Abstract
Respiratory syncytial virus (RSV) is the major leading cause of infantile viral bronchiolitis. However, cellular phenotypes contributing to the RSV protection and vaccine-enhanced disease remain largely unknown. Upon RSV challenge, we analyzed phenotypes and cellularity in the lung of mice that were naïve, immunized with formalin inactivated RSV (FI-RSV), or re-infected with RSV. In comparison with naïve and live RSV re-infected mice, the high levels of eosinophils, neutrophils, plasmacytoid and CD11b+ dendritic cells, and IL-4+ CD4+ T cells were found to be contributing to pulmonary inflammation in FI-RSV immune mice despite lung viral clearance. Alveolar macrophages appeared to play differential roles in protection and inflammation upon RSV infection of different RSV immune mice. These results suggest that multiple innate and adaptive immune components differentially contribute to RSV disease and inflammation.
Keywords: Respiratory syncytial virus (RSV), alveolar macrophages, formalin-inactivated RSV, vaccine, clodronate liposome
Introduction
Respiratory syncytial virus (RSV) is the major leading cause of bronchiolitis in infants and in older populations (Stott and Taylor, 1985). In the 1960s, vaccination of young children was conducted using formalin inactivated RSV (FI-RSV) vaccines with alum adjuvant. Most vaccinated children were hospitalized due to severe pulmonary disease during next epidemic season and 2 children died (Kapikian et al., 1969; Kim et al., 1969a). Histological analysis revealed dramatically increased eosinophilic infiltration in the lungs (Kim et al., 1969a). Others demonstrated that vaccine-enhanced disease was resulted from the induction of T helper type 2 (Th2) cytokines, including interleukin-4 (IL-4), IL-5 and IL-13 (Johnson and Graham, 1999; Johnson et al., 2003). However, it is still challenging to develop an effective and safe vaccine which does not cause RSV disease despite several decades of studies (Rudraraju et al., 2013).
Dendritic cells (DCs) as sentinel cells in the lung constantly monitor for incoming foreign pathogens or antigens, playing a pivotal role in the induction of RSV specific immune responses. Different subsets of DCs influence the magnitude and quality of the host response to RSV infection as well as RSV pathogenesis. Murine DCs can be divided into conventional DCs and plasmacytoid DCs (pDCs) (Lambrecht and Hammad, 2012). The conventional DCs (cDCs) in the lung are further divided into CD11b+ DCs and CD103+ DCs. CD11b+ DCs are mainly involved in recruiting leukocytes by producing proinflammatory cytokines and in priming effector CD4+ T cells. Whereas CD103+ DCs are known to be effective in sampling foreign antigens in the airway lumen and in priming naïve CD8+ and CD4+ T cells (Lukens et al., 2009). Lung pDCs are an important producer of type I interferons, granting them to activate antiviral functions of other cell types including myeloid DCs, B cells, T cells, and natural killer cells (Colonna et al., 2004; Fitzgerald-Bocarsly and Feng, 2007; Takagi et al., 2011). A high level of infiltrating eosinophils is known to be associated with inflammatory RSV vaccine-enhanced disease although direct roles of eosinophilia in the RSV pathogenesis still remain controversial (Castilow et al., 2008; Kim et al., 1969b)
Alveolar macrophages (AMs) are the first line of innate immune cells responding to incoming pathogens in the airways and play a key role in regulating inflammatory responses (Sibille and Reynolds, 1990). In response to RSV infection, AMs in humans are responsible for producing numerous proinflammtory cytokines including TNF-Α, IL-6, IL-8, and IL-10 (Becker et al., 1991; Panuska et al., 1995). A recent study has shown that AMs can regulate recruitment of inflammatory monocytes and monocyte-derived DCs via type I interferon dependent manner upon RSV infection, which play an important role in RSV disease severity (Goritzka et al., 2015). AMs as antigen presenting cells in the lungs were also known to modulate DC migration and antigen presentation (Holt, 2000; Jakubzick et al., 2006). In addition, AMs are required for protection against several pathogens such as influenza virus (Tumpey et al., 2005) and Klebsiella Pneumonia (Broug-Holub et al., 1997). In contrast, improved protection against Mycobacterium tuberculosis was observed in the absence of AMs (Leemans et al., 2001).
The roles of AMs as a major airway cell type in the RSV protection and disease as well as in the modulation innate and adaptive immune cells largely remain unknown. One of major goals in this study is to better understand potential roles of multiple innate and adaptive cellular components in the RSV protection and disease. Here we investigated cellular phenotypes in the lung microenvironment of mice that were naïve, previously immunized with FI-RSV, or re-infected with live RSV upon RSV challenge. To understand the roles of AMs in protection and inflammation upon RSV infection, the in vivo effects of AM-depleting clodronate liposome (CL) treatment prior to RSV challenge were analyzed.
Results
RSV infection causes differential effects on alveolar macrophages in RSV pre-immune mice
To investigate cellular phenotypes and cellularity in the protection and pathogenesis of vaccine-enhanced RSV disease (ERD), mice (n=10) were i.m. immunized with FI-RSV or i.n. inoculated with RSV (1 × 106 PFU). Both FI-RSV immunization and live RSV infection induced similar levels of serum IgG antibodies specific for RSV (Fig. S1A). Live RSV reinfection but not FI-RSV immunization also raised IgA antibodies in bronchoalveolar lavage fluids (BALF) (Fig. S1B). RSV specific antibody responses suggest that FI-RSV immunization and RSV infections were properly performed as expected.
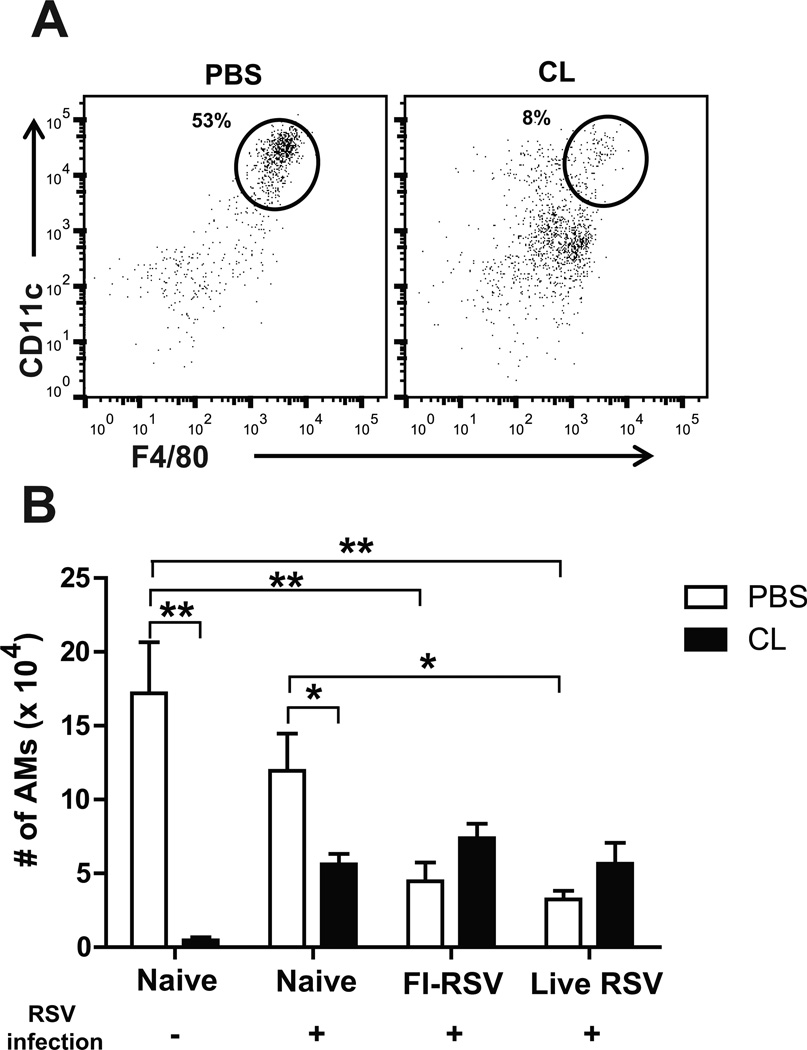
Clodronate liposome (CL) preferentially depletes alveolar macrophages (AMs) without affecting other cell types in the lung as fast as within 4 h post treatment (Thepen et al., 1989; van Rooijen et al., 1997). Mock control mice (no immunization, no infection) were sacrificed to determine the efficacy of depleting AMs in BAL 5 days after CL administration (100µl of 30% CL) (Fig. 1A). The surface markers (CD11c+CD11b−F4/80+) were used to analyze a population of AMs by flow cytometry (Hall et al., 2008). Consistent with a previous study (Pribul et al., 2008), the percentage of F4/80+ AMs was reduced to 8% in CL-treated naïve mice compared to 53% in the untreated mice (Fig. 1A). As a result of CL treatment, the cellularity of AMs was depleted up to 97% in naïve (Fig. 1B), FI-RSV and live RSV immune mice prior to RSV challenge (data not shown), suggesting that AM populations in naïve and RSV immune mice could be depleted by CL administration.
Fig. 1. Depletion of alveolar macrophages by clodronate liposomes.
Bronchoalveolar lavage fluids (BALF) were performed to collect immune cells by passing through PBS through the trachea after sacrificing the mice day 5 post-challenge (n=5). (A) CD11c, CD11b, and F4/80 antibodies for flow cytometry were used to analyze the depletion of alveolar macrophages (AMs). (B) Total numbers of AMs in BAL fluids were analyzed in each group of mice. CL (n=5): Intranasal treatment with 30% of CL (100µl) 4 hours prior to RSV infection. The data are representative out of two independent experiments with reproducible results. PBS: PBS control groups (instead of CL). Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. *, P<0.05; **, P<0.01.
Interestingly, RSV-infected naïve mice showed a lower level of AM cellularity (~ 12 × 104/mouse) compared to uninfected naive mice (~ 17 × 104/mouse) (Fig. 1B). FI-RSV and live RSV immune mice showed a significantly lower level (~ 4.5 × 104/mouse and ~3.2 × 104/mouse, respectively) of AMs in BAL upon RSV challenge compared to those in infected naïve mice (~ 12 × 104/mouse, Naïve, Fig. 1B). CL treatment 4 h prior to RSV infection of naïve mice resulted in approximately 50% reduction in AM cellularity (~ 5.6 × 104/mouse Fig. 1B). Paradoxically, FI-RSV and live RSV immune mice showed moderate increases in AM cellularity (~ 7.5 × 104/mouse and ~ 5.7 × 104/mouse, respectively) rather than depletion as a result of CL treatment (Fig. 1B). It is an unexpected finding that the FI-RSV and live RSV groups with CL treatment showed a trend of increasing the AM population after RSV challenge. Taken together, when RSV challenge was followed, the AM populations in the airways of FI-RSV immune and live RSV reinfection mice were significantly lower than those in naïve mice with RSV infection.
Neutrophils are highly infiltrated into the airways of FI-RSV immune mice
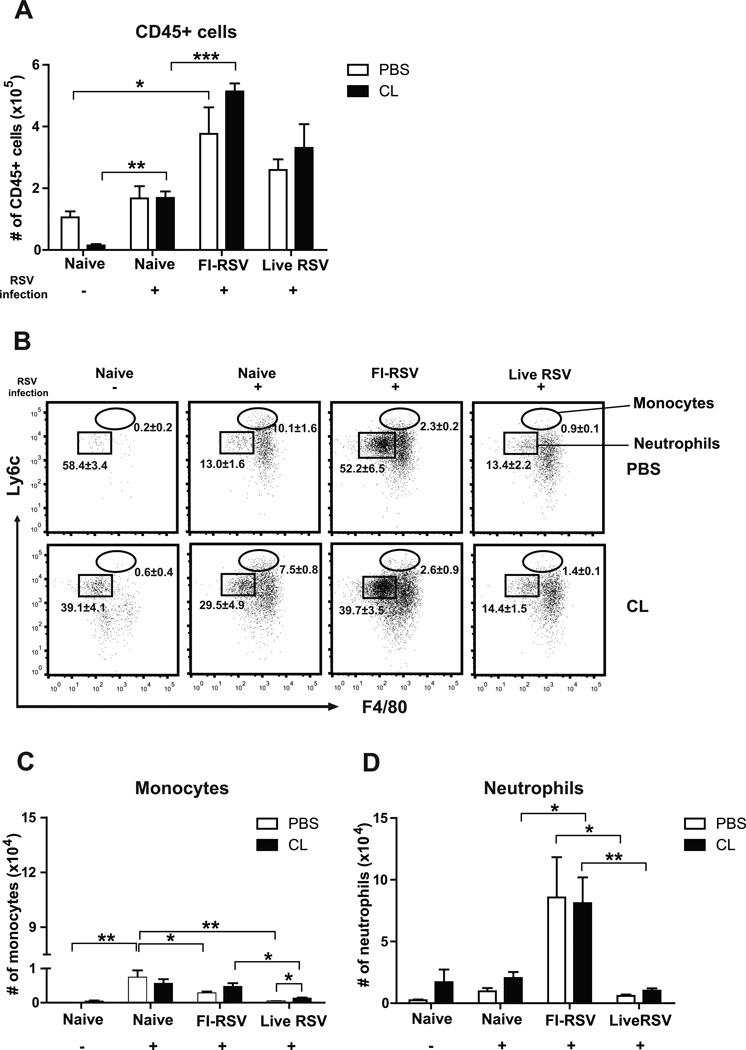
In contrast to low cellularity of CD45+ cells in uninfected naïve mice, CD45+ leukocytes were significantly increased to the highest levels in the airways of FI-RSV immune mice, followed by live RSV reinfection mice day 5 after RSV challenge, and AM-depleted naïve uninfected mice lowered CD45+ cells but not in other groups (Fig. 2A). Monocytes (CD11b+Ly6chiF4/80+) and neutrophils (CD11b+Ly6c+F4/80−) were determined based on their surface markers as described (Daley et al., 2008). The percentage and number of monocytes were found to be highest in the airways of naïve mice after RSV infection and substantial levels in the FI-RSV group (Fig. 2B and C). It is noticeable that monocytes were lowest in the live RSV group and increased after AM depletion (Fig. 2B and C). In contrast, naïve mice showed the highest levels of monocytes after RSV infection but the scale (×103) is approximately 10 to 100 fold lower than that (×105) of CD45+ leukocytes (Fig. 2C). Most significantly, percentage and cellularity of neutrophils was found to be highest in the FI-RSV group, low in other naïve or live RSV groups (Fig. 2C). Thus, these results suggest that high levels of neutrophils recruited into the airways of FI-RSV immune mice might have contributed to inflammatory lung disease (Fig. 2B and D).
Fig. 2. FI-RSV immune mice recruit CD45+ leukocytes and neutrophils into the airways upon RSV infection.
The cells from the airways (BALF) of individual mice (n=5 per group) were collected 5 days after RSV challenges. Distinct cell subsets were analyzed using specific markers such as CD45, CD11c, CD11b, F4/80 and Ly6c. Total numbers of (A) CD45+ leukocytes were analyzed in BAL fluids, (B) The percentages of monocytes (CD11b+Ly6chiF4/80+) and neutrophils (CD11b+Ly6c+F4/80−) were shown on flow cytometry plots. The absolute numbers of (C) monocytes and (D) neutrophils were analyzed in BAL fluids. The data were reproducible with two independent experiments. Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. *, P<0.05; **, P<0.01; ***, P<0.001.
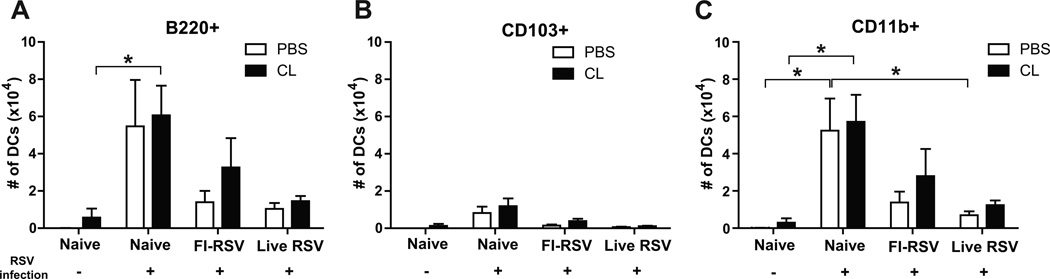
CD11b+ and plasmacytoid dendritic cells are significantly recruited into the lungs of mice with FI-RSV immunization and RSV infection
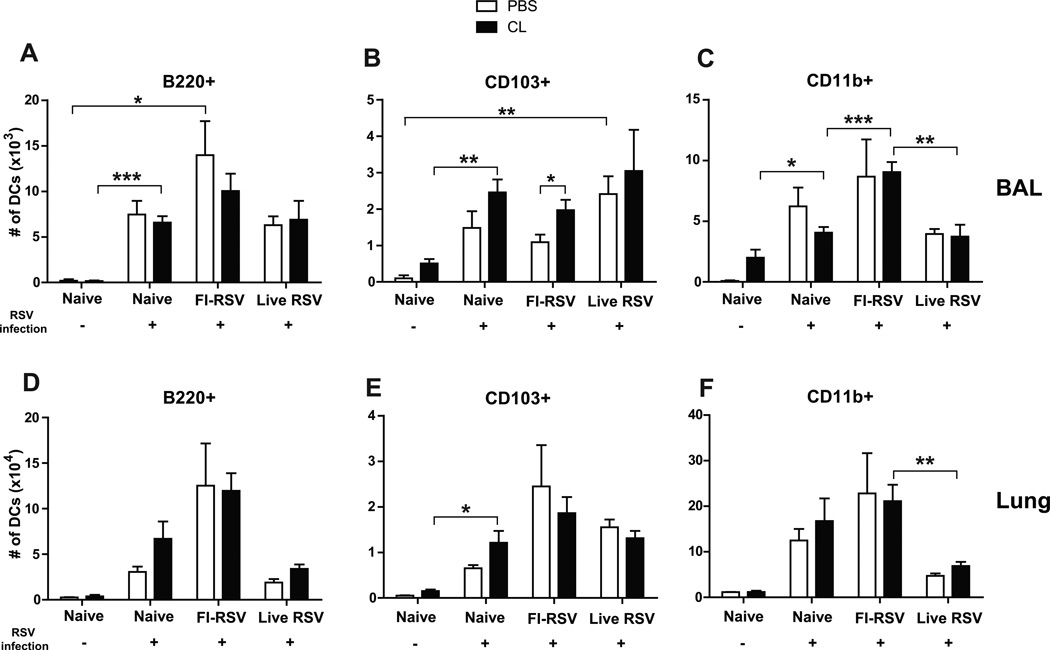
The markers such as CD11c, CD11b, CD103, B220, and MHCII for flow cytometry were used to separate different dendritic cells (DCs) such as CD11c+CD11b+ DCs, CD11c+CD103+ DCs, and CD11c+B220+ DCs (Beaty et al., 2007; Sung et al., 2006). In contrast to high cellularity of AMs, uninfected naïve mice did not show DCs in lungs (Fig. 3). After RSV infection, different types of dendritic cells (DCs) were differentially and significantly recruited into the airways and lungs of naïve unimmunized mice or immune mice with FI-RSV immunization or RSV reinfections (Fig. 3). Plasmacytoid DCs (pDCs, B220+CD11c+F4/80−CD45+) were observed at higher levels in the airways and lungs of FI-RSV immune mice than those in the naïve and live RSV groups day 5 post RSV challenge (Fig. 3A and D, respectively). As a result of AM depletion, an increase in lung pDCs was observed in naïve mice with RSV infection but there was no statistical significance (Fig. 3D). CD103+ DCs (CD103+CD11c+F4/80−CD45+) were observed in the airways of the live RSV group relatively at high levels (Fig. 3B). There was a moderate increase of CD103+ DCs in the airways of the naïve and FI-RSV groups with CL treatment upon RSV infection (Fig. 3B). CD11b+ DCs (CD11b+CD11c+F4/80−CD45+) were observed at highest levels in the airways and lungs of the FI-RSV group, and at substantial levels in naïve mice upon RSV infection compared to those in the live RSV reinfection group (Fig. 3C and F). Analysis of DCs in the airways and lungs suggest that high cellular infiltrations of B220+ pDCs and CD11b+ DCs are likely contributing to FI-RSV vaccination-associated pulmonary disease.
Fig. 3. Plasmacytoid and CD11b+ dendritic cells are highly recruited into the lungs after FI-RSV immunization and RSV infection.
The BALF and lung tissues from individual mice (n=5) were collected 5 days after RSV infection of immune mice. Distinct dendritic cell subsets were separated by using the surface markers CD45, CD11c, CD11b, B220, and F4/80. (A and D) Plasmacytoid DCs (pDCs, B220+CD11c+F4/80− CD45+), (B and E) CD103+ DCs (CD103+CD11c+F4/80−CD45+), and (C and F) CD11b+ DCs (CD11b+CD11c+F4/80−CD45+) were analyzed in the BALF (top graphs, A–C) and lungs (bottom graphs, D-F). Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. *, P<0.05; **, P<0.01; ***, P<0.001.
FI-RSV immune mice show significant body weight loss upon RSV infection
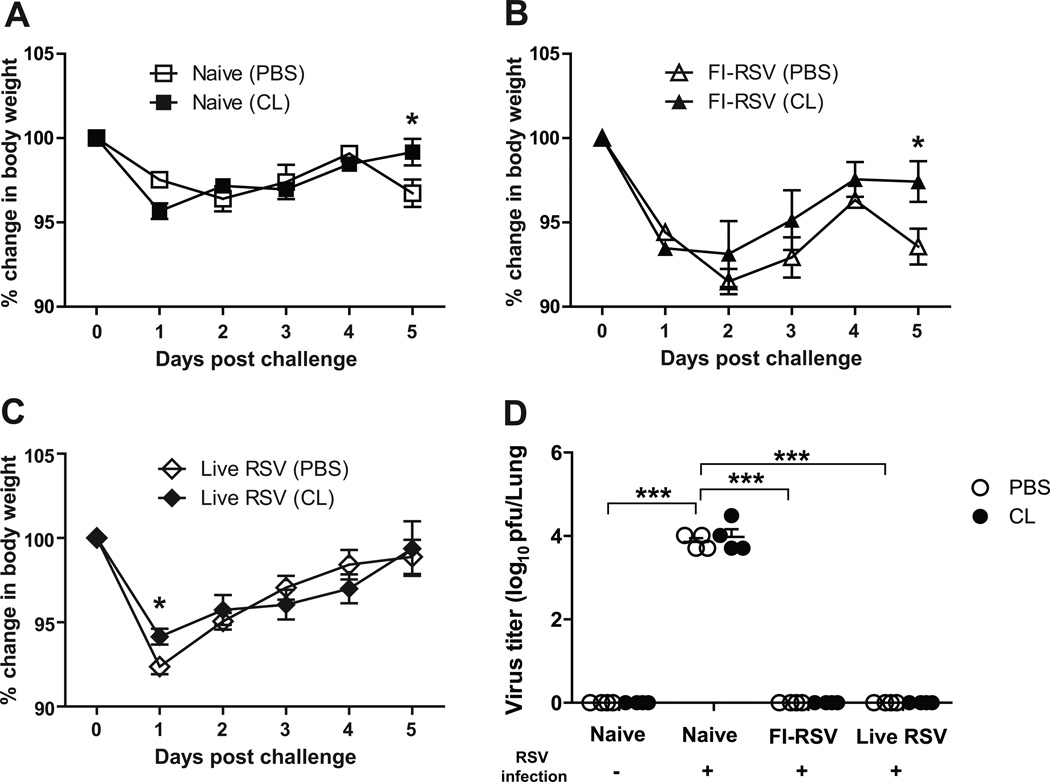
To elucidate the possible effects of AM depletion on body weight changes of FI-RSV immunized or live RSV re-infected mice after RSV challenge (2 × 106 PFU), body weights of mice were monitored (Fig. 4). The unimmunized naive group challenged with RSV displayed approximately 2.5% weight loss in PBS-treated mice and AM-depleted naïve mice showed a better recovery in body weight than PBS-treated mice day 5 post RSV infection (Fig. 4A). The FI-RSV group revealed prominent weight loss (approximately 9%) until day 2 post challenge compared to the naive or live RSV groups (Fig. 4B). The CL-treated FI-RSV group displayed a pattern of progressive weight recovery from day 2 post challenge. In contrast, PBS-treated FI-RSV immunized mice revealed a second wave of body weight loss day 5 post challenge (Fig. 4B). The live RSV group showed an early loss in body weight loss and a progressive recovery although PBS-treated live RSV mice showed more weight loss (approximately 7%) day 1 post RSV re-infection (Fig. 4C). These results suggest that FI-RSV immunization and live RSV infection cause significant illness of weight loss respectively upon RSV challenge. An increase in AMs and CD103+ DC populations as a result of CL treatment prior to RSV infection might have protective effects on preventing severe weight loss in the FI-RSV immune mice.
Fig. 4. Live RSV and FI-RSV immune mice show differential body weight loss after RSV infection.
PBS or CL-treated mice (n=5) were challenged with RSV (2 × 106 PFU) 6 weeks after boost FI-RSV immunization or live RSV reinfections. Changes in body weight (A–C) were monitored daily for 5 days. (D). RSV titers in lungs. The lung samples harvested day 5 post-infection were used to determine RSV titers using the immune-plaque assay. Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. *, P<0.05.
To determine whether AM depletion influenced lung viral clearance, lung RSV titers were determined day 5 post-challenge. High RSV titers were observed in the lungs of the unimmunized naive group whereas the FI-RSV and live RSV groups cleared RSV titers to a detection limit, and no difference was observed in viral clearance with or without CL treatment (Fig. 4D). Also, there was no difference in IgA antibody responses between PBS- and CL-treated live RSV mice (Fig. S2).
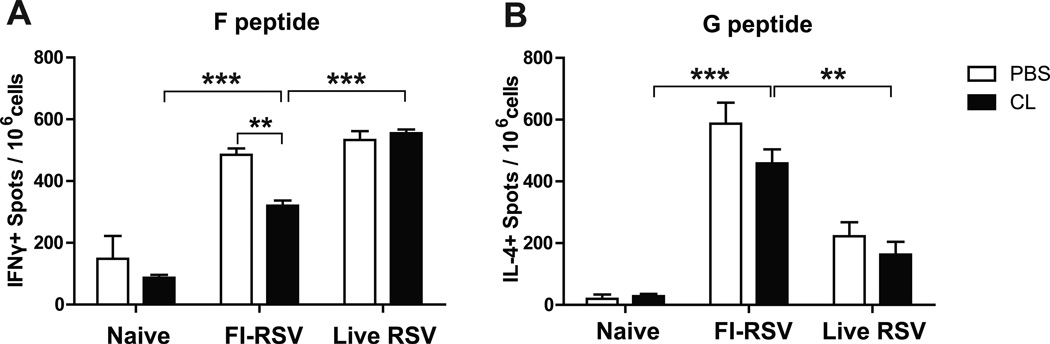
FI-RSV immunized mice show G peptide-specific CD4+ T cells producing IL-4 cytokine
It has been shown that antigen-specific T cells play dual roles in both protection and immunopathology during RSV infection (Olson and Varga, 2008). To investigate whether AM population would modulate IFN-Γ-producing T cell responses, lung T cells from unimmunized naïve and immunized mice day 5 post-challenge were stimulated with the synthetic F85–93 (KYKNAVTEL) (Chang et al., 2001) and G183–195 (WAICKRIPNKKPG) (Varga et al., 2000) peptides specific for CD8 and CD4, respectively. High numbers of IFN-Γ positive spots with stimulation of F peptides were similarly detected in the FI-RSV and live RSV groups (Fig. 5A). CL-treatment resulted in reduced numbers of IFN-Γ-producing cell spots specific for F peptides in FI-RSV immunized mice, which might be associated with a better recovery in weight loss (Fig. 5A). In addition, total numbers of IL-4+ spots with stimulation of G peptides in FI-RSV immunized mice were observed at significantly higher levels than those in unimmunized naïve and live RSV infected mice (Fig. 5B). These results suggest that high levels of RSV G specific IL-4 producing CD4+ T cells contribute to vaccine-enhanced disease in FI-RSV immune mice upon RSV infection.
Fig. 5. FI-RSV immune mice show high levels of RSV-G specific T cells producing IL-4 cytokine in the lungs after RSV infection.
Lung cells harvested 5 days after RSV infection were stimulated in the presence of (A) RSV F85–93 (KYKNAVTEL) peptide or (B) RSV G183–195 (WAICKRIPNKKPG) peptide. Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals (n=5). **, P<0.01; ***, P<0.001.
DC migration into the draining lymph nodes is correlated with lung viral loads
To further investigate whether respiratory DC migration to draining lymph nodes (dLNs) would be correlated with viral loads and influenced by AM population, the numbers of migratory lung DCs were analyzed in the mediastinal lymph node (MLN) of immunized mice 5 days after RSV challenge. There were no significant differences in PBS- and CL-treated mice despite a trend of increasing DC migration into MLN in CL-treated FI-RSV immunized mice (Fig. 6). Cellularity of CD103+ DCs, CD11b+ DCs, and B220+ pDCs in the MLNs was observed at significantly higher levels in naïve mice after RSV infection than those in the FI-RSV and live RSV groups, which is different from a pattern of various DC populations in the airways and lungs (Fig. 3). These results suggest a significant correlation between lung viral loads and various subsets of DC migration into MLN.
Fig. 6. Naïve mice have high levels of respiratory DC migration to the draining lymph nodes after RSV infection.
Distinct subsets of dendritic cells were analyzed in the MLN 5 days after RSV infection using specific markers such as CD11c, CD11b and CD103. (A) Plasmacytoid DCs (pDCs, B220+CD11c+F4/80−CD45+), (B) CD103+ DCs (CD103+CD11c+F4/80−CD45+), and (C) CD11b+ DCs (CD11b+CD11c+F4/80−CD45+) were analyzed in the MLN from individual mice (n=5). Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. *, P<0.05.
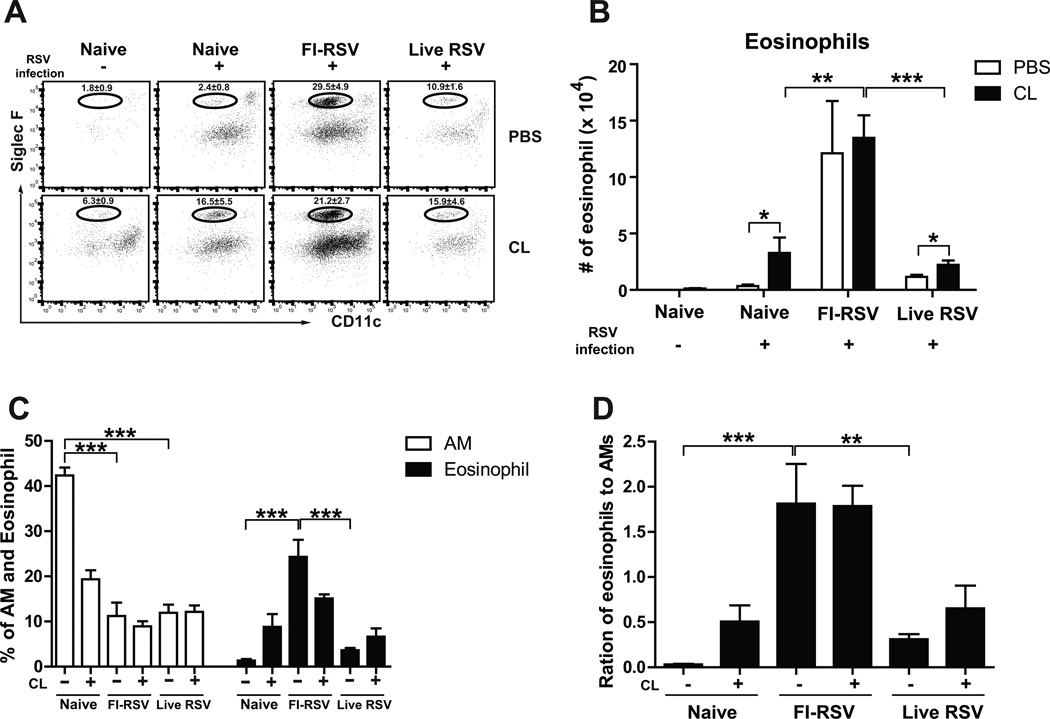
FI-RSV immune mice show extensive eosinophil infiltration in lungs upon RSV infection
We analyzed the possible effects of AMs on eosinophil infiltration into the airways using eosinophil markers (CD11b+CD11c−SiglecF+) (Stevens et al., 2007). Interestingly, depletion of AMs in the airways of naïve mice resulted in approximately a four-fold increase in eosinophils 5 days after RSV infection (Fig. 7A and B). The live RSV group with CL treatment showed a moderate increase in the cellularity of eosinophils in the airways (Fig. 7B). Eosinophils were observed at the highest level in the FI-RSV group compared to those of unimmunized and live RSV immunized groups (Fig. 7B and C, respectively). Furthermore, a ratio of eosinophils relative to AMs in the airways of FI-RSV immunized group was higher (approximately 1.8 fold) than those in unimmunized and live RSV immunized groups (Fig. 7D). These results suggest that an unbalance in inducing high levels of eosinophils and decreasing AMs plays a role in causing severe pulmonary inflammation upon RSV infection.
Fig. 7. FI-RSV immune mice have high eosinophil infiltration into the airways after RSV infection.
The cells from the airways of individual mice (n=5) were collected 5 days after RSV infection. Eosinophils were analyzed using specific markers such as CD11c, CD11b, F4/80, and siglec F. (A) Frequencies of eosinophils. (B) Eosinophil cellularity. (C) The percentages of eosinophils and AMs. (D) Ratios of eosinophils relative to AMs. Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. *, P<0.05; **, P<0.01; ***, P<0.001.
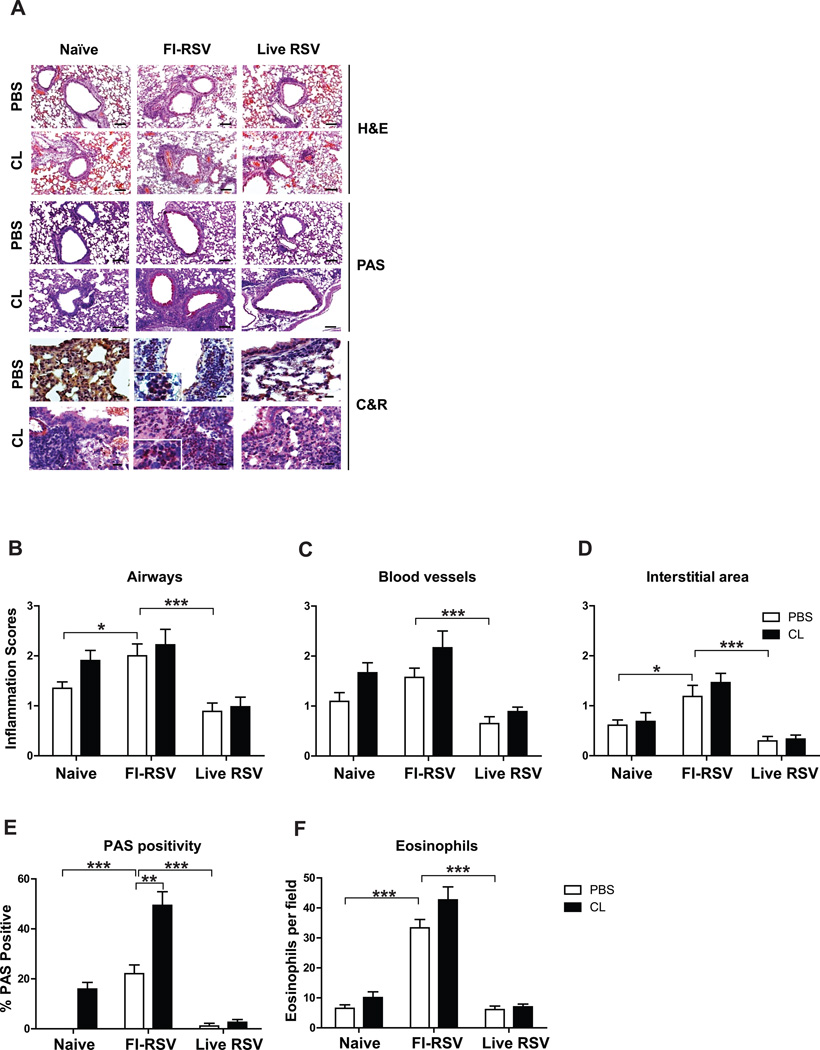
FI-RSV immunization causes severe pulmonary inflammation and mucus production upon RSV infection
To further understand the possible effects of CL treatment on pulmonary histopathology, histological analysis was performed in the lung tissue sections day 5 post challenge. In the analysis of H&E staining, leukocyte infiltrates and histopathological inflammation were observed at the highest levels in the lung tissues from FI-RSV immune mice (Fig. 8A). Histopathological scores showed an increasing trend around the airways, blood vessels and interstitial area of FI-RSV immune mice but there was no significant difference as a result of CL treatment prior to RSV infection (Fig. 8B, C and D, respectively). In particular, highest levels of PAS positivity and eosinophils were detected in FI-RSV immune mice and CL treatment resulted in a further increase in PAS positivity, indicating more mucus production (Fig. 8E and F, respectively). Importantly, as a result of AM depletion, naïve mice showed a pattern of increasing inflammation in the airways, blood vessels and PAS positivity after RSV challenge (Fig. 8B, C and E). These data implicate that FI-RSV immunization causes severe pulmonary inflammation and mucus production and that AM population plays a differential role in modulating pulmonary inflammation in nave and immune mice upon RSV infection.
Fig. 8. FI-RSV immunization causes severe histopathology, mucus production, and eosinophilia.
The lung tissues harvested 5 days after RSV infection were fixed with 10% neutral buffered formalin solution. (A) The tissues were embedded in paraffin and 5µm sections were stained with hematoxylin and eosin (H&E), PAS, and congo red (C&R). Histophathology scores were determined in the airways (B), blood vessels (C), and interstitial area (D). PAS positivity (E) and eosinophil infiltration (F) were determined in stained lung sections. Photographs were taken at a magnification of 5 and 20 and scale bars indicate 100µm. The data were reproducible with two independent experiments (n=3–5). Statistical significance was determined using one-way ANOVA or an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. *, P<0.05; **, P<0.01.
Discussion
A better understanding of vaccine-enhanced RSV disease is expected to provide insights into developing a safe vaccine against RSV. However, cellular phenotypes and cellularity in the protection and pulmonary RSV disease after vaccination are poorly understood. In this study, we have focused on comparative analysis of cellular phenotypes in the airways (BAL) and lungs of mice that were under different immune status (naïve, naïve+RSV, FI-RSV+RSV, live RSV reinfections+RSV). We found highly dynamics in cellular phenotypes and cellularity, which were differentially modulated depending on the pre-existing immune status of mice with immunization and infections. AMs were found to be high in naïve mice, but low in naïve mice after RSV infection, and further reduced in FI-RSV or live RSV mice. The airways of FI-RSV immune mice were found to contain the highest levels of CD45+ leukocyte cellularity, neutrophils, and eosinophils as well as plasmacytoid and CD11b+ conventional DCs. Our results suggest that multiple granulocytes and DCs as well as RSV specific CD4+ T cells infiltrated into the lung of FI-RSV immune mice at high levels might have contributed to vaccine-enhanced pulmonary inflammation.
RSV infection resulted in differential effects on lowering AM populations in the airways of mice depending on the pre-existing immune status. Infection of naïve mice with RSV led to a moderate decrease in AMs in the airways. Interestingly, upon RSV infection, the cellularity of AMs in the airways of mice with previous FI-RSV immunization or live RSV reinfection was found to be further reduced to approximately 30% of those in naïve mice with RSV infection. It is unclear how pre-existing RSV immunity leads to significant decreases in AMs in the airways of immune mice compared to those in naïve mice upon RSV challenge infection. Decreases in AM populations were due to RSV infection and not because of immunization since AMs were not affected by immunization and maintained in immune mice at a level similar to that of naïve mice before RSV infection (data not shown). One possibility is that AMs sensing virus and virus-immune complexes are preferentially depleted or moving away from the airways. CL is a well-known chemical in depleting macrophage phenotypic cells at a fast kinetics (Van Rooijen and Sanders, 1994). As a result of CL treatment, AM populations were efficiently depleted in the airways from naïve mice (Fig. 1B). Before RSV challenge, FI-RSV and live RSV immune mice showed high levels of AMs in bronchoalveolar lavage (BAL) fluids, which were found to be sensitive to CL treatment in an independent set of mouse experiments. After RSV challenge, FI-RSV and live RSV immune mice showed significantly lower levels of AMs in BAL fluids even in the absence of CL treatment compared to those of naïve mice with RSV infection. We expected that CL treatment would effectively deplete AM populations in naïve mice regardless of RSV infection. However, it may be possible that intrinsic properties of AMs might have changed to be less sensitive to CL as a result of RSV infection. Alternatively, CL treatment and RSV infection might have induced pulmonary inflammation which resulted in influx of new AM population until day 5.
In naïve mice after RSV infection, AM depletion resulted in an increase in different dendritic cells (lung pDCs, CD103+ DCs), and eosinophils, all or some of which together or independently might have contributed to a better recovery without a second wave of weight loss. Different strains of RSV were also known to influence a differential weight loss pattern (Stokes et al., 2011). Depletion of AMs in naïve mice also resulted in decreases in Th2 cytokines and an increase in chemokine MIP-1β which is chemoattractant for natural killer cells, monocytes, and eosinophils (data not shown). Meanwhile, there was no difference in lung viral clearance in naïve mice with and without AM populations. A previous study reported that early proinflammatory IL-6 and TNF-Α cytokines were markedly reduced in naïve mice with AM depletion during RSV infection (Pribul et al., 2008). Similar to RSV infection, a number of proinflammatory cytokines were shown to be dramatically reduced by depleting AMs prior to influenza virus infection (Tumpey et al., 2005).
As a result of CL treatment, we observed an unexpected outcome of a moderate increase rather than decrease in AM populations in the airways from FI-RSV immune mice after RSV challenge infection. It is not clear yet how RSV infection causes differential effects on the airway AMs in RSV pre-immune mice. In line with this change in AM cellularity in the FI-RSV group, we also found an increase in monocytes, in CD103+ DCs in the airways, and in various respiratory DC migrations (CD103+, CD11b+, B220+) into the draining lymph nodes, and a moderate decrease in airway plasmacytoid DCs. These multicellular changes with CL treatment of the FI-RSV group together with reduced T helper type 2 (Th2) cytokines (data not shown) and lower RSV specific T cell responses might have resulted in a better recovery without experiencing the second wave of weight loss. In line with a finding (Stokes et al., 2011), RSV infection resulted in the differential weight loss in mice vaccinated with FI-RSV after RSV challenge. The early release of cytokines and innate immune cell infiltrates may contribute to the first wave of loss in body weight, but IL-4 or IFN-Γ producing T cells may have impacts on the second wave. However, CL treatment of FI-RSV immune mice resulted in a trend of increasing multiple cells including leukocytes, monocytes, and CD103+ DCs, all of which might have contributed to a moderate increase in histopathology including PAS positive mucus production. Similar or different effects were observed in the live RSV group although these changes were minimal. After CL treatment of the live RSV group, there was a pattern of increasing monocytes and eosinophils in the airways, but cytokine ELISA with BAL fluids revealed a moderate decrease in Th2 cytokines (data not shown). Naïve mice with AM depletion showed a profile of increasing chemokines, eosinophils, pDCs and CD103+ DCs in lungs as well as better recovery in body weight and less inflammation around blood vessels. A balanced and low level of inflammation would have beneficial effects in naïve mice upon infection. In support of this, activated mouse eosinophils were recently shown to protect against lethal respiratory virus (Percopo et al., 2014). The protection and disease outcomes observed in the naïve, FI-RSV, and live RSV groups might need to be differentially interpreted depending on the pre-existing immune conditions. Therefore, it is important to carefully consider changes in multiple cellular phenotypes as well as protection and pathology together.
This study reveals dynamic changes in granulocyte types and cellularity in the airways of the naïve, FI-RSV, and live RSV groups upon RSV challenge compared to naïve mice (no immunization, no infection). (1) AM populations were significantly reduced in the airways of naïve mice with RSV infection, and further reduced in the FI-RSV and live RSV groups. (2) CD45+ leukocyte populations were highest in the FI-RSV group and then followed by live RSV mice with reinfections. (3) Monocytes were recruited at the highest level in the naïve group upon RSV infection and followed by the FI-RSV group. (4) Most prominently, neutrophils and eosinophils as well as IL-4 cytokine secreting T cells were recruited at the highest levels in the FI-RSV group. Our results suggest that induction of Th2 cytokines and infiltration of neutrophils and eosinophils appear to be an independent outcome because CL-mediated reduction of Th2 cytokine production did not influence the recruitment of these cells.
The possible roles of respiratory DCs in the RSV inflammatory disease largely remain unknown although DCs play a central role in the induction of the RSV-specific adaptive immune response. Depletion of pDCs prior to RSV infection was shown to increase pulmonary inflammation and mucus production (Smit et al., 2006; Wang et al., 2006). Also, lung T cells from pDC-depleted mice were demonstrated to secrete more Th2 proinflammatory cytokines (IL-4, IL-5, IL-13) as well as Th1 proinflammatory cytokine IFN-Γ upon in vitro stimulation (Smit et al., 2006). However, our results of differential recruitment of various DCs in the airways and the MLN suggest alternative roles of DCs in the RSV protection and disease. The FI-RSV group recruited pDCs and CD11b+ DCs into the airways and lungs at the highest level whereas the live RSV groups showed a low level of both DC populations. Meanwhile CD103+ DCs were observed at high levels in the airways of live RSV mice. Naïve mice with RSV infection showed a similar level of pDCs and moderately higher levels of CD11b+ DC compared to the live RSV group. In contrast to pDCs and CD11b+ DCs, relatively low levels of CD103+ DCs were observed in the airways of FI-RSV mice compared to live RSV mice. Since the FI-RSV group showed significantly more pulmonary histopathology, high levels of pDCs and CD11b+ DCs together with eosinophils and neutrophils in the lungs might have a role in the progress of FI-RSV vaccine-induced pulmonary inflammation. In contrast, the naïve RSV group showed the highest levels of monocytes into lungs as well as different phenotypic respiratory DCs (CD103+, CD11b+, B220+) migrating into the MLN, exhibiting a correlation with lung viral loads. The FI-RSV group showed high levels of IL-4+ T cell responses stimulated with RSV G peptide compared to those in the live RSV group, which may be associated with an accelerated pulmonary immunopathology as consistent with a previous study using a recombinant RSV vaccine (Bukreyev et al., 2005). A comparative analysis implicates that high cellularity of infiltrates (neutrophils, eosinophils, plasmacytoid and CD11b+ DCs, G-specific T cell responses) seems to be major contributors to FI-RSV vaccination enhanced pulmonary inflammation.
In conclusion, this study demonstrates multiple immune parameters that might be involved in the RSV protection and pulmonary inflammatory disease progression. Lung viral clearance, body weight loss, innate immune cell infiltration, Th1 and Th2 cell responses, and pulmonary inflammation may be interdisciplinary protection and independent disease parameters in mice. Lung viral clearance seems to be mainly dependent on pre-existing RSV specific (humoral) immune responses. Pulmonary inflammation can be observed in mice even with lung viral clearance. AMs, monocytes, neutrophils, eosinophils, and different phenotypic DCs are highly dynamic in the lung microenvironment depending on the status of mice with naïve, first RSV infection, repeated RSV infections, or FI-RSV immunization and RSV infection. High levels of eosinophils and neutrophils as well as certain respiratory DCs (pDCs, CD11b+ DCs) and Th2 cells are likely to be the major contributing cellular parameters to pulmonary inflammation in FI-RSV immune mice after RSV infection. The results from this study provide important observations on possible protective immune cellular correlates inducing protection but also preventing RSV pulmonary disease. This study further provides new insight into developing a safe and effective RSV vaccine which confers protections against RSV inducing protective immune correlates but without causing pulmonary inflammatory disease. A comparison with RSV vaccine candidates would further validate the findings of cellular immune parameters contributing to safe RSV vaccination. According to a previous finding, we found that mice vaccinated with a cocktail vaccine of F DNA and virus-like particles containing RSV fusion and attachment glycoprotein induced low bronchoalveolar cellularity, low levels of granulocytes, no sign of eosinophilia upon RSV challenge, which were similar to those in live RSV-reinfected mice (Ko et al., 2015). This implies that comparing immune cellular correlates between FI-RSV and live RSV reinfection might provide useful parameters to predict protection against RSV and to prevent RSV disease.
Materials and methods
Mice and FI-RSV
Female BALB/c mice aged 6 to 8 weeks were purchased from the Harlan (Indianapolis, IN). In order to obtain a stock of respiratory syncytial virus (type A2 strain), RSV was grown in HEp-2 cells which were cultured in dulbecco’s modified eagle medium (DMEM) containing 2 % fetal bovine serum, penicillin and streptomycin. RSV was then harvested after sonication of infected HEp-2 cells and inactivated with 10% formalin (1:400 vol/vol) as previously described (Quan et al., 2011). Formalin inactivated RSV (FI-RSV) was purified by ultracentrifugation. Inactivated RSV was adsorbed to aluminium hydroxide adjuvant (alum, 4mg/ml) and used for immunization.
Immunization and viral challenge
Female BALB/c mice were intranasally (i.n.) infected with live RSV A2 strain (1 × 106 PFU) or intramuscularly (i.m.) immunized with FI-RSV (2µg) at weeks 0 and 4 (n=10). Immunized mice were anesthetized by isoflurane (Baxter, Deerfield, IL) and i.n. challenged with RSV A2 strain (2 × 106 PFU) in 50 µl of phosphate-buffered saline (PBS) at 6 weeks after boost immunization. Half of the mice in each group (n=5) were i.n. given with 30% of clodronate liposome (CL; Clodronate Liposomes, Netherlands) 4 h prior to RSV infection and 5 days later, depletion of AMs was analyzed by flow cytometry. RSV challenged mice were daily monitored to record body weight changes for 5 days. To compare the efficacy of vaccination, unimmunized naive mice were used as a negative-control group. Experimental data were confirmed by two independent experiments. All animal studies were approved and conducted under the guidelines by Georgia State University’s IACUC (A11026).
Serum preparation and humoral immune responses
The blood samples after prime and boost immunization were collected from naïve mice and immune mice with FI-RSV or live RSV. The specific serum antibody titers against FI-RSV as a coating antigen were determined by enzyme-linked immunosorbent assay (ELISA) as described (Lee et al., 2014; Quan et al., 2007). Briefly, FI-RSV was used to determine the amount of specific antibodies. FI-RSV (4µg/ml) in coating buffer was coated on 96-well microtiter plates (Nunc, Rochester, NY) and incubated for overnight at 4°C. The 96-well ELISA plates were washed with PBS containing 0.05 % Tween 20 (PBST) and 3% BSA in PBST was used for blocking reagents for 90 minutes at 37 °C. The blood samples were serially diluted in PBS and incubated for 90 minutes at 37 °C. Secondary goat anti-mouse IgG (Sourthern Biotech, Birmingham, AL) antibodies conjugated with horseradish peroxidase (HRP) were incubated for 90 minutes and then the tetramethybenzidine (TMB) peroxidase substrate (Sigma-Aldrich, St. Louis, Mo) as used as the a substrate. The optical density (OD) values were measured using optical spectrophotometer reader at 450nm. The total antibody concentration was determined with respect to quantitative standard antibody concentration by using purified mouse IgG and IgA antibodies.
Cell preparations and flow cytometry
Bronchoalveolar lavage fluids (BALF) were harvested using PBS to collect non-adherent cells from the lung airways. After collecting BALF, lung tissues were homogenated and cells were then passed through strainer and spun on 44/67% Percoll gradients at 2800 rpm for 20 minutes. A lung cell band which contained most immune cells was harvested. The frosted glass microscope slides were used to make cell suspensions from the mediastinal lymph nodes (MLN) and spleens. Red blood cells were lysed with ammonium chloride and lymphocytes were filtered through cell strainers. Flow cytometric analysis were carried out using cell surface marker antibodies specific for CD3, CD4, CD8, CD11b, CD11c, CD45, F4/80, Siglec F and Ly6c (eBioscience or BD Pharmingen). The Becton-Dickinson LSR-II/Fortessa flow cytometer (BD, San Diego, CA) was used to analyze distinct populations from the tissues and acquired samples were further analyzed by using Flowjo software (Tree Star Inc.).
Enzyme-linked immunospot (EISPOT) assay
Cytokine secreting cell spots were determined on Multi-screen 96 well plates. Briefly, lymphocytes (5 × 105 cells/well) from lung tissues were cultured in the presence of the synthetic F85–93 (KYKNAVTEL) (Chang et al., 2001) and G183–195 (WAICKRIPNKKPG) (Varga et al., 2000) peptides specific for CD8 and CD4, respectively. Interferon gamma (IFN-Γ) producing cell spots were counted using BioSys ELISpot reader.
RSV immuno-plaque assay
RSV lung viral titers were determined by an immune plaque assay using anti-F monoclonal antibody (Millipore) as described previously (Quan et al., 2011).
Lung histopathology
The lung tissues were harvested from RSV infected animals and fixed with 10% neutral buffered formalin for 48hr. Formalin fixed lung tissues were transferred into 70% ethanol and followed by the routine histology staining processes of hematoxylin and eosin (H&E), periodic acid-Schiff stain (PAS) or hematoxylin and congo red (H&CR) as described (Meyerholz et al., 2009). To analyze pneumonia in enhanced RSV disease model, the inflammation scoring system was applied in the bronchi, blood vessels, and interstitial space as described previously (Melendi et al., 2007).
Statistics
Unless otherwise stated, all results were presented as means ± SEM (standard error of mean). One-ANOVA or an unpaired two-tailed Student’s t test was used for statistical significance for all experiments. We analyzed all data with statistical Prism software (GraphPad software Inc, San Diego, CA). The comparisons that were used to generate P values are indicated by horizontal lines (*P<0.05, ** P<0.01, *** P<0.001).
Supplementary Material
Mice (n = 10) were intramuscularly immunized with FI-RSV (2µg) or intranasally inoculated with live RSV (1 × 106 PFU) at week 0 and boosted at week 4. Immune sera were collected 3 weeks after prime and boost immunization from mice. (A and B) RSV specific IgG and IgA levels were analyzed by ELISA using FI-RSV as a coating antigen. Total concentrations of IgG (µg/ml) and IgA (ng/ml) antibodies against FI-RSV were also determined. Statistical significance was determined using an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. ***, P<0.001.
Bronchoalveolar lavage fluids (BALF) were used to determine mucosal antibody levels 5 days after RSV infection. RSV-specific IgG and IgA levels were analyzed by ELISA against using FI-RSV as a coating antigen.
Research highlights.
FI-RSV immunization induced infiltration of eosinophils and neutrophils in the lungs.
Subsets of dendritic cells were found to contribute to RSV vaccine-enhanced disease.
RSV G specific IL-4+ CD4+ T cells were found to be an important factor in disease.
Alveolar macrophages differentially modulated inflammation and protection.
Acknowledgments
Funding
This work was supported by the National Institute of Allergy and Infectious Disease [NIH NIAID grants AI105170, AI093772, AI119366 to SMK].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no commercial conflicts of interest.
References
- Beaty SR, Rose CE, Jr, Sung SS. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. Journal of immunology. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. Journal of immunology. 1991;147:4307–4312. [PubMed] [Google Scholar]
- Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, 3rd, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infection and immunity. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A, Belyakov IM, Prince GA, Yim KC, Harris KK, Berzofsky JA, Collins PL. Expression of interleukin-4 by recombinant respiratory syncytial virus is associated with accelerated inflammation and a nonfunctional cytotoxic T-lymphocyte response following primary infection but not following challenge with wild-type virus. Journal of virology. 2005;79:9515–9526. doi: 10.1128/JVI.79.15.9515-9526.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilow EM, Legge KL, Varga SM. Cutting edge: Eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. Journal of immunology. 2008;181:6692–6696. doi: 10.4049/jimmunol.181.10.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Srikiatkhachorn A, Braciale TJ. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J Immunol. 2001;167:4254–4260. doi: 10.4049/jimmunol.167.8.4254. [DOI] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nature immunology. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of leukocyte biology. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie. 2007;89:843–855. doi: 10.1016/j.biochi.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritzka M, Makris S, Kausar F, Durant LR, Pereira C, Kumagai Y, Culley FJ, Mack M, Akira S, Johansson C. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. The Journal of experimental medicine. 2015;212:699–714. doi: 10.1084/jem.20140825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infection and immunity. 2008;76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG. Antigen presentation in the lung. American journal of respiratory and critical care medicine. 2000;162:S151–S156. doi: 10.1164/ajrccm.162.supplement_3.15tac2. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. Journal of immunology. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Graham BS. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 1999;73:8485–8495. doi: 10.1128/jvi.73.10.8485-8495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TR, Parker RA, Johnson JE, Graham BS. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 2003;170:2037–2045. doi: 10.4049/jimmunol.170.4.2037. [DOI] [PubMed] [Google Scholar]
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969a;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969b;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Ko EJ, Kwon YM, Lee JS, Hwang HS, Yoo SE, Lee YN, Lee YT, Kim MC, Cho MK, Lee YR, Quan FS, Song JM, Lee S, Moore ML, Kang SM. Virus-like nanoparticle and DNA vaccination confers protection against respiratory syncytial virus by modulating innate and adaptive immune cells. Nanomedicine : nanotechnology, biology, and medicine. 2015;11:99–108. doi: 10.1016/j.nano.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annual review of immunology. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kwon YM, Hwang HS, Lee YN, Ko EJ, Yoo SE, Kim MC, Kim KH, Cho MK, Lee YT, Lee YR, Quan FS, Kang SM. Baculovirus-expressed virus-like particle vaccine in combination with DNA encoding the fusion protein confers protection against respiratory syncytial virus. Vaccine. 2014;32:5866–5874. doi: 10.1016/j.vaccine.2014.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Juffermans NP, Florquin S, van Rooijen N, Vervoordeldonk MJ, Verbon A, van Deventer SJ, van der Poll T. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. Journal of immunology. 2001;166:4604–4611. doi: 10.4049/jimmunol.166.7.4604. [DOI] [PubMed] [Google Scholar]
- Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, van Bleek GM. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. Journal of virology. 2009;83:7235–7243. doi: 10.1128/JVI.00452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendi GA, Hoffman SJ, Karron RA, Irusta PM, Laham FR, Humbles A, Schofield B, Pan CH, Rabold R, Thumar B, Thumar A, Gerard NP, Mitzner W, Barnum SR, Gerard C, Kleeberger SR, Polack FP. C5 modulates airway hyperreactivity and pulmonary eosinophilia during enhanced respiratory syncytial virus disease by decreasing C3a receptor expression. Journal of virology. 2007;81:991–999. doi: 10.1128/JVI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicologic pathology. 2009;37:249–255. doi: 10.1177/0192623308329342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Varga SM. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert review of vaccines. 2008;7:1239–1255. doi: 10.1586/14760584.7.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuska JR, Merolla R, Rebert NA, Hoffmann SP, Tsivitse P, Cirino NM, Silverman RH, Rankin JA. Respiratory syncytial virus induces interleukin-10 by human alveolar macrophages. Suppression of early cytokine production and implications for incomplete immunity. The Journal of clinical investigation. 1995;96:2445–2453. doi: 10.1172/JCI118302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, Lee JJ, Lee NA, Domachowske JB, Rosenberg HF. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123:743–752. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribul PK, Harker J, Wang B, Wang H, Tregoning JS, Schwarze J, Openshaw PJ. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. Journal of virology. 2008;82:4441–4448. doi: 10.1128/JVI.02541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. Journal of virology. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. Virus like particle vaccine induces protection against respiratory syncytial virus infection in mice. The Journal of infectious diseases. 2011;204:987–995. doi: 10.1093/infdis/jir474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudraraju R, Jones BG, Sealy R, Surman SL, Hurwitz JL. Respiratory syncytial virus: current progress in vaccine development. Viruses. 2013;5:577–594. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. The American review of respiratory disease. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. The Journal of experimental medicine. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. Journal of immunological methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS, Jr, Moore ML. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. Journal of virology. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott EJ, Taylor G. Respiratory syncytial virus. Brief review. Archives of virology. 1985;84:1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. Journal of immunology. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, Otsuka H, Hijikata A, Watanabe T, Ohara O, Kaisho T, Malissen B. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. The Journal of experimental medicine. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. Journal of virology. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends in biotechnology. 1997;15:178–185. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. Journal of immunological methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Varga SM, Wissinger EL, Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. Journal of immunology. 2000;165:6487–6495. doi: 10.4049/jimmunol.165.11.6487. [DOI] [PubMed] [Google Scholar]
- Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. Journal of immunology. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mice (n = 10) were intramuscularly immunized with FI-RSV (2µg) or intranasally inoculated with live RSV (1 × 106 PFU) at week 0 and boosted at week 4. Immune sera were collected 3 weeks after prime and boost immunization from mice. (A and B) RSV specific IgG and IgA levels were analyzed by ELISA using FI-RSV as a coating antigen. Total concentrations of IgG (µg/ml) and IgA (ng/ml) antibodies against FI-RSV were also determined. Statistical significance was determined using an unpaired two-tailed Student’s t test. Error bars are means ± SEM of concentration or ratios from individual animals. ***, P<0.001.
Bronchoalveolar lavage fluids (BALF) were used to determine mucosal antibody levels 5 days after RSV infection. RSV-specific IgG and IgA levels were analyzed by ELISA against using FI-RSV as a coating antigen.