Abstract
Introduction
Measurement of energy expenditure (EE) is recommended to guide nutrition in critically ill patients. Availability of a gold standard indirect calorimetry is limited, and continuous measurement is unfeasible. Equations used to predict EE are inaccurate. The purpose of this study was to provide proof of concept that EE can be accurately assessed on the basis of ventilator-derived carbon dioxide production (VCO2) and to determine whether this method is more accurate than frequently used predictive equations.
Methods
In 84 mechanically ventilated critically ill patients, we performed 24-h indirect calorimetry to obtain a gold standard EE. Simultaneously, we collected 24-h ventilator-derived VCO2, extracted the respiratory quotient of the administered nutrition, and calculated EE with a rewritten Weir formula. Bias, precision, and accuracy and inaccuracy rates were determined and compared with four predictive equations: the Harris–Benedict, Faisy, and Penn State University equations and the European Society for Clinical Nutrition and Metabolism (ESPEN) guideline equation of 25 kcal/kg/day.
Results
Mean 24-h indirect calorimetry EE was 1823 ± 408 kcal. EE from ventilator-derived VCO2 was accurate (bias +141 ± 153 kcal/24 h; 7.7 % of gold standard) and more precise than the predictive equations (limits of agreement −166 to +447 kcal/24 h). The 10 % and 15 % accuracy rates were 61 % and 76 %, respectively, which were significantly higher than those of the Harris–Benedict, Faisy, and ESPEN guideline equations. Large errors of more than 30 % inaccuracy did not occur with EE derived from ventilator-derived VCO2. This 30 % inaccuracy rate was significantly lower than that of the predictive equations.
Conclusions
In critically ill mechanically ventilated patients, assessment of EE based on ventilator-derived VCO2 is accurate and more precise than frequently used predictive equations. It allows for continuous monitoring and is the best alternative to indirect calorimetry.
Introduction
The optimal energy target in the first days of critical illness remains controversial [1–3]. Nonetheless, measurement of energy expenditure (EE) is important to prevent early overfeeding and later underfeeding, as both are associated with increased mortality [4–6]. EE can be accurately assessed with indirect calorimetry, which measures oxygen consumption (VO2) and carbon dioxide production (VCO2) from respiratory gases [7, 8]. EE is then calculated using the abbreviated formula published by Weir [9]:
Indirect calorimetry is often not available and is resource- and time-consuming. Daily assessment of EE is not feasible but could be important because EE is known to vary widely over time as a result of changing metabolic rate [10–12]. In the absence of indirect calorimetry, numerous predictive equations are used to estimate EE, including the Harris–Benedict equation and the European Society for Clinical Nutrition and Metabolism (ESPEN) guideline equation of 25 kcal/kg/day [13, 14]. These equations are notoriously inaccurate for individual critically ill patients, owing to large disease-, treatment-, and interindividual-related differences in metabolic rate [15–17]. The Penn State University and Faisy equations were especially developed for mechanically ventilated critically ill patients and include temperature and minute ventilation in the calculation of EE [15, 18]. The Penn State University equation is recommended by the Academy of Nutrition and Dietetics when indirect calorimetry is not feasible [19]. Validation studies for both equations are limited.
An alternative method to assess EE in mechanically ventilated critically ill patients could be the use of VCO2 measurements only. This is practical, as most mechanical ventilators provide the option to measure VCO2 continuously. When VCO2 is known, the Weir formula can be used to calculate VO2, assuming the respiratory quotient (RQ), which is the ratio between VCO2 and VO2. Its physiologic range of 0.67–1.2 depends on the type of the actually metabolized substrate provided that ventilation and acid–base balance are stable [20]. Although the latter vary during critical illness, in prolonged measurement periods metabolic CO2 production equals its excretion. Given these limitations, we hypothesized that EE could be assessed on the basis of ventilator-derived VCO2 using RQ of the administered nutrition and the rewritten Weir formula.
The aim of this study was to provide proof of concept that EE can be accurately assessed on the basis of ventilator-derived VCO2 and nutritional RQ and to determine whether this method is more accurate than frequently used predictive equations.
Material and methods
Study design and setting
This prospective observational study was conducted in the mixed medical-surgical adult intensive care unit (ICU) of the VU University Medical Center in Amsterdam, The Netherlands. The study was approved by the Medical Ethics Committee of the VU University Medical Center. The need for written informed consent was waived because indirect calorimetry is part of routine care in our ICU and imposes no burden on patients.
Subjects
Inclusion criteria were age 18 years or older, mechanical ventilation, ICU stay of 3 days or more, and enteral or parenteral nutrition reaching at least two-thirds of calculated nutritional target. According to the standard practice of the unit, the initial nutritional target was an energy delivery as calculated with the revised Harris–Benedict equation [21], adding 20 % for stress and 10 % for activity [22, 23] and protein delivery of 1.2–1.5 g/kg preadmission body weight per day [24]. This target was adjusted based on indirect calorimetry measurements. An algorithm was used to determine the optimal nutritional product and amount needed to meet both protein and energy requirements [25]. Patients were excluded if they failed to meet accuracy criteria or safety criteria for indirect calorimetry, being a fraction of inspired oxygen (FiO2) greater than 0.6, air leakage through cuff or chest tubes, or a positive end-expiratory pressure (PEEP) greater than 14 cmH2O (arbitrary limit).
Our patient data management system (PDMS) (MetaVision; iMDsoft, Düsseldorf, Germany) was used to routinely record demographic and clinical data; Acute Physiology and Chronic Health Evaluation (APACHE) II and III scores and APACHE IV predicted mortality [26–28]; diagnosis group; type, amount, and composition of feeding; and ventilation characteristics. Sedation was assessed by using the Ramsay Sedation Scale [29].
Study protocol
Patient weight and height were recorded upon ICU admission. Preadmission weight and height were obtained, and, if not available, they were measured or estimated by a clinician. Indirect calorimetry was performed for 24 h. Simultaneously, 24-h minute-by-minute ventilator-derived VCO2, which is routinely exported to the PDMS, was recorded. After the first hour of measurement, type and amount of nutrition were adjusted to meet EE as measured with indirect calorimetry. All macronutrient intake during the study period, including propofol and glucose infusions, were routinely stored in the PDMS.
Methods used to assess energy expenditure
Energy expenditure from indirect calorimetry
Twenty-four–hour indirect calorimetry was performed with a Deltatrac II MBM-200 Metabolic Monitor (Datex, Helsinki, Finland) connected to the ventilator. Before this study, an alcohol-burning test was performed to calibrate the metabolic monitor. Before each 24-h measurement, the metabolic monitor was prepared and calibrated according to the manufacturer’s instructions. Artifact suppression was turned on. For each patient, VCO2, VO2, RQ, and energy expenditure from indirect calorimetry (EE:Calorimetry) were recorded minute by minute and exported to a computer. For comparison, the mean 24-h value was calculated for each patient.
Energy expenditure from ventilator-derived volume of carbon dioxide and nutritional respiratory quotient
We use SERVO-i mechanical ventilators (Maquet, Rastatt, Germany) in our ICU. These have mainstream CO2 sensors connected to the airway adapter that measure end-tidal CO2. Sensors were calibrated before every study period and subsequently at 8-h intervals or more often if necessary. The SERVO-i ventilator calculates VCO2 from the product of CO2 concentration in expiratory air and the expiratory volume (VCO2 = volume × fraction of expired CO2).
VCO2 is displayed breath by breath and exported to the PDMS once each minute. For each patient, 24-h minute-by-minute VCO2 values were collected. To calculate energy expenditure from ventilator-derived volume of carbon dioxide and nutritional respiratory quotient (EE:VCO2), the average 24-h VCO2 (ml/min) was used.
Nutritional RQ was calculated considering 24-h macronutrient delivery, including calories provided by propofol (1.1 kcal/ml) and glucose (4 kcal/g). We assumed RQs of 1 for carbohydrates, 0.7 for fat, and 0.8 for protein. Nutritional RQ was calculated from the weighted average RQ for intake during the study period. For example, if the composition of the enteral formula was 16 % protein, 49 % carbohydrates, and 35 % fat, the nutritional RQ was calculated as 0.16 × 0.8 + 0.49 × 1 + 0.35 × 0.7 = 0.86.
After calculating nutritional RQ for each patient, EE:VCO2 was subsequently calculated using the following rewritten Weir formula:
Energy expenditure derived from predictive equations
EE was calculated using four predictive equations: the Harris–Benedict equation [21], the ESPEN guideline equation [14], the Penn State University 2003b equation [15], and the Faisy equation [18].
Energy expenditure was calculated with the Harris–Benedict equation (EE:HB) as follows:
Energy expenditure was calculated with the European Society for Clinical Nutrition and Metabolism guideline of 25 kcal/kg/day (EE:Esp25).
Energy expenditure was calculated with the Penn State University 2003b equation (EE:PSU) as follows:
The Mifflin-St Jeor calculation is as follows [30]:
Tmax is highest body temperature during the 24-h study period, and Ve is mean minute ventilation during the 24-h study period.
Energy expenditure was calculated with the Faisy equation (EE:Faisy) as follows:
Ve is mean minute ventilation during the 24-h study period, and T is mean temperature during the 24-h study period.
Endpoints
The primary endpoint was accuracy of EE:VCO2 using EE:Calorimetry as a gold standard. Secondary endpoints were the accuracy of EE:HB, EE:Esp25, EE:Faisy, and EE:PSU.
Data analysis
Descriptive data are reported as mean [standard deviation (SD)], median (25th–75th percentile), or number (percentage) as appropriate. Student’s t test was used for comparison of paired data. Correlations were calculated using Pearson’s test, and strength of correlation was expressed as r. The accuracy of the different measurement methods was assessed in accordance with the ISO 5725 standard [31], which describes how accuracy can be defined in terms of bias and precision. Bias is the systematic error as compared with the gold standard (in this case EE:Calorimetry), whereas precision is the random (non-systematic) error of individual measurements. The inaccuracy of a measurement method can thus be due to a large bias (the systematic component), low precision (the random component), or both. Bias was calculated as the mean difference of EE:VCO2 (or equation-based EEs) and gold standard EE (EE:Calorimetry). EE was considered unbiased if the bias was less than 10 % of the gold standard EE [32]. Precision was quantified as the SD of the bias and the limits of agreement (2 SD). SDs of the different methods were compared using Levene’s test for equality of variances. Bland–Altman plots were used to graphically represent bias and limits of agreement [33]. Accuracy was further quantified by accuracy rates, which we defined as the proportion of patients for which the EE:VCO2 (or equation-based EE) predicted EE within 10 % and 15 % of gold standard EE:Calorimetry. We calculated greater than 25 % and greater than 30 % inaccuracy rates to quantify the occurrence of large errors, as the proportion of patients for which the EE:VCO2 (or equation-based EE) differed by more than 25 % or more than 30 % from gold standard EE:Calorimetry.
In a post hoc analysis, we calculated for which stress and activity factor the bias of the Harris–Benedict equation was lowest and used this equation in further data analysis (EE:HB15).
IBM SPSS 20 software (IBM, Armonk, NY, USA) was used for statistical analysis. A p value less than 0.05 was considered statistically significant.
Results
During the study period (20 March to 5 December 2013), 1172 patients were admitted to our ICU. Among them, 163 (13.9 %) were mechanically ventilated for more than 3 days with FiO2 60 % or less and PEEP 14 cmH2O or less. Among these 163 patients, 123 (75 %) received about two-thirds of their nutritional energy target (defined by Harris–Benedict +30 %) and 92 of those 123 had no thoracic drains. Of the 92 eligible patients, 84 patients (91 %) were included (see Fig. 1). The main reason for missed inclusion was absence of a researcher. The included patients’ demographic, clinical, and nutritional characteristics are shown in Table 1. Twenty-six patients (31 %) were female. The most prevalent ICU admission diagnoses were post–cardiac arrest, postsurgery, and trauma. Twelve patients (14 %) had sepsis. The mean APACHE II score was 23.9 ± 8.4. Most patients were on pressure support ventilation (82 %). The mean total macronutrient intake during the 24-h study period was 1835 ± 627 kcal, including caloric intake from glucose and propofol infusions.
Fig. 1.
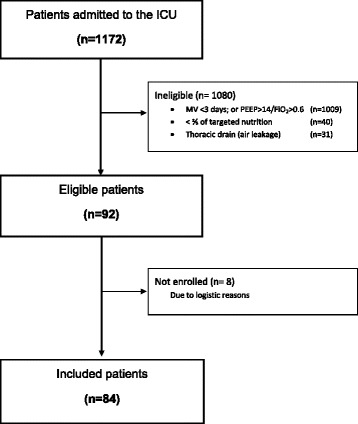
Consolidated Standards of Reporting Trials diagram representing the inclusion of patients. FiO 2 fraction of inspired oxygen, ICU intensive care unit, MV mechanical ventilation, PEEP positive end-expiratory pressure
Table 1.
Demographic, clinical, and nutritional characteristics of the study population
| Characteristics | Data |
|---|---|
| Number of patients | 84 |
| Male, n (%) | 58 (69) |
| Female, n (%) | 26 (31) |
| Age, yr (mean ± SD) | 63.5 ± 14.9 |
| Height, cm (mean ± SD) | 173.7 ± 7.8 |
| Weight, kg (mean ± SD) | 79.1 ± 16.0 |
| BMI, kg/m2 (mean ± SD) | 22.7 ± 4.4 |
| APACHE II score (mean ± SD) | 23.9 ± 8.4 |
| APACHE III score (mean ± SD) | 91.0 ± 34.3 |
| APACHE IV predicted mortality (mean ± SD) | 0.47 ± 0.31 |
| ICU admission diagnosis, n (%) | |
| Trauma | 15 (17.9) |
| Sepsis | 12 (14.3) |
| Respiratory insufficiency | 10 (11.9) |
| Postsurgery | 18 (21.4) |
| Neurologic | 4 (4.8) |
| Post–cardiac arrest | 21 (25) |
| Cardiovascular | 4 (4.8) |
| Length of ICU stay at time of study, days, median (IQR) | 4.0 (3–6) |
| Ramsay Sedation Scale score,a median (IQR) | 5 (4–6) |
| Body temperature, °C (mean ± SD) | 36.8 ± 0.8 |
| Heart rate, beats/minute (mean ± SD) | 91 ± 20 |
| MAP, mmHg (mean ± SD) | 84 ± 14 |
| Norepinephrine, n (%) | 33 (39.3) |
| CVVH, n (%) | 8 (9.5) |
| Respiratory rate, breaths/min, median (IQR) | 19 (15–24) |
| Minute volume, L/min (mean ± SD) | 9.3 ± 3.2 |
| Tidal volume, ml (mean ± SD) | 462 ± 121 |
| PaO2/FiO2 ratio, median (IQR) | 220 (180–263) |
| PEEP, cmH2O, median (IQR) | 8 (5–10) |
| Mechanical ventilation mode, n (%) | |
| PS/CPAP | 69 (81.4) |
| PC | 15 (17.9) |
| Type of nutrition, n (%) | |
| Enteral | 73 (86.9) |
| Parenteral | 4 (4.8) |
| Combination enteral and parenteral | 7 (8.3) |
| Total nutritional intake, kcal/24 h (mean ± SD) | 1748 ± 621 |
| Total macronutrient intake,b kcal/24 h (mean ± SD) | 1835 ± 627 |
| Length of mechanical ventilation, days, median (IQR) | 8 (6–15) |
| Length of stay ICU, days, median (IQR) | 11 (7–18) |
| Length of stay hospital, days, median (IQR) | 23 (13–45) |
| ICU mortality, n (%) | 29 (30.9) |
| Hospital mortality, n (%) | 36 (38.3) |
APACHE acute physiology and chronic health evaluation, BMI body mass index, CPAP continuous positive airway pressure, CVVH continuous venovenous hemofiltration, ICU intensive care unit IQR interquartile range, MAP mean arterial pressure, PaO 2 /FiO 2 ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, PC pressure control, PEEP positive end-expiratory pressure, PS pressure support, SD standard deviation
aRamsay Sedation Scale scoring system: 1 = patient anxious and agitated or restless, or both; 2 = patient cooperative, orientated, and tranquil; 3 = patient responds to commands only; 4 = brisk response to a light glabellar tap or auditory stimulus; 5 = sluggish response to light glabellar tap or auditory stimulus; 6 = no response to the stimuli mentioned for scores 4 and 5
bIncluding intake from intravenous propofol and glucose
Energy expenditure, VO2, VCO2, and RQ
Mean 24-h results for EE, VO2, VCO2, and RQ are presented in Table 2. Mean 24-h EE:Calorimetry was 1823 ± 408 kcal. Mean 24-h EE:VCO2 was 1963 ± 431 kcal, which was significantly higher than EE:Calorimetry (p < 0.001) (see Table 2).
Table 2.
Mean 24-h results of VCO2, VO2, RQ, and EE measurements
| Mean ± SD | p value (vs. calorimetry) | |
|---|---|---|
| VCO2 (ml/min) | ||
| Calorimetry | 225 ± 47 | |
| Ventilator | 240 ± 52 | <0.001 |
| VO2 (ml/min) | ||
| Calorimetry | 265 ± 59 | |
| RQ | ||
| Calorimetry | 0.8592 ± 0.0473 | |
| Nutrition | 0.8636 ± 0.0119 | 0.410 |
| Nutritiona | 0.8629 ± 0.0151 | 0.485 |
| Energy expenditure (kcal/24 h) | ||
| Calorimetry | 1823 ± 408 | |
| VCO2-derived | 1963 ± 431 | <0.001 |
| HB equation | 1576 ± 257 | <0.001 |
| Esp25 | 1979 ± 400 | <0.001 |
| Faisy equation | 1999 ± 269 | <0.001 |
| PSU | 1801 ± 314 | 0.431 |
| HB15 | 1813 ± 295 | 0.724 |
Calorimetry measured with indirect calorimetry, Esp25 European Society for Clinical Nutrition and Metabolism -guideline equation of 25 kcal/kg/day, HB15 Harris–Benedict equation with 15 % added; PSU Penn State University 2003b equation, RQ respiratory quotient, SD standard deviation, VCO 2 -derived carbon dioxide production, VCO 2 -derived from ventilator-derived carbon dioxide production and nutritional respiratory quotient, VO 2 oxygen consumption
aIncluding macronutrient intake from intravenous propofol and glucose
Correlation
EE:VCO2 correlated strongly with EE:Calorimetry (r = 0.935). The equation-based EEs correlated less strongly and the correlation coefficient was lowest for EE:Esp25 (r = 0.639) (see Fig. 2).
Fig. 2.
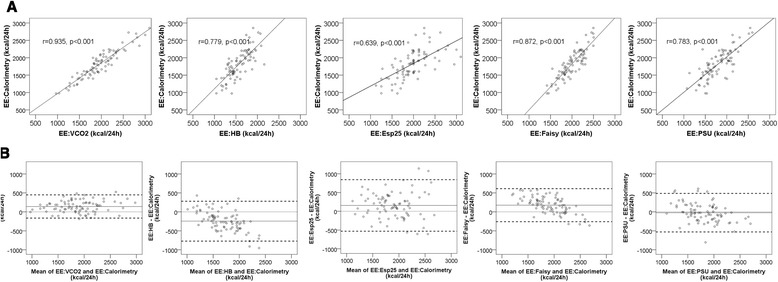
Correlation and agreement between the methods used to assess energy expenditure (EE) and gold standard indirect calorimetry. a Regression plots showing the correlation between the different methods used to assess EE and gold standard indirect calorimetry. b Bland–Altman plots showing the agreement between the methods used to assess EE and gold standard indirect calorimetry. The solid lines indicate the bias (mean difference with indirect calorimetry). The thick dashed lines indicate the limits of agreement (bias ±2 standard deviations). Every dot represents 1 of 84 patients. The x-axis represents the mean of the method used to assess EE and gold standard indirect calorimetry. The y-axis represents the difference in EE in kilocalories per 24 h between the method used and gold standard indirect calorimetry. EE:Esp25, Energy expenditure calculated with the European Society for Clinical Nutrition and Metabolism guideline equation of 25 kcal/kg/day; EE:Faisy, Energy expenditure calculated with the Faisy equation; EE:HB, Energy expenditure calculated with the Harris–Benedict equation; EE:PSU, Energy expenditure calculated with the Penn State University 2003b equation; EE:VCO2, Energy expenditure from ventilator-derived volume of carbon dioxide and nutritional respiratory quotient
Bias (mean difference of EE:VCO2 and predictive equations with EE:Calorimetry)
Bland–Altman plots are shown in Fig. 2. The bias of EE:VCO2 was +141 ± 153 kcal/24 h (7.7 % of EE:Calorimetry). This was significantly lower than the bias of EE:HB (−246 ± 263 kcal/24 h, p < 0.001), comparable to the bias of EE:Faisy (+176 ± 218 kcal/24 h, p = 0.226) and EE:Esp25 (+156 ± 344 kcal/24 h, p = 0.709), but higher than the bias of EE:PSU (−22 ± 254 kcal/24 h, p < 0.001). In post hoc analysis, we calculated that the bias of the Harris–Benedict equation was lowest with a stress and activity factor of +15 % (−10 ± 257 kcal/24 h). See Table 3 for detailed results. The bias of ventilator-derived VCO2 was 14.7 ml/min (6.5 % of VCO2:Calorimetry). The bias of nutritional RQ was 0.0037 (0.4 % of RQ:Calorimetry).
Table 3.
Accuracy of the methods used to assess EE, expressed as bias, precision, and accuracy and inaccuracy rates
| Bias | Precision | Accuracy quantified | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean difference in kcal/day, 95% CI | Mean difference (% of gold standard EE) | SD of bias (Levene’s F-testa) | Bland–Altman limits of agreement | Accuracy rates | Inaccuracy rates | |||
| <10 % | <15 % | >25 % | >30 % | |||||
| Method | ||||||||
| EE:VCO2 b | +141, +107 to +174 | 7.7 % | 153 | −166 to +447 | 61 % | 79 % | 2 % | 0 % |
| EE:HBc | +246 | 13.5 % | 263 | −722 to +280 | 31 % | 52 % | 13 % | 5 % |
| −303 to −189 | p < 0.001 | (F 14.1) | p = 0.001 | p < 0.001 | p < 0.002 | p < 0.01 | ||
| p < 0.001 | ||||||||
| EE:Esp25d | +156, | 8.6 % | 344 | −531 to +843 | 40 % | 56 % | 25 % | 14 % |
| +81 to +230 | p = 0.709 | (F 31.1) | p = 0.009 | p = 0.002 | p < 0.001 | p < 0.001 | ||
| p < 0.001 | ||||||||
| EE:Faisye | +176, | 9.7 % | 218 | −260 to + 612 | 45 % | 61 % | 17 % | 12 % |
| +129 to +233 | p = 0.226 | (F 9.0) | p = 0.01 | p = 0.003 | p < 0.001 | p < 0.001 | ||
| p = 0.003 | ||||||||
| EE:PSUf | −22, | 1.2 % | 254 | −529 to +458 | 54 % | 75 % | 10 % | 6 % |
| −77 to +33 | p < 0.001 | (F 11.9) | p = 0.341 | p = 0.582 | p = 0.05 | p = 0.023 | ||
| p < 0.001 | ||||||||
| Post hoc calculation | ||||||||
| EE:HB15g | −10 | 0.5 % | 257 | −524 to +504 | 55 % | 71 % | 10 % | 6 % |
| −66 to +46 | p < 0.001 | (F 12.3) | p = 0.435 | p = 0.285 | p = 0.05 | p = 0.023 | ||
| p < 0.001 | ||||||||
EE energy expenditure, EE:Esp25 energy expenditure calculated with the European Society for Clinical Nutrition and Metabolism guideline equation of 25 kcal/kg/day, EE:Faisy energy expenditure calculated with the Faisy equation, EE:HB15 energy expenditure calculated with the Harris–Benedict equation with 15 % added, EE:PSU energy expenditure calculated with the Penn State University 2003b equation, EE:VCO 2 energy expenditure from ventilator-derived volume of carbon dioxide and nutritional respiratory quotient, HB Harris–Benedict equation
Less than 10 % and less than 15 % accuracy rates represent the proportion of patients for which EE:VCO2 (or equation-based EE) predicted EE within 10 % and within 15 %, respectively, of gold standard EE:Calorimetry. Greater than 25 % and greater than 30 % inaccuracy rates represent the proportion of patients for whom EE:VCO2 (or equation-based EE) differed by more than 25 % and more than 30 %, respectively, from gold standard EE:Calorimetry
All p values are relative to EE:VCO2.
F-test and p value reflect the comparison of the variance of the mean difference of EE:VCO2 and EE from equations. The higher the F-value, the higher the difference of the variances. p < 0.05 indicates that the variance of the mean difference is significantly different from EE:VCO2.
aLevene’s F-test on similar variances
Bias: b vs. c p < 0.001; b vs. d p = 0.709; b vs. f p = <0.001; b vs. e p < 0.001; b vs. g p = 0.226; c vs. d p < 0.001; c vs. f p < 0.001; c vs. e p < 0.001; c vs. g p < 0.001; d vs. f p = 0.001; d vs. e p < 0.001; d vs. g p = 0.652; f vs. e p = 0.762; e vs. g p < 0.001
Precision
Limits of agreement were smallest for EE:VCO2 (−166 to +447 kcal/24 h) The SD of the bias of EE:VCO2 was significantly smaller than that of all equation-based EE values (see Table 3 and Figs. 2 and 3).
Fig. 3.
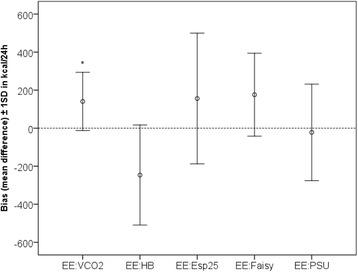
Bias and precision of the methods used to assess energy expenditure (EE). The x-axis shows the different methods used to assess EE. The y-axis represents the bias (mean difference with gold standard indirect calorimetry) and the precision (±1 standard deviation) in kilocalories per day. *Variance of the bias significantly smaller than that of the predictive equations. EE:Esp25, Energy expenditure calculated with the European Society for Clinical Nutrition and Metabolism guideline equation of 25 kcal/kg/day; EE:Faisy, Energy expenditure calculated with the Faisy equation; EE:HB, Energy expenditure calculated with the Harris–Benedict equation; EE:PSU, Energy expenditure calculated with the Penn State University 2003b equation; EE:VCO2, Energy expenditure from ventilator-derived volume of carbon dioxide and nutritional respiratory quotient
Accuracy and inaccuracy rates
Less than 10 % and less than 15 % accuracy rates of EE:VCO2 were 61 % and 79 %, respectively. These were significantly higher than those of EE:HB, EE:Esp25, and EE:Faisy but not significantly different from EE:PSU and EE:HB15. Less than 25 % and less than 30 % inaccuracy rates of EE:VCO2 were 2 % and 0 %, respectively. The less than 30 % inaccuracy rate of EE:VCO2 was significantly lower than that of all equation-based EE values (Table 3 and Fig. 4).
Fig. 4.
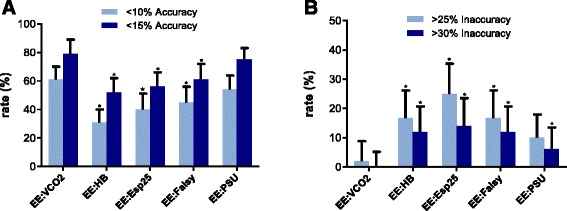
Accuracy and inaccuracy of the different methods quantified in less than 10 % and less than 15 % accuracy rates and greater than 25 % and greater than 30 % inaccuracy rates. a Less than 10 % and less than 15 % accuracy rates were defined as the proportion of patients for whom energy expenditure (EE) was predicted within 10 % and within 15 % of gold standard EE:Calorimetry. b Greater than 25 % and greater than 30 % inaccuracy rates were defined as the proportion of patients for whom EE differed by more than 25 % and more than 30 % from gold standard EE:Calorimetry. The x-axis shows the different methods used to assess EE. The y-axis represents the accuracy rates or inaccuracy rates in percentages. The error bars reflect upper bounds of 95 % confidence intervals. *Significantly different from EE:VCO2 (p values are shown in Table 3). EE:Esp25, Energy expenditure calculated with the European Society for Clinical Nutrition and Metabolism guideline equation of 25 kcal/kg/day; EE:Faisy, Energy expenditure calculated with the Faisy equation; EE:HB, Energy expenditure calculated with the Harris–Benedict equation; EE:PSU, Energy expenditure calculated with the Penn State University 2003b equation; EE:VCO2, Energy expenditure from ventilator-derived volume of carbon dioxide and nutritional respiratory quotient
Discussion
The present prospective observational study in critically ill mechanically ventilated patients provides proof of concept that EE can be accurately calculated from EE:VCO2. Furthermore, it shows that this method is more precise than frequently used predictive equations. The bias or systematic error of EE:VCO2 was 141 kcal/24 h, indicating that EE:VCO2 as derived from the ventilator systematically overestimates EE compared with gold standard EE:Calorimetry. However, this bias corresponds to a relative error of only 7.7 % of the gold standard, whereas up to 10 % is considered acceptable according to a consensus statement [32]. The precision or random error component of EE:VCO2, expressed as the SD of the bias and compared between methods by using Levene’s test, is visualized by the width of the limits of agreement in the Bland–Altman plots and in Fig. 2. The precision of EE:VCO2 was significantly better than that of the equations.
The accuracy rates of EE:VCO2 were higher than those of all predictive equations, but not significantly so for EE:PSU. However, the inaccuracy of EE:PSU was higher, with greater than 25 % and greater than 30 % inaccuracy rates of 10 % and 6 %, respectively, indicating that in more than half of the patients with inaccuracy of greater than 25 %, the error was even larger—namely, more than 30 % difference from EE as measured by indirect calorimetry.
High inaccuracy rates were found for EE:HB and EE:Esp25, making these equations unacceptable for use in critically ill patients. In all, EE:VCO2 appears to be the most precise equation and EE:PSU and EE:HB15 the most unbiased equations. Despite a better estimation of the mean EE of the study population, the inferior precision of EE:PSU and EE:HB15 led to higher inaccuracy rates, which may result in severe over- or underfeeding in a considerable number of patients. Thus, for the individual patient, EE:VCO2 performs best.
We further explored the source of the bias of EE:VCO2, which can be due to inaccuracy of the VCO2 measurement or the RQ estimation. We found an unexpected bias of ventilator-derived VCO2 of 14.7 ml/min (6.5 % of VCO2:Calorimetry). Assuming an RQ of 0.86, which is the RQ of most nutritional products, this systematic error accounts for 120 kcal/24 h (i.e., 85 % of the bias of EE:VCO2). We noted the largest differences between ventilator-derived and calorimetry-derived VCO2 in patients with extreme variations in respiratory rate and tidal volume. Rapid and irregular breathing may lead to errors in ventilator-derived VCO2 due to dyssynchrony between the flow and the CO2 measurement. Furthermore, the ventilator exports a single-breath VCO2 value once each minute to the PDMS, which can lead to high variability in patients with irregular breathing. One way of improving the accuracy of the EE:VCO2 method is the development of more accurate VCO2 analyzers in mechanical ventilators, such as by more frequent sampling and data export.
A second source of error and an important limitation of our study is that the actual RQ of the patients was not known. In the present study, we used nutritional RQ. However, during critical illness, RQ is influenced not by actual nutritional intake alone. An unknown and uninhibitable part of energy is derived from endogenous sources, and there are different illness-related degrees of protein synthesis or catabolism, lipogenesis or lipolysis, and gluconeogenesis or glycolysis. Because of the uncertainty of actual RQ when endogenous sources are used for energy, we could not correct RQ if nutritional intake was less than EE. However, our patients received more than two-thirds of actual EE, and this is the time point when measurement of EE becomes relevant. RQ is also influenced by periods of hypo- and hyperventilation (e.g., induced by stress or sedation or in respiratory compensation for metabolic acidosis or alkalosis). This will temporarily modulate VCO2 [34]; however, over 24 h, mean VCO2 reflects CO2 produced by metabolism. Although nutritional RQ did indeed not correlate with measured RQ:Calorimetry, only 15 % of the bias of EE:VCO2 was attributable to the difference between nutritional RQ and RQ:Calorimetry.
In our study, additional calories provided during the study period by glucose and propofol were taken into account. With a single exception in a patient who received large amounts of glucose 40 %, these additional calories did not substantially change nutritional RQ and subsequently EE:VCO2.
Mehta et al. tested the accuracy of a VCO2 based equation to calculate EE in critically ill children [35]. Metabolic data from mechanically ventilated children was used to derive this equation. The equation was then applied to a second dataset of critically ill children to test accuracy. They found superiority of the simplified equation over standard equations. These findings further strengthen the concept of using VCO2 measurement instead of estimating equations to calculate EE in critically ill adults and children. It should be noted, however, that the VCO2 in the Mehta study was not independently measured; it was derived from the metabolic monitor. Thus, a direct comparison between EE:Calorimetry and a ventilator-derived or separate module-derived EE:VCO2 was not performed. Mehta et al. mentioned this as a limitation of their study. Also, measurement periods were relatively short. We were able to perform simultaneous 24-h VCO2 and indirect calorimetric measurements in a large and representative population of ICU patients ventilated for more than 3 days, providing information on real-time total EE.
Indirect calorimetry remains the gold standard. However, the most validated system, the Deltatrac, is no longer being manufactured. While we await new, accurate, affordable metabolic monitors, EE:VCO2 could be of great benefit for ICUs that do not have indirect calorimetry available. The method can also be used to monitor fluctuations in EE over time and to identify patients at risk for being over- or underfed. EE:VCO2 allows for daily adjustment of nutrition in ventilated patients. This may be important because metabolic rate and associated energy requirements vary widely during the day and during the course of disease [11, 12, 36, 37]. Another major advantage of EE:VCO2 is that the calculation of EE is independent of body length and weight, thereby reducing error.
We are aware of the fact that not all ICUs have mechanical ventilators that measure VCO2 continuously. Most modern ventilators do have this option available and cost less than a metabolic monitor. Of note, the present validation was performed with one type of mechanical ventilator. We do not know the accuracy of VCO2 measurements with other ventilators.
We excluded patients with FiO2 exceeding 0.6 for reliability reasons and patients with PEEP above 14 cmH2O because of risks associated with disconnection when connecting the indirect calorimeter to the ventilator. Therefore, our method was not validated in this population. Nonetheless, we suppose that EE:VCO2 is reliable in all mechanically ventilated patients, regardless of ventilator settings, provided that air leakage is not present.
The most important message of this study is that EE (kcal/day) can be calculated at the bedside as 8.19 × VCO2 (ml/min). This equation is derived from the rewritten Weir formula using an RQ of 0.86, which is the RQ of most nutritional products, and after converting liters per minute to milliliters per minute.
Conclusions
In critically ill mechanically ventilated adult patients, the assessment of EE from ventilator-derived VCO2 is accurate and more precise than frequently used predictive equations. It allows for continuous monitoring and provides the best alternative to gold standard indirect calorimetry. Future studies are necessary to improve accuracy of the VCO2 measurement, to detect sources of error, and to investigate whether daily adjustment of nutrition based on ventilator-derived EE improves the outcome of ICU patients.
Key messages
EE from ventilator-derived VCO2 is accurate and more precise than predictive equations.
This method allows for continuous monitoring and is the best alternative to indirect calorimetry.
EE (kcal/day) can be calculated at the bedside as 8.19 × VCO2 (ml/min).
Acknowledgements
We thank Ronald Driessen of the Department of Adult Intensive Care Medicine at VU University Medical Center for his contribution.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- BMI
Body mass index
- CPAP
Continuous positive airway pressure
- CVVH
continuous venovenous hemofiltration, EE, Energy expenditure
- EE:Esp25
Energy expenditure calculated with the European Society for Clinical Nutrition and Metabolism guideline equation of 25 kcal/kg/day
- EE:Faisy
Energy expenditure calculated with the Faisy equation
- EE:HB
Energy expenditure calculated with the Harris–Benedict equation
- EE:PSU
Energy expenditure calculated with the Penn State University 2003b equation
- EE:VCO2
Energy expenditure from ventilator-derived volume of carbon dioxide and nutritional respiratory quotient
- ESPEN
European Society for Clinical Nutrition and Metabolism
- FiO2
Fraction of inspired oxygen
- ICU
Intensive care unit
- IQR
Interquartile range
- MAP
mean arterial pressure
- PaO2
Partial pressure of arterial oxygen
- PC
Pressure control
- PDMS
Patient data management system
- PEEP
Positive end-expiratory pressure
- PS
Pressure support
- RQ
Respiratory quotient
- SD
Standard deviation
- T
Mean temperature during the 24-h study period
- Tmax
Maximum body temperature during the 24-h study period
- VCO2
carbon dioxide production
- Ve
Mean minute ventilation during the 24-h study period
- VO2
oxygen consumption
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SNS, ARJG, PJMW, PWGE, and HMOvS designed the study. SNS and HA obtained the data. SNS, HJSdG, HMOvS, and PJMW analyzed the data. HMOvS had primary responsibility for final content. All authors contributed to the drafting of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Sandra N. Stapel, Phone: +31204444444, Email: s.stapel@vumc.nl
Harm-Jan S. de Grooth, Email: h.degrooth@vumc.nl
Hoda Alimohamad, Email: hoda.04@gmail.com.
Paul W G Elbers, Email: p.elbers@vumc.nl.
Armand R J Girbes, Email: arj.girbes@vumc.nl.
Peter J M Weijs, Email: p.weijs@vumc.nl.
Heleen M. Oudemans-van Straaten, Email: h.oudemans@vumc.nl
References
- 1.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 2.Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med. 2011;39:967–974. doi: 10.1097/CCM.0b013e31820a905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer P, Anbar R, Cohen J, Shapiro H, Shalita-Chesner M, Lev S, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37:601–609. doi: 10.1007/s00134-011-2146-z. [DOI] [PubMed] [Google Scholar]
- 4.Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux RNM, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502–509. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Weijs PJM, Looijaard GPM, Beishuizen A, Girbes ARJ, Oudemans-van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care. 2014;18:701. doi: 10.1186/s13054-014-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer P, Pichard C, Heidegger CP, Wernerman J. Considering energy deficit in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2010;13:170–176. doi: 10.1097/MCO.0b013e3283357535. [DOI] [PubMed] [Google Scholar]
- 7.Cooney RN, Frankenfield DC. Determining energy needs in critically ill patients: equations or indirect calorimeters. Curr Opin Crit Care. 2012;18:174–177. doi: 10.1097/MCC.0b013e3283514bbc. [DOI] [PubMed] [Google Scholar]
- 8.Branson RD, Johannigman JA. The measurement of energy expenditure. Nutr Clin Pract. 2004;19:622–636. doi: 10.1177/0115426504019006622. [DOI] [PubMed] [Google Scholar]
- 9.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. Nutrition. 1990;6:213–221. [PubMed] [Google Scholar]
- 10.McClave SA, Martindale RG, Kiraly L. The use of indirect calorimetry in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2013;16:202–208. doi: 10.1097/MCO.0b013e32835dbc54. [DOI] [PubMed] [Google Scholar]
- 11.Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response to the stress of critical illness. Br J Anaesth. 2014;113:945–954. doi: 10.1093/bja/aeu187. [DOI] [PubMed] [Google Scholar]
- 12.Berger MM, Pichard C. Best timing for energy provision during critical illness. Crit Care. 2012;16:215. doi: 10.1186/cc11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–223. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Frankenfield DC, Coleman A, Alam S, Cooney RN. Analysis of estimation methods for resting metabolic rate in critically ill adults. J Parenter Enteral Nutr. 2009;33:27–36. doi: 10.1177/0148607108322399. [DOI] [PubMed] [Google Scholar]
- 16.Weijs PJ. Validity of predictive equations for resting energy expenditure in US and Dutch overweight and obese class I and II adults aged 18–65 y. Am J Clin Nutr. 2008;88:959–970. doi: 10.1093/ajcn/88.4.959. [DOI] [PubMed] [Google Scholar]
- 17.Walker RN, Heuberger RA. Predictive equations for energy needs for the critically ill. Respir Care. 2009;54:509–521. [PubMed] [Google Scholar]
- 18.Faisy C, Guerot E, Diehl J, Labrousse J, Fagon J. Assessment of resting energy expenditure in mechanically ventilated patients. Am J Clin Nutr. 2003;78:241–249. doi: 10.1093/ajcn/78.2.241. [DOI] [PubMed] [Google Scholar]
- 19.Academy of Nutrition and Dietetics. Critical illness evidence-based nutrition practice guideline. Chicago: Author; 2012. http://www.guideline.gov/content.aspx?id=39404. Accessed 15 Oct 2015.
- 20.Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22:377–388. doi: 10.1177/0115426507022004377. [DOI] [PubMed] [Google Scholar]
- 21.Roza AM, Shizgal HM. The Harris Benedict equation re-evaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40:168–182. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 22.Alexander E, Susla GM, Burstein AH, Brown DT, Ognibene FP. Retrospective evaluation of commonly used equations to predict energy expenditure in mechanically ventilated, critically ill patients. Pharmacotherapy. 2004;24:1659–1667. doi: 10.1592/phco.24.17.1659.52342. [DOI] [PubMed] [Google Scholar]
- 23.van Lanschot JJ, Feenstra BW, Vermeij CG, Bruining HA. Calculation versus measurement of total energy expenditure. Crit Care Med. 1986;14:981–985. doi: 10.1097/00003246-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Sauerwein HP, Strack van Schijndel RJ. Perspective: How to evaluate studies on peri-operative nutrition? Considerations about the definition of optimal nutrition for patients and its key role in the comparison of the results of studies on nutritional intervention. Clin Nutr. 2007;26:154–158. doi: 10.1016/j.clnu.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 25.van Strack van Schijndel RJM, Weijs PJM, Sauerwein HP, de Groot SDW, Beishuizen A, Girbes ARJ. An algorithm for balanced protein/energy provision in critically ill mechanically ventilated patients. E Spen Eur E J Clin Nutr Metab. 2007;2:69–74. doi: 10.1016/j.eclnm.2007.05.001. [DOI] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1936. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 29.Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 31.International Organization for Standardization (ISO). Accuracy (trueness and precision) of measurement methods and results. Part 1: general principles and definitions. ISO 5725-1:1994. https://www.iso.org/obp/ui/#iso:std:iso:5725:-1:ed-1:en. Accessed 15 Oct 2015.
- 32.Frankenfield D, Hise M, Malone A, Russell M, Gradwell E, Compher C. Prediction of resting metabolic rate in critically ill adult patients: results of a systematic review of the evidence. J Am Diet Assoc. 2007;107:1552–1561. doi: 10.1016/j.jada.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 34.McClave SA, Lowen CC, Kleber MJ, McConnell JW, Jung LY, Goldsmith LJ. Clinical use of the respiratory quotient obtained from indirect calorimetry. J Parenter Enteral Nutr. 2003;27:21–26. doi: 10.1177/014860710302700121. [DOI] [PubMed] [Google Scholar]
- 35.Mehta NM, Smallwood CD, Joosten KFM, Hulst JM, Tasker RC, Duggan CP. Accuracy of a simplified equation for energy expenditure based on bedside volumetric carbon dioxide elimination measurement – a two-center study. Clin Nutr. 2015;34:151–155. doi: 10.1016/j.clnu.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraipont V, Preiser JC. Energy estimation and measurement in critically ill patients. J Parenter Enteral Nutr. 2013;37:705–713. doi: 10.1177/0148607113505868. [DOI] [PubMed] [Google Scholar]
- 37.Elia M. Insights into energy requirements in disease. Public Health Nutr. 2005;8:1037–1052. doi: 10.1079/PHN2005795. [DOI] [PubMed] [Google Scholar]


