Abstract
Background
Successful management of cardiovascular disease (CVD) is impaired by poor adherence to clinical practice guidelines. The objective of our review was to synthesize evidence about the effectiveness of interventions that target healthcare providers to improve adherence to CVD guidelines and patient outcomes.
Methods
We searched PubMed, EMBASE, Cochrane Library, PsycINFO, Web of Science and CINAHL databases from inception to June 2014, using search terms related to adherence and clinical practice guidelines. Studies were limited to randomized controlled trials testing an intervention to improve adherence to guidelines that measured both a patient and adherence outcome. Descriptive summary tables were created from data extractions. Meta-analyses were conducted on clinically homogeneous comparisons, and sensitivity analyses and subgroup analyses were carried out where possible. GRADE summary of findings tables were created for each comparison and outcome.
Results and Discussion
We included 38 RCTs in our review. Interventions included guideline dissemination, education, audit and feedback, and academic detailing. Meta-analyses were conducted for several outcomes by intervention type. Many comparisons favoured the intervention, though only the adherence outcome for the education intervention showed statistically significant improvement compared to usual care (standardized mean difference = 0.58 [95 % confidence interval 0.35 to 0.8]).
Conclusions
Many interventions show promise to improve practitioner adherence to CVD guidelines. The quality of evidence and number of trials limited our ability to draw conclusions.
Electronic supplementary material
The online version of this article (doi:10.1186/s12875-015-0341-7) contains supplementary material, which is available to authorized users.
Keywords: Clinical practice guidelines, Cardiovascular disease, Adherence, Systematic review
Background
Cardiovascular disease (CVD) is a leading cause of death in Canada [1]. Successful management of CVD involves not only the treatment of a specific disease, but also treating and preventing risk factors for CVD, including diabetes, dyslipidemia and hypertension [1–3]. However, the management of CVD is complicated by the large number of clinical practice guidelines available for conditions that contribute to this disease. An article by Ray et al. noted there are also discrepancies in recommendations across guidelines, potentially contributing to low adherence rates [2, 4, 5]. A harmonized guideline by Tobe et al. (2011) found there are over 400 recommendations for managing risk factors for heart disease [3].
Given the complexity of the management of this illness, it is imperative that practitioners use guidelines, and the most appropriate guidelines, in caring for patients with CVD and risk factors for CVD. The impact of guideline implementation has been illustrated previously; a review by Grimshaw and Russell found that using guidelines improved clinical practice [6]. Despite evidence to support the use of guidelines, there remains a gap in their implementation [7].
The dissemination of guidelines alone has little to no effect on practice [8], thus many studies have investigated interventions of varying intensity to increase the uptake of clinical practice guidelines. Numerous studies of interventions to improve the uptake of guidelines in CVD prevention are available. However, their overall impact on guideline adherence and clinical outcomes is unclear. Unverzagt et al. [9] published a systematic review on a similar topic that focused on primary care physicians’ adherence to guidelines, wherein they demonstrated these interventions can have an impact on adherence outcomes. It is important to determine the effect of these interventions on other healthcare providers, as well as determine the impact of these interventions on clinical outcomes, which is yet to be addressed in the literature to our knowledge.
We identified and synthesized the available research evidence about the effectiveness of interventions that target healthcare providers to improve adherence to CVD prevention and treatment guidelines and clinical outcomes. Our secondary objective was to explore characteristics of guideline implementation interventions and contexts that are associated with increased effectiveness. This leads to our research question: what is the most effective intervention to improve the implementation of, uptake of, or adherence to cardiovascular disease-related clinical practice guidelines by healthcare providers in randomized controlled trials?
Methods
As this research did not involve the collection of primary data, we did not seek ethics approval. This review has been registered with PROSPERO 2014:CRD42014010111. Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014010111
Search
A systematic search was conducted using search terms related to “adherence” and “clinical practice guidelines”, which was refined with the help of a medical librarian. We searched the following databases: PubMed, EMBASE, Cochrane Library (including CENTRAL, DARE and HTAs), PsycINFO, Web of Science and CINAHL (all available years, up to June 2014). Grey literature was also searched, including clinicaltrials.gov to identify potential new studies, ICTRP registry database, and ProQuest thesis database. Our search strategy did not impose any limits on language of publication (Additional file 1).
Inclusion criteria
Study design
The included studies were limited to randomized controlled trials (RCTs). We included all types of RCTs, including cluster RCTs, and nested designs.
Population
Studies that enrolled any registered healthcare providers were included. Subgroups of interest for our analyses included comparing physician participants to other healthcare providers (non-physicians). We excluded trials if less than 75 % of the participants included were certified, regulated healthcare providers.
Intervention
All studies that evaluated the impact of an intervention on the implementation of, uptake of, or adherence to a clinical practice guideline by a health care provider were included. The guideline of concern had to relate to the prevention or management of CVD, including risk factor management for any of: diabetes, dyslipidemia or hypertension. Guideline definitions were based on authors stating a guideline to be such. A study was deemed to be about the implementation or adherence to a guideline if the trial report explicitly stated that improving use of a clinical practice guideline was the focus of the intervention. Types of interventions included: academic detailing, audit and feedback, educational sessions, continuing medical education (CME) sessions, and ‘other’ (such as reminders or decision support systems).
Comparison group
We selected studies that included at least one control group. Comparison groups included usual care, a similar guideline implementation intervention of differing intensity or duration than the main intervention group, or no intervention (receipt of the intervention at a different time than the intervention group, such as after data collection).
Primary outcomes
We included trials that reported both a measure of guideline adherence and at least one clinical outcome. Measures of adherence included self-reported adherence, prescription review, and chart review. We included studies reporting any relevant clinical outcomes and considered the following groups of outcomes for analyses: mortality, hospitalizations, quality of life, and disease targets. Outcomes assessed at similar time points were combined in our analysis as short term (3–6 months), and long term (7 months or longer).
Study selection and data extraction
Articles were screened based on title and abstract using the inclusion criteria, then based on full text by two independent reviewers. Discrepancies were resolved by consensus.
Data from included articles was extracted in duplicate by independent extractors. We extracted study characteristics (study design, setting and population), a description of the intervention (the type of intervention, providers, and resources involved), comparison intervention, risk of bias, outcome measurement and results, and funding for the study. Risk of bias was assessed using the Cochrane Risk of Bias tool for RCTs [10]. All discrepancies between extractors were resolved through consensus. Data was managed using spreadsheets created for each extractor. Authors were contacted after data extraction and consensus meetings were completed to request missing data and to check the accuracy of our extractions.
Data analysis
We conducted descriptive analyses of included studies. We conducted meta-analyses (MA) for outcome results when there was sufficient clinical homogeneity across the studies. Clinical homogeneity was based on similar study characteristics (intervention type, outcome and follow-up point of interest). Meta-analyses were conducted in Review Manager (RevMan 5), using a random effects model and forest plots were generated. Intraclass correlation coefficient (ICC) for cluster RCTs were used in our meta-analyses to calculate the effective sample size to ensure the effect of clustering was taken into account in our analyses, as per the Cochrane Handbook [11]. A Z-test was used to assess statistical significance of meta-analysis results and a p < 0.05 was considered significant.
The adherence and patient outcomes were measured as both dichotomous and continuous outcome measures. Odds ratios (OR) and 95 % confidence intervals (CI) were calculated for use in the MA for dichotomous outcomes. Standardized mean differences (SMD) were used for continuous outcomes, as outcomes measuring the same construct were measured on different scales. Most continuous outcome analyses looked at the differences in mean change of each group from baseline, and this value was used in the MA, though some trials reported follow up results in each group, wherein we calculated the change score for each group to use in our MA. In order to impute the standard deviation for the change score in this instance, the standard deviation of the change score from another similar study was used.
We conducted sensitivity analyses to determine the robustness of our results, comparing the results of our analyses including and excluding studies with imputed standard deviations, and excluding studies with high risk of bias (greater than 3 domains rated as high risk of bias). We conducted subgroup analyses considering participant subgroups (physician participants and other healthcare providers), and considering the condition that was the focus of the guideline in the study (acute and chronic CVD conditions or risk factors). We planned to create funnel plots to investigate potential publication bias if at least ten studies were included for a given outcome, however this was not possible. We present a summary of the overall strength of evidence available using GRADE Summary of Findings tables produced using GRADEpro.
Results
Results of the search
We identified 12,255 potentially relevant unique citations. We excluded 12,033 citations during the initial abstract and title screening. We reviewed 222 full text publications and included 38 studies in the review [12–54] (Fig. 1).
Fig. 1.
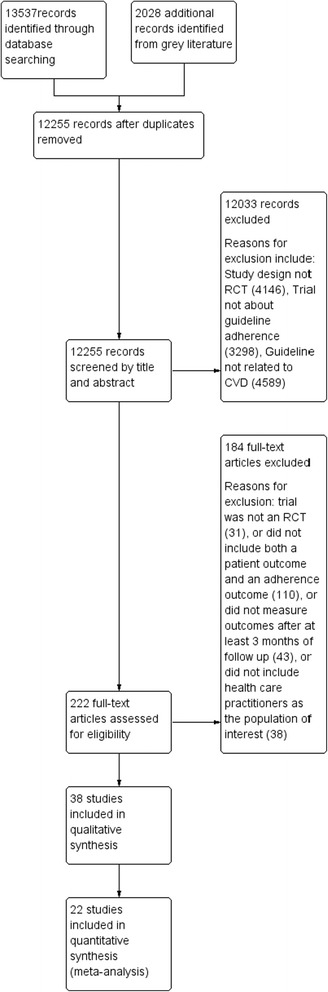
PRISMA flow chart of study inclusions
Included studies (Table 1)
Table 1.
Characteristics of included studies
| Study ID | Topic of trial | Study Design | Population description | Setting | Intervention Description; Intervention 2 description (if applicable) | Type | Duration of treatment period | Comparison intervention | Outcomes measured | Risk of bias ratinga |
|---|---|---|---|---|---|---|---|---|---|---|
| Ansari, 2003 | Use of beta-blockers in congestive heart failure | cRCT | Specialist doctors and nurse practitioners, patients with CHF | USA, urban medical centre | Nurse facilitator plus healthcare provider educational sessions; provider and patient reminder letters | Other type: Nurse facilitator; notifications | 1 year | Educational sessions, no nurse facilitator | Mortality, hospitalization, adherence (prescription review, chart review) | High risk of bias |
| Baker, 2003 | Guidelines in prioritised review criteria | cRCT | Family doctors, patients with angina | England, general practices | Review criteria; criteria plus feedback | Other type: review criteria | 12 months | Guideline dissemination alone | Disease target (cholesterol), adherence (prescription review, chart review) | Low risk of bias |
| Bertoni, 2009 | Physician adherence to ATP III guidelines | cRCT | Family doctors | USA, primary care practices | CDSS, educational sessions, academic detailing, CME sessions | Education + audit and feedback + academic detailing + CME session | 2 years | educational sessions, CME sessions, guideline mailed to participants | Disease target (cholesterol), adherence (prescription review, chart review) | High risk of bias |
| Berwanger, 2012 | Multifaceted quality improvement intervention in ACS patients | cRCT | Patients with ACS at general public hospitals | Brazil, public hospitals | Training, reminders, checklists, case management, educational sessions | Education | 8 months | Routine care | Mortality, major adverse cardiac events, adherence (prescription review) | Low risk of bias |
| Bonds, 2009 | Compliance to JNC 7 guidelines to improve blood pressure | cRCT | Family doctors | USA, primary care practices | Educational sessions, dissemination of guidelines, academic detailing for physicians, feedback on blood pressure control | Education + audit and feedback + academic detailing + CME sessions | 2 years | Similar to intervention but focused on ATPIII guidelines | Disease target (BP), adherence (prescription review, chart review) | Low risk of bias |
| Browner, 1994 | CME and follow up to improve detection and treatment of high cholesterol | cRCT | Family and internal medicine doctors | USA, general practices | CME seminar; Intensive CME (office visits and educational materials) | Education + CME sessions | 18 months | Educational sessions | Disease target (cholesterol), adherence (chart review) | High risk of bias |
| Carter, 2009 | Physician and pharmacist collaborative model to improve blood pressure | cRCT | Family doctors, patients with hypertension | USA, community based family medicine | Collaborative model, team building exercises, training sessions, educational sessions | Education + other (collaborative model) | 6 months | Collaborative model | Disease target (BP), guideline adherence tool | High risk of bias |
| De Lusignan, 2013 | Audit based education to reduce blood pressure | cRCT | Mixed health care professionals | United Kingdom, primary care | Audit based education consisting of workshops; academic detailing plus workshops | Education + audit and feedback; academic detailing | 2 years | Usual care | Mortality, major adverse cardiac events, disease target (BP), adherence (prescription review) | Low risk of bias |
| Deales, 2014 | Team based approach to disease and care management | cRCT | Mixed health care professionals | Italy, primary care groups | Recommendations as textbooks and decision algorithms, education sessions | Education | 12 months | Usual care | Disease target (HbA1c), adherence (chart review) | High risk of bias |
| Dijkstra, 2006 | Implementation strategies for diabetes guidelines | cRCT | T1D and T2D patients | The Netherlands, hospitals | Educational meetings, feedback, reminder card; diabetes passport, education | Education + audit and feedback | 1 year | Usual care | Disease target (HbA1c), adherence (chart review) | High risk of bias |
| Eaton, 2011 | Multimodal intervention to improve screening and management of hyperlipidemic patients | cRCT | Family doctors | USA, primary care practices | PDA with decision support and education toolkit and academic detailing | Academic detailing | 12 months | PDA with decision support but minimal follow up | Disease target (cholesterol), adherence (chart review) | Low risk of bias |
| Eccles, 2002 | Computerised decision support system to implement angina guidelines | cRCT | Family doctors | England, general practices | Computer decision support that provided access to guidelines | Other: CDSS | 12 months | Same intervention but asthma guideline provided | Quality of life, adherence (chart review) | Low risk of bias |
| Feldman, 2009 | Simplified algorithm for treatment of hypertension | cRCT | Family practices, patients with hypertension | Canada, family practices | Algorithm, aids, one follow up meeting, educational materials and sessions | Education + Other (algorithms) | 6 months | Educational sessions and guidelines | Mortality, disease target (BP), adherence (chart review) | Low risk of bias |
| Fihn, 2011 | Collaborative care model based intervention to improve angina management | cRCT | Family doctors, patients with angina | USA, academic primary care clinics | Expert advice, progress evaluations, education | Education | 12 months | Usual care | Mortality, disease target, adherence (chart review) | Low risk of bias |
| Fretheim, 2006 | Tailored intervention to support implementation of CVD guidelines | cRCT | Family practices, hypertensive or hypercholesterolemic patients | Norway, general practices | Tailored intervention including reminders, audit and feedback and education | Education + audit and feedback | 12 months | Passive dissemination | Disease target (cholesterol, BP), adherence (prescription review, chart review) | Low risk of bias |
| Gill, 2009 | EMR-based intervention for lipid management | cRCT | Family doctors, general internists | USA, academic family practice | EMR disease management tool | Other (integration into EMR) | 12 months | Usual care | Disease target (cholesterol), adherence (chart review) | High risk of bias |
| Goldstein, 2005 | Intervention on drug choice for hypertension | cRCT | Family doctors, nurse practitioners | USA, multiple sites | Education, individual drug profiles, follow up | Education | 9 months | Education on guidelines | Disease target (BP), adherence (prescription and chart review) | Low risk of bias |
| Harris, 2005 | Teleconferenced educational detailing for diabetes | cRCT | Family doctors | Canada, family practices | Eight one hour small group educational sessions with opinion leaders | Education | 3 months | CME session after intervention period | Disease target (HbA1c), adherence (chart review) | High risk of bias |
| Hayes, 2002 | Quality improvement and written feedback for CHF management | cRCT | Hospitals, CHF patients | USA, hospitals | Education, quality improvement tools from liaisons, chart reminders | Education + audit and feedback | 6 months | Mailed quality improvement tools | Disease target (ventricular fxn), adherence (chart review) | High risk of bias |
| Headrick, 1992 | Education and feedback strategies to improve compliance with NCEP-PCEP guidelines | RCT | Resident doctors | USA. Academic hospital | Lecture, chart reminders; Lecture, patient specific feedback and chart reminder | Education + Other (reminders) | 20 weeks | Lecture alone | Disease targets (cholesterol), adherence (chart review) | Low risk of bias |
| Hendriks, 2012 | Nurse led guideline based software supported ICCP | RCT | Family doctors, specialists, patients with atrial fibrillation | Netherlands, academic center | Nurse specialist educated patients and CDSS | Other (nurse specialist) | 12 months | Usual care | Mortality, hospitalizations, quality of life, adherence (chart review) | Low risk of bias |
| Kiessling, 2011 | Case based training to optimize hyperlipidemia care | RCT | Family doctors, patients with CHD | Sweden, primary health care centres | Case based training seminars and guideline provided | Education | 2 years | Usual care | Mortality, disease target (cholesterol), adherence (prescription review) | High risk of bias |
| Leonardis, 2012 | Multimodal intervention to improve adherence to targets | cRCT | Specialists, CKD patients | Italy, renal clinics | Education session, follow up and audits | Education + audit and feedback | 3 years | Education and standard care | Mortality, hospitalizations, quality of life, disease target (cholesterol), adherence (prescription/ chart review) | Low risk of bias |
| Levine, 2011 | Multicomponent internet delivered intervention improve CHD guideline adherence | cRCT | Family doctors, MI patients | Virgin Islands and Puerto Rico, community primary care clinics | Educational cases, guidelines, monthly update, reminders | Education + Other (reminders) | 27 months | Passive dissemination | Disease target (cholesterol), adherence (chart review) | High risk of bias |
| Ornstein, 2004 | Multimethod quality improvement intervention for adherence to quality indicators in CVD and stroke | cRCT | Practice based research network of practices | USA, primary care practices | Education, performance reports quarterly, practice site visits and network meetings (6–7 1–2 day visits) with pharmacist (academic detailing) | Education + academic detailing | 2 years | Education, performance reports quarterly | Disease target (BP), adherence (prescription, chart review) | High risk of bias |
| Petersen, 2013 | Effect of financial incentives to reward guideline based hypertension care | cRCT | Family doctors | USA, primary care clinics | Physician level incentives; practice levels incetives; combined (both) incentives | Other (incentives) | 20 months | Usual care | Disease target (BP), adherence (prescription, chart review) | High risk of bias |
| Peters-Klimm, 2009 | Educational model for GPs for the management of CHF | cRCT | Family doctors, CHF patients | Germany, general practitioner clinics | “Train the trainer” = multidisciplinary andragogic and didactic educational sessions | Education + Other (feedback) | 7 months | Single educational session by cardiologist | Mortality, hospitalizations, quality of life, disease target (course), adherence (prescription review) | Low risk of bias |
| Reutens, 2012 | Education of GPs on the IDF-WPR guidelines to improve metabolic control | cRCT | Family doctors, T2D patients | Asia-Pacfic, general practitioner clinics | Education meetings (two 3 months apart), reminder letters and cards, flowsheet on patient notes, patient diabetes passport | Education + Other (reminders, diabetes passport) | 12 months | Instructed on assessments in study but no information on guidelines | Disease target (BP), adherence (chart review) | High risk of bias |
| Rood, 2005 | Computer based guidelines to improve nurse measurement of patient glucose | RCT | ICU patients | The Netherlands, teaching hospital | Guideline based advice via computer decision support software | Other (decision support tool) | 10 weeks | Paper based guideline flowchart | Disease target (glucose), adherence (chart review) | High risk of bias |
| Rossi, 1997 | Guideline reminders to improve prescribing based on JNC V guideline | cRCT | Nurse practitioners, hypertension patients | USA, GIM clinic | Guideline reminder for prescription and alternatives | Other (reminder) | 5 months | Usual care | Disease target (BP), adherence (prescription review) | High risk of bias |
| Roumie, 2006 | Multifactorial intervention to improve quality of care of hypertension patients | cRCT | Physicians and nurse practitioners, hypertension patients | USA, community and hospital clinics | Alert on medical record; Educational sessions and alert on medical record | Education + other (alerts) | 6 months | Providers received email with guideline | Mortality, hospitalizations, disease target (BP), adherence (prescription review) | High risk of bias |
| Simon, 2005 | Academic detailing individually or group to increase diuretic use in hypertension patients | cRCT | Family doctors, hypertension patients | USA, community health plan | Academic detailing meeting one-on-one; small group academic detailing session | Academic detailing | 3 months | Passive dissemination | Hospitalizations, disease target(BP), adherence (chart review) | High risk of bias |
| Steyn, 2013 | Structured clinical record and training health care providers to control diabetes and hypertension | cRCT | Nurses, patients with diabetes and hypertension | South Africa, community health centres | Structured record with guideline embedded added to patient folders, educational package | Education | 1 year | Passive dissemination | Disease target (HbA1c), adherence (chart review) | High risk of bias |
| Svetkey, 2009 | Intervention to increase physician adherence to BP guideline | cRCT | Physicians, hypertension patients | USA, community practice | CME courses, treatment algorithm, quarterly feedback on adherence | Education + CME session + other (feedback) | 18 months | Usual care | Disease target (BP), adherence (chart review) | Low risk of bias |
| Tierney, 2003 | Decision support system with guideline for managing ischemic heart disease and CHF patients | RCT | Pharmacists, CHF patients | USA, academic primary care practice | Physicians received patient specific feedback; pharmacist system to send feedback to physicians; both | Education + audit and feedback + other (decision support system) | 1 year | Usual care | Mortality, hospitalizations, quality of life, adherence (chart review) | High risk of bias |
| Van Bruggen, 2008 | Facilitator enhanced multifaceted intervention for T2D guideline implementation | cRCT | Family doctors and nurses and practice assistants, T2D patients | The Netherlands, primary care practices | Facilitators visited twice a month to train staff on guidelines, performance feedback, | Education + audit and feedback | 1 year | Usual care | Disease target (HbA1c), adherence (prescription and chart review) | Low risk of bias |
| Van Steenkiste, 2007 | Decision support tool for risk management improving CVD guideline performance | cRCT | Family doctors, patients without CVD | The Netherlands, hospital | Education, decision support tool, | Other (decision support tool) | 8 months | Educational materials on guideline | Disease target (lifestyle), adherence (chart review) | High risk of bias |
| Verweij, 2013 | Effectiveness of guideline based care on weight, CVD risk | cRCT | Occupational physicians | The Netherlands, occupational medicine | Environment scan, patient counselling training, patient toolkit | Other (environment scan, toolkit) | 18 months | Usual care | Quality of life, disease target (BP), adherence (chart review) | High risk of bias |
Footnote: a Risk of bias rated as high or low risk of bias based on overall domains, where high risk of bias designated if greater than 3 domains rated as high risk of bias
Eighteen studies took place in the USA, 14 were completed in Europe (the Netherlands, Italy, England, and Norway), two took place in Canada, one in South Africa, one in Brazil, one in Asia-Pacific area, and one in the Virgin Islands. Thirty-five studies included an intervention to improve physician use of guidelines and ten of those studies included a nurse as a target for the intervention; two studies focused on nurses alone, and one study focused on pharmacists. The most common intervention type was educational focused intervention (18/38), followed by audit and feedback (9/38), academic detailing focused interventions (4/38), comprehensive interventions that included education, audit and feedback and an academic detailing component (2/38), and “other” interventions that did not fall into any pre-designated category (8/38). Seven trials included more than one intervention group. All studies included an adherence outcome, as per our inclusion criterion. Disease target was the most common clinical outcome reported (33/38 trials), followed by mortality (11/38). Hospitalization and quality of life data were also reported in 8/38 and 6/38 trials, respectively.
Risk of bias in included studies
Risk of bias summary graphs and tables were created using RevMan (Figs. 2 and 3). Risk of bias was often assessed as unclear due to poor reporting of a methodological procedure. The majority of trials (33/38) were cluster RCTs, therefore additional risk of bias criteria were included for these studies. Random sequence generation was most often assessed to be low risk of bias, while blinding of participants was most commonly rated as high risk of bias.
Fig. 2.

Risk of bias summary table for each study. Green indicates a low risk of bias, yellow indicates unclear risk of bias and red indicates high risk of bias, as assessed by reviewers using the Cochrane risk of bias tool
Fig. 3.
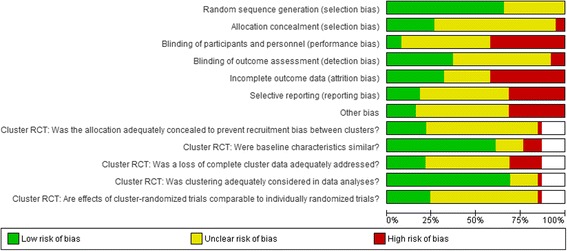
Risk of bias summary graph, summarized for each domain. Green indicates a low risk of bias, yellow indicates unclear risk of bias and red indicates high risk of bias, as assessed by reviewers using the Cochrane risk of bias tool
Effects of interventions
Education intervention
Seventeen trials tested an education-focused intervention and were included in a meta-analysis. These trials overall favoured the intervention, and one meta-analysis was statistically significant. Seven trials (2545 subjects) reported mortality outcomes, three of which reported mortality at a short term time point with an overall odds ratio of 0.54 (95 % CI 0.2 to 1.42). Four trials reported mortality at a long term time point with an overall odds ratio of 0.48 (95 % CI 0.11 to 1.98). Four trials (979 subjects) reported hospitalizations as an outcome at a long term time point. The overall odds ratio for this outcome was 0.88 (95 % CI 0.54 to 1.41). Six trials (2145 subjects) reported disease target results at a short term time point (SMD = −0.32 (95 % CI −0.71 to 0.07)) and five trials (2732 subjects) reported this outcome at a long term time point (SMD = −0.09 (95 % CI −0.24 to 0.07)) (Fig. 5). Seventeen trials reported adherence outcome data, six (2306 subjects) reported dichotomous data at a short term time point (OR = 2.11 (95 % CI 0.90 to 4.97)), four trials (322 subjects) reported continuous data at a short term time point (Fig. 4) and eight trials (6019 subjects) reported dichotomous data at a long term time point (OR = 1.05 (95 % CI 0.82 to 1.34)) (Fig. 6).
Fig. 5.

Summary disease target outcome forest plot for three comparisons measured by standardized mean difference, with point estimate and 95 % CIs
Fig. 4.

Forest plot of education intervention comparison for continuous adherence outcome at a short term time point (<6 months)
Fig. 6.

Summary adherence outcome forest plot for five comparisons measured by odds ratio, with point estimates and 95 % CIs
Audit and feedback
Nine trials included an intervention that focused on audit and feedback, and seven of those trials reported data sufficient to be included in meta-analyses. Three trials (2240 subjects) reported disease target results at a long term time point with an overall effect of −0.44 SMD (95 % CI −1.05 to 0.17) (Fig. 5). Six trials (2983 subjects) reported adherence data at a long term time point with an overall odds ratio of 1.39 (95 % CI 0.88 to 2.21) (Fig. 6).
Academic detailing
Four trials (6017 subjects) included academic detailing as the focus of the intervention and all of these trials reported data that was included in a meta-analysis for adherence outcome. The overall odds ratio for this comparison was 1.32 (95 % CI 0.88 to 1.96) (Fig. 6).
Other interventions
Eight trials included an intervention whose focus did not fit these previous groups. Four trials (1782 subjects) included a decision support tool as the focus of their intervention. The overall odds ratio for this comparison was 1.19 (95 % CI 0.83 to 1.70) (Fig. 6).
Sensitivity and subgroup analyses
Sensitivity analysis investigating the impact of imputed standard deviations in continuous data was possible in the education intervention outcome for the disease target outcome at a short term time point. The pooled SMD from six studies in this comparison was −0.32 (95 % CI −0.71 to 0.07), while the estimate from the sensitivity analysis, with studies that included imputed standard deviation removed, was −0.27 (95 % CI −0.71 to 0.17). Another sensitivity analysis, investigating the impact of high risk of bias studies on the overall estimate was possible for the meta-analysis of the effect of education on short term adherence outcomes. The pooled odds ratio was 2.36 (95 % CI 0.86 to 6.51) before studies with high risk of bias were excluded, and 3.65 (95 % CI 0.53 to 25.15) after studies with high risk of bias were excluded.
We compared results in studies that targeted physicians only in their intervention to interventions that involved non-physician healthcare providers alone or in addition to physicians with subgroup analysis. This subgroup analysis was possible in seven comparisons, and the subgroups of physician participants alone frequently had less heterogeneity than when grouped with all studies, suggesting participants may be a source of heterogeneity (Additional file 2: Figure S1). Another subgroup analysis we conducted compared results in studies that focused on an acute cardiovascular condition to a chronic cardiovascular condition. Five comparisons showed inconsistent results although the heterogeneity was reduced in at least one of the two subgroups in all comparisons.
GRADE summary of findings tables
The overall quality of evidence identified in this systematic review was moderate to very low due to high risk of bias, imprecision, and heterogeneity (Table 2). The most patient important outcome of mortality had moderate quality of evidence associated, indicating the results may be interpreted with some confidence.
Table 2.
Summary of findings table for educational interventions
| Education compared to control for improving adherence to cardiovascular disease guidelines | ||||||
|---|---|---|---|---|---|---|
| Patient or population: patients with improving adherence to cardiovascular disease guidelines | ||||||
| Settings: | ||||||
| Intervention: Education | ||||||
| Comparison: control | ||||||
| Outcomes | Illustrative comparative risksa (95 % CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk control | Corresponding risk Education | (95 % CI) | (studies) | (GRADE) | ||
| Mortality | Study population | OR 0.54 | 2190 | ⊕ ⊕ ⊕⊝ | ||
| Follow-up: median 6 months | 40 per 1000 | 22 per 1000 | (0.2 to 1.42) | (3 studies) | moderatec | |
| (8 to 56) | ||||||
| Moderate | ||||||
| 26 per 1000 | 14 per 1000 | |||||
| (5 to 37)b | ||||||
| Disease Targets | The mean disease targets in the intervention groups was0.32 standard deviations lower | 2145 | ⊕⊝⊝⊝ | SMD −0.32 (−0.71 to 0.07) | ||
| Follow-up: 3–6 months | (6 studies) | very lowc,e,f | ||||
| (0.71 lower to 0.07 higher) | ||||||
| Adherence | The mean adherence in the intervention groups was 0.58 standard deviations higher | 322 | ⊕ ⊕ ⊕⊕ | SMD 0.58 (0.35 to 0.8) | ||
| Follow-up: 6–24 months | (4 studies) | high | ||||
| (0.35 to 0.8 higher) | ||||||
| Mortality | Study population | OR 0.48 | 355 | ⊕ ⊕ ⊝⊝ | ||
| Follow-up: 7 months - 10 years | 182 per 1000 | 96 per 1000 | (0.11 to 1.98) | (4 studies) | lowg | |
| (24 to 306) | ||||||
| Moderate | ||||||
| 146 per 1000 | 76 per 1000 | |||||
| (18 to 253)b | ||||||
| Hospitalizations | Study population | OR 0.88 | 979 | ⊕ ⊕ ⊕⊕ | ||
| Follow-up: 7–22 months | 188 per 1000 | 170 per 1000 | (0.54 to 1.41) | (4 studies) | high | |
| (111 to 246) | ||||||
| Moderate | ||||||
| 191 per 1000 | 172 per 1000 | |||||
| (113 to 250)b | ||||||
| Disease Targets | The mean disease targets in the intervention groups was 0.09 standard deviations lower | 2732 | ⊕ ⊕ ⊝⊝ | SMD −0.09 (−0.24 to 0.07) | ||
| Follow-up: 7–27 months | (5 studies) | lowf,h | ||||
| (0.24 lower to 0.07 higher) | ||||||
| Adherence | Study population | OR 1.05 | 6019 | ⊕ ⊕ ⊝⊝ | ||
| Follow-up: 7–27 months | 609 per 1000 | 620 per 1000 | (0.82 to 1.34) | (8 studies) | lowc,i | |
| (561 to 676) | ||||||
| Moderate | ||||||
| 236 per 1000 | 245 per 1000 | |||||
| (202 to 293)b | ||||||
| Adherence | Study population | OR 2.36 | 2145 | ⊕⊝⊝⊝ | ||
| Follow-up: median 6 months | 288 per 1000 | 489 per 1000 | (0.86 to 6.51) | (5 studies) | very lowc,j,k | |
| (258 to 725) | ||||||
| Moderate | ||||||
| 326 per 1000 | 533 per 1000 | |||||
| (294 to 759)b | ||||||
aThe basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95 % confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95 % CI)
CI Confidence interval, OR Odds ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
bAssumed Risk is based on the default calculation within GRADEpro (mean control group risk, and median control group risk)
cAssessment based on three studies thus precision cannot be accurately determined
dSeveral included studies had 3 or more high risk of bias assessments
eStatistical heterogeneity I2 = 94 %
fDisease targets are an indirect estimate of patient important outcomes
gStatistical heterogeneity I2 = 70 %
hStatistical heterogeneity I2 = 41 %
iStatistical heterogeneity I2 = 60 %
jStatistical heterogeneity I2 = 95 %
kOverall estimate has large range for 95 % confidence interval
Discussion
Statement of principal findings
We have focused on interventions aimed at improving adherence to CVD guidelines. Overall studies are variable in their conclusions on whether the intervention was effective, though our quantitative analysis supports that interventions trend towards having an impact on adherence to guidelines and patient outcomes. One comparison of an education intervention for the adherence outcome was statistically significant, indicating this area of study deserves further consideration, as these interventions may help improve both adherence to guidelines, and more importantly, patient outcomes. Our results were robust where sensitivity analyses were possible. Subgroup analyses (participant and condition) reduced the statistical heterogeneity but there was inconsistency in the subgroup with the larger effect for each analysis. In some cases, the physician subgroup favoured the intervention to a greater degree than the non-physician subgroup, but in other comparisons the opposite was true. The same results were found for the condition subgroup (acute vs. chronic condition). The confidence in these recommendations ranged from moderate to very low based on a GRADE summary of findings due to imprecision, risk of bias, and inconsistency.
Strengths and weaknesses of the review
Our systematic review has several strengths, including that it was comprehensive in inclusion of studies. We included all types of healthcare providers in order to illustrate the impact these interventions can have on both physicians and non-physicians, which is increasingly important for multidisciplinary teams required for complex diseases such as CVD. We limited our study inclusion to those that reported both adherence and a patient outcome, as interventions must improve both in order to be clinically useful. All screening, data extraction, and risk of bias assessment was done in duplicate with trained reviewers to ensure the reproducibility of these results. Our quantitative analysis was pre-specified to avoid finding spurious results due to post hoc analyses. We minimized the number of comparisons that were made while ensuring comparisons had fairly good clinical homogeneity to maintain the strength of those conclusions. We also contacted authors for missing data and to verify the accuracy of our data extractions of their trial, thus we have confidence in this data.
However, this review has limitations. The first relates to the quality of reporting in trials. Reporting of risk of bias domains was poor in many trials, making it difficult to assess risk of bias. There was also significant heterogeneity in the studies’ interventions and characteristics making combining results in a meta-analysis difficult, leading to small numbers of studies included in each comparison. Meta-analyzing results was further complicated by uncertainty of the exact nature of some interventions due to limited descriptions of interventions available in publications. This also limited our ability to assess publication bias, so we were unable to determine the effect that might have on our confidence in these results.
Comparison to similar reviews
A systematic review on CVD guideline implementation strategies in primary care physicians by Unverzagt et al. reported similar conclusions on the effectiveness of education and reminder system interventions to improve adherence [9]. Our review extends these findings, illustrating the impact at the patient level on mortality, hospitalizations, quality of life and disease targets, and to different healthcare providers.
Similar to our findings, a review by Grimshaw et al. on guideline implementation noted overall the most effective interventions tend to include specific educational interventions and patient specific reminders at point of care [6].
Meaning of the review results
These results indicate there is some evidence to support the use of some interventions to improve healthcare provider adherence to CVD guidelines. Despite the limitations in the studies in this review, a trend of interventions improving adherence and patient outcomes was noted, supporting that these interventions may be more effective than passive guideline dissemination strategies. However, more studies are needed to strengthen these conclusions.
The majority of interventions included were multifaceted, which some reviews have suggested provide positive outcomes more frequently than single interventions [53–55]. However, our results were not consistent with these; we found these interventions have limited effects, which may be related to the number of components in a given intervention, as only two interventions included all of the types of interventions. A review by Squires et al. found there is ambiguity in the evidence of whether multifaceted interventions are more effective than single interventions, which is in agreement with our inconsistent findings [55].
Another possible reason for the overall small effect sizes may relate to the complexity of the management of CVD. This includes treating and preventing multiple risk factors in patients, such as diabetes, hypertension and dyslipidemia [56]. Most guidelines address only one of these diseases, and this may contribute to the small improvements found in this review. Given the multifactorial nature of CVD, it needs to be treated with guidelines that acknowledge this. Using harmonized CVD guidelines such as C-CHANGE is an important step that needs to be taken in CVD guideline implementation intervention trials to ensure the best, most comprehensive care is provided to patients [1]. This is also an important consideration as to why CVD guideline implementation strategies must differ from strategies used in treating simpler diseases such as pneumonia or asthma [57, 58].
Unanswered questions and future research
It would be beneficial for more high quality studies on this topic to be conducted to improve the strength of our recommendations, given the low confidence in most of these estimates due to a small number of studies included in each MA. Interventions should be fully described so they are not only reproducible, but future reviews are able to confidently determine homogeneous groups for meta-analyses. Future reviews on this topic should also define clinically important differences to determine whether the effects are not only statistically significant, but clinically significant as well.
Conclusions
Interventions to improve adherence to CVD guidelines can be effective at improving both adherence and patient outcomes, and are often more effective than guideline dissemination alone. Interventions that focused on healthcare provider education demonstrated statistically significant improvements. The overall quality of evidence available in this review was low, but several patient important outcomes including mortality were supported by moderate to high quality evidence.
Acknowledgements
We acknowledge the assistance of Robin Parker for advice during the creation of our search strategy. RAJ received funding from the DMRF Leo Alexander Summer Research Studentship. JH held funding from the Nova Scotia Health Research Foundation to support local systematic review activities. MJT and GHC received funding through the RIM summer research program. CVZ has received a research grant through Boehringer-Ingelheim. All funding bodies had no influence on the conception, design or interpretation of data.
Funding
RAJ received funding from the DMRF Leo Alexander Summer Research Studentship. JAH held funding from the Nova Scotia Health Research Foundation to support local systematic review activities. MJT and GHC received funding through the RIM summer research program. CVZ has received a research grant through Boehringer-Ingelheim. All funding bodies had no influence on the conception, design or interpretation of data
Abbreviations
- CVD
Cardiovascular disease
- CPG
Clinical practice guideline
- MA
Meta-analysis
- RCT
Randomized controlled trials
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- CME
Continuing medical education
- OR
Odds ratio
- SMD
Standardized mean difference
- CI
Confidence interval
Additional files
Supplementary: Medline search strategy. (DOCX 14 kb)
Figure A: Subgroup analysis of physician participants compared to other participants in education focused intervention trials disease target outcome at a short term time point. (DOCX 30 kb)
Footnotes
Competing Interests
CVZ received a research grant through Boehringer-Ingelheim. All other authors declare no conflicts of interest.
Authors’ contributions
RAJ conceived of the study and its design, coordinated and participated in data extraction and carried out statistical analyses, and drafted the manuscript. MJT participated in screening and data extraction. GHC participated in screening. CT participated in screening. AC participated in data extraction. CVZ helped conceive of the study. JH helped plan the design and data analysis, and helped draft the manuscript. All authors read, revised, and approved the final manuscript.
Authors’ information
RAJ, GHC and MJT are medical students at Dalhousie University. CT is an undergraduate science student at St. Mary’s University. JH is an associate professor in Epidemiology at Dalhousie University and is head of the Nova Scotia Cochrane Resource Centre. CVZ is a general internist and an assistant professor in the Department of Medicine at Dalhousie University.
Availability of data and materials
Not applicable.
Contributor Information
Rebecca A. Jeffery, Phone: 902-719-7299, Email: rjeffer@dal.caAQ3
Matthew J. To, Email: mt303402@dal.ca
Gabrielle Hayduk-Costa, Email: gb980142@dal.ca.
Adam Cameron, Email: adam.cameron@live.ca.
Cameron Taylor, Email: camtaylor85@eastlink.ca.
Colin Van Zoost, Email: colin.vanzoost@cdha.nshealth.ca.
Jill A. Hayden, Email: jhayden@dal.ca
References
- 1.Tobe SW, Stone JA, Brouwers M, Bhattacharyya O, Walker KM, Dawes M, et al. Harmonization of guidelines for the prevention and treatment of cardiovascular disease: the C-CHANGE Initiative. Can Med Assoc J. 2011;183(15):e1135–50. [DOI] [PMC free article] [PubMed]
- 2.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): Addenda The Fifth Joint Task Force of the European Society of Cardiology. Eur Heart J. 2012. doi:10.1093/eurheart/ehs165. [DOI] [PubMed]
- 3.Indio do Brasil CKO, Avezum A, Uint L, Del Monaco MI, De Barros VM, Campos SYR, et al. Cardiovascular prevention in coronary heart disease patients: guidelines implementation in clinical practice. Rev Bras Cir Cardiovasc. 2013;28(2):238–47. [DOI] [PubMed]
- 4.Ray KK, Kastelein JJP, Boekholdt SM, Nicholls SJ, Khaw KT, Ballantyne CM, et al. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: The good the bad and the uncertain: A comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur Heart J. 2014;35:960–8. [DOI] [PubMed]
- 5.Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, et al. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129 suppl 2:S1–S45. [DOI] [PubMed]
- 6.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317–1322. doi: 10.1016/0140-6736(93)92244-N. [DOI] [PubMed] [Google Scholar]
- 7.Feifer C, Ornstein SM, Jenkins RG, Wessell A, Corley ST, Nemeth LS, et al. The logic behind a multimethod intervention to improve adherence to clinical practice guidelines in a nationwide network of primary care practices. Eval Health Prof. 2006;29:65–88. [DOI] [PubMed]
- 8.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. Can Med Assoc J. 1997;157:408–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Unverzagt S, Oemler M, Braun K, Klement A. Strategies for guideline implementation in primary care focusing on patients with cardiovascular disease: a systematic review. Fam Pract. 2014;31(3):247–66. doi: 10.1093/fampra/cmt080. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- 11.McKenzie J, Ryan R, Di Tanna GL; Cochrane Consumers and Communication Review Group. ‘Cochrane Consumers and Communication Review Group: cluster randomised controlled trials’. http://cccrg.cochrane.org, March 2014 (December 20, 2014).
- 12.Ansari M, Shlipak MG, Heidenreich PA, Van Ostaeyen D, Pohl EC, Browner WS, et al. Improving guideline adherence: A randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation. 2003;107:2799–804. [DOI] [PubMed]
- 13.Baker R, Fraser RC, Stone M, Lambert P, Stevenson K, Shiels C. Randomised controlled trial of the impact of guidelines, prioritised review criteria and feedback on implementation of recommendations for angina and asthma. Br J Gen Pract. 2003;53:284–291. [PMC free article] [PubMed] [Google Scholar]
- 14.Bertoni AG, Bonds DE, Chen H, Hogan P, Crago L, Rosenberger E, et al. Impact of a multifaceted intervention on cholesterol management in primary care practices. Public Health. 2009;169(7):678–86. [DOI] [PMC free article] [PubMed]
- 15.Browner WS, Baron RB, Solkowitz S, Adler LJ, Gullion DS. Physician management of hypercholesterolemia. A randomized trial of continuing medical education. West J Med. 1994;161:572–578. [PMC free article] [PubMed] [Google Scholar]
- 16.Carter BL, Ardery G, Dawson JD, James P, Bergus GR, Doucette WR, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169(21):1996–2002. [DOI] [PMC free article] [PubMed]
- 17.Deales A, Fratini M, Romano S, Rappelli A, Penco M, Perna GP, et al. Care manager to control cardiovascular risk factors in primary care: The Raffaello cluster randomized trial. Nutr Metab Cardiovasc Dis. 2014;24(5):563–71. [DOI] [PubMed]
- 18.de Lusignan S, Gallagher H, Chan T, Thomas N, van Vlymen J, Nation M, et al. The QICKD study protocol: a cluster randomised trial to compare quality improvement interventions to lower systolic BP in chronic kidney disease (CKD) in primary care. Implement Sci. 2009;4:39–54. [DOI] [PMC free article] [PubMed]
- 19.Dijkstra RF, Niessen LW, Braspenning JCC, Adang E, Grol R. Patient-centred and professional-directed implementation strategies for diabetes guidelines: A cluster-randomized trial-based cost-effectiveness analysis. Diabet Med. 2006;23:164–170. doi: 10.1111/j.1464-5491.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- 20.Eccles M, Hawthorne G, Whitty P, Steen N, Vanoli A, Grimshaw J, et al. A randomised controlled trial of a patient based Diabetes Recall and Management System: the DREAM trial: a study protocol [ISRCTN32042030]. BMC Health Serv Res. 2002;2(1):5. [DOI] [PMC free article] [PubMed]
- 21.Feldman RD, Zou GY, Vandervoort RK, Wong CJ, Nelson SE, Feagan BG. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. doi: 10.1161/HYPERTENSIONAHA.108.123455. [DOI] [PubMed] [Google Scholar]
- 22.Fihn SD, Bucher JB, McDonell M, Diehr P, Rumsfeld JS, Doak M, et al. Collaborative Care Intervention for Stable Ischemic Heart Disease. Arch Intern Med. 2011;171(16):1471–9. [DOI] [PubMed]
- 23.Fretheim A, Oxman AD, Håvelsrud K, Treweek S, Kristoffersen DT, Bjørndal A. Rational prescribing in primary care (RaPP): A cluster randomized trial of a tailored intervention. PLoS Med. 2006;3(6):0783–0791. doi: 10.1371/journal.pmed.0030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fretheim A, Oxman AD, Treweek S, Bjørndal A. Rational Prescribing in Primary Care (RaPP-trial). A randomised trial of a tailored intervention to improve prescribing of antihypertensive and cholesterol-lowering drugs in general practice [ISRCTN48751230] BMC Health Serv Res. 2003;3:5–13. doi: 10.1186/1472-6963-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fretheim A, Oxman AD, Flottorp S. Improving prescribing of antihypertensive and cholesterol-lowering drugs: a method for identifying and addressing barriers to change. BMC Health Serv Res. 2004;4(1):23. doi: 10.1186/1472-6963-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill JM, Chen YX, Glutting JJ, Diamond JJ, Lieberman MI. Impact of decision support in electronic medical records on lipid management in primary care. Popul Health Manag. 2009;12(5):221–226. doi: 10.1089/pop.2009.0003. [DOI] [PubMed] [Google Scholar]
- 27.Harris SB, Leiter LA, Webster-Bogaert S, Van DM, O’Neill C. Teleconferenced educational detailing: diabetes education for primary care physicians. J Contin Educ Health Prof. 2005;25:87–97. doi: 10.1002/chp.13. [DOI] [PubMed] [Google Scholar]
- 28.Hayes RP, Baker DW, Luthi JC, Baggett RL, McClellan W, Fitzgerald D, et al. The effect of external feedback on the management of medicare inpatients with congestive heart failure. Am J Med Qual. 2002;17:225–35. [DOI] [PubMed]
- 29.Headrick LA, Speroff T, Pelecanos H, Cebul RD. Efforts to improve compliance with the national cholesterol education program guidelines. Arch Intern Med. 1992;152:2490–96. doi: 10.1001/archinte.1992.00400240104017. [DOI] [PubMed] [Google Scholar]
- 30.Hendriks JML, De Wit R, Crijns H, Vrijhoef H, Prins M, Pisters R, et al. Nurse-led care vs. usual care for patients with atrial fibrillation: Results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33:2692–9. [DOI] [PubMed]
- 31.Leonardis D, Mallamaci F, Enia G, Postorino M, Tripepi G, Zoccali C. The MAURO study: Baseline characteristics and compliance with guidelines targets. J Nephrol. 2012;25(5):1081–90. doi: 10.5301/jn.5000239. [DOI] [PubMed] [Google Scholar]
- 32.Levine DA, Funkhouser EM, Houston TK, Gerald JK. Improving Care After Myocardial Infarction Using a 2-Year Internet-Delivered Intervention. Arch Intern Med. 2014;171(21):1910–7. doi: 10.1001/archinternmed.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ornstein S, Jenkins RG, Nietert PJ, Feifer C, Roylance LF, Nemeth L. Improving Patient Care A Multimethod Quality Improvement Intervention To Improve Preventive Cardiovascular Care. Ann Intern Med. 2004;141:523–32. doi: 10.7326/0003-4819-141-7-200410050-00008. [DOI] [PubMed] [Google Scholar]
- 34.Petersen LA, Simpson K, Pietz K, Urech TH, Hysong SJ, Profit J, et al. Effects of individual physician-level and practice-level financial incentives on hypertension care: a randomized trial. JAMA. 2013;310(10):1042–50. [DOI] [PMC free article] [PubMed]
- 35.Petersen LA, Urech L, Simpson K, Pietz K, Hysong SJ, Profit J, et al. Design, rationale, and baseline characteristics of a cluster randomized controlled trial of pay for performance for hypertension treatment: study protocol. Implement Sci. 2011;6(1):114. [DOI] [PMC free article] [PubMed]
- 36.Peters-Klimm F, Campbell S, Müller-Tasch T, Schellberg D, Gelbrich G, Herzog W, et al. Primary care-based multifaceted, interdisciplinary medical educational intervention for patients with systolic heart failure: lessons learned from a cluster randomised controlled trial. Trials. 2009;10:68. [DOI] [PMC free article] [PubMed]
- 37.Peters-Klimm F, Müller-Tasch T, Remppis A, Szecsenyi J, Schellberg D. Improved guideline adherence to pharmacotherapy of chronic systolic heart failure in general practice - Results from a cluster-randomized controlled trial of implementation of a clinical practice guideline. J Eval Clin Pract. 2008;14:823–9. doi: 10.1111/j.1365-2753.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 38.Reutens AT, Hutchinson R, Van Binh T, Cockram C, Deerochanawong C, Ho LT, et al. The GIANT study, a cluster-randomised controlled trial of efficacy of education of doctors about type 2 diabetes mellitus management guidelines in primary care practice. Diabetes Res Clin Pract. 2012;98(1):38–45. [DOI] [PubMed]
- 39.Rood E, Bosman R, Van der Spoel J, Taylor P, Zandstra D. Use of a computerized guideline for glucose regulation in the ICU improved both guideline adherence and glucose regulation. J Am Med Inform Assoc. 2005;12:172–80. doi: 10.1197/jamia.M1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi RA, Every NR. A computerized intervention to decrease the use of calcium channel blockers in hypertension. J Gen Intern Med. 1997;12:672–8. doi: 10.1046/j.1525-1497.1997.07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon SR, Majumdar SR, Prosser LA, Salem-Schatz S, Warner C, Kleinman K, et al. Group versus individual academic detailing to improve the use of antihypertensive medications in primary care: A cluster-randomized controlled trial. Am J Med. 2005;118:521–8. [DOI] [PubMed]
- 42.Steyn K, Lombard C, Gwebushe N, Fourie JM, Everett-Murphy K, Zwarenstein M, et al. Implementation of national guidelines, incorporated within structured diabetes and hypertension records at primary level care in Cape Town, South Africa: A randomised controlled trial. Glob Health Action. 2013;6:1–9. [DOI] [PMC free article] [PubMed]
- 43.Svetkey LP, Pollak KI, Yancy WS, Dolor RJ, Batch BC, Samsa G, et al. Hypertension improvement project: Randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension. 2009;54:1226–33. [DOI] [PMC free article] [PubMed]
- 44.Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou X, Eckert GJ, et al. Effects of computerized guidelines for managing heart disease in primary care. J Gen Intern Med. 2003;18:967–76. [DOI] [PMC free article] [PubMed]
- 45.van Bruggen R, Gorter KJ, Stolk RP, Verhoeven RP, Rutten GE. Implementation of locally adapted guidelines on type 2 diabetes. Fam Pract. 2008;25:430–7. doi: 10.1093/fampra/cmn045. [DOI] [PubMed] [Google Scholar]
- 46.van Steenkiste B, van der Weijden T, Stoffers HEJH, Kester ADM, Timmermans DRM, Grol R. Improving cardiovascular risk management: a randomized, controlled trial on the effect of a decision support tool for patients and physicians. Eur J Cardiovasc Prev Rehabil. 2007;14:44–50. doi: 10.1097/01.hjr.0000239475.71805.1e. [DOI] [PubMed] [Google Scholar]
- 47.Verweij LM, Proper KI, Weel AN, Hulshof CTJ, van Mechelen W. Long-term effects of an occupational health guideline on employees’ body weight-related outcomes, cardiovascular disease risk factors, and quality of life: results from a randomized controlled trial. Scand J Work Environ Health. 2013;39(3):284–94. doi: 10.5271/sjweh.3341. [DOI] [PubMed] [Google Scholar]
- 48.Verweij LM, Proper KI, Weel ANH, Hulshof CTJ, van Mechelen W. Design of the Balance@Work project: systematic development, evaluation and implementation of an occupational health guideline aimed at the prevention of weight gain among employees. BMC Public Health. 2009;9:461. doi: 10.1186/1471-2458-9-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiessling A, Lewitt M, Hendriksson P. Case-based training of evidence-based clinical practice in primary care with decreased mortality in patients with coronary heart disease. Ann Fam Med. 2011;9:211–18. doi: 10.1370/afm.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roumie C, Elasy T, Greevy R, Griffin M, Liu X, Stone W, et al. Improving Blood Pressure Control through Provider Education, Provider Alerts, and Patient Education. Ann Intern Med. 2006;145:165–75. [DOI] [PubMed]
- 51.Bonds D, Hogen P, Bertoni A, Chen H, Clinch C, Hiott A, et al. A multifaceted intervention to improve blood pressure control: The Guideline Adherence for Health Health (GLAD) study. Am J Manag Care. 2010;157(2):278–84. [DOI] [PMC free article] [PubMed]
- 52.Eaton C, Parker D, Borkan J, McMurray J, Roberts M, Lu B, et al. Translating Cholesterol Guidelines Into Primary Care Practice: A Multimodal Cluster Randomized Trial. Ann Fam Med. 2011;9:528–37. [DOI] [PMC free article] [PubMed]
- 53.Berwanger O, Guimaraes H, Larangeira L, Cavalcanti A, Kodama A, Zazula A, et al. Effect of a multifaceted intervention on use of evidence-based therapies in patients with acute coronary syndromes in Brazil: The BRIDGE-ACS randomized trial. JAMA. 2012;307(19):2041–49. [DOI] [PubMed]
- 54.Goldstein M, Lavori P, Coleman R, Advani A, Hoffman B. Improving adherence to guidelines for hypertension drug prescribing: cluster-randomized controlled trial of general versus patient-specific recommendations. Am J Manag Care. 2005;11:677–85. [PubMed] [Google Scholar]
- 55.Squires JE, Sullivan K, Eccles MP, Worswick J, Grimshaw JM. Are multifaceted interventions more effective than single-component interventions in changing health-care professionals’ behaviours? An overview of systematic reviews. Implement Sci. 2014;9:152. doi: 10.1186/s13012-014-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reiner Z, Sonicki Z, Tedeschi-Reiner R. Physicians perception, knowledge and awareness of cardiovascular risk factors and adherence to prevention guidelines: The PERCRO-DOC survey. Atherosclerosis. 2010;213:598–603. doi: 10.1016/j.atherosclerosis.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Okelo SO, Butz AM, Sharma R, Diette GB, Pitts SI, King TM, et al. Interventions to modify health care provider adherence to asthma guidelines. Rockville (MD): AHRQ Publication. 2013;95. [DOI] [PMC free article] [PubMed]
- 58.Ambroggio L, Thomson J, Murtagh Kurowski E, Courter J, Statile A, Graham C, et al. Quality improvement methods increase appropriate antibiotic prescribing for childhood pneumonia. Pediatrics. 2013;131:e1623–31. [DOI] [PMC free article] [PubMed]


