Abstract
Background
Allosensitization can be a significant barrier to transplantation for some patients, and previous studies suggested that pre-transplant allosensitization was associated with worse outcomes after lung transplantation. However, human leukocyte antigen (HLA) antibody testing has evolved significantly over the past 10 years and current assays are highly sensitive and specific.
Methods
We examined the impact of pre-transplant allosensitization on post-transplant outcomes in the era of solid-phase multiplex HLA antibody detection assays in this retrospective, single-center study of 304 adult recipients between 1/1/2006 and 12/31/2012. We accepted donor organs for allosensitized patients if a virtual crossmatch was compatible with all previously identified antibodies.
Results
In univariate and multivariate Cox proportional hazards models, pre-transplant allosensitization, the calculated panel reactive antibody (CPRA), and the number of pre-transplant HLA antibodies were not associated with the development of acute cellular rejection, lymphocytic bronchiolitis, donor-specific HLA antibodies, chronic lung allograft dysfunction, or graft failure.
Conclusions
We conclude that pre-transplant allosensitization does not adversely affect outcomes after lung transplantation when the potentially reactive HLA are avoided in the donor by a virtual crossmatch with the recipient.
Keywords: lung transplantation, allosensitization, panel reactive antibody
INTRODUCTION
Lung transplantation is the ultimate treatment option for patients with end-stage lung disease. However, long-term survival after transplantation remains disappointing, and the leading cause of death is chronic lung allograft dysfunction (CLAD) (1). Multiple studies have identified the development of donor-specific human leukocyte antigen (HLA) antibodies (DSA) after transplantation as an important risk factor for the development of CLAD, lymphocytic bronchiolitis (LB), acute cellular rejection (ACR), antibody-mediated rejection (AMR), and death (2–9). However, the impact of pre-transplant HLA antibodies, or allosensitization, on post-transplant outcomes is less clear, and previous studies have generated conflicting results.
An early study using the complement-dependent cytotoxicity (CDC) assay concluded that pre-transplant allosensitization was uncommon, and a modestly elevated panel reactive antibody (PRA) was not a risk factor for CLAD, ACR, or death (11). In contrast, another study showed that patients who had a PRA > 10% required prolonged mechanical ventilation immediately after transplantation, were more likely to develop CLAD, and had a trend to worse survival (12). A subsequent multicenter study using the CDC assay showed that recipients with a PRA > 25% were more likely to have a positive crossmatch and had a higher risk of death in the early post-transplant period (13).
The increased morbidity and mortality associated with allosensitization after transplantation suggests that recipients may have had pre-existing DSA that were not detected by the CDC assay, ultimately resulting in HLA-incompatible transplants. An analysis of the United Network for Organ Sharing (UNOS) registry found that a PRA > 25% was an independent risk factor for death after transplantation between 1987 and 1997, but not between 1998 and 2005 (14). The authors proposed that advancements in HLA antibody detection methods improved donor selection and minimized the effects of allosensitization on post-transplant outcomes in the more recent era. Indeed, antibody analysis using solid-phase multiplex methods has allowed precise identification of antibody specificity, and potential donors with unacceptable HLA that would be expected to result in a positive direct crossmatch can then be avoided. Use of this virtual crossmatch can expand the donor pool and improve waitlist outcomes (15).
The impact of pre-transplant allosensitization on long-term outcomes after transplantation in the era of solid-phase multiplex HLA antibody detection assays and virtual crossmatching has not been evaluated. We hypothesized that virtual crossmatching based on sensitive and specific HLA antibody detection assays would ameliorate the impact of pre-transplant allosensitization on post-transplant outcomes.
METHODS
Study design
We conducted a retrospective cohort study including all patients listed for lung transplantation at our program between 1/1/2006 and 12/31/2011. During this time period, 368 patients were listed for transplantation; 3 were subsequently transplanted at another program and were excluded. Of the remaining 365 patients, 304 were transplanted at our center before 12/31/2012 and comprise this cohort. The remaining 61 patients died on the waitlist, were removed from the waitlist before transplantation, or were still waiting on 12/31/2012. We conducted a separate study examining the impact of pre-transplant allosensitization on waitlist outcomes, and those results are not presented here (16). Our institutional review board approved this study as part of our lung transplant registry protocol.
Clinical management
At listing, we screened all patients for pre-formed HLA antibodies using the LABScreen® Single Antigen assay. Thereafter, we repeated antibody testing every 3 months while on the waitlist and 2–4 weeks after a potentially allosensitizing event. Our center’s histocompatibility lab defines HLA antibody positivity as reactivity with a mean fluorescence intensity (MFI) ≥ 2000. We used this cut-off for antibody detection before and after transplantation, and computed the calculated PRA (CPRA) using the UNOS calculator (17). We defined allosensitization as any HLA antibodies, either historical or current, with an MFI ≥ 2000, and accepted donor lungs if a virtual crossmatch was compatible with all previously identified antibodies. At the time of transplant, we performed a direct CDC crossmatch in all patients.
We treated recipients with antithymocyte globulin or basiliximab for induction immunosuppression and used tacrolimus, azathioprine or mycophenolate mofetil, and prednisone for maintenance immunosuppression. We performed surveillance bronchoscopies at 1, 2, 3, 6, and 12 months after transplantation, when clinically indicated, and 3–6 weeks after an episode of ACR. We screened recipients for DSA using the LABScreen® Single Antigen assay at 1, 2, 3, 6, and 12 months after transplantation, and when clinically indicated. We examined bronchoscopy and nasopharyngeal swab samples for community-acquired respiratory viruses (CARV) using a fluorescent-antibody (FA) assay and culture until 3/1/2013. After 3/1/2013, we used a multiplex viral PCR assay, and defined any positive result as a CARV infection. We measured spirometry weekly for the first 12 weeks, monthly for the remainder of the first year, then every 1–3 months thereafter. We diagnosed CLAD according to the International Society for Heart and Lung Transplantation (ISHLT) guidelines (18, 19).
Statistical analysis
We compared categorical variables using the chi-squared test and continuous variables using the Student’s t-test for normally distributed data and the Wilcoxon Rank-Sum test for skewed data. We defined graft failure as death or re-transplantation. We examined freedom from ACR, lymphocytic bronchiolitis, CLAD, DSA, and graft failure using the Kaplan-Meier method and compared groups using the log rank test. We constructed univariate and multivariate Cox proportional hazards models to evaluate the impact of different variables on the development of DSA, CLAD, and graft failure. In all models, we evaluated DSA, ACR, lymphocytic bronchiolitis, CLAD, and CARV infections as time-dependent variables to avoid assigning risk before their development. In addition, we included only 1 time-dependent variable in each multivariate model to avoid risk overinflation; this resulted in 4 multivariate models. We conducted statistical analysis using SPSS 22.0.
RESULTS
Baseline characteristics and histocompatibility
Follow-up was complete through 12/31/2013, and the study included 974 patient-years of follow-up with a mean follow-up of 3.2 ± 1.9 years. Among the 304 recipients, 108 (35.5%) were allosensitized before transplantation, and 196 (64.5%) were not (Table 1). Overall, there was no significant difference in baseline characteristics between recipients who were allosensitized and those who were not (Table 1). Among the 108 allosensitized recipients, 29 (27%) had a CPRA > 50%, and 10 (9%) had a CPRA ≥ 80%.
Table 1.
Baseline characteristics of transplant recipients (n = 304).
| Variable | Not allosensitized (n = 196) |
Allosensitized (n = 108) |
p value |
|---|---|---|---|
| Male recipient, n (%) | 115 (59%) | 60 (56%) | 0.599 |
| Age, mean ± SD | 52.5 ± 14.4 | 50.2 ± 14.9 | 0.430 |
| Diagnosis, n (%) | 0.144 | ||
| COPD | 55 (28%) | 29 (27%) | |
| Cystic fibrosis | 34 (17%) | 24 (22%) | |
| Interstitial lung disease | 85 (43%) | 37 (34%) | |
| Re-transplant | 5 (3%) | 8 (7%) | |
| Pulmonary hypertension | 3 (2%) | 0 | |
| Other | 14 (7%) | 10 (9%) | |
| Race, n (%) | 0.531 | ||
| White | 182 (93%) | 101 (93.5%) | |
| African American | 9 (4.5%) | 5 (4.5%) | |
| Asian | 1 (0.5%) | 0 | |
| Hispanic | 4 (2%) | 1 (1%) | |
| Other | 0 | 1 (1%) | |
| Operation | 0.164 | ||
| Bilateral, n (%) | 186 (95%) | 106 (98%) | |
| Single, n (%) | 10 (5%) | 2 (2%) | |
| CMV mismatch (D+/R-), n (%) | 56 (29%) | 29 (27%) | 0.929 |
| PGD 3 at 2 hours, n (%) | 9 (5%) | 9 (8%) | 0.186 |
None of the allosensitized recipients had DSA at the time of transplant. The direct CDC crossmatch was negative in 173 of the 196 (88%) patients who were not allosensitized and 95 of the 108 (88%) who were allosensitized (Table 2). Further delineation of the crossmatch results is detailed in Table 2. The crossmatch results were not available for 7 patients. None of the recipients with a positive crossmatch had hyperacute rejection clinically.
Table 2.
Direct donor-recipient crossmatch results.
| Not allosensitized (n = 196) |
Allosensitized (n = 108) |
p value |
|
|---|---|---|---|
| Crossmatch results | 0.161 | ||
| Negative CDC, n (%) | 173 (88%) | 95 (88%) | |
| Positive CDC, reduced to negative with DTT, n (%) | 15 (7.7%) | 8 (7.4%) | |
| B-cell positive, reduced to negative with DTT, n (%) | 13 (6.6%) | 8 (7.4%) | |
| T- and B-cell positive, reduced to negative with DTT, n (%) | 2 (1%) | 0 | |
| Positive CDC B-cell crossmatch, positive with DTT, n (%) | 0 | 2 (2%) | |
| Positive CDC B-cell crossmatch, positive with DTT, negative flow cytometry crossmatch, n (%) | 1 (0.5%) | 2 (2%) | |
| Positive CDC B-cell crossmatch, reduced to negative with DTT, positive flow cytometry T- and B-cell crossmatch, n (%) | 0 | 1 (1%) | |
| Crossmatch results not available, n (%) | 7 | 0 |
Acute cellular rejection and lymphocytic bronchiolitis
There was no significant difference in freedom from ACR grade ≥ A2 between recipients who were allosensitized and those who were not (Figure 1A). In addition, there was no significant difference in the mean number of episodes of ACR grade ≥ A2 between recipients who were allosensitized and those who were not (1.18 ± 1.03 vs. 1.22 ± 1.08, respectively; p = 0.82). Likewise, there was no significant difference in freedom from ACR grade ≥ A2 between recipients who were allosensitized with a CPRA > 50%, those with a CPRA between 1–50%, and those who were not allosensitized (Figure 1B). In addition, there was no significant difference in freedom from ACR when the allosensitized group was stratified by HLA antibody class (data not shown). Similarly, there was no significant difference in freedom from LB grade ≥ B1R between recipients who were allosensitized and those who were not (Figure 1C), and there was no significant difference in the mean number of episodes of LB grade ≥ B1R between those who were allosensitized and those who were not (1.51 ± 1.30 vs. 1.58 ± 1.22, respectively; p = 0.74). Lastly, there was no significant difference in freedom from LB grade ≥ B1R between recipients with a CPRA > 50%, those with a CPRA between 1–50%, and those who were not allosensitized (Figure 1D).
Figure 1.
Freedom from acute cellular rejection (ACR) and lymphocytic bronchiolitis (LB). A. There was no significant difference in freedom from ACR grade ≥ A2 between allosensitized recipients and those who were not. B. There was no significant difference in freedom from ACR grade ≥ A2 between recipients who were allosensitized with a CPRA > 50%, those with a CPRA between 1–50%, and those who were not allosensitized. C. There was no significant difference in freedom from LB grade ≥ B1R between recipients who were allosensitized and those who were not. D. There was no significant difference in freedom from LB grade ≥ B1R between recipients with a CPRA > 50%, those with a CPRA between 1–50%, and those who were not allosensitized.
Donor-specific antibodies
Patients had a mean 6.0 ± 2.1 DSA screens in the first year after transplantation and 4.2 ± 3.8 screens beyond the first year. Among the 304 recipients, 155 (51%) developed DSA at 134±275 days after transplantation; 18 developed class I DSA only, 91 developed class II DSA only, and 46 developed both class I and II DSA. One hundred thirty five recipients developed DSA in the first year after transplantation, and 20 developed DSA beyond the first year. There was no significant difference in freedom from DSA between recipients who were allosensitized before transplantation and those who were not (Figure 2A). Similarly, there was no significant difference in freedom from DSA between recipients with a CPRA between 1–50%, those with a CPRA > 50%, and those who were not allosensitized (Figure 2B). Univariate Cox proportional hazards models of risk factors for DSA are shown in Table 3. Primary graft dysfunction (PGD) grade 3 at 72 hours, ACR grade ≥ A2, and CLAD were associated with a significantly increased risk of DSA (Table 3). Among the 75 recipients who had DSA and ACR grade ≥ A2, DSA was detected first in 20 recipients, ACR was detected first in 19 recipients, and both ACR and DSA were detected concurrently in 36 recipients. However, allosensitization, CPRA, and the number of HLA antibody specificities before transplantation were not associated with an increased risk of DSA development after transplantation (Table 3). Multivariate models of risk factors for DSA are shown in Table 4. In model 1, ACR grade ≥ A2 was associated with a significantly increased risk of DSA, and PGD 3 at 72 hours was associated with a trend to an increased risk of DSA, but there was no association between pre-transplant CPRA and DSA development (Table 4). In model 2, CLAD was associated with a significantly increased risk of DSA, and PGD 3 at 72 hours was associated with a trend to an increased risk of DSA, but there was no association between pre-transplant CPRA and DSA (Table 4). Finally, there was no association between allosensitization or the number of pre-transplant HLA antibodies and DSA when we substituted these variables for CPRA in these models (data not shown). During the study period, DSA persisted in 65 of the 155 (42%) recipients who developed DSA and cleared in 90 (58%); however, we did not evaluate the impact of treatment on DSA depletion or clinical outcomes as our clinical practice evolved significantly during the study period.
Figure 2.
Freedom from the development of donor-specific HLA antibodies (DSA). A. There was no significant difference in freedom from DSA between recipients who were allosensitized before transplantation and those who were not. B. There was no significant difference in freedom from DSA between recipients with a CPRA > 50%, those with a CPRA between 1–50%, and those who were not allosensitized.
Table 3.
Univariate Cox proportional hazards models for risk factors for DSA, CLAD, and graft failure.
| Variable | DSA | CLAD | Graft failure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI |
p | HR | 95% CI |
p | HR | 95% CI |
p | |
| Male recipient | 1.06 | 0.77–1.46 | 0.72 | 0.96 | 0.69–1.34 | 0.83 | 0.99 | 0.69–1.42 | 0.97 |
| Recipient age | 0.99 | 0.99–1.01 | 0.36 | 1.00 | 0.99–1.01 | 0.87 | 0.99 | 0.98–1.01 | 0.27 |
| Diagnosis (reference: COPD) | 0.99 | 0.87 | 0.26 | ||||||
| Cystic fibrosis | 1.15 | 0.72–1.82 | 0.55 | 0.87 | 0.53–1.41 | 0.57 | 1.10 | 0.67–1.81 | 0.70 |
| Interstitial lung disease | 1.10 | 0.73–1.63 | 0.66 | 0.92 | 0.61–1.37 | 0.68 | 0.70 | 0.44–1.09 | 0.12 |
| Re-transplant | 0.97 | 0.43–2.17 | 0.94 | 0.63 | 0.25–1.60 | 0.33 | 0.63 | 0.22–1.77 | 0.38 |
| Pulmonary hypertension | 1.61 | 0.22–11.7 | 0.64 | 1.11 | 0.15–8.19 | 0.91 | 2.13 | 0.51–8.85 | 0.30 |
| Other | 1.12 | 0.59–2.14 | 0.73 | 0.71 | 0.35–1.43 | 0.34 | 1.08 | 0.56–2.07 | 0.83 |
| Allosensitized pre-transplant | 1.11 | 0.80–1.54 | 0.53 | 1.04 | 0.74–1.47 | 0.82 | 1.06 | 0.73–1.54 | 0.78 |
| Pre-transplant CPRA | 1.00 | 0.99–1.01 | 0.65 | 0.99 | 0.99–1.01 | 0.56 | 0.99 | 0.99–1.01 | 0.79 |
| Number of pre-transplant HLA antibodies | 0.99 | 0.96–1.03 | 0.67 | 0.99 | 0.95–1.02 | 0.60 | 0.99 | 0.94–1.03 | 0.59 |
| Pre-transplant HLA antibody class | 0.67 | 0.76 | 0.66 | ||||||
| Class I only | 1.14 | 0.76–1.71 | 0.52 | 1.16 | 0.78–1.74 | 0.46 | 0.89 | 0.55–1.45 | 0.64 |
| Class II only | 0.83 | 0.42–1.64 | 0.59 | 0.78 | 0.38–1.61 | 0.50 | 1.28 | 0.66–2.48 | 0.46 |
| Class I and II | 1.27 | 0.76–2.13 | 0.36 | 0.98 | 0.52–1.83 | 0.94 | 1.31 | 0.69–2.46 | 0.41 |
| Positive direct crossmatch | 0.99 | 0.57–1.73 | 0.98 | 0.95 | 0.52–1.71 | 0.85 | 1.28 | 0.72–2.28 | 0.40 |
| PGD 3 at 72 hours | 2.50 | 1.16–5.38 | 0.02 | 1.97 | 0.94–4.14 | 0.07 | 2.06 | 0.92–4.62 | 0.08 |
| DSA* | 1.55 | 1.11–2.16 | 0.01 | 2.06 | 1.41–3.00 | <0.001 | |||
| Acute rejection grade ≥ A2* | 1.86 | 1.25–2.76 | 0.002 | 1.11 | 0.79–1.55 | 0.55 | 0.95 | 0.65–1.38 | 0.77 |
| Lymphocytic bronchiolitis ≥ B1R* | 1.27 | 0.86–1.87 | 0.23 | 0.93 | 0.66–1.30 | 0.67 | 0.99 | 0.68–1.45 | 0.98 |
| CARV infection* | 0.17 | 0.02–1.25 | 0.10 | 1.46 | 0.91–2.36 | 0.12 | 1.19 | 0.70–2.02 | 0.52 |
| CLAD* | 4.59 | 2.02–10.4 | <0.001 | 8.92 | 5.61–14.2 | <0.001 | |||
Analyzed as a time-dependent variable
Table 4.
Multivariate Cox proportional hazards models for risk factors for DSA, CLAD, and graft failure.
| Model | Variable | DSA | CLAD | Graft failure | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
| 1 | PGD 3 at 72 hours | 1.65 | 0.87–3.15 | 0.13 | 2.17 | 1.13–4.15 | 0.02 | 1.63 | 0.83–3.22 | 0.15 |
| Pre-transplant CPRA | 1.00 | 0.99–1.01 | 0.49 | 1.00 | 0.99–1.01 | 0.52 | 1.00 | 0.99–1.01 | 0.74 | |
| Acute rejection grade ≥ A2* | 1.93 | 1.30–2.88 | 0.001 | 1.10 | 0.79–1.54 | 0.57 | 0.95 | 0.65–1.38 | 0.77 | |
| 2 | PGD 3 at 72 hours | 1.45 | 0.76–2.77 | 0.25 | 1.26 | 0.64–2.49 | 0.51 | |||
| Pre-transplant CPRA | 1.00 | 0.99–1.01 | 0.58 | 1.00 | 0.99–1.01 | 0.75 | ||||
| CLAD* | 4.59 | 2.02–10.5 | <0.001 | 8.91 | 5.60–14.2 | <0.001 | ||||
| 3 | PGD 3 at 72 hours | 2.08 | 1.09–3.99 | 0.03 | 1.70 | 0.86–3.34 | 0.13 | |||
| Pre-transplant CPRA | 1.00 | 0.99–1.01 | 0.45 | 1.00 | 0.99–1.01 | 0.76 | ||||
| DSA* | 1.54 | 1.10–2.14 | 0.01 | 2.07 | 1.42–3.01 | <0.001 | ||||
| 4 | PGD at 72 hours | 1.48 | 0.78–2.82 | 0.23 | 2.19 | 1.14–4.18 | 0.02 | 1.66 | 0.84–3.28 | 0.14 |
| Pre-transplant CPRA | 1.00 | 0.99–1.01 | 0.74 | 0.99 | 0.98–1.01 | 0.49 | 1.00 | 0.99–1.01 | 0.77 | |
| CARV infection* | 0.18 | 0.04–1.28 | 0.10 | 1.48 | 0.92–2.40 | 0.10 | 1.22 | 0.72–2.07 | 0.47 | |
Analyzed as a time-dependent variable.
Chronic lung allograft dysfunction (CLAD)
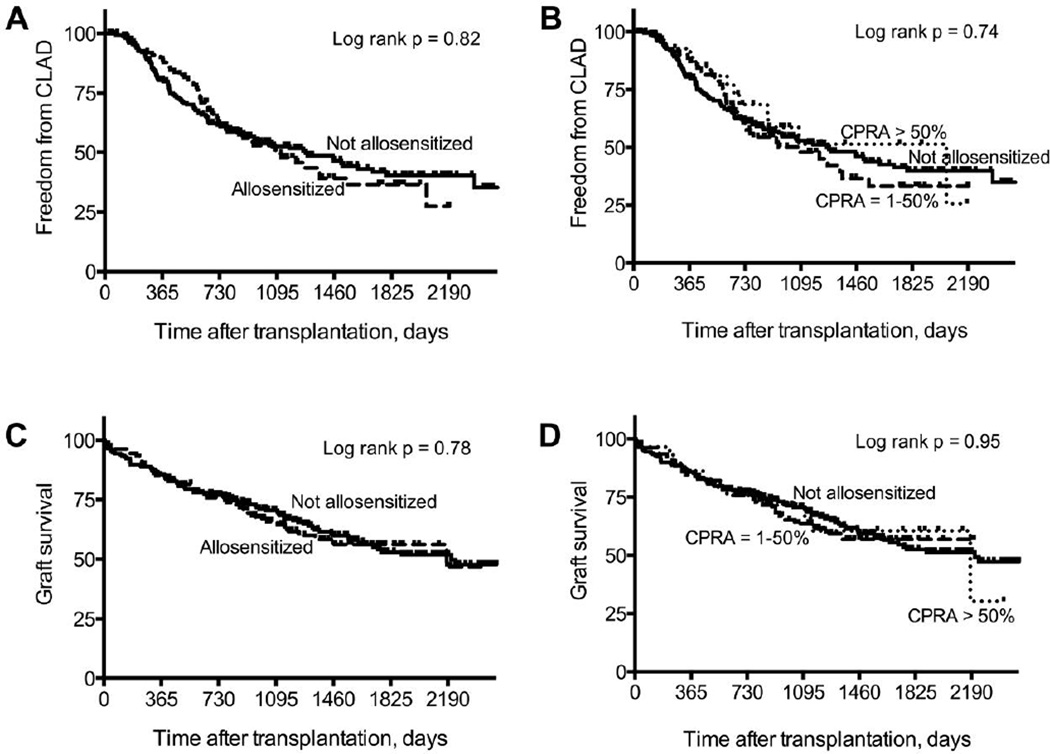
There was no significant difference in freedom from CLAD between recipients who were allosensitized and those who were not (Figure 3A). In addition, there was no significant difference in freedom from CLAD between recipients who were allosensitized with a CPRA between 1–50%, those with a CPRA > 50%, and those who were not allosensitized (Figure 3B). Univariate Cox proportional hazards models of risk factors for CLAD are shown in Table 3. DSA was associated with a significantly increased risk of CLAD, and PGD 3 at 72 hours and CARV infections were associated with a trend to an increased risk of CLAD (Table 3). Among the 87 recipients who developed DSA and CLAD, DSA was detected first in 67 recipients, CLAD was diagnosed before the detection of DSA in 10 recipients, and DSA was detected concurrently with the diagnosis of CLAD in 10 recipients. However, allosensitization, CPRA, and the number of HLA antibodies were not associated with an increased risk of CLAD (Table 3). Similarly, a positive crossmatch result was not associated with an increased risk of CLAD. Multivariate models of risk factors for CLAD are shown in Table 4. In model 1, PGD 3 at 72 hours was associated with an increased risk of CLAD, but there was no association between ACR or CPRA and CLAD (Table 4). In model 3, PGD 3 at 72 hours and DSA were associated with a significantly increased risk of CLAD, but there was no association between CPRA and CLAD (Table 4). There was no association between allosensitization or the number of pre-transplant HLA antibodies and CLAD when we substituted these variables for CPRA in these models (data not shown).
Figure 3.
Freedom from chronic lung allograft dysfunction (CLAD) and graft survival. A. There was no significant difference in freedom from CLAD between recipients who were allosensitized and those who were not. B. There was no significant difference in freedom from CLAD between recipients who were allosensitized with a CPRA > 50%, those with a CPRA between 1–50%, and those who were not allosensitized. C. There was no significant difference in graft survival between recipients who were allosensitized and those who were not. D. There was no significant difference in graft survival between those with a CPRA > 50%, those with a CPRA between 1–50%, and those who were not allosensitized.
Graft failure
There was no significant difference in freedom from graft failure (defined as death or re-transplantation) between recipients who were allosensitized and those who were not (Figure 3C). In addition, there was no significant difference in freedom from graft failure between those with a CPRA between 1–50%, those with a CPRA > 50%, and those who were not allosensitized (Figure 3D). Univariate Cox proportional hazards models of risk factors for graft failure are shown in Table 3. CLAD and DSA were associated with a significantly increased risk of graft failure, and PGD 3 at 72 hours was associated with a trend to an increased risk of graft failure (Table 3). However, allosensitization, CPRA, and the number of pre-transplant HLA antibodies were not associated with an increased risk of graft failure (Table 3). Similarly, a positive crossmatch result was not associated with an increased risk of graft failure. Multivariate models of risk factors for graft failure are shown in Table 4. In model 2, CLAD was associated with a significantly increased risk of graft failure, but CPRA was not (Table 4). Similarly, in model 3, DSA was associated with a significantly increased risk of graft failure, but CPRA was not (Table 4). In addition, there was no association between allosensitization or the number of pre-transplant HLA antibodies and graft failure when we substituted these variables for CPRA in these models (data not shown).
DISCUSSION
In this study, we examined the impact of pre-transplant allosensitization on post-transplant outcomes in the era of solid-phase multiplex HLA antibody detection assays and virtual crossmatching. Our analysis did not reveal any association between pre-transplant allosensitization and adverse outcomes after lung transplantation. Specifically, we found no association between pre-transplant allosensitization and the development of ACR, lymphocytic bronchiolitis, DSA, CLAD, or graft failure. Moreover, we found no association between the degree of allosensitization, as gauged by the CPRA and the number of pre-transplant HLA antibodies, and these post-transplant adverse outcomes. Our findings differ from prior studies that identified an increased risk of early morbidity and mortality associated with pre-transplant allosensitization (12, 13). We attribute this to advancements in HLA antibody testing that improved the detection of antibodies and the precise identification of their specificities. The use of the solid-phase multiplex assays and virtual crossmatching likely minimized HLA incompatibilities between the donor and recipient and reduced the impact of allosensitization on post-transplant outcomes. Indeed, pre-transplant DSA may not be detected by less sensitive assays, yet these antibodies are associated with an increased risk of CLAD and death after transplantation (20, 21). Furthermore, our findings corroborate the results of the analysis of the modern era in the UNOS registry and a recent single-center study that used a solid-phase antibody detection assay (14, 22). Our data extend these results by demonstrating that there is no association between pre-transplant allosensitization and ACR, LB, CLAD, and long-term graft survival.
Our data confirm significant associations between ACR, DSA, and CLAD, as previously reported (2–8). However, these associations are complex, and it is difficult to reach conclusions about causality. Although we evaluated ACR and CLAD as time-dependent variables in the analysis of risk factors for DSA, because of the time interval between DSA testing, some patients could have had DSA for some time before it was detected. In addition, concurrent development of DSA and ACR as well as DSA and CLAD suggests a potential link between humoral and cellular immune responses. Alternatively, the development of ACR or CLAD before DSA suggests that graft injury including the resulting inflammatory cascade and remodeling characteristic of ACR or CLAD may increase the expression of HLA molecules and promote DSA development. Regardless, we are unable to make conclusions about causality from our data because of these intrinsic limitations and potential biases. PGD 3 at 72 hours was associated with a trend to an increased risk of DSA and graft failure in multivariate models, but this was not statistically significant. We suspect that this is related to the small sample size. In addition, CARV infections were associated with an increased risk of CLAD, but this was not statistically significant. We suspect that this may be related to a relatively small sample size and an insensitive CARV detection assay used during most of the study period.
There are several limitations inherent to this study’s design. First, this was a retrospective single-center study. Importantly, we used a relatively low MFI cut-off to define HLA antibody positivity, and all historical and current HLA antibodies were included in the virtual crossmatch with prospective donors. Clearly, this is a conservative approach, and there was strong concordance between the virtual crossmatch and the CDC crossmatch. While few patients had a positive direct crossmatch, this was likely due to non-HLA antibodies and did not result in hyperacute rejection or an increased risk of CLAD or graft failure. Indeed, most positive crossmatch results were reduced to negative after treatment with dithiothreitol (DTT), indicating IgM antibodies. However, this conservative strategy is associated with longer waiting times for transplantation and increased waitlist mortality for highly allosensitized patients (16). More aggressive strategies such as using a higher MFI cut-off to define antibody positivity, using the C1q binding assay to identify unacceptable antigens, or implementing an antibody depletion protocol may expand the donor pool and improve the likelihood of transplantation for allosensitized patients (22–24). Importantly, we can only conclude that pre-transplant allosensitization does not adversely affect post-transplant outcomes when a conservative virtual crossmatch is used to accept donors. Lastly, there were only 10 highly allosensitized (CPRA > 80%) patients in this cohort, and it is possible that this small sample size may have been underpowered to detect an impact on post-transplant outcomes if this degree of allosensitization carries an increased risk.
In conclusion, our results demonstrate that pre-transplant allosensitization does not adversely affect post-transplant outcomes when a sensitive and specific antibody detection assay is used in conjunction with a conservative virtual crossmatch approach. However, allosensitization remains a significant barrier to transplantation for some patients.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (HL056643 to T.M. and R.R.H., HL105412 to R.D.Y., T.M., and R.R.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
REFERENCES
- 1.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report – 2014, focus theme: retransplatnation. J Heart Lung Transplant. 2014;33:1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Sundaresan S, Mohankumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65:648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo A, Smith MA, Phelan D, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155–1161. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 4.Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74:799–804. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 5.Safavi S, Robinson DR, Soresi S, Carby M, Smith JD. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:1273–1281. doi: 10.1016/j.healun.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33:1288–1294. doi: 10.1016/j.healun.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Girnita AL, Duquesnoy R, Samuel A, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 8.Girnita AL, McCurry KR, Iacono AT, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23:1135–1141. doi: 10.1016/j.healun.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32:1034–1040. doi: 10.1016/j.healun.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder LD, Wang Z, Chen DF, et al. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest. 2013;144:226–233. doi: 10.1378/chest.12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gammie JS, Pham SM, Colson YL, et al. Influence of panel-reactive antibody on survival and rejection after lung transplantation. J Heart Lung Transplant. 1997;16:408–415. [PubMed] [Google Scholar]
- 12.Lau CL, Palmer SM, Posther KE, et al. Influence of panel-reactive antibodies on posttransplant outcomes in lung transplant recipients. Ann Thorac Surg. 2000;69:1520–1524. doi: 10.1016/s0003-4975(00)01224-8. [DOI] [PubMed] [Google Scholar]
- 13.Hadjiliadis D, Chaparro C, Reinsmoen NL, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005;24:S249–S254. doi: 10.1016/j.healun.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Shah AS, Nwakanma L, Simpkins C, Williams J, Chang DC, Conte JV. Pretransplant panel reactive antibodies in human lung transplantation: an analysis of over 10,000 patients. Ann Thorac Surg. 2008;85:1919–1924. doi: 10.1016/j.athoracsur.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Appel JZ, Hartwig MG, Cantu E, Palmer SM, Reinsmoen NL, Davis RD. Role of flow cytometry to define unacceptable HLA antigens in lung transplant recipients with HLA-specific antibodies. Transplantation. 2006;81:1059–1057. doi: 10.1097/01.tp.0000204046.89396.c5. [DOI] [PubMed] [Google Scholar]
- 16.Witt CA, Byers DE, Yusen RD, et al. Allosensitization increases the risk of death on the lung transplant waiting list. J Heart Lung Transplant. 2014;33:S299. [Google Scholar]
- 17. Optn.transplant.hrsa.gov [homepage on the Internet] U.S. Department of Health and Human Services. Optn.transplant.hrsa.gov/converg/resources/allocationcalculators.asp?index=78.
- 18.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 19.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Brugiere O, Suberbielle C, Thabut G, et al. Lung transplantation in patients with pretransplant donorspecific antibodies detected by luminex assay. Transplantation. 2013;95:761–765. doi: 10.1097/TP.0b013e31827afb0f. [DOI] [PubMed] [Google Scholar]
- 21.Smith JD, Ibrahim MW, Newell H, et al. Pre-transplant donor HLA-specific antibodies: characteristics causing detrimental effects on survival after lung transplantation. J Heart Lung Transplant. 2014;33:1074–1082. doi: 10.1016/j.healun.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Kim M, Townsend KR, Wood IG, et al. Impact of pretransplant anti-HLA antibodies on outcomes in lung transplant candidates. Am J Respir Crit Care Med. 2014;189:1234–1239. doi: 10.1164/rccm.201312-2160OC. [DOI] [PubMed] [Google Scholar]
- 23.Tinckam KJ, Keshavjee S, Chaparro C, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. 2015;15:417–426. doi: 10.1111/ajt.13076. [DOI] [PubMed] [Google Scholar]
- 24.Hachem RR, Reinsmoen NL. What is the definition of a clinically relevant donor HLA-specific antibody (DSA)? Am J Transplant. 2015;15:299–300. doi: 10.1111/ajt.13079. [DOI] [PubMed] [Google Scholar]