Abstract
Purpose
To analyze the effect of neglecting non-zero echo times (TEs) in the conventional model of multicomponent driven equilibrium single pulse observation of T1 and T2 (mcDESPOT).
Theory and Methods
Formulations of the two-component spoiled gradient recalled echo (SPGR) and balanced steady state free precession (bSSFP) models that incorporate non-zero TE effects are presented in the context of mcDESPOT and compared to the conventionally-used SPGR and bSSFP models which ignore non-zero TEs. Relative errors in derived parameter estimates from conventional mcDESPOT, omitting TE effects, are assessed using simulations over a wide range of experimental and sample parameters.
Results
The neglect of non-zero TE leads to an overestimate of the SPGR signal and an underestimate of the bSSFP signal. These effects can introduce large errors in parameter estimates derived from conventional mcDESPOT under realistic imaging conditions.
Conclusion
SPGR and bSSFP signal models accounting for non-zero TE effects should be incorporated into quantitative mcDESPOT analyses.
Keywords: mcDESPOT, SPGR, bSSFP, Quantitative MRI
INTRODUCTION
Combining spoiled gradient recalled echo (SPGR) and balanced steady state free precession (bSSFP) imaging sequences, as in multicomponent driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) (1–2), results in relatively high signal-to-noise ratio (SNR) and rapid image acquisitions in studies of multicomponent systems. This facilitates, in principle, bicomponent, high-resolution, maps of component fractions and corresponding relaxation times across the entire human brain or knee (3–6), overcoming the main limitations of conventional multi-spin-echo pulse sequences (7–9).
Although it is often restricted to a two-pool model (1–2, 9), mcDESPOT has been extended to include a third pool to account for either partial volume effects (10) or magnetization transfer (11). However, in all variants of mcDESPOT, the magnetization relaxation that occurs between the pulse preceding signal acquisition and the acquisition itself is neglected; that is, non-zero TE effects are neglected in analysis of both the SPGR and bSSFP signals. In a recent study (12), it was shown that the neglect of non-zero TE in the two-component SPGR signal model introduces considerable errors into derived parameter estimates from mcDESPOT. However, neglect of nonzero TE effects in the bSSFP model, and the resulting errors in mcDESPOT parameter estimates, were not investigated.
mcDESPOT is a multicomponent extension of the well established DESPOT1 and DESPOT2 methods used for accurate and rapid whole-brain T1 and T2 mapping (13–15). It is clear that, given the long mean relaxation times in brain or other soft tissues, the neglect of non-zero TE effects in DESPOT1 and DESPOT2 signal models will have negligible impact on the derived parameter estimates. However, it remains unclear if this assumption still holds for multicomponent studies, since underlying signal components will exhibit differential amplitude weighting during relaxation.
Here, we evaluate formulations of the SPGR and bSSFP signal models that account for nonzero TE in the context of mcDESPOT analysis. Next, numerical simulations are presented to determine the impact of neglecting non-zero TEs on derived parameter estimates from conventional two-pool mcDESPOT (1–2, 5–6, 9, 12). Since our focus is on the effect of underlying signal models, we restricted our analyses to the case of infinite SNR.
THEORY
Under conditions of chemical equilibrium and in the presence of two-pool exchange between a slowly relaxing species S and a fast-relaxing species F, the steady-state (SS) bSSFP magnetization, MSS, immediately preceding each radio-frequency (RF) pulse is given by (1–2, 10, 16)
| [1] |
where , C = M0[0 0 0 0 fS/T1,S fF/T1,F]T,
and
where M0 represents the signal amplitude at TE = 0 ms and incorporates proton density and various machine factors, I is the 6 × 6 identity matrix, TRbSSFP is the repetition time that corresponds to the time between two successive RF pulses, θRF is the phase increment of the RF excitation pulse, fF and fS are the fractions of the fast and slow T2 components, respectively, with fF = 1 − fS, T2 and T1 are the transverse and longitudinal relaxation times, respectively, kFS is the exchange rate from F to S, kSF is the exchange rate from S to F, Δω is the off-resonance frequency, with the assumption that both components exhibit the same chemical shift, and α represents flip angle (FA) with pulse phase taken arbitrarily as x.
In the conventional implementation of mcDESPOT (1–2, 10), the theoretical two-component bSSFP signal is taken as
| [2] |
or, in the absence of chemical exchange, by the following summation (17)
| [3] |
where
and
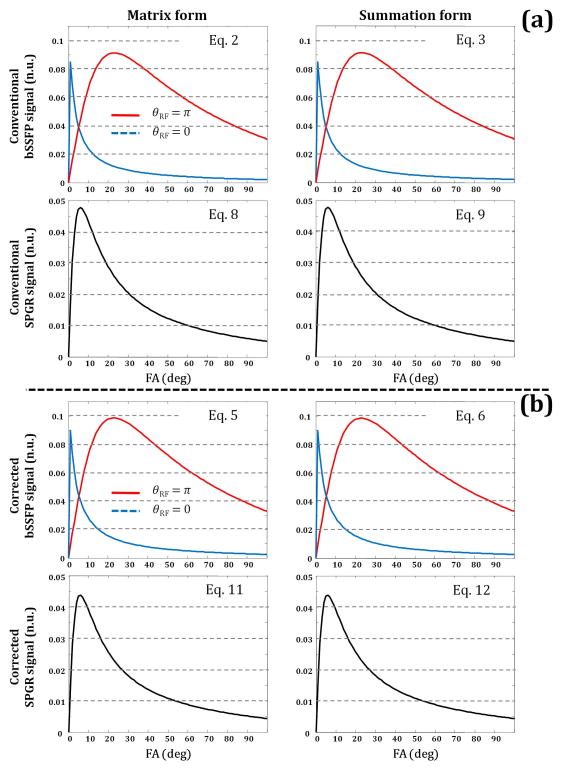
where φj = θRF + TRΔωj, E2,j = exp(−TRbSSFP/T2,j) and E1,j = exp(−TRbSSFP/T1,j), and j stands for the jth component (i.e. S or F). The equivalence of Eqs. 2 and 3 is shown in Fig. 1a, upper row. However, Eqs. 1–3 do not account for the relaxation occurring between a bSSFP excitation pulse and the subsequent acquisition that occurs at TEbSSFP = TRbSSFP/2 (18–19). The corrected bSSFP magnetization, , is described by application of Eq. 1 to the magnetization MSS prior to the pulse that precedes echo acquisition
Fig. 1.
Comparison of the matrix (left column) and summation (right column) forms of the two-component bSSFP and SPGR signals in the absence of chemical exchange as a function of flip angle (FA) when non-zero TE effects were (a) omitted or (b) incorporated in the signal models. The modeled equations are indicated in the figure panels. Signals were generated using the following underlying input and experimental parameters: fF = 0.2, T2,F = 10 ms, T2,S = 90 ms, T1,F = 450 ms, T1,S = 2000, kFS = kSF = 0 ms−1, Δω = 0 Hz, TESPGR = 2.5 ms, and TRbSSFP = TRSPGR = 7 ms. Note the increase in the corrected bSSFP and the decrease in the corrected SPGR signal when these non-zero TE effects were incorporated as compared to the conventional bSSFP and SPGR signal forms used in mcDESPOT that neglect TEs.
| [4] |
where the matrix A* is identical to the matrix A as defined above, but with TRbSSFP replaced by TEbSSFP throughout. Then the corrected theoretical two-component bSSFP signal is given by
| [5] |
where the x- and y-components of the transverse magnetization are indicated by subscripts, or, in the absence of chemical exchange, by the following summation
| [6] |
where
and
This form of the numerator, in which appears instead of E2,j, reflects the fact that relaxation prior to echo formation occurs over a time period TEbSSFP = TRbSSFP/2 (18–19), rather than over the time period TRbSSFP. The equivalence of Eqs. 5 and 6 is shown in Fig. 1b, upper row.
Similarly, for spoiled steady state (SSS) transverse magnetization prior to each RF pulse as in the case of SPGR, the magnetization MSSS immediately following each RF pulse is given by (1–2, 16)
| [7] |
where I is the 2 × 2 identity matrix, D = M0[fS fF]T and
In the conventional implementation of mcDESPOT (1–2, 10), the theoretical two-component SPGR signal is taken as
| [8] |
which, in the absence of chemical exchange, reduces to the following summation (Fig. 1a, second row) (20)
| [9] |
Eqs. 7–9 do not account for the relaxation occurring between an excitation pulse and the subsequent acquisition (12). The corrected two-component SPGR magnetization, , is described by (21)
| [10] |
where the matrix ASPGR is given by
and , I is the 6 × 6 identity matrix, and S is the spoiler matrix given by
from which the corrected theoretical two-component SPGR signal is given by
| [11] |
or, in the absence of chemical exchange, by the following simple summation (Fig. 1b, second row)
| [12] |
where and , and where describes mesoscopic or macroscopic field inhomogeneities common to both relaxation components (12).
METHODS
Effect of non-zero TESPGR on SPGR and non-zero TEbSSFP on bSSFP signals
Corrected SPGR and bSSFP signals generated using Eq. 11 and Eq.5, respectively, explicitly accounting for transverse relaxation during non-zero TESPGR and TEbSSFP were compared to conventional SPGR and bSSFP signals generated using Eq. 8 and Eq. 2, respectively, which neglect non-zero TESPGR and TEbSSFP effects. Signal differences were calculated as 100 * (SConv − SCorr)/SCorr, where SConv and SCorr are conventional and corrected signals, respectively, as a function of FA. Analyses were performed for corrected SPGR signals generated with TRSPGR = 7 ms, and over a range of TESPGR from 0.5 to 6 ms in 0.25 ms increments and for FAs ranging from 1° to 100° in increments of 1°. The signal difference between conventional and corrected bSSFP signals was likewise calculated for values of TEbSSFP (= TRbSSFP/2) ranging from 0.5 to 6 ms in 0.25 ms increments for FAs ranging from 1° to 100° in increments of 1°. To correspond to actual practice (2–6, 9–12), two corrected bSSFP signals were generated with θRF of 0 or π(bSSFP0 or bSSFPπ), respectively. All simulations used input parameters fF = 0.2, T2,F = 10 ms, T2,S = 90 ms, T1,F = 450 ms, T1,S = 2000, kFS = kSF = 0 ms−1 and Δω = 0 Hz; these are based on reported values from human brain imaging (1–4, 8–10).
Effects of non-modeled non-zero TESPGR and TEbSSFP on mcDESPOT-derived parameter estimates
Effects of non-zero TESPGR
Corrected SPGR signals were simulated using Eq. 11 for values of TESPGR ranging from 0.5 to 6 ms in 0.25 ms increments and corrected bSSFP signals were generated using Eq. 5 with fixed TEbSSFP value of 3.5 ms and for θRF of 0 or π. Corrected SPGR and bSSFP were generated for values of fF ranging from 0.025 to 0.5 in increments of 0.025. For each TESPGR and fF combination, corrected SPGR and bSSFP signals were then fitted simultaneously to Eqs. 11 and 5, explicitly accounting for non-zero TESPGR and TEbSSFP (TE-Corrected mcDESPOT; TEC-mcDESPOT), or to Eqs. 8 and 2, neglecting non-zero TESPGR and TEbSSFP (Conventional mcDESPOT). Using typical values of experimental parameters (1–6, 9–12, 22–28), SPGR signals were generated for αo = {2, 4, 6, 8, 10, 12, 14, 16, 18, 20}, while, bSSFP signals were generated for αo = {2, 6, 14, 22, 30, 38, 46, 54, 62, 70}. The other experimental and input parameters were identical to those noted above. Relative parameter errors were calculated according to 100 * |P̂ − P|/P, where P̂ and P are the estimated and true parameter values, respectively.
Parameter estimates were derived using the stochastic region contraction (SRC) algorithm (1–2, 9, 12, 29). For each iteration of the SRC algorithm, 20000 random samples were generated within specified parameter bounds. The 50 solutions with the smallest least squares residual were used to construct bounds for the next iteration. Iterations were terminated after convergence was achieved, defined as a difference between the minimum and maximum values for all parameters of less than 1%, or after 30 iterations. The initial parameter bounds were: 0 ≤ fF ≤ 0.8, 1 ms ≤ T2,F ≤ 40 ms, 40 ms ≤ T2,S ≤ 200 ms, 100 ms ≤ T1,F ≤ 700 ms and 700 ms ≤ T1,S ≤ 3000 ms. To avoid the need to explicitly fit for M0, each dataset was normalized with respect to its mean value over the full range of FA (1–2, 9, 12).
Effects of non-zero TEbSSFP
Corrected SPGR signals were simulated using Eq. 11 with a fixed TESPGR value of 2 ms. Corrected bSSFP signals were generated using Eq. 5 for values of TEbSSFP (= TRbSSFP/2) ranging from 1.5 to 6 ms in 0.25 ms increments and for θRF of 0 or π. Corrected SPGR and bSSFP were generated for values of fF ranging from 0.025 to 0.5 in increments of 0.025. For each TEbSSFP and fF combination, corrected SPGR and bSSFP signals were then fitted simultaneously to Eqs. 11 and 5, explicitly accounting for non-zero TESPGR and TEbSSFP (TEC-mcDESPOT), or to Eqs. 8 and 2, neglecting non-zero TESPGR and TEbSSFP (Conventional mcDESPOT). The other experimental and underlying input parameters were identical to those used in the previous analyses. Parameter estimates were derived using the SRC algorithm with relative parameter errors calculated as described above.
RESULTS
Figure 2 shows the relative differences between conventional (Eqs. 8 and 2) and corrected (Eqs. 11 and 5) two-component SPGR and bSSFP signals for different values of TESPGR and TEbSSFP as a function of FA. As seen, conventional SPGR signals are overestimated to an extent that increases with increasing TESPGR or FA. In contrast, conventional bSSFP0 and bSSFPπ are underestimated to an extent increasing with increasing TEbSSFP and with increasing FAs for bSSFP0, and decreasing with respect to increasing FA for bSSFPπ.
Fig. 2.
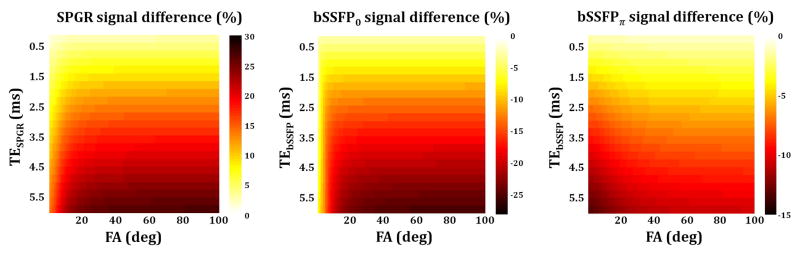
Relative difference between corrected and conventional SPGR and bSSFP signals. The corrected signals incorporate non-zero TESPGR and TEbSSFP as discussed in the text, while the conventional signals omit these. Results are shown for different values of TE and as a function of flip angle (FA). Signals were generated using the following underlying input and experimental parameters: fF = 0.2, T2,F = 10 ms, T2,S = 90 ms, T1,F = 450 ms, T1,S = 2000, kFS = kSF = 0 ms−1, Δω = 0 Hz, and TRbSSFP = TRSPGR = 7 ms. As shown, for all TEs and FA combinations, the conventional SPGR signal was overestimated while the conventional bSSFP signal was underestimated.
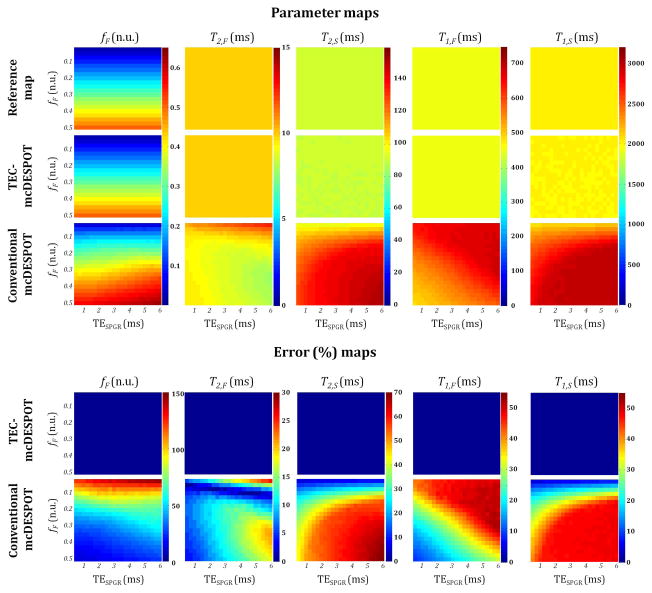
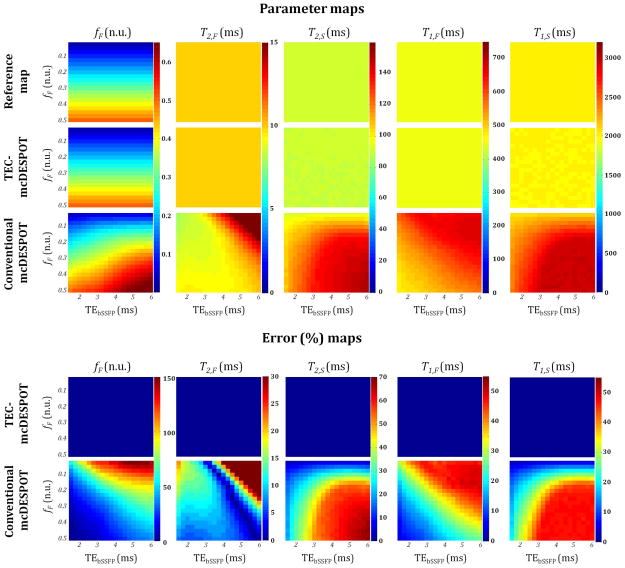
Parameter estimate errors resulting from conventional mcDESPOT are shown in Figs. 3 and 4. When data were simulated with non-zero TEs (i.e. TESPGR or TEbSSFP) using Eqs. 11 and 5 and fit with the correct models (TEC-mcDESPOT), Eqs. 11 and 5, true input values were obtained for all parameters, shown as zero errors in derived parameter estimates. This indicates the ability of the SRC fitting procedure to determine the correct least-squares minima for these multiparametric signal models at infinite SNR. Figures 3 and 4 also show parameter estimates and the corresponding relative error maps for conventional mcDESPOT-derived parameter estimates obtained by fitting data generated from Eqs. 11 and 5, correctly incorporating non-zero TE effects, with Eqs. 8 and 2, which do not include appropriate correction for TEs. Results are shown for different combinations of fF and TEs. For all TEs and fF combinations, estimated parameter values of fF, T2,S, T1,F and T1,S were clearly overestimated, while estimated parameter values of T2,F showed more complex patterns. In all cases, conventional mcDESPOT led to substantial errors in derived parameter estimates over the entire ranges of TEs and fF. Relative error was seen to increase with increasing TESPGR or TEbSSFP for all parameters, while error increased for T2,S and T1,S and decreased for fF and T1,F as a function of fF. However, the relative error in the estimated values of T2,F showed more complex patterns as a function of TEs and fF.
Fig. 3.
Parameter value and relative error maps obtained from fitting simulated data with TEC-mcDESPOT (Eqs. 5 and 11) or conventional mcDESPOT (Eqs. 2 and 8) signal models for different combinations of TESPGR and fF. Analyses were performed at infinite SNR with, in all cases, corrected bSSFP signals generated using Eq. 5 for a fixed TEbSSFP value of 3.5 ms. Note that the color bars were set to correspond to the dynamic range of each parameter, and therefore differ between parameters.
Fig. 4.
Parameter and relative error maps obtained from fitting simulated data with TEC-mcDESPOT (Eqs. 5 and 11) or conventional mcDESPOT (Eqs. 2 and 8) signal models for different combinations of TEbSSFP and fF. Analyses were performed at infinite SNR with, in all cases, corrected SPGR signals were generated using Eq. 11 for a fixed TESPGR value of 2 ms. Note that the color bars were set to correspond to the dynamic range of each parameter, and therefore differ between parameters.
DISCUSSION
The introduction of mcDESPOT in 2008 (1) permitted for the first time high-resolution whole-brain analysis of myelin water fraction in human brain (1–4, 10, 22–28), and proteoglycan associated water fraction in human knee cartilage (5–6, 11). In light of the importance of this method, we have re-examined the signal models used in mcDESPOT and noted that the approximations used in single-component analyses, that is in DESPOT1 and DESPOT2, may not hold for the multicomponent mcDESPOT analysis. For DESPOT1 and DESPOT2, non-zero TE effects may be neglected due to the long mean relaxation times in brain and other soft tissues. However, while the resulting errors in signal amplitudes may appear to be negligible for mcDESPOT as well (Figs. 1–2), our detailed analysis has indicated that in fact these small deviations may result in large errors in parameter estimates under realistic imaging conditions (Figs. 3–4). This is likely due to the inherent and unavoidable sloppiness of this high dimensional signal model (12, 30–31), and to the fact that differential relaxation between the two components can introduce substantial errors.
Our simulation analyses showed that, as expected, neglect of non-zero TESPGR in the SPGR model leads to overestimates of signal values (Figs. 1–2), due to the omission of the signal decay between the RF pulse and the read-out gradient (12, 21, 32). In contrast, neglect of non-zero TE in the bSSFP model leads to signal underestimates (Figs. 1–2) since, in effect, this neglect incorporates transverse relaxation over a period of TRbSSFP rather than TRbSSFP/2 prior to echo acquisition (18–19, 33). That is, the incorrect signal model evaluates the signal after an echo time of TR, rather than after the shorter echo time of TR/2. Thus, in this case, incorrect modeling of transverse relaxation results in an underestimate of signal intensity. More importantly, neglect of non-zero TEs in mcDESPOT leads to significant errors in derived parameter estimates (Figs. 3–4).
The impact of noise on the accuracy and precision of derived parameter estimates has been the subject of extensive previous investigations. It was demonstrated that high quality parameter estimates may be obtainable from mcDESPOT with SNR that is clinically achievable (12, 34). However, the errors in parameter estimation described herein and due to the neglect of TE effects were considerably larger than those introduced due to noise (12, 34). In fact, the relative error calculated over 1000 noise realizations in the estimation of fF was ~10% with the above underlying and experimental parameters and SNR = M0/σ = 1000; achievable in clinical setting (12, 34), where σ represents the standard deviation of zero-mean Gaussian noise. The error due to neglect of TEs under comparable circumstances with TESPGR = 2 ms and TEbSSFP = 3.5 ms was however ~60% (Figs. 3–4). Since our focus is on the effect of underlying signal models, we restricted our analyses to the case of infinite SNR and evaluated simulation results to explicitly determine the magnitude of the effects, without the manifold additional complications inherent in analysis of experimental data.
We assumed no exchange in the mcDESPOT signal model for several reasons. First, a recent study suggests that the incorporation of two-site exchange has a minimal effect on the estimated values of the rapidly-relaxing fraction of a two-component model (9), which is the main parameter of interest for characterization of cartilage and brain (3–11). In addition, we have found (data not shown) that the inclusion of exchange as an additional unknown parameter requires further constraints on the initial parameter bounds of the SRC algorithm for reliable parameter estimates, even at infinite SNR. This is likely due to the increased complexity of the local minima structure or the further flatness of the parameter energy surfaces as compared to the five unknown parameters model adopted in our analysis and analyzed elsewhere (12). In addition, we neglected finite RF pulse length effects in the current study in order to correspond to the conventional analysis of mcDESPOT. However, it has been shown that the neglect of relaxation during RF pulses can lead to further deviations of the measured signals from the theoretical SPGR and bSSFP models (21, 35–38). Moreover, again as with the conventional mcDESPOT approach, we assumed perfect spoiling of transverse magnetization prior to each RF pulse in the SPGR sequence. However, it has been shown that there may be a degree of preservation of transverse coherence in fast RF spoiled sequences with concomitant deviation of the signal behavior from the idealized model (32, 39). In the experimental setting, all of these additional effects would presumably be additive to those discussed here, which are based on considerations of the underlying signal models used in mcDESPOT analysis. Finally, we note that our results are equally applicable to other steady-state approaches such as mcRISE (11).
CONCLUSIONS
Neglect of non-zero TESPGR and TEbSSFP leads to signal models that overestimate SPGR signal intensity and underestimate bSSFP signal intensity. We have shown that this can result in substantial errors in mcDESPOT-derived parameter estimates and propose the use of the SPGR and bSSFP signal models described herein for quantitative analyses using mcDESPOT.
Acknowledgments
We gratefully acknowledge the comments of an anonymous reviewer of a previous publication, which led to this analysis. This work was supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Deoni SCL, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. 2008;60:1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- 2.Deoni SCL. Correction of Main and Transmit Magnetic Field (B0 And B1) Inhomogeneity Effects in Multicomponent-driven Equilibrium Single-pulse Observation of T1 And T2. Magn Reson Med. 2011;65:1021–1035. doi: 10.1002/mrm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deoni SCL, Dean DC, O’Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63:1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean DC, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Walker L, Piryatinsky I, Deoni SCL. Estimating the Age of Healthy Infants From Quantitative Myelin Water Fraction Maps. Hum Brain Mapp. 2015;36:1233–1244. doi: 10.1002/hbm.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Choi K, Samsonov A, Spencer RG, Wilson JJ, Block WF, Kijowski R. In-Vivo Multi-Component T2 Relaxation Time Analysis of the Articular Cartilage of the Human Knee Joint at 3.0T. Radiology. 2015 doi: 10.1148/radiol.2015142201. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Chaudhary R, Hurley SA, Munoz Del Rio A, Alexander AL, Samsonov A, Block WF, Kijowski R. Rapid multicomponent T2 analysis of the articular cartilage of the human knee joint at 3. 0 T. J Magn Reson Imaging. 2014;39:1191–1197. doi: 10.1002/jmri.24290. [DOI] [PubMed] [Google Scholar]
- 7.Prasloski T, Rauscher A, Mackay AL, Hodgson M, Vavasour IM, Laule C, Madler B. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. Neuroimage. 2012;63:533–539. doi: 10.1016/j.neuroimage.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 8.MacKay A, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med. 1994;31:673–677. doi: 10.1002/mrm.1910310614. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Kolind SH, Laule C, MacKay AL. Comparison of myelin water fraction from multiecho T2 decay curve and steady-state methods. Magn Reson Med. 2015;73:223–232. doi: 10.1002/mrm.25125. [DOI] [PubMed] [Google Scholar]
- 10.Deoni SCL, Matthews L, Kolind SH. One Component? Two Components? Three? The Effect of Including a Non-Exchanging ‘Free” Water Component in Multicomponent Driven Equilibrium Single Pulse Observation of T1 & T2 (mcDESPOT) Magn Reson Med. 201(70):147–154. doi: 10.1002/mrm.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Block WF, Kijowski R, Samsonov A. Rapid multicomponent relaxometry in steady state with correction of magnetization transfer effects. Magn Reson Med. doi: 10.1002/mrm.25672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouhrara M, Reiter DA, Celik H, Fishbein KW, Kijowski R, Spencer RG. Analysis of mcDESPOT- and CPMG-derived Parameter Estimates for Two-component Non-exchanging Systems. Magn Reson Med. doi: 10.1002/mrm.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deoni SCL, Peters TM, Rutt BK. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med. 2005;53:237–241. doi: 10.1002/mrm.20314. [DOI] [PubMed] [Google Scholar]
- 14.Deoni SCL, Rutt BK, Peters TM. Rapid Combined T1 and T2 Mapping Using Gradient Recalled Acquisition in the Steady State. Magn Reson Med. 2003;49:515, 526. doi: 10.1002/mrm.10407. [DOI] [PubMed] [Google Scholar]
- 15.Deoni SCL, Catani M. Visualization of the deep cerebellar nuclei using quantitative T1 and ρ magnetic resonance imaging at 3 Tesla. Neuroimage. 2007;37:1260–1266. doi: 10.1016/j.neuroimage.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Spencer RGS, Fishbein KW. Measurement of spin-lattice relaxation times and concentrations in systems with chemical exchange using the one-pulse sequence: breakdown of the Ernst model for partial saturation in nuclear magnetic resonance spectroscopy. J Magn Reson. 2000;142:120–135. doi: 10.1006/jmre.1999.1925. [DOI] [PubMed] [Google Scholar]
- 17.Deoni SCL, Rutt BK, Jones DK. Investigating exchange and multicomponent relaxation in fully-balanced steady-state free precession imaging. J Magn Reson Imaging. 2008;27:1421–1429. doi: 10.1002/jmri.21079. [DOI] [PubMed] [Google Scholar]
- 18.Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur Radiol. 2003;13:2409–2418. doi: 10.1007/s00330-003-1957-x. [DOI] [PubMed] [Google Scholar]
- 19.Scheffler K, Hennig J. Is TrueFISP a gradient-echo or a spin-echo sequence? Magn Reson Med. 2003;49:395–397. doi: 10.1002/mrm.10351. [DOI] [PubMed] [Google Scholar]
- 20.Deoni SCL, Rutt BK, Jones DK. Investigating the effect of exchange and multicomponent T1 relaxation on the short repetition time spoiled steady-state signal and the DESPOT1 T1 quantification method. J Magn Reson Imaging. 2007;25:570–578. doi: 10.1002/jmri.20836. [DOI] [PubMed] [Google Scholar]
- 21.Murase K. Generalized equation for describing the magnetization in spoiled gradient-echo imaging. Magn Reson Imaging. 2011;29:723–730. doi: 10.1016/j.mri.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Deoni SCL, Dean DC, Remer J, Dirks H, O’Muircheartaigh J. Cortical maturation and myelination in healthy toddlers and young children. Neuroimage. 2015;115:147–161. doi: 10.1016/j.neuroimage.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deoni SCL, Zinkstok JR, Daly E, Ecker C, Williams SCR, Murphy DGM. White-matter relaxation time and myelin water fraction differences in young adults with autism. Psych Med. 2015;45:795–805. doi: 10.1017/S0033291714001858. [DOI] [PubMed] [Google Scholar]
- 24.Deoni SCL, O’Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Dirks H, Piryatinsky I, Dean DC, Jumbe NL. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Muircheartaigh J, Dean DC, Ginestet CE, Walker L, Waskiewicz N, Lehman K, Dirks H, Piryatinsky I, Deoni SCL. White matter development and early cognition in babies and toddlers. Hum Brain Mapp. 2014;35:4475–4487. doi: 10.1002/hbm.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean DC, O’Muircheartaigh J, Dirks H, Waskiewicz N, Walker L, Doernberg E, Piryatinsky I, Deoni SCL. Characterizing longitudinal white matter development during early childhood. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deoni SCL, Dean DC, Piryatinsky I, O’Muircheartaigh J, Waskiewicz N, Lehman K, Han M, Dirks H. Breastfeeding and early white matter development: A cross-sectional study. Neuroimage. 2013;82:77–86. doi: 10.1016/j.neuroimage.2013.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolind S, Matthews L, Johansen-Berg H, Leite MI, Williams SCR, Deoni SCL, Palace J. Myelin water imaging reflects clinical variability in multiple sclerosis. Neuroimage. 2012;60:263–270. doi: 10.1016/j.neuroimage.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger MF, Silverman HF. Microphone array optimization by stochastic region contraction. IEEE Trans Signal Process. 1991;39:2377–2386. [Google Scholar]
- 30.Gutenkunst RN, Waterfall JJ, Casey FP, Brown KS, Myers CR, Sethna JP. Universally sloppy parameter sensitivities in systems biology models. PLOS Comput Biol. 2007;3:1871–1878. doi: 10.1371/journal.pcbi.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waterfall JJ, Casey FP, Gutenkunst RN, Brown KS, Myers CR, Brouwer PW, Elser V, Sethna JP. Sloppy model universality class and the Vandermonde matrix. Phys Rev Letters. 2006;97:150601. doi: 10.1103/PhysRevLett.97.150601. [DOI] [PubMed] [Google Scholar]
- 32.Yarnykh VL. Optimal Radiofrequency and Gradient Spoiling for Improved Accuracy of T1 and B1 Measurements Using Fast Steady-State Techniques. Magn Reson Med. 2010;63:1610–1626. doi: 10.1002/mrm.22394. [DOI] [PubMed] [Google Scholar]
- 33.Scheffler K. On the Transient Phase of Balanced SSFP Sequences. Magn Reson Med. 2003;49:781–783. doi: 10.1002/mrm.10421. [DOI] [PubMed] [Google Scholar]
- 34.Deoni SCL, Kolind SH. Investigating the stability of mcDESPOT myelin water fraction values derived using a stochastic region contraction approach. Magn Reson Med. 2015;73:161–169. doi: 10.1002/mrm.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bieri O, Scheffler K. SSFP Signal With Finite RF Pulses. Magn Reson Med. 2009;62:1232–1241. doi: 10.1002/mrm.22116. [DOI] [PubMed] [Google Scholar]
- 36.Bieri O. An Analytical Description of Balanced Steady-State Free Precession With Finite Radio-Frequency Excitation. Magn Reson Med. 2011;65:422–431. doi: 10.1002/mrm.22626. [DOI] [PubMed] [Google Scholar]
- 37.Boulant N. T1 and T2 effects during radio-frequency pulses in spoiled gradient echo sequences. J Magn Reson. 2009;197:213–218. doi: 10.1016/j.jmr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Lenz C, Klarhöfer M, Scheffler K. Limitations of rapid myelin water quantification using 3D bSSFP. MAGMA. 2010;23:139–151. doi: 10.1007/s10334-010-0211-1. [DOI] [PubMed] [Google Scholar]
- 39.Preibisch C, Deichmann R. Influence of RF Spoiling on the Stability and Accuracy of T1 Mapping Based on Spoiled FLASH With Varying Flip Angles. Magn Reson Med. 2009;61:125–135. doi: 10.1002/mrm.21776. [DOI] [PubMed] [Google Scholar]