Abstract
Double-stranded DNA bacteriophages are highly pressurized, providing a force driving ejection of a significant fraction of the genome from its capsid. In P22-like Podoviridae, internal proteins (“E proteins”) are packaged into the capsid along with the genome, and without them the virus is not infectious. However, little is known about how and when these proteins come out of the virus. We employed an in vitro osmotic suppression system with high-molecular-weight polyethylene glycol to study P22 E protein release. While slow ejection of the DNA can be triggered by lipopolysaccharide (LPS), the rate is significantly enhanced by the membrane protein OmpA from Salmonella. In contrast, E proteins are not ejected unless both OmpA and LPS are present and their ejection when OmpA is present is largely complete before any genome is ejected, suggesting that E proteins play a key role in the early stage of transferring P22 DNA into the host.
Keywords: bacteriophage infectivity, genome ejection, internal protein ejection, receptors, osmotic suppression
Introduction
The detailed mechanisms by which bacteriophages deliver their genomes into their bacterial hosts are not fully understood. In general, for tailed double-stranded (ds) DNA phages, proteins located in the phage tail first contact the surface of the bacterium and the phage diffuses along the surface until it finds a specific receptor. Upon binding to the receptor, the phage tail undergoes a series of conformational changes that result in release of the DNA and in its translocation from the capsid into the cytoplasm of the host (Poranen et al., 2002; Bhardwaj et al., 2014). In order to overcome the defense barriers of the bacteria – e.g., the outer and inner membranes along with the periplasmic space in between – bacteriophages use different strategies based on their tail morphology. Compared to the long-tailed Myoviridae (with contractile tails) and Siphoviridae (non-contractile tails) (Leiman et al., 2012; Davidson et al., 2012), Podoviridae, a family of bacteriophages with tails shorter than the width of the periplasm, cannot directly use their tails to penetrate both membranes. While studies of the particular podoviruses phi29 and T7 (González-Huici et al., 2006; Molineux, 2001) have shed much light on their mechanism of infection; to date no generalized mechanism of infection for phages has been identified.
P22 is a member of Podoviridae that infects Salmonella enterica. It is a dsDNA phage with a 43.5 kbp genome that is packaged via a headful mechanism (Casjens et al., 1988) into an icosahedral procapsid formed from assembly of the coat, scaffolding, and portal proteins (King et al., 1976). There are three internal proteins (called “pilot, “ejection”, or “E” proteins) packaged inside P22 procapsids, all incorporated by the scaffolding protein in the early stages of assembly: gp16, gp20 and gp7 (“gp” = gene product), each with 10-20 copies (Israel, 1977). A short tail “machine” is then connected at a unique five-fold vertex to complete the P22 virion. Several of these structural proteins have been identified in recent cryoEM investigations (Tang et al., 2011; Lander et al., 2009). Although density in these reconstructions had been ascribed to the E proteins (Chang et al., 2006; Lander et al., 2006), a crystal structure of the complete portal, as well as the most recent asymmetric reconstructions (Olia et al., 2011; Tang et al., 2011) show this to be incorrect. Accordingly, the location of the three E proteins within the mature virion remains unknown.
Along with their DNA, most phages eject proteins that have been packaged into the capsid, and this is generally essential for infection (Molineux et al., 2013). Although the exact roles that each of these E proteins plays in infection have not been determined, some information is available. For example, cells infected with gp16-deficient phage (a mutant lacking a functional gp16 E protein) continue to divide normally and do not replicate the P22 genome, but co-infections show that gp16 can work in trans to complement a gp16-deficient particle, indicating an early function (Hoffman et al., 1975). Furthermore, gp16-deficient phages do not induce the superinfection-exclusion response and gp16 is not part of the replication machinery (Israel et al., 1972). Gp7 and gp20 often co-purify with gp16, and gp7 ejection does not occur when gp16 is absent (Israel, 1977). Indirect evidence supports a membrane-breaching role because purified gp16 disrupts dye-loaded lipid vesicles (Perez et al., 2009). It has been proposed that the E proteins protect the DNA in the periplasm during infection (Israel, 1977), but their function and location post-infection has yet to be determined experimentally. Taken together, these facts suggest that the E proteins, gp7, gp16, and gp20, may be linked to the efficiency and dynamics of DNA ejection across host membranes.
If the receptor is known and can be solubilized, phage ejection can be triggered in vitro, in which case the extent of DNA ejection can be controlled by the presence of an osmolyte in the surrounding buffer solution (Tzlil et al., 2003; Evilevitch et al., 2003; Castelnuovo and Evilevitch, 2007; Bauer et al., 2013). More explicitly, each concentration of high-molecular-weight polyethylene glycol (PEG8000) in the host (“external”) solution corresponds to a certain amount of water being drawn out of the phage capsid (from which PEG is excluded). As a result, the water inside is under tension, thereby producing a force resisting the ejection of DNA. Such osmotic suppression studies done with dsDNA phages like lambda (Evilevitch et al., 2003) and T5 (Leforestier et al., 2008), both members of Siphoviridae, have shown that the virus capsid is highly pressurized as a result of the electrostatic self-repulsion and bending of the densely-packed, negatively-charged, semi-rigid DNA (Riemer and Bloomfield, 1978; Tzlil et al., 2003). In these in vitro experiments, in which pressure is the only driving force for DNA ejection, the length of DNA ejected can be tuned by the osmotic pressure difference between the outside and inside of the capsid (Evilevitch et al., 2003). In the present study we use osmotic suppression to examine for the first time the ejection of both the DNA and the E proteins from P22, which sheds light on ejection mechanisms in Podoviridae.
Results/Discussion
For P22, the O-antigen portion of lipopolysaccharide (LPS) located on the surface of the host Salmonella enterica serovar Typhimurium works as a primary receptor for infection (Iwashita and Kanegasaki, 1973). Further, it has been demonstrated (Andres et al., 2010) that LPS can trigger a slow ejection of DNA from P22 in vitro, which makes it possible to study the process with the osmotic suppression technique.
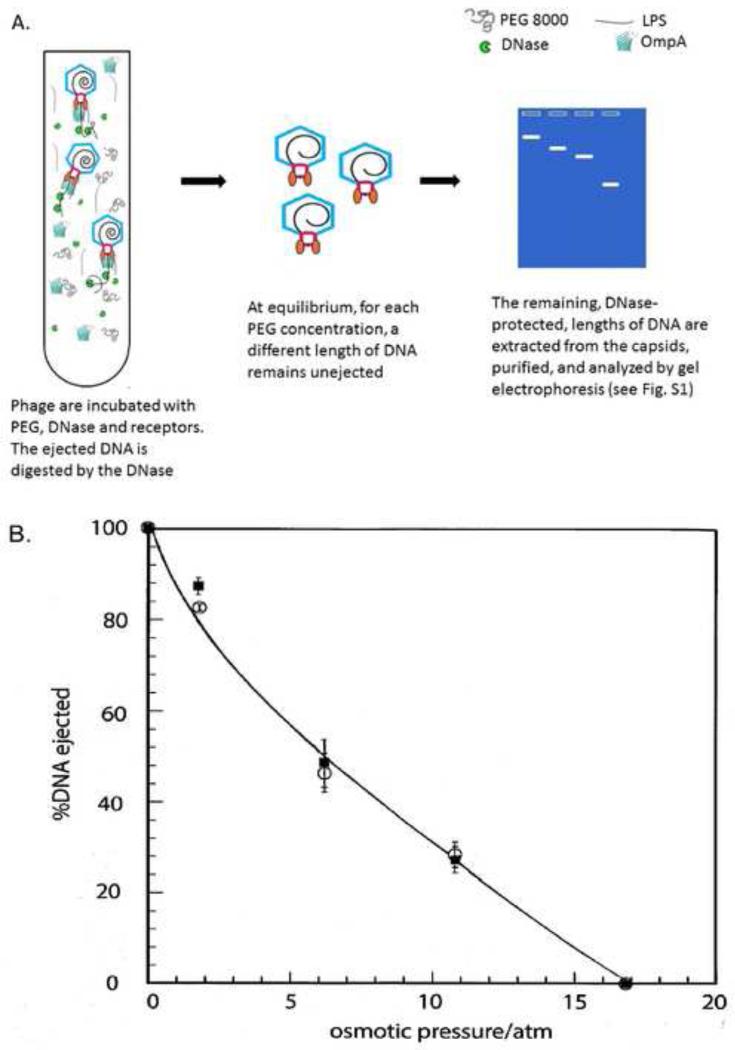
Osmotic suppression experiments show release of DNA from P22 is inhibited at a pressure of 16.8 atm
Using LPS to trigger ejection, we determined the fraction of the DNA remaining unejected in the presence of an osmotic pressure by separating the capsids from the ejected DNA, which was degraded by DNase, and then recovering and analyzing the DNA remaining in the capsids as in the experiment of Evilevitch et al. (2005). Fig. 1A is a cartoon showing the ejection behavior of P22 in the presence of LPS under different osmotic pressures. Ejected DNA is digested into nucleotides, but the DNA that is kept inside the capsids by the PEG pressure is protected; this DNA is extracted (see Materials and Methods) and its length analyzed by gel electrophoresis. As a control, we have demonstrated that the isolated P22 genome can be degraded by DNAse in the presence of PEG8000, as is consistent with previously published data (Andres et al., 2010).
Figure 1. The extent of genome ejection is controlled by osmotic pressure.
A. cartoon of P22 in vitro ejection. The purified phage is incubated in PEG8000 and DNase. Receptor is then added, triggering ejection of progressively less DNA as the osmotic pressure increases; ejected DNA is digested by the DNase, and the protected DNA that remains in the capsid is extracted and its length analyzed by gel electrophoresis. B. Measured DNA ejection percentage from P22 at various osmotic pressures under different receptor conditions: P22+LPS (○); P22+LPS+OmpA (■). Ejection is triggered by addition of receptor (LPS, or LPS and OmpA) in the presence of PEG and DNase I; the DNase is inactivated; and the DNA remaining in the capsids is extracted, run on an agarose gel, and the unejected length calculated from an accompanying DNA ladder. % DNA ejection is relative to the full-length DNA. The solid curve is drawn to aid the eye.
The results are indicated by the open circles in Fig. 1B. A representative agarose gel showing unejected DNA from the capsid under different osmotic pressure conditions is included in the supplemental materials (Fig. S1); the length of DNA remaining in the capsid is seen to increase with increasing PEG concentration until ejection is completely suppressed at 16.8 atm. Because of the jump from 10.8 to 16.8 atm in our osmotic pressure measurements, it is possible that complete suppression occurs anywhere between these two pressures. But it is clear from the variation of ejection fraction with pressure for the set of measured points that 16.8 atm is an upper bound (and very close) to the value at which the suppression is complete. This is significant because, as we shall see in the following sections, ejection of the E proteins is largely complete at 16.8 atm in the presence of OmpA. For all samples, more than 80% of the capsids were triggered open as was confirmed by plating experiments in which the number of remaining plaque-forming units (PFUs) was counted after treatment with receptor, as previously described (Parent et al., 2014). Note that partially-ejected capsids, and even capsids triggered open, but for which DNA ejection was completely suppressed (those bound by LPS in high [PEG]), lose their infectivity, i.e., are unable to form a plaque. As an additional control, we incubated P22 in increasing [PEG] in the absence of LPS and analyzed the samples by agarose gels, confirming that PEG alone does not trigger capsid opening (data not shown).
E protein ejection does not occur when P22 is treated with LPS alone
Using the osmotic-suppression method described above to control the extent of ejection for a series of phage samples, we centrifuged 35S-labeled samples (see Materials and Methods) to separate capsids from ejected protein and DNA. We then analyzed by SDS-PAGE, autoradiography and densitometry the protein content in the pellet, i.e., those proteins still associated with the capsid/receptor macromolecular complex. Fig. 2 shows the results for pellet fractions corresponding to different osmotic pressures, with LPS used to trigger ejection. For all the pressures reported in Fig. 1B, we assayed a portion of each reaction and counted PFUs before and after treatment with LPS to confirm that more than 80% of virions were triggered for ejection (Fig. S2).
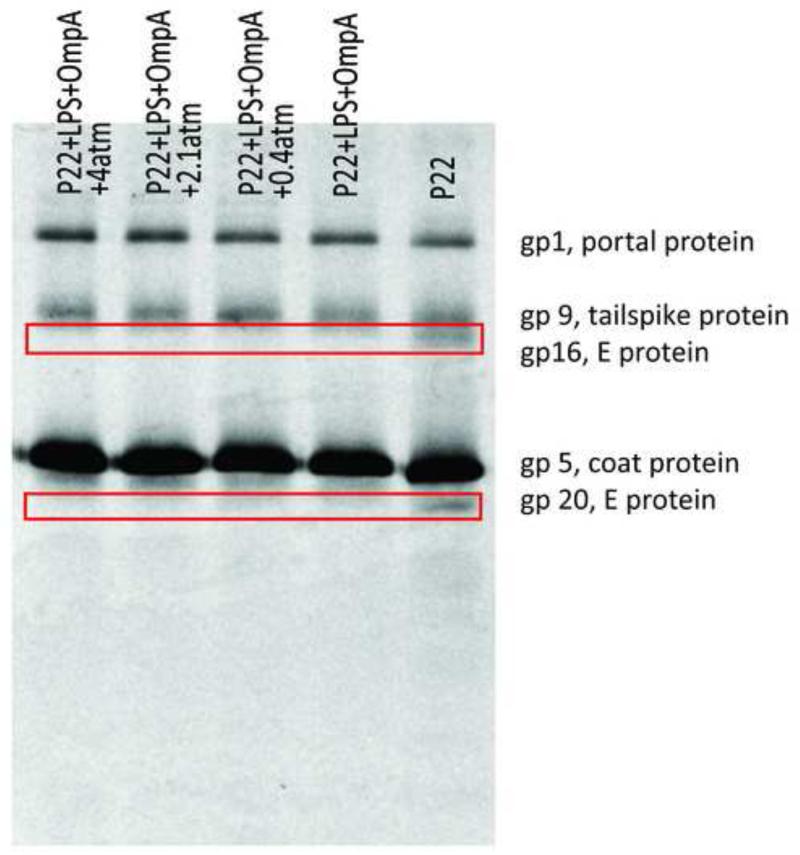
Figure 2. LPS alone is insufficient to eject E proteins.
SDS-PAGE visualized by autoradiography showing the protein content in the pellet after triggering ejection by LPS under different external osmotic pressures. The left-most lane is the control which does not contain any receptor. Boxes highlight the three E proteins.
If any (or all) of the E proteins had been ejected, they would be present in the supernatant and consequently the bands representing those proteins would become less intense or even disappear, depending on the fraction ejected. As is evident, each lane has the same viral protein pattern with similar band intensities, independent of the presence of LPS and the amount of PEG. The same result was found in 10 independent trials. Similar results were also found when we performed a trypsin digestion in parallel samples to digest any protein that was ejected and hence no longer protected by the capsid (data not shown). Therefore, we can exclude the possibility that protein was ejected but pelleted along with the capsid. Trypsin digestion was also performed with purified E proteins, and they were completely and rapidly degraded (Carol Teschke, personal communication), indicating that these proteins are highly sensitive to protease when they are outside of the capsid. We can thus conclude that LPS alone did not trigger E protein ejection in vitro.
P22 ejection efficiency increases in vitro in the presence of outer membrane protein A (OmpA)
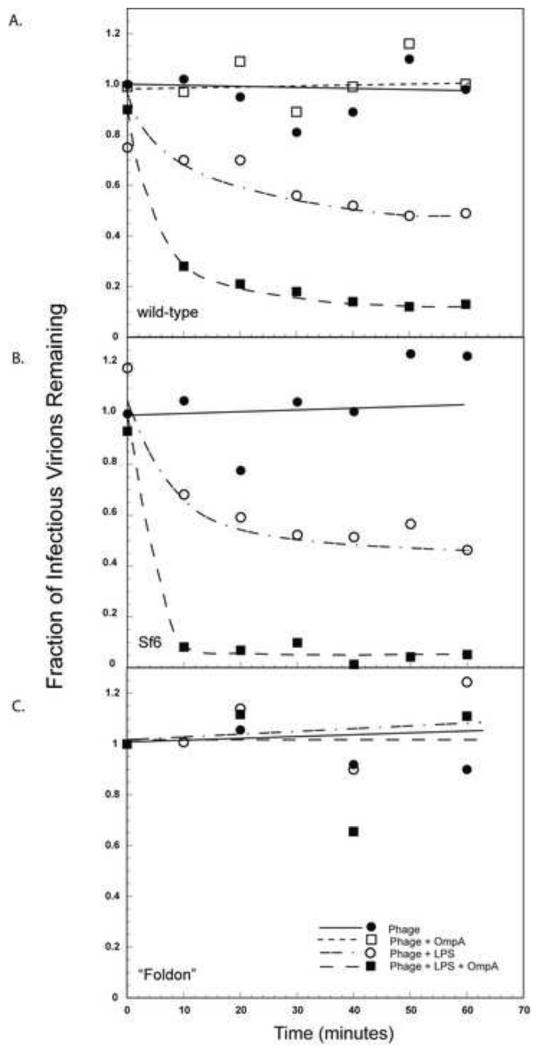
Studies by Seckler and colleagues have shown that while LPS from Salmonella can trigger P22 ejection in vitro, the kinetics are very slow (~ 5 hours) compared to the phage life cycle (~1 hour) (Andres et al., 2010; 2012). Because of the close relationship between Sf6 and P22 (Casjens et al., 2011), and the fact that both the outer membrane protein OmpA and LPS are required for Sf6 ejection in vitro (Parent et al., 2014), we examined if OmpA was able to enhance the rate of ejection in P22. As seen in Fig. 3A, as early as 10 min after incubation with both LPS and OmpA, around 75% of the virions in the sample have lost infectivity versus only a 30% infectivity loss upon incubation with LPS alone. Samples incubated with OmpA alone showed essentially no loss of infectivity, indicating that OmpA does not trigger ejection without LPS (Fig. S2).
Figure 3. Loss of infectivity is enhanced by OmpA.
In vitro genome ejection of (A) wild type P22, and of two P22 hybrids, one with an Sf6 tail (B) and the other with a T4 “foldon” tail (C). The “fraction of infectious virions remaining” was calculated at each time point as the number of PFU remaining after incubation with LPS, OmpA or LPS and OmpA, divided by the number of PFU when incubated with buffer at t = 0 min.
We also performed the same experiment with two P22 hybrids (Leavitt et al., 2013) where the head of the phage is comprised of P22 proteins but the tail needle tip is from another phage type. These hybrids are constructs where the tips were fused downstream of the helical core of the P22 tail needle (includes P22 residues 1-140). For one such hybrid, containing an Sf6 tail needle knob (Sf6 residues 132-282) fused to the P22 helical core, we obtained results (Fig. 3B) similar to P22. But for a mutant with a T4 “foldon” tail needle (Fig. 3C), neither LPS with OmpA nor LPS alone was able to trigger loss of infectivity. The “foldon” is a 25-residue trimer found at the C terminus of T4 fibritin (Tao et al., 1997) and all of the foldon residues are added to the 140 amino acid P22 helical core. It appears then that for LPS and OmpA to function as receptors, the tail needle structure must be closely similar to that of P22, consistent with the earlier suggestion that the P22 tail needle is part of the trigger that determines DNA ejection (Leavitt, et al., 2013).
P22 E proteins are ejected in vitro in the presence of both LPS and an outer membrane protein
Since OmpA enhanced P22 ejection kinetics and overall efficiency (Fig. 3A), we asked if P22 capsids would release the E proteins in vitro when both receptors were present. The pressure driving DNA ejection was determined again, but this time in the presence of both LPS and OmpA (Fig. 1B—filled squares). Within the precision of the measurements, the data obtained for fast ejection, i.e., with the two receptors, are identical to those for slow ejection, i.e., for LPS alone.
However, when both LPS and OmpA were present, the E protein behavior was different. Fig. 4 is an autoradiograph showing pellets from samples with both receptors at osmotic pressures from 0 to 4 atm. Comparing lanes with receptors (lane 1-4) and the lane without receptors (lane 5), we can clearly see that bands representing gp16 and gp20 disappear in the presence of the two receptors, independent of the osmotic pressure. Similar results were found in more than 10 independent trials, but in most of them the E protein bands for samples of virus with both receptors – while many times weaker than the band for pure virus – were still visible and measurable. From these 10 trials we obtain (see discussion below) an average intensity of the E protein band associated with the pelleted phage in the presence of both receptors, for each of increasing osmotic pressures. A test with trypsin digestion, but without separation by centrifugation, gave consistent results for the autoradiograph – confirming that the E proteins were rapidly digested when the capsids were triggered “open” with both LPS and OmpA, indicating their release into the solution (data not shown). As above, all samples were analyzed by measuring PFU, and a large fraction of the phages (>90%) were triggered for ejection (Fig. S2).
Figure 4. E proteins are ejected in the presence of both LPS and OmpA.
SDS-PAGE gel visualized by autoradiography showing the protein content in the pellet after ejection was triggered by LPS and OmpA at different external osmotic pressures. The right-most lane is the control which does not contain any receptor. This is a representative film that most clearly shows the release of gp 16 and gp 20.
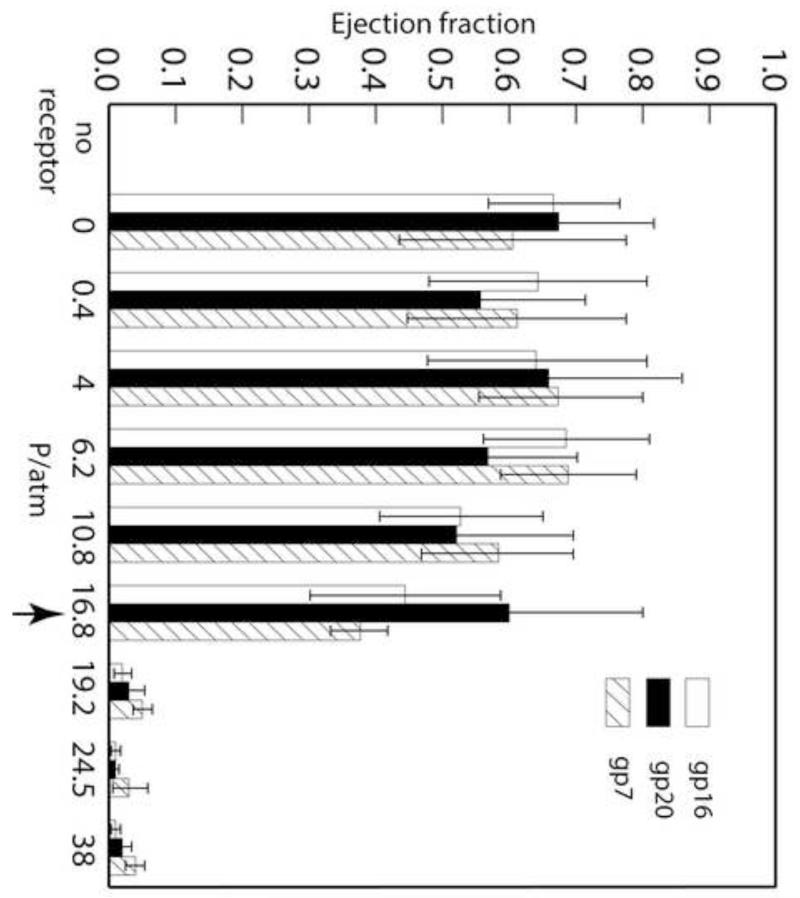
The percentages of ejection of all three E proteins against osmotic pressures ranging from 0 to 38 atm were calculated as follows: For each autoradiograph, there is one lane involving receptor-free virus at a concentration identical to that of the other samples in the gel. The intensity of each E protein band for each sample is determined relative to the coat protein band from the same sample (see Materials and Methods). We also compared E protein intensity to that of portal and tailspike protein bands in the same lanes. In all instances, the ratios were consistent (data not shown). These results are presented in Fig. 5: 60 – 70% of each of the three E proteins is ejected from the capsid, independent of osmotic pressures ranging from 0 to 16.8 atm, whereas no E protein ejection can be detected at higher pressures (Fig. S3).
Figure 5. E proteins are ejected in the presence of LPS + OmpA, independent of osmotic pressures up to 16.8 atm.
In vitro E protein ejection fractions at different osmotic pressures were calculated from the intensity of E protein band in each sample divided by the intensity of that band in the absence of receptors. Each value is an average of at least five experiments and the error bars represent one standard deviation. The arrow is to emphasize the pressure at which it was first observed that, after triggered ejection by LPS and OmpA, all of the DNA remained inside the capsid but the majority of the E proteins ejected.
In comparison with the incomplete (≈70%) ejection of E proteins that we have observed, in vivo studies of P22 E protein ejection performed in the early 1970s showed that more than 90% of gp16 and gp20, as well as 70-90% of gp7 were ejected from the capsid during infection. In our system LPS and OmpA are present as free molecules in solution while in vivo the LPS and OmpA are both in the outer membrane of the bacterium and closely situated. In contrast, in our in vitro solution studies it is possible that virus particles interact with LPS without simultaneously being in contact with OmpA, thereby triggering DNA ejection only and leaving E proteins inside the capsid. An alternative explanation is that perhaps OmpA is not as functional when purified in detergent micelles as it is in vivo. However, our data show very clearly that virtually no E protein release occurs with LPS alone, whereas near-physiological amounts of protein ejection occurs when OmpA is also present.
If we compare the DNA partial ejection data (Fig. 1B) with the E protein ejection data (Fig. 5), we see that while at 16.8 atm DNA ejection is completely suppressed, most of the E proteins are still ejected. From this we conclude that in the presence of LPS and OmpA all three E proteins are ejected prior to DNA ejection. It is only at an osmotic pressure 2 atm higher that the ejection of the E proteins is inhibited. However, we were not able to determine the order of E protein ejection. It is possible that all the proteins bind together and are ejected together, or that they are ejected in a random sequence that is not detectable in our bulk population measurements. Even if an order exists, it is also likely that, because all the proteins are ejected before the highly stressed DNA is ejected, their order cannot be resolved using the osmotic suppression method because the contribution of each protein to the overall pressure is too small.
The force driving ejection of the protein from P22 arises from the confined DNA and is expected to be similar to that associated with the ejection of DNA from λ phage, which like P22 has a T=7 capsid and is about the same size. Grayson et al. (2006) carried out osmotic suppression measurements on λ for the 48.5 kb wild-type genome and a 37.7 kb mutant and found that the ejection was completely inhibited at pressures of 20-25 and 10-15 atm, respectively. The pressure required to inhibit the E proteins that we have observed for P22 originates from 43.5kb-length of DNA (longer than the 41.7 kb genome because of head-full packaging) confined to a volume slightly smaller than the capsid interior because of the presence of the E proteins. The fraction of the internal volume occupied by the E proteins can be estimated from their number and molecular masses, using a typical protein density of 1.4g/cm3. Taking the internal volume to be 1.1 × 105 nm3 (Patterson et al., 2012), one estimates that the E proteins occupy about 2% of the volume. This decrease in volume available to the DNA is quite small. Nevertheless because of the exponential dependence of osmotic pressure of highly condensed DNA (Rau et al., 1984 and Tzlil et al., 2003) it would be expected to increase the ejection pressure by about 10%, comparable to the difference we observe between the pressures (16.8atm and 19.2atm, respectively) that inhibit the ejection of the DNA and proteins. Finally we note that the turgor pressure in both the periplasm and cytoplasm of Salmonella is about 3.5 atm (Stock et al., 1977), so that pressure-driven ejection from P22 can be expected to transfer only about 60% of the DNA from the phage to the host (see Fig. 1B); another mechanism is necessary to account for the remaining 40%, and this remains to be clarified.
Conclusions
Productive virus infection requires accurate recognition of the host cell to avoid wasted genome release. This process is largely controlled by specific interactions between the virus and the receptors from the host. It has been shown previously (Andres et al., 2010; 2012) that LPS from the host Salmonella can trigger slow DNA ejection of P22 in vitro. In our present work, we have demonstrated for the first time that, together with LPS, purified OmpA can: (1) dramatically increase the rate and the efficiency of DNA ejection of P22 in vitro; and (2) facilitate the in vitro ejection of P22’s internal (E) proteins. OmpA is one of the major outer membrane proteins in bacteria, with ~100,000 copies in each cell (Koebnik et al., 2000). Our results suggest that it can serve as a potential secondary receptor during P22 infection. This finding is similar to the case of Sf6 (another Podovirus closely related to P22) for which LPS from the host Shigella cannot trigger DNA release by itself. Only when a secondary receptor like OmpA or OmpC is also present can the virus trigger DNA release (Parent et al., 2014). It remains to be determined how LPS alone or LPS and OmpA interact with the portal complex. Perhaps interaction with both receptors present induces a conformational change that promotes effective ejection of both DNA and E proteins.
Another open question, especially for short-tailed Podoviridae, is the following: after specific binding to the outer membrane, how is viral DNA translocated across both membranes of the host to initiate the infection? Compared to some phages whose long tails are in principle long enough to span the space between the outer surface and the cytoplasm of their host, Podoviridae may require a more complex mechanism to deliver their genome. One of the best studied Podoviridae, T7, likely utilizes its internal proteins to further extend its tail across the membranes to facilitate genome delivery as seen in cryo tomograms of T7 infecting mini cells (Garcia, 1995; Hu et al., 2013; Molineux et al., 2013). A recent example of a non-tailed phage, phiX174, has shown that this icosahedral ssDNA phage can use an internal protein to build a structure required for DNA translocation. These results suggest that even in the absence of a tail machine, a protein tube is still formed by internal phage proteins (Sun et al., 2014). So far there is no such structural evidence for P22. Our finding that in in vitro experiments with OmpA that all E proteins are ejected before the DNA, and the fact that all of them are essential for infectivity, along with the finding that gp16 can work in trans (Hoffman et al., 1975; Israel; 1977), suggest that the these proteins may play a key role in vivo in transferring P22 DNA through the periplasm and inner membrane by either protecting the DNA from periplasmic nuclease digestion or disrupting the membrane.
We have shown that the ejection of the proteins precedes that of the DNA but we have been unable to determine if they are ejected in a specific order. The question might be resolved if there were information about the location of the proteins in the capsid, or about their specific functions after ejection. One possibility for elucidating more about these mechanisms is to determine by cryo-EM high-resolution structures during ejection, i.e., distinguishing “before” and “after” conformations. More generally, it will be interesting to compare and contrast these issues for short- vs long- tailed phages and to unravel the roles of primary and secondary receptors in the genome ejection and delivery processes.
Materials and Methods
Plaque assay for determining the virus ejection rate
For all experiments, the P22 strain used was a clear plaque mutant that is an obligate lytic strain (Casjens et al., 1987) and was purified from Salmonella enterica serovar Typhimurium DB7136 (leuA414am, hisC525am, su0) LT2 cell lysates. S. enterica serovar Typhimurium strain DB7136 (Winston et al., 1979) was used for all phage plaque assays. Hybrid P22 with either an Sf6 tail needle knob or a T4 foldon tail were induced from prophage, and were a gift from Sherwood Casjens (University of Utah). LPS was purified using a kit (Bulldog Bio), and OmpA was purified as described (Parent et al., 2014; Porcek and Parent, 2015). Each type of virus with final concentration of 109 PFU/ml was incubated with LPS (0.25 mg/ml), or OmpA (0.2 mg/ml), or both LPS and OmpA, all in the presence of detergent Triton X-100 [1.06% (w/v)]. All plating experiments were performed in phage dilution buffer (10 mM Tris, 10 mM MgCl2, pH = 7.6). At each time point (0, 10, 20, 30, 60 min post mixing), an aliquot was removed for titering. The percentage of infectious virus remaining was determined using the PFU from each sample divided by that for a sample containing no receptor.
Genome ejection assay
P22 labeled with radioactive 35S was purified as described in (Parent et al., 2004). The virus sample (1010 PFU/ml) was first treated with DNase I (New England Biolabs) to remove any free DNA. Samples of P22 and LPS in phage dilution buffer were mixed with PEG 8000 (at concentrations of PEG corresponding to osmotic pressures of 0, 1.78, 6.2, 10.8 or 16.8 atm, respectively) (Stanley and Strey, 2003) and were incubated overnight at 37°C to ensure maximum genome ejection. The ratios between virus and the receptors were the same as those employed in the plaque assays. For samples containing both LPS and OmpA as receptors, the same concentrations of P22, LPS, OmpA, Triton X-100 and PEG used in the plaque assays were mixed and incubated at 37°C for 1 hr. In both cases, DNase I (5 units) was added to the sample after ejection was triggered, and the mixture was kept at 37°C for 4 hr to digest the DNA ejected from the capsid. The procedure for determining the amount of the genome remaining in the capsids after partial ejection is essentially that described by Evilevitch et al. (2005). Before recovering the unejected DNA from the capsid, 1mM EDTA was added and the sample heated for 10 min at 75°C to denature the DNase I. After addition of an equal volume of lysis buffer (25mM EDTA, 200mM Tris, 250mM NaCl, 1%SDS, pH 7.5) and 1 unit of Proteinase K, the samples were incubated at 65°C for 1 hr to disrupt the capsids, allowing the DNA inside to be released. Phenol / chloroform extraction was carried out twice to separate protein from DNA, followed by ethanol precipitation to concentrate the DNA. The DNA was then resuspended in TE buffer and analyzed by gel electrophoresis. In order to resolve the relatively long DNA, a low-percentage agarose gel (0.3%) was used and the running condition was 6 hr at 3V/cm; SYBR gold was used to stain the gel in the last step (Fig. S1).
SDS-PAGE for determining E protein ejection
We used samples prepared as described above for the plaque assays. Samples with LPS as the only receptor were incubated overnight to ensure maximum ejection. DNase I was then added followed by another 2 hr incubation at 37°C to completely digest the ejected DNA. An aliquot of each sample was used to determine the efficiency of genome ejection. The remainder of the sample was centrifuged at 27,000 × g for 1.5 hr to separate the virus capsids from possible ejected proteins and free nucleotides. Resuspended pellets containing capsids were TCA-precipitated and loaded into a 10% SDS-PAGE gel. The gel was then fixed, dried and exposed to HyBlot CL autoradiography Xray film (Denville). Gel densitometry on the developed film was performed with a BIORAD Gel Doc XR+ documentation system.
The ejection fraction (f) for each E protein is calculated from the intensity (I) of that band, corrected by the receptor efficiency (α) found from the plaque assay, divided by that of pure virus sample (I0). More explicitly:
Supplementary Material
Highlights.
P22 in vitro genome ejection was monitored by osmotic suppression measurements
Ejection proteins do not exit the capsid when triggered with LPS only
Both LPS and an outer membrane protein were needed for Ejection protein release
Ejection proteins, gp7, gp16, and gp20 are ejected prior to DNA release
Acknowledgements
Thanks are extended to Dr. Sherwood Casjens (U. of Utah) for stimulating discussions and the gift of P22 phage with hybrid tail needles. The authors would also like to thank Shelagh Ferguson-Miller (MSU) for the gift of the gel dryer. This work was supported in part by NSF grant CHE1051507 to CMK and WMG and NIH grant R01GM110185 to KNP.
Abbreviations
- LPS
lipopolysaccharide
- PFU
plaque forming units
- PEG
polyethylene glycol
- dsDNA
double-stranded DNA
- ssDNA
single-stranded DNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres D, Hanke C, Baxa U, Seul A, Barbirz S, Seckler R. Tailspike interactions with lipopolysaccharide effect DNA ejection from phage P22 particles in vitro. J Biol Chem. 2010;285(47):36768–36775. doi: 10.1074/jbc.M110.169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres D, Roske Y, Doering C, Heinemann U, Seckler R, Barbirz S. Tail morphology controls DNA release in two Salmonella phages with one lipopolysaccharide receptor recognition system. Mol Microbiol. 2012;83(6):1244–1253. doi: 10.1111/j.1365-2958.2012.08006.x. [DOI] [PubMed] [Google Scholar]
- Bauer DW, Huffman JB, Homa FL, Evilevitch A. Herpes virus genome, the pressure is on. J Am Chem Soc. 2013;135(30):11216–11221. doi: 10.1021/ja404008r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A, Olia AS, Cingolani G. Architecture of viral genome-delivery molecular machines. Curr Opin Struct Biol. 2014;25:1–8. doi: 10.1016/j.sbi.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, Huang WM, Hayden M, Parr R. Initiation of bacteriophage P22 DNA packaging series Analysis of a mutant that alters the DNA target specificity of the packaging apparatus. J Mol Biol. 1987;194:411–422. doi: 10.1016/0022-2836(87)90671-1. [DOI] [PubMed] [Google Scholar]
- Casjens S, Hayden M. Analysis in vivo of the bacteriophage P22 headful nuclease. J Mol Biol. 1988;199(3):467–474. doi: 10.1016/0022-2836(88)90618-3. [DOI] [PubMed] [Google Scholar]
- Casjens SR, Thuman-Commike PA. Evolution of mosaically related tailed bacteriophage genomes seen through the lens of phage P22 virion assembly. Virology. 2011;411(2):393–415. doi: 10.1016/j.virol.2010.12.046. [DOI] [PubMed] [Google Scholar]
- Castelnovo M, Evilevitch A. DNA ejection from bacteriophage: towards a general behavior for osmotic-suppression experiments. Eur Phys J E Soft Matter. 2007;24(1):9–18. doi: 10.1140/epje/i2007-10205-5. [DOI] [PubMed] [Google Scholar]
- Chang J, Weigele P, King J, Chiu W, Jiang W. Cryo-EM asymmetric reconstruction of bacteriophage P22 reveals organization of its DNA packaging and infecting machinery. Structure. 2006;14(6):1073–1082. doi: 10.1016/j.str.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Davidson AR, Cardarelli L, Pell LG, Radford DR, Maxwell KL. Long noncontractile tail machines of bacteriophages. Adv Exp Med Biol. 2012;726:115–142. doi: 10.1007/978-1-4614-0980-9_6. [DOI] [PubMed] [Google Scholar]
- Evilevitch A, Lavelle L, Knobler CM, Raspaud E, Gelbart WM. Osmotic pressure inhibition of DNA ejection from phage. Proc Natl Acad Sci U S A. 2003;100(16):9292–9295. doi: 10.1073/pnas.1233721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evilevitch A, Gober JW, Phillips M, Knobler CM, Gelbart WM. Measurements of DNA lengths remaining in a viral capsid after osmotically suppressed partial ejection. Biophys J. 2005;88:751–756. doi: 10.1529/biophysj.104.045088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García LR, Molineux IJ. Rate of translocation of bacteriophage T7 DNA across the membranes of Escherichia coli. J Bacteriol. 1995;177(14):4066–4076. doi: 10.1128/jb.177.14.4066-4076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Huici V, Salas M, Hermoso JM. Requirements for Bacillus subtilis bacteriophage phi29 DNA ejection. Gene. 2006;374:19–25. doi: 10.1016/j.gene.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Grayson P, Evilevitch A, Inamdar MM, Purohit PK, Gelbart WM, Knobler CM, Phillips R. The effect of genome length on ejection forces in bacteriophage lambda. Virology. 2006;348(2):430–436. doi: 10.1016/j.virol.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B, Levine M. Bacteriophage P22 virion protein which performs an essential early function I. analysis of 16-ts mutants. J Virol. 1975a;16(6):1536–1546. doi: 10.1128/jvi.16.6.1536-1546.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B, Levine M. Bacteriophage P22 virion protein which performs an essential early function II. characterization of the gene 16 function. J Virol. 1975b;16(6):1547–1559. doi: 10.1128/jvi.16.6.1547-1559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Margolin W, Molineux IJ, Liu J. The bacteriophage t7 virion undergoes extensive structural remodeling during infection. Science. 2013;339(6119):576–579. doi: 10.1126/science.1231887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V, Woodworth-Gutai M, Levine M. Inhibitory effect of bacteriophage P22 infection on host cell deoxyribonuclease activity. J Virol. 1972;9(5):752–757. doi: 10.1128/jvi.9.5.752-757.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V. E proteins of bacetriophage P22 I. Identification and Ejection from Wild-Type and Defective Particles. J Virol. 1977;23(1):91–97. doi: 10.1128/jvi.23.1.91-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S, Kanegasaki S. Smooth specific phage adsorption endorhamnosidase activity of tail parts of P22. Biochem Biophys Res Commun. 1973;55(2):403–409. doi: 10.1016/0006-291x(73)91101-7. [DOI] [PubMed] [Google Scholar]
- King J, Botstein D, Casjens S, Earnshaw W, Harrison S, Lenk E. Structure and assembly of the capsid of bacteriophage P22. Phil Trans R Soc Lond B. 1976;276:37–49. doi: 10.1098/rstb.1976.0096. [DOI] [PubMed] [Google Scholar]
- Koebnik R, Locher KP, Van Gelder P. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol. 2000;37(2):239–253. doi: 10.1046/j.1365-2958.2000.01983.x. [DOI] [PubMed] [Google Scholar]
- Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, Poliakov A, Potter CS, Carragher B, Johnson JE. The structure of an infectious P22 virion shows the signal for headful DNA packaging. Science. 2006;312(5781):1791–1795. doi: 10.1126/science.1127981. [DOI] [PubMed] [Google Scholar]
- Lander GC, Khayat R, Li R, Prevelige PE, Potter CS, Carragher B, Johnson JE. The P22 tail machine at subnanometer resolution reveals the architecture of an infection conduit. Structure. 2009;17(6):789–799. doi: 10.1016/j.str.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt JC, Gogokhia L, Gilcrease EB, Bhardwaj A, Cingolani G, Casjens SR. The tip of the tail needle affects the rate of DNA delivery by bacteriophage P22. PLoS One. 2013;8(8):e70936. doi: 10.1371/journal.pone.0070936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leforestier A, Brasilès S, de Frutos M, Raspaud E, Letelier L, Tavares P, Livolant F. Bacteriophage T5 DNA Ejection under Pressure. J Mol Biol. 2008;384(3):730–739. doi: 10.1016/j.jmb.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Leiman PG, Shneider MM. Contractile tail machines of bacteriophages. Adv Exp Med Biol. 2012;726:93–114. doi: 10.1007/978-1-4614-0980-9_5. [DOI] [PubMed] [Google Scholar]
- Molineux IJ. No syringes please, ejection of phage T7 DNA from the virion is enzyme driven. Mol Microbiol. 2001;40(1):1–8. doi: 10.1046/j.1365-2958.2001.02357.x. [DOI] [PubMed] [Google Scholar]
- Molineux IJ, Panja D. Popping the cork: mechanisms of phage genome ejection. Nat Rev Microbiol. 2013;11(3):194–204. doi: 10.1038/nrmicro2988. [DOI] [PubMed] [Google Scholar]
- Olia AS, Prevelige PE, Jr., Johnson JE, Cingolani G. Three-dimensional structure of a viral genome-delivery portal vertex. Nat Struct Mol Biol. 2011;18(5):597–603. doi: 10.1038/nsmb.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent KN, Ranaghan MJ, Teschke CM. A second-site suppressor of a folding defect functions via interactions with a chaperone network to improve folding and assembly in vivo. Mol Microbiol. 2004;54(4):1036–1050. doi: 10.1111/j.1365-2958.2004.04326.x. [DOI] [PubMed] [Google Scholar]
- Parent KN, Erb ML, Cardone G, Nguyen K, Gilcrease EB, Porcek NB, Pogliano J, Baker TS, Casjens SR. OmpA and OmpC are critical host factors for bacteriophage Sf6 entry in Shigella. Mol Microbiol. 2014;92(1):47–60. doi: 10.1111/mmi.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DP, Prevelige PE, Douglas T. Nanoreators by Programmed Enzyme Encapsulation Inside the Capsid of the Bacteriophage P22. ACS Nano. 2012;6(6):5000–5009. doi: 10.1021/nn300545z. [DOI] [PubMed] [Google Scholar]
- Perez GL, Huynh B, Slater M, Maloy S. Transport of phage P22 DNA across the cytoplasmic membrane. J Bacteriol. 2009;191(1):135–140. doi: 10.1128/JB.00778-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poranen MM, Daugelavicius R, Bamford DH. Common principles in viral entry. Annu Rev Microbiol. 2002;56:521–538. doi: 10.1146/annurev.micro.56.012302.160643. [DOI] [PubMed] [Google Scholar]
- Porcek NB, Parent KN. Key residues of S. flexneri OmpA mediate infection by bacteriophage Sf6. J Mol Biol. 2015 doi: 10.1016/j.jmb.2015.03.012. doi:10.1016/j.jmb.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau DC, Lee B, Parsegian VA. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution Hydration forces between parallel DNA doubles helices. Proc Natl Acad Sci U S A. 1984;81:2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer SC, Bloomfield VA. Packaging of DNA in bacteriophage heads some considerations on energetics. Biopolymers. 1978;17:785–794. doi: 10.1002/bip.1978.360170317. [DOI] [PubMed] [Google Scholar]
- Stanley CB, Strey HH. Measuring Osmotic Pressure of Poly(ethylene glycol) Solutions by Sedimentation Equilibrium Ultracentrifugation. Macrolulecules. 2003;36:6888–6893. [Google Scholar]
- Stock JB, Rauch B, Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 1977;252(21):7850–7861. [PubMed] [Google Scholar]
- Sun L, Young LN, Zhang X, Boudko SP, Fokine A, Zbornik E, Roznowski AP, Molineux IJ, Rossmann MG, Fane BA. Icosahedral bacteriophage PhiX174 forms a tail for DNA transport during infection. Nature. 2014;505(7483):432–435. doi: 10.1038/nature12816. [DOI] [PubMed] [Google Scholar]
- Tang J, Lander GC, Olia AS, Li R, Casjens S, Prevelige P, Jr., Cingolani G, Baker TS, Johnson JE. Peering down the barrel of a bacteriophage portal: the genome packaging and release valve in p22. Structure. 2011;19(4):496–502. doi: 10.1016/j.str.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Strelkov SV, Mesyanzhinov VV, Rossmann MG. Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain. Stucture. 1997;5(6):789–798. doi: 10.1016/s0969-2126(97)00233-5. [DOI] [PubMed] [Google Scholar]
- Tzlil S, Kindt J, Gelbart WM, Ben-Shaul A. Forces and pressures in DNA packaging and release from viral capsids. Bioiphys J. 2003;84(3):1616–27. doi: 10.1016/S0006-3495(03)74971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F, Botstein D, Miller JH. Characterization of amber and ochre suppressors in Salmonella typhimurium. J Bacteriol. 1979;137(1):433–439. doi: 10.1128/jb.137.1.433-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.