed for the first time that androgens directly stimulate muscle protein synthesis and stimulate mRNA expression of the androgen receptor in skeletal muscle (44–46). These findings shifted the focus to using testosterone in men (47, 48) and women with muscle wasting diseases such as HIV-AIDS (49). The use of anabolic agents as replacement therapies for diseased-caused muscle wasting is clinically justified (36) based on preexisting evidence for the anti-inflammatory inhibition of cytokines such as IL-6 and TNF-α by these agents (50). The primary goal of androgen therapy is to improve or maintain muscle mass, muscle strength and functional capability (36).
Anti-catabolic Properties of Androgens
Relatively few interventional therapies have shown much promise against cancer cachexia, although almost 30 years ago Chlebowski et al. (51) showed that plasma total and free testosterone levels were decreased by 43% and 66% in adult males with disseminated cancer prior to chemotherapy. Additionally, 82% of these patients were less than 90% of ideal body weight showing that preserving or restoring muscle mass and improving nutritional status is critical in providing patients with opportunities to withstand the detrimental metabolic effects of aggressive chemotherapeutics (52, 53).
The use of anabolic steroids to stimulate protein anabolism in muscle of cancer patients is undoubtedly promising and clinically relevant, yet data are scant and warrant further controlled clinical trials (1, 54). A weight loss study conducted in cancer patients involving a randomized controlled trial of weekly nandrolone decanoate for 4 weeks in combination with standard chemotherapy (57, 58) showed that survival time was significantly longer in the group receiving androgen therapy (median survival 5.5 months without treatment and 8.2 months with nandrolone treatment) with a trend for less severe weight loss with nandrolone decanoate. Indeed, testosterone has been found capable of reducing systemic inflammatory cytokines such as TNF-α, IL-6 and IL-1β in humans (55), and stimulating the anti-inflammatory cytokine IL-10 (55). While testosterone appears to exert anticatabolic effects, the mechanism has yet to be discovered as to whether testosterone functions upstream of inflammatory cytokines or at downstream markers such as MuRF-1 and NF-κB. For a more in-depth review of the mechanisms of action of testosterone see a review by Bhasin et al. (56).
The regulation of skeletal muscle hypertrophy has been linked to both the direct actions of testosterone and its indirect actions on IGF-1. We have demonstrated that during the first month of androgen therapy, the primary effect of testosterone on skeletal muscle was increased muscle protein synthesis shifting to a reduction in protein breakdown by 6 months (45). Therefore, the immediate and sustained elevation of IGF-1 with testosterone therapy and the subsequent suppression of muscle protein breakdown provide evidence that testosterone may exert anti-catabolic properties via the IGF-1 pathway. Additionally, IGF-1 has recently been shown to block the transcriptional upregulation of key mediators of skeletal muscle atrophy, the ubiquitin E3 ligases MuRF-1 and MAFbx/Atrogin-1 (29). Although, proteolysis in the ubiquitin-proteasome system does not account for all protein loss that occurs with catabolic conditions such as cancer. While a reconstituted ubiquitin-proteasome system rapidly degrades actin or myosin, it cannot degrade actomyosin or myofibrils (59, 60). Thus, before the ubiquitin-proteasome system can fully break down the complex structure of muscle protein, another protease must act to initiate proteolysis. Work by Du et al. (61) found that recombinant caspase-3 cleaves actomyosin and leaves a “footprint” in the form of a 14-kD actin fragment that can be identified by Western blot analysis. Undoubtedly, work in the coming years will uncover the specific mechanisms that trigger cancer-induced muscle wasting and the exact mechanisms by which agents such as testosterone work to counter the devastating catabolic consequences on skeletal muscle.
Targeted role of Amino Acids
During convalescence from an illness such as cancer, the anabolic stimulus provided by nutrient ingestion represents a primary means of ameliorating the loss of muscle protein. Studies of healthy elderly and young individuals during acute (38, 62–67) and bedrest-induced catabolism (68) demonstrated that essential amino acids (EAAs) are largely responsible for the amino acid induced stimulation of muscle protein anabolism. Of particular interest is a study by Dillon et al. (69) that showed oral amino acid supplementation could induce muscle protein synthesis in ovarian cancer patients. This study showed that even in instances of systemic inflammation and during chemotherapy, amino acid supplements can improve net protein balance in skeletal muscle.
Unfortunately, weight loss due to cachexia is not likely reversible with regular mixed meals alone. Branched-chain amino acids (BCAAs), and leucine in particular, have received considerable scientific study, focusing on their ability to stimulate protein synthesis in in vitro preparations of skeletal muscle (70). Likewise, BCAAs are largely responsible for the stimulation of protein synthesis in skeletal muscle produced by ingestion of a mixed meal (70). Leucine and other BCAAs are known to stimulate protein synthesis in muscle by initiating signal-transduction pathways that moderate translation initiation (71) through a number of mechanisms (70). Much of the work in our laboratory (67, 72) has focused on tailoring the proper amount and type of amino acids needed to most efficiently stimulate muscle protein synthesis in age-related sarcopenia (67, 68, 72, 73), and leucine’s ability to reverse anabolic signaling defects was recently shown (67, 74).
In cancer, BCAAs have been recognized for many years for their prophagic effects (75) and have been found to stimulate food intake and counteract muscle wasting in anorectic, weight-losing patients (76). A study using BCAAs in hepatic cancer patients demonstrated marked improvements in metabolic parameters, morbidity and quality of life (77). We recently showed, for the first time, in vivo evidence in elderly humans that a relatively small bolus of ingested leucine (2.8 g) can acutely improve muscle protein retention and reverse a lack of stimulation of muscle protein synthesis following the ingestion of a small amount of EAA (74). Whether the effects of leucine on muscle protein anabolism can be sustained over longer periods of time in the presence of cancer cachexia in humans remains to be studied.
Severe and chronic illnesses can induce peripheral muscle proteolysis early in the course of the disease to provide the amino acids required for the synthesis of various liver proteins. Accordingly, the BCAAs (leucine, isoleucine, and valine) have been used to improve positive nitrogen balance. BCAAs also compete for tryptophan across the blood–brain barrier. Through this action, BCAAs can block increased hypothalamic activity of serotonin and counteract anorexia. Studies are needed to test more targeted nutritional interventions such as BCAAs and androgens combined in patients with cachexia.
Conclusion
The mechanisms behind cancer-related cachexia show heavy involvement of TNF-α induced activation of NF-κB and the proteasomal degradation pathway. Initial studies attempting to restore positive nitrogen balance have shown promise. Targeted anabolic therapies aimed at preventing or reversing cancer-induced muscle loss might involve the combined use of androgens and amino acids, but additional focused clinical trials are needed to identify muscle-specific targets or biomarkers for defined therapeutic approaches to slow or prevent cancer cachexia.
Acknowledgments
This study was funded by a National Cancer Institute grant to M. Sheffield-Moore (RO1CA127971)
References
- 1.Muscaritoli M, Bossola M, Aversa Z, Bellantone R, Rossi Fanelli F. Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer. 2006 Jan;42(1):31–41. doi: 10.1016/j.ejca.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002 Mar-Apr;52(2):72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald N, Easson AM, Mazurak VC, Dunn GP, Baracos VE. Understanding and managing cancer cachexia. J Am Coll Surg. 2003 Jul;197(1):143–61. doi: 10.1016/S1072-7515(03)00382-X. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009 Apr;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 5.Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda) 2005 Oct;20:340–8. doi: 10.1152/physiol.00019.2005. [DOI] [PubMed] [Google Scholar]
- 6.Argiles JM, Busquets S, Garcia-Martinez C, Lopez-Soriano FJ. Mediators involved in the cancer anorexia-cachexia syndrome: past, present, and future. Nutrition. 2005 Sep;21(9):977–85. doi: 10.1016/j.nut.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Figueras M, Busquets S, Carbo N, Almendro V, Argiles JM, Lopez-Soriano FJ. Cancer cachexia results in an increase in TNF-alpha receptor gene expression in both skeletal muscle and adipose tissue. Int J Oncol. 2005 Sep;27(3):855–60. [PubMed] [Google Scholar]
- 8.Saini A, Faulkner S, Al-Shanti N, Stewart C. Powerful signals for weak muscles. Ageing Res Rev. 2009 Oct;8(4):251–67. doi: 10.1016/j.arr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003 Jan 24;278(4):2294–303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- 10.Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–86. doi: 10.1042/EB0410173. [DOI] [PubMed] [Google Scholar]
- 11.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. Faseb J. 1998 Jul;12(10):871–80. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 12.Choudry HA, Pan M, Karinch AM, Souba WW. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr. 2006 Jan;136(1 Suppl):314S–8S. doi: 10.1093/jn/136.1.314S. [DOI] [PubMed] [Google Scholar]
- 13.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994 Jun;124(6):906–10. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 14.Harvie MN, Campbell IT. Energy balance, cancer and the sympathetic nervous system. Eur J Cancer. 2000 Feb;36(3):289–92. doi: 10.1016/s0959-8049(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks RE, Forbes MA, Storr M, Higginson J, Thompson D, Raynes J, et al. The acute phase protein response in patients receiving subcutaneous IL-6. Clin Exp Immunol. 1995 Oct;102(1):217–23. doi: 10.1111/j.1365-2249.1995.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeJong CH, Busquets S, Moses AG, Schrauwen P, Ross JA, Argiles JM, et al. Systemic inflammation correlates with increased expression of skeletal muscle ubiquitin but not uncoupling proteins in cancer cachexia. Oncol Rep. 2005 Jul;14(1):257–63. [PubMed] [Google Scholar]
- 18.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999 Jan;129(1S Suppl):227S–37S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 19.Lecker SH. Ubiquitin-protein ligases in muscle wasting: multiple parallel pathways? Curr Opin Clin Nutr Metab Care. 2003 May;6(3):271–5. doi: 10.1097/01.mco.0000068963.34812.e5. [DOI] [PubMed] [Google Scholar]
- 20.Adams V, Linke A, Gielen S, Erbs S, Hambrecht R, Schuler G. Modulation of Murf-1 and MAFbx expression in the myocardium by physical exercise training. Eur J Cardiovasc Prev Rehabil. 2008 Jun;15(3):293–9. doi: 10.1097/HJR.0b013e3282f3ec43. [DOI] [PubMed] [Google Scholar]
- 21.Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am J Physiol Endocrinol Metab. 2005 Dec;289(6):E1007–14. doi: 10.1152/ajpendo.00109.2005. [DOI] [PubMed] [Google Scholar]
- 22.Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004 Jun;18(9):1025–7. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- 23.Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol. 2008 Oct;295(4):C986–93. doi: 10.1152/ajpcell.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004 Apr 30;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004 Oct 15;119(2):285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Durham WJ, Dillon EL, Sheffield-Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 2009 Jan;12(1):72–7. doi: 10.1097/MCO.0b013e32831cef61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Chen X, Fan M. Signaling mechanisms involved in disuse muscle atrophy. Med Hypotheses. 2007;69(2):310–21. doi: 10.1016/j.mehy.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 28.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004 Dec 29;119(7):907–10. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005 Oct;37(10):1974–84. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Tanca FM, Madeddu C, Macciò A, Serpe R, Panzone F, Antoni G, et al. New perspective on the nutritional approach to cancer-related anorexia/cachexia: preliminary results of a randomised phase III clinical trial with five different arms of treatment. Mediterr J Nutr Metab. 2009;2:29–36. [Google Scholar]
- 31.Mantovani G, Maccio A, Madeddu C, Mura L, Massa E, Mudu MC, et al. Serum values of proinflammatory cytokines are inversely correlated with serum leptin levels in patients with advanced stage cancer at different sites. J Mol Med. 2001 Jul;79(7):406–14. doi: 10.1007/s001090100234. [DOI] [PubMed] [Google Scholar]
- 32.Reid MB, Li YP. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res. 2001;2(5):269–72. doi: 10.1186/rr67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Y, Weinman S, Boldogh I, Walker RK, Brasier AR. Tumor necrosis factor-alpha-inducible IkappaBalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. J Biol Chem. 1999 Jan 8;274(2):787–94. doi: 10.1074/jbc.274.2.787. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, et al. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007 Jan;69(1):113–7. doi: 10.1016/j.urology.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Strassmann G, Fong M, Freter CE, Windsor S, D’Alessandro F, Nordan RP. Suramin interferes with interleukin-6 receptor binding in vitro and inhibits colon-26-mediated experimental cancer cachexia in vivo. J Clin Invest. 1993 Nov;92(5):2152–9. doi: 10.1172/JCI116816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheffield-Moore M, Urban RJ. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab. 2004 Apr;15(3):110–5. doi: 10.1016/j.tem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Fryburg DA, Barrett EJ, Louard RJ, Gelfand RA. Effect of starvation on human muscle protein metabolism and its response to insulin. Am J Physiol. 1990 Oct;259(4 Pt 1):E477–82. doi: 10.1152/ajpendo.1990.259.4.E477. [DOI] [PubMed] [Google Scholar]
- 38.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003 Aug;78(2):250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003 Jan;88(1):358–62. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- 40.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997 Jul;273(1 Pt 1):E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 41.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001 Sep 12;286(10):1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001 Oct;29(10):1936–42. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Lim VS, Yarasheski KE, Crowley JR, Fangman J, Flanigan M. Insulin is protein-anabolic in chronic renal failure patients. J Am Soc Nephrol. 2003 Sep;14(9):2297–304. doi: 10.1097/01.asn.0000085590.83005.a0. [DOI] [PubMed] [Google Scholar]
- 44.Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999 Aug;84(8):2705–11. doi: 10.1210/jcem.84.8.5923. [DOI] [PubMed] [Google Scholar]
- 45.Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000 Apr;32(3):181–6. doi: 10.3109/07853890008998825. [DOI] [PubMed] [Google Scholar]
- 46.Wannenes F, Caprio M, Gatta L, Fabbri A, Bonini S, Moretti C. Androgen receptor expression during C2C12 skeletal muscle cell line differentiation. Mol Cell Endocrinol. 2008 Sep 24;292(1–2):11–9. doi: 10.1016/j.mce.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Grinspoon S, Corcoran C, Anderson E, Hubbard J, Stanley T, Basgoz N, et al. Sustained anabolic effects of long-term androgen administration in men with AIDS wasting. Clin Infect Dis. 1999 Mar;28(3):634–6. doi: 10.1086/515162. [DOI] [PubMed] [Google Scholar]
- 48.Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. Jama. 2000 Feb 9;283(6):763–70. doi: 10.1001/jama.283.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grinspoon S, Corcoran C, Miller K, Biller BM, Askari H, Wang E, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1997 May;82(5):1332–7. doi: 10.1210/jcem.82.5.3907. [DOI] [PubMed] [Google Scholar]
- 50.Witte KK, Clark AL. Nutritional abnormalities contributing to cachexia in chronic illness. Int J Cardiol. 2002 Sep;85(1):23–31. doi: 10.1016/s0167-5273(02)00231-0. [DOI] [PubMed] [Google Scholar]
- 51.Chlebowski RT, Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res. 1982 Jun;42(6):2495–8. [PubMed] [Google Scholar]
- 52.Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer-related cachexia. Nutrition. 2001 Jan;17(1 Suppl):S1–20. doi: 10.1016/s0899-9007(01)80001-0. [DOI] [PubMed] [Google Scholar]
- 53.Griffiths RD, Hinds CJ, Little RA. Manipulating the metabolic response to injury. Br Med Bull. 1999;55(1):181–95. doi: 10.1258/0007142991902204. [DOI] [PubMed] [Google Scholar]
- 54.Muscaritoli M, Bossola M, Bellantone R, Rossi Fanelli F. Therapy of muscle wasting in cancer: what is the future? Curr Opin Clin Nutr Metab Care. 2004 Jul;7(4):459–66. doi: 10.1097/01.mco.0000134366.07148.2e. [DOI] [PubMed] [Google Scholar]
- 55.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004 Jul;89(7):3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 56.Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, et al. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006 Mar;2(3):146–59. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bossola M, Pacelli F, Tortorelli A, Doglietto GB. Cancer cachexia: it’s time for more clinical trials. Ann Surg Oncol. 2007 Feb;14(2):276–85. doi: 10.1245/s10434-006-9179-5. [DOI] [PubMed] [Google Scholar]
- 58.Chlebowski RT, Herrold J, Ali I, Oktay E, Chlebowski JS, Ponce AT, et al. Influence of nandrolone decanoate on weight loss in advanced non-small cell lung cancer. Cancer. 1986 Jul 1;58(1):183–6. doi: 10.1002/1097-0142(19860701)58:1<183::aid-cncr2820580131>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 59.Du J, Hu Z, Mitch WE. Molecular mechanisms activating muscle protein degradation in chronic kidney disease and other catabolic conditions. Eur J Clin Invest. 2005 Mar;35(3):157–63. doi: 10.1111/j.1365-2362.2005.01473.x. [DOI] [PubMed] [Google Scholar]
- 60.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996 Oct 25;271(43):26690–7. doi: 10.1074/jbc.271.43.26690. [DOI] [PubMed] [Google Scholar]
- 61.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004 Jan;113(1):115–23. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paddon-Jones D, Sheffield-Moore M, Creson DL, Sanford AP, Wolf SE, Wolfe RR, et al. Hypercortisolemia alters muscle protein anabolism following ingestion of essential amino acids. Am J Physiol Endocrinol Metab. 2003 May;284(5):E946–53. doi: 10.1152/ajpendo.00397.2002. [DOI] [PubMed] [Google Scholar]
- 63.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998 May 1;101(9):2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999 Sep;277(3 Pt 1):E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 65.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, et al. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006 Sep;84(3):623–32. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 66.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006 Aug 15;575(Pt 1):305–15. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005 Nov;82(5):1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 68.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004 Mar;286(3):E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 69.Dillon EL, Volpi E, Wolfe RR, Sinha S, Sanford AP, Arrastia CD, et al. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin Nutr. 2007 Dec;26(6):736–43. doi: 10.1016/j.clnu.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006 Jan;136(1 Suppl):227S–31S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 71.Yoshizawa F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem Biophys Res Commun. 2004 Jan 9;313(2):417–22. doi: 10.1016/j.bbrc.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab. 2005 Mar;90(3):1453–9. doi: 10.1210/jc.2004-1702. [DOI] [PubMed] [Google Scholar]
- 73.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006 Feb;41(2):215–9. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006 Aug;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 75.Laviano A, Muscaritoli M, Cascino A, Preziosa I, Inui A, Mantovani G, et al. Branched-chain amino acids: the best compromise to achieve anabolism? Curr Opin Clin Nutr Metab Care. 2005 Jul;8(4):408–14. doi: 10.1097/01.mco.0000172581.79266.19. [DOI] [PubMed] [Google Scholar]
- 76.Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol Dial Transplant. 2001 Sep;16(9):1856–62. doi: 10.1093/ndt/16.9.1856. [DOI] [PubMed] [Google Scholar]
- 77.Cangiano C, Laviano A, Meguid MM, Mulieri M, Conversano L, Preziosa I, et al. Effects of administration of oral branched-chain amino acids on anorexia and caloric intake in cancer patients. J Natl Cancer Inst. 1996 Apr 17;88(8):550–2. doi: 10.1093/jnci/88.8.550. [DOI] [PubMed] [Google Scholar]