Abstract
Progressive multifocal leukoencephalopathy (PML) is caused by JC polyomavirus (JCPyV). Because a reciprocal relationship has been described between antibody levels to JCPyV and BK polyomavirus (BKPyV), we performed a nested case control study with pre-diagnostic serum samples from HIV infected subjects to examine the relationship between BKPyV capsid antibodies and the risk of PML. Serum samples collected 0·5–2 years before PML diagnosis from 25 cases (66 samples) and 80 matched controls (204 samples) were tested in ELISA for JCPyV, BKPyV type 1 and type 4 capsid antibodies. High levels of BKPyV 1 and 4 antibodies were associated with a lower risk of PML (BKPyV 1 OR, 0·56, 95% CI, 0·35–0·89; BKPyV 4 OR, 0·40, 95% CI, 0·24–0·0.67). Our study demonstrates that antibodies to BKPyV capsids are an immunological marker of protection against development of PML. Further studies are needed to define the mechanism.
Keywords: Human immunodeficiency virus, progressive multifocal leukoencephalopathy, BK polyomavirus, JC polyomavirus, viral serology, cohort study
Introduction
Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disorder of the central nervous system caused by lytic infection of oligodendrocytes with JC polyomavirus (JCPyV) (Brew et al., 2010). PML occurs on a background of conditions associated with T-cell deficiencies (Viscidi RP and Shah KV, 2010). The majority of cases reported in the past 3 decades have occurred in human immunodeficiency virus (HIV) infected patients (Christensen et al., 2010). More recently, the immunomodulatory drug, natalizumab, and less commonly other monoclonal antibodies targeting immune modulatory antigens, have been associated with rare cases of PML (Rossi et al., 2014b).
Exposure to JCPyV is common in the general population but PML is a rare disease, even among patients at risk due to an underlying predisposing condition. Thus, most individuals must be protected against the development of PML. Protection is likely to be immunologically mediated since all conditions associated with PML involve an immunodeficiency state. However, the nature of protective immunity is unknown. As a measure of exposure to JCPyV, antibodies to the VP1 major capsid protein have been shown to be a risk predictor for PML associated with natalizumab therapy (Gorelik et al., 2010), and we have shown that JCPyV seropositivity is associated with a non-significant trend for increased risk of PML in HIV-infected patients (Viscidi et al., 2011a). JCPyV is genetically related to a second human polyomavirus, BK polyomavirus (BKPyV) (Bennett et al., 2012). A negative correlation has been observed between levels of antibody to JCPyV and BKPyV capsids (Kean et al., 2009; Egli et al., 2009; Hamilton et al., 2000; Knowles et al., 2003). This unexplained phenomenological observation lead us to hypothesize that high levels of antibody to BKPyV will be a biomarker for protection against the development of PML. An opportunity to investigate pre-diagnostic markers is provided by prospective cohort studies of HIV infected subjects. We performed a nested case control study, conducted within the Multicenter AIDS Cohort Study (MACS), to examine whether antibody to BKPyV capsids are predictors of the subsequent diagnosis of PML. BKPyV genotypes are known to be serologically distinct and the major genotypes circulating in human populations are type 1 and 4; therefore, we tested for antibody to the capsid proteins of these two major serotypes (Pastrana et al., 2013).
Methods and Materials
Study population
The MACS is an ongoing, prospective cohort study of HIV/AIDS in homosexual and bisexual men in the United States (Detels et al., 2012). Initial recruitment for the MACS began in April 1983 and up to January 2001, a total of 2,239 HIV-positive men have been enrolled and 584 HIV-negative men at study entry have become HIV-1 infected. Collectively, these men have contributed over 34,491 HIV-positive person-years of observation. The study was approved by local ethical review boards, and written informed consent was obtained from all participants. Demographic and clinical data and plasma samples were collected at follow-up visits every 6 months. A total of 32 patients were diagnosed with PML between 1985 and 2000. Seven patients were excluded from the present study because plasma samples were not available during the time interval of interest, 0.5 to 2 years prior to PML diagnosis. The time interval was selected based on sample availability and the desire to evaluate biomarkers in a clinically useful time frame. PML was diagnosed by histological examination of brain tissue in 7 of 25 (28%), a combination of radiographic and clinical signs in 8 (32%), radiology alone in 2 (8%), and clinical diagnosis alone in 8 (32%) patients. Each case was matched to three HIV seropositive participants, who did not develop PML. Matching criteria were (1) CD4+ T-cell count (+50 cells per microliter) at the study entry visit to account for duration of HIV infection prior to entry into the study and (2) PML-free time from baseline. Cases for which PML was the AIDS defining event were further matched to HIV-positive controls on rate of CD4+ T-cell decline from baseline (+50cells per microliter per year). If PML was not the AIDS-defining event of a case, an HIV-positive control was selected who had an AIDS-defining event within one calendar year of the AIDS-defining event of the case and comparable PML-free time from AIDS. A total of 81 controls were identified. The 25 cases contributed a total of 66 plasma specimens with 16 (64%) cases providing three or more specimens. The 80 controls contributed a total of 204 plasma specimens with 52 (65%) controls providing three or more specimens.
Serological assays
To construct a virus-like particle (VLP) for a BKPyV genotype 4 strain, a recombinant baculovirus expressing a codon optimized VP1 gene of BKPyV genotype 4 strain MMR-29 was constructed and VLPs were purified from insect cells infected with the recombinant virus using methods previously developed for purification of polyomavirus VLPs (Viscidi et al., 2003). A VLP-based enzyme-linked immunosorbent assay (ELISA) was used to detect serum antibody to JCPyV, BKPyV genotype I and BKPyV genotype 4 capsids, as described previously, with minor modifications (Viscidi et al., 2011a). A subset of serum samples was tested in a competitive inhibition (blocking) ELISA assay to determine the specificity of BKPyV 4 seroreactivity. Percent inhibition was calculated as follows: optical density (OD) value of blocking VLP/OD value of buffer control × 100.
Statistical analyses
The distributions of characteristics in cases and controls were compared by the Wilcoxon rank-sum test or chi-square test. The distributions of JCPyV, BKPyV 1 and BKPyV 4 seroreactivity by case control status by time interval before PML diagnosis were displayed by box plots and levels of antibodies to BKPyV 1, BKPyV 4, and JCPyV between cases and controls at each time interval were compared by Wilcoxon test. Logistic regression with generalized estimating equation and matched case-control-pair number as strata was used to investigate the relationship between PML and levels of log2-transformed BKPyV 1, BKPyV 4, and JCPyV OD values by using SAS GENMOD procedure (SAS Institute, Cary, North Carolina, USA). Separate univariate and multivariate analyses (adjusted for age at diagnosis, CD4+ T-cell counts, and log2 transformed JCPyV OD value) were conducted for antibodies to BKPyV 1 and BKPyV 4. Another multivariate logistic model was also performed including all possible predictors: age at diagnosis, CD4+ T-cell counts, and log2-transformed JCPyV, BKPyV 1, and BKPyV 4 OD values. A Wilcoxon test was used to compare differences between cases and controls in percent seroreactivity blocked by pre-incubation with BKPyV 4 VLP antigen (percent blocked) at 1–1.5 year before PML. The proportion of samples from cases and controls that were blocked by at least or more than 40% in the competitive inhibition assay were compared by Fisher’s exact test. Conditional logistic regression, with adjustment for age and CD4+ T-cell, was used to investigate the association between the percent blocked and the risk of subsequent development of PML. Classification tree analysis predicting PML was performed by using Splus software (TIBCO Spotfire S+® 8.2, TIBCO Software Inc.). Predictors in the classification tree analysis included CD4+ T-cell count, and OD values of BKPyV 1, BKPyV 4, and JCPyV seroreactivity where the average value for each biomarker over 0.5 -2 years before PML were used. Exploratory pruning of trees was done by examining deviance with addition of nodes and effects of pruning on misclassification rate.
Results
Demographic characteristics of cases and controls were similar (Table1). The two populations were matched for age, race/ethnicity, intravenous drug use, average years on the study, and level of CD4+ T-cells at entry into the study. Cases were more likely than controls to have been smokers and had a lower median CD4+ T-cell count at the time of PML diagnosis, but these differences did not reach statistical significance. Seven cases were excluded from the study because plasma samples were not available during the time interval of interest, 0.5 to 2 years prior to PML diagnosis. The excluded cases had similar demographics characteristics to the cases included in the study, with the exception that the excluded cases were less likely to have been smokers (P=0.01).
Table 1.
Characteristics of Study Populations
| Variable | Cases in analysis (n=25) |
Controls (n=80) |
p-value1 | Cases excluded (n=7) |
p-value2 |
|---|---|---|---|---|---|
| Age, median (IQR) | 39 (36, 44) | 41 (36, 46) | 0.575 | 46(37, 61) | 0.202 |
| Race/ethnicity, N (%) | 0.281 | 0.395 | |||
| White | 24(96) | 78(98) | 6(86) | ||
| Black | 0(0) | 2(3) | 1(14) | ||
| Other | 1(4) | 0(0) | 0(0) | ||
| Ever IV drug use, N (%) | 4(16) | 9(11) | 0.504 | 0(0) | 0.552 |
| Ever Smoking, N (%) | 21(84) | 51(64) | 0.083 | 2(29) | 0.010 |
| Years on study, median (IQR) | 6.8(4.3, 8.6) | 6.8(4.8, 9.3) | 0.717 | 6(2.5, 12.0) | 0.906 |
| Calendar Yr Dx, median (IQR) | 1992 (1989, 1994) | NA | 1991(1989, 1997) | 1.0 | |
| Entry CD4, median (IQR) | 551(359, 643) | 484(376, 636) | 0.861 | 506(162, 579) | 0.211 |
| CD4 at Dx, median (IQR) | 123(87, 199) | 182(90, 280) | 0.086 | 155(100, 252) | 0.715 |
P-values were obtained by non-parametric Wilcoxon test or Fisher exact test, as appropriate.
Comparing cases and controls.
Comparing 25 cases in analysis and 7 cases excluded from analysis.
The distribution of IgG seroreactivity to BKPyV 1, BKPyV 4, and JCPyV capsids in cases and controls during six-month time intervals from 0.5 to 2 years prior to PML diagnosis is shown in figure 1. In all three time intervals before PML, BKPyV 1 and BKPyV 4 IgG levels were significantly (p<0.05) lower in cases than in controls, indicating that a high BK antibody level is a biomarker for protection against the risk of developing PML. For example, 1 to 1.5 years prior to diagnosis, the median seroreactivity to BK serotype 1 and serotype 4 among cases was 0.186 optical density (OD) units and 0.153 OD units, respectively, while the corresponding values for controls were 0.443 OD units and 0.578 OD units, respectively. Similar differences between cases and controls were observed 0.5 to 1 year and 1.5 to 2 years before PML diagnosis. In contrast, JCPyV antibody levels were higher among cases than controls, although the difference was not statistically significant. The strength of the association was similar for all three 6-month time intervals indicating that the relationship between the biomarkers and risk of PML is stable up to 2 years before PML diagnosis.
Figure 1.
Distribution of IgG absorbance values to BKPyV 1, BKPyV 4, and JCPyV capsids in sera from PML cases and HIV disease matched controls at time intervals before the diagnosis of PML. The summary statistics of each distribution are displayed in a box plot. The length of the box corresponds to the interquartile range with the upper boundary of the box representing the 75th percentile and the lower boundary the 25th percentile. The horizontal clear line in the box represents the median value. The 10th and 90th percentiles are shown by the small bar at the end of the line extending downward or upward, respectively, from the box. Each outlier value is shown individually by a solid line. The number of outliers that are not displayed in the graph space is indicated at the top of each graph. The number of samples contributing to each distribution is indicated at the bottom of the figure.
Logistic regression analysis were performed in an univariate model for BKPyV 1 and BKPyV 4 seroreactivity, and in multivariate models for each biomarker after adjusting for age, CD4+ T-cell count, JCPyV seroreactivity, and seroreactivity to the heterologous BKPyV serotype (Multivariate Models 1 and 2) and after adjusting for all the variables (Multivariate Model 3)(Table 2). In multivariate models 1 and 2, pre-diagnostic BKPyV 1 and BKPyV 4 seroreactivity were significantly associated with a lower risk of subsequent development of PML, with a BKPyV 1 odds ratio (OR) of 0.56 and 95% confidence limits (CI) of 0.35–0.89, and a BKPyV 4 OR of 0.40 and 95% CI of 0.24–0.67. After adjusting for all variables (model 3), BKPyV 4 seroreactivity was associated with a statistically significant lower risk of subsequent PML (OR, 0.45, 95% CI, 0.24–0.83) and BKPyV 1 seroreactivity was associated with a lower, but non-significant risk of PML (OR, 0.75, 95% CI, 0.41–1.4). These data provide strong support for the value of BKPyV serology, particularly seroreactivity to BKPyV 4 capsids, as a pre-diagnostic marker for the risk of developing PML in patients with human immunodeficiency virus infection. In an univariate model restricted to the 7 histologically confirmed cases of PML, BKPyV 4 seroreactivity was also significantly associated with a lower risk of subsequent PML (OR, 0.27, 95% CI, 0.09–0.86) and BKPyV 1 seroreactivity was associated with a lower, but non-significant risk of PML (OR, 0.48, 95% CI, 0.22–1.04).
Table 2.
| Possible predictors | Univariate Model |
Multivariate Model 1 |
Multivariate Model 2 |
Multivariate Model 3 |
|---|---|---|---|---|
| Log2(BKPyV 1), per +1.6 log2 | 0.54(0.34,0.85) | 0.56(0.35,0.89) | - | 0.75(0.41,1.40) |
| Log2(BKPyV 4), per +1.4 log2 | 0.42(0.26,0.69) | - | 0.40(0.24,0.67) | 0.45(0.25,0.83) |
| Age, per +6.7 years | 0.93(0.59,1.49) | 0.86(0.53,1.41) | 0.87(0.52,1.45) | 0.86(0.51,1.43) |
| CD4 cell count, per +180 cells | 0.75(0.52,1.08) | 0.78(0.53,1.14) | 0.65(0.44,0.97) | 0.68(0.45,1.03) |
| Log2(JCPyV), per +1.6 log2 | 1.27(0.8,2.01) | 1.20(0.73,1.96) | 1.38(0.84,2.26) | 1.31(0.79,2.18) |
ORs (95% CIs) were based on 1 standard deviation increase in all predictors in models
Logistic regression models using combined data from 0.5 to 2 years before PML diagnosis with general estimating equations to account for repeated measures.
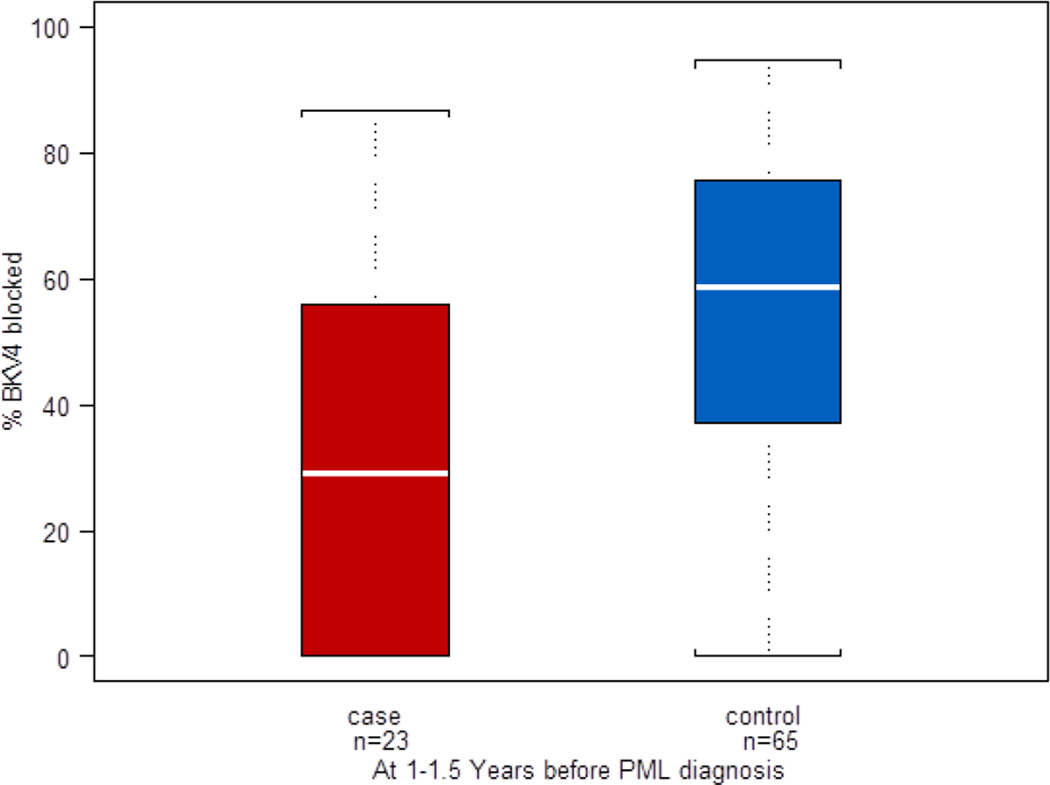
Because the multivariate model adjusting for all variables highlighted the importance of BKPyV 4 seroreactivity, we investigated the specificity of this reactivity by performing a competitive inhibition assay. A reduction in BKPyV 4 seroreactivity by pre-incubation of serum samples with BKPyV 4 VLP protein is evidence for specificity of the reactivity, while failure to inhibit the reactivity indicates that uncharacterized cross reactive antibodies are responsible for the seroreactivity. The distribution of BKPyV 4 percent inhibition among cases and controls is shown in figure 2. Mean percent inhibition of BKPyV 4 seroreactivity was greater in serum samples from controls than cases with a median percent inhibition of control and case serum samples of 59% and 29%, respectively (nonparametric Wilcoxon test p<0·001), indicating that BKPyV 4 seroreactivity in control sera was more specific than that in case sera. In a multivariate conditional logistic model, percent BKPyV 4 inhibition after adjustment for age and CD4+ T-cell count was associated with a lower risk of subsequent development of PML (hazard ratio, 0.30, 95% CI, 0.12–0.71). Using a conventional definition of specific seroreactivity of inhibition greater than 40%, 46 of 65 (71%) control serum samples as compared to eight of 23 (35%) case serum samples were BKPyV 4 reactive (Fisher’s exact, p=0.005), showing the value of specific BKPyV 4 seroreactivity as a biomarker for protection against PML.
Figure 2.
Distribution of percent BKPyV 4 seroreactivity blocked by pre-incubation with BKPyV 4 VLP protein among 23 PML cases and 65 HIV disease matched controls. Data are displayed in a box plot as described in the legend to figure 1.
To develop an analytical framework that would combine information from the three antibody biomarkers and CD4+ T-cell count into an algorithm to predict the risk of PML, we implemented a classification tree analysis. In this analysis data are iteratively stratified into dichotomous categories to achieve the optimal differentiation of cases from controls. The analytical results are displayed as a tree-like structure with all data points falling within terminal nodes. Ideally every node would have a probability of 100% for classification as a case or control. Where probabilities are less than 100%, the node will contain a mixture of cases and controls. For the classification tree analysis we used the average value across all 6-month time intervals for each variable so the number of data points corresponds to the number of subjects. As shown in the optimally pruned tree (figure 3), subjects were initially stratified by BKPyV 4 seroreactivity greater or less than 0.140 OD units. Only 12 subjects had an OD value <0.140 and 10 of these subjects were cases, resulting in a terminal node with a probability of being a case of 83%. The result highlights the importance of a low BKPyV 4 antibody level for the risk of subsequent PML. The remaining 93 subjects, which included 15 cases, had a BKPyV 4 seroreactivity >0.140 OD units and could be further stratified based on a CD4+ T-cell count greater or less than 336 cell per microliter. There were 29 subjects with a CD4+ T cell count greater than 336 cells per microliter and all of them were controls, resulting in a terminal node with 100% probability of being a control. Of the remaining 64 subjects, including 15 cases, stratification based on a JCPyV antibody level greater or less than 0.56 OD units placed 11 cases in the group with the higher JCPyV antibody level. Among the 28 subjects and 11 cases in this group, 9 of the 11 cases had a CD4+ T cell count above 195.5 cells per microliter (and less than 336 cells per microliter as noted above), resulting in a terminal node with a probability of being a case of 64%. Two cases fell in the group with a CD4+ T cell count less than 195.5 cells per microliter suggesting that a very low CD4+ T cell count was not a primary determinant of risk in the sub group of subjects with a high JCPyV antibody level and CD4+ T cell count below 336 cell per microliter. Among the 36 subjects with a JCPyV antibody level less than 0.56 OD units the risk of being a case was low (11%), but further stratification into a group with a BKPyV 1 antibody level between 0.496 and 0.65 OD units resulted in a terminal node with 5 subjects, 3 of whom were cases (probability of being a case 60%). The results of a classification analysis can also be expressed as the proportion of data points that are misclassified. For our analysis 12 (11.4%) of 105 subjects were misclassified as either controls falling in terminal nodes categorized as case or cases in control-designated terminal nodes.
Figure 3.
Classification tree analysis predicting PML among 25 cases and 80 matched controls. Classification tree analysis was performed by using Splus software. Predictors in the analysis included CD4+ T-cell count, and BKPyV 1, BKPyV 4, and JCPyV OD values. The predictor and cut off used to stratify subjects is shown above the horizontal line and the number of subjects and percentage that is cases is indicated below the line. Terminal nodes (vertical lines ending without further division) are labeled Case or Ctrl (control) based on a majority of classified subjects falling into that strata. For each terminal node, the number of subjects, percentage of cases, and absolute number of cases is indicated.
Discussion
PML is always observed in the setting of cellular immunodeficiency. However, the incidence of PML among immunosuppressed patients is low. Among HIV patients, the incidence of PML was calculated between 3–7% before the advent of combined antiretroviral therapy and has decreased in the current therapy era (Cinque et al., 2009). The overall incidence of PML in patients treated with natalizumab is 3.78 per 1000 (Kornek, 2015). Few prognostic factors, other than immunosuppression, have been identified to predict the risk of PML. In patients receiving natalizumab, prior immunosuppressive therapy, a longer duration of natalizumab therapy, and JCPyV seropositivity, particularly high levels of antibody, convey a risk of 1% as compared to less than 0·01% in seronegative individuals (Biogen Idec, 2015; Bloomgren et al., 2012; Gorelik et al., 2010; Plavina et al., 2014). Among HIV infected patients, JCPyV seropositivity is associated with a non-significant trend for increased risk of subsequent PML (Viscidi et al., 2011a). The reason for the modest predictive value of JCPyV serology in HIV patients as compared to natalizumab-associated PML is unclear but may reflect an effect of immunosuppression on antibody levels. We also cannot exclude the possibility that long term storage of serum samples interfered with the performance of the serological assay.
Our nested-case-control study of patients enrolled in MACS provides evidence that higher levels of antibody to BKPyV capsids are associated with a decreased risk of PML up to 2 years prior to the clinical diagnosis. Why should individuals with high levels of antibody to BKPyV capsids be protected against PML? BKPyV and JCPyV are genetically closely related, with greater than 85% similarity in protein sequences, raising the possibility of immunological cross protection. A direct protective role for antibody to BKPyV capsids is highly unlikely because adsorption studies have shown there is no serological cross reactivity between the capsid proteins of the viruses (Viscidi and Clayman, 2006). Additionally, mechanisms involved in lysis of infected cells are more likely to be important in control of reactivation of a latent or persistent viral infection. Capsid antibodies could be a correlative marker for antibodies to other JCPyV antigens that share cross reactive epitopes with the corresponding BKPyV antigens. Studies with the animal polyomavirus SV40 have shown that antibodies to the large T antigen can mediate lysis of SV40-transformed cells by a mechanism involving antibody dependent cellular cytotoxicity ADCC (Bright et al., 1994). Such a mechanism of BKPyV-mediated cross protection against JCPyV infection is possible, but to date ADCC responses to JCPyV and BKPyV large T antigen have not been investigated. Capsid antibodies could also be a correlative marker for cellular immunity. We speculate that cross reactive cellular immunity may explain our findings. JCPyV-specific T-cell immunity has been associated with a better outcome following the diagnosis of PML (Khanna et al., 2009; Du Pasquier et al., 2004). We performed a bioinformatics analysis of predicted HLA-A*02 epitopes in JCPyV and BKPyV large T antigen and capsid protein amino acid sequences and found several putative T cell epitopes with identical amino acid sequences (data not shown). Cross reactive T cell epitopes in the major capsid proteins of BKPyV and JCPyV have also been experimentally demonstrated (Li et al., 2006). Additional indirect evidence for BKPyV cross protection against JCPyV infection is provided by the observation that the presence of one virus in the urine makes viruria with the other virus less likely and co-detection of both viruses in urine is rare (Cheng et al., 2011; Rossi et al., 2014a). We also note that exposure to BKPyV occurs earlier in life than exposure to JCPyV, as documented by age seroprevalence studies (Viscidi et al., 2011b). Prior exposure to BKPyV might confer partial immunity to JCPyV, resulting in a mild primary infection and low levels of serum antibody to JCPyV capsids. In contrast, unprotected individuals would have a severe primary JCPyV infection, resulting in high levels of JCPyV capsid antibody and a future risk of PML. Irrespective of the mechanism of protection, we are unaware of other examples where exposure to one virus is associated with protection against disease with a genetically related virus, making this a novel mechanism of disease prevention.
Our study has several limitations. It is not clear if the findings of our study are applicable to patients with other immunodeficiency states associated with PML or patients treated with natalizumab. While the majority of cases in our study were diagnosed clinically and without the aid of virological testing, the findings were replicated in the subset of patients with a histologically confirmed diagnosis. Although the classification tree analysis shows that BKPyV serology together without other variables has utility for risk prediction, these findings need to be confirmed in an independent cohort. In addition to BKPyV serotypes 1 and 4, there are two other BKPyV serotypes. We cannot exclude cross reactivity in our ELISA assay and thus we cannot attribute the risk of PML to a particular serotype.
Highlights.
Progressive multifocal leukoencephalopathy (PML) is caused by JC virus.
We performed a nested case control study of pre-diagnostic serological markers.
Antibody to genetically-related BK virus was associated with lower risk of PML.
The viruses are serologically distinct but may share cross reactive T cell epitopes.
Acknowledgements
We thank Barbara Silver for technical assistance with ELISA assays. This study was sponsored in part by a grant from the PML Consortium (RPV). Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick, Lisa P. Jacobson), Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles R. Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute; [UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett SM, Broekema NM, Imperiale MJ. BK polyomavirus: emerging pathogen. Microbes. Infect. 2012;14:672–683. doi: 10.1016/j.micinf.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biogen Idec. Biogen Idec website. 2015 Ref Type: Online Source. [Google Scholar]
- Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat. Rev. Neurol. 2010;6:667–679. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- Bright RK, Shearer MH, Kennedy RC. Immunization of BALB/c mice with recombinant simian virus 40 large tumor antigen induces antibody-dependent cell-mediated cytotoxicity against simian virus 40- transformed cells. An antibody-based mechanism for tumor immunity. J. Immunol. 1994;153:2064–2071. [PubMed] [Google Scholar]
- Cheng XS, Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Randhawa P, Hardinger KL, Brennan DC. Inhibitory interactions between BK and JC virus among kidney transplant recipients. J. Am. Soc. Nephrol. 2011;22:825–831. doi: 10.1681/ASN.2010080877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KL, Holman RC, Hammett TA, Belay ED, Schonberger LB. Progressive multifocal leukoencephalopathy deaths in the USA, 1979–2005. Neuroepidemiology. 2010;35:178–184. doi: 10.1159/000311014. [DOI] [PubMed] [Google Scholar]
- Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect. Dis. 2009;9:625–636. doi: 10.1016/S1473-3099(09)70226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detels R, Jacobson L, Margolick J, Martinez-Maza O, Munoz A, Phair J, Rinaldo C, Wolinsky S. The multicenter AIDS Cohort Study, 1983 to …. Public Health. 2012;126:196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Lerner M, Bixler S, Crossman M, Schlain B, Simon K, Pace A, Cheung A, Chen LL, Berman M, Zein F, Wilson E, Yednock T, Sandrock A, Goelz SE, Subramanyam M. Anti-JC virus antibodies: implications for PML risk stratification. Ann. Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- Hamilton RS, Gravell M, Major EO. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J Clin. Microbiol. 2000;38:105–109. doi: 10.1128/jcm.38.1.105-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS. Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna N, Wolbers M, Mueller NJ, Garzoni C, Du Pasquier RA, Fux CA, Vernazza P, Bernasconi E, Viscidi R, Battegay M, Hirsch HH. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J. Virol. 2009;83:4404–4411. doi: 10.1128/JVI.02657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, Miller E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- Kornek B. An update on the use of natalizumab in the treatment of multiple sclerosis: appropriate patient selection and special considerations. Patient. Prefer. Adherence. 2015;9:675–684. doi: 10.2147/PPA.S20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Melenhorst J, Hensel N, Rezvani K, Sconocchia G, Kilical Y, Hou J, Curfman B, Major E, Barrett AJ. T-cell responses to peptide fragments of the BK virus T antigen: implications for cross-reactivity of immune response to JC virus. J Gen. Virol. 2006;87:2951–2960. doi: 10.1099/vir.0.82094-0. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Cuburu N, Buck CB. BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J. Virol. 2013;87:10105–10113. doi: 10.1128/JVI.01189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, Schlain B, Campagnolo D, Belachew S, Ticho B. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann. Neurol. 2014;76:802–812. doi: 10.1002/ana.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AP, Anderson KL, Brennan DC. JC polyoma virus and kidney disease. Kidney Int. 2014a;85:1242. doi: 10.1038/ki.2014.38. [DOI] [PubMed] [Google Scholar]
- Rossi F, Newsome SD, Viscidi R. Molecular diagnostic tests to predict the risk of progressive multifocal leukoencephalopathy in natalizumab-treated multiple sclerosis patients. Mol. Cell Probes. 2014b doi: 10.1016/j.mcp.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Viscidi RP, Shah KV. Polyomaviurses. In: Cohen J, Opal SM, Powderly WG, editors. Infectious Diseases. Mosby Elsevier; 2010. pp. 1570–1572. [Google Scholar]
- Viscidi RP, Clayman B. Serological cross reactivity between polyomavirus capsids. Adv. Exp. Med. Biol. 2006;577:73–84. doi: 10.1007/0-387-32957-9_5. [DOI] [PubMed] [Google Scholar]
- Viscidi RP, Khanna N, Tan CS, Li X, Jacobson L, Clifford DB, Nath A, Margolick JB, Shah KV, Hirsch HH, Koralnik IJ. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin. Infect. Dis. 2011a;53:711–715. doi: 10.1093/cid/cir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi RP, Rollison DE, Sondak VK, Silver B, Messina JL, Giuliano AR, Fulp W, Ajidahun A, Rivanera D. Age-specific seroprevalence of Merkel cell polyomavirus, BK virus, and JC virus. Clin. Vaccine Immunol. 2011b;18:1737–1743. doi: 10.1128/CVI.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi RP, Rollison DE, Viscidi E, Clayman B, Rubalcaba E, Daniel R, Major EO, Shah KV. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-likeparticle- based enzyme immunoassays. Clin. Diagn. Lab Immunol. 2003;10:278–285. doi: 10.1128/CDLI.10.2.278-285.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]