Abstract
Functional magnetic resonance imaging (fMRI) was employed to examine the effects of a study task manipulation on pre-stimulus activity in the hippocampus predictive of later successful recollection. Eighteen young participants were scanned while making either animacy or syllable judgments on visually presented study words. Cues presented before each word denoted which judgment should be made. Following the study phase, a surprise recognition memory test was administered in which each test item had to be endorsed as ‘Remembered’, ‘Known’ or ‘New’. As expected, ‘deep’ animacy judgments led to better memory for study items than did ‘shallow’ syllable judgments. In both study tasks, pre-stimulus subsequent recollection effects were evident in the interval between the cue and the study item in bilateral anterior hippocampus. However, the direction of the effects differed according to the study task: whereas pre-stimulus hippocampal activity on animacy trials was greater for later recollected items than items judged old on the basis of familiarity (replicating prior findings), these effects reversed for syllable trials. We propose that the direction of pre-stimulus hippocampal subsequent memory effects depends on whether an optimal pre-stimulus task set facilitates study processing that is conducive or unconducive to the formation of contextually rich episodic memories.
Keywords: Encoding, Episodic Memory, fMRI, Preparation, Recollection
Functional neuroimaging studies of episodic memory encoding have often employed the subsequent memory procedure, when electrophysiological or fMRI BOLD activity associated with a series of study events is segregated and contrasted according to whether the events go on to receive accurate as opposed to inaccurate judgments on a later memory test (for reviews, see Paller and Wagner, 2002; Kim, 2011; Rugg et al., in press). Whereas most fMRI studies of episodic encoding that have employed the subsequent memory procedure have focused on neural activity elicited by study items (‘post-stimulus’ activity), a few have used the procedure to examine pre-stimulus activity, that is, neural activity leading up to item onset (Adcock et al., 2006; Mackiewicz et al, 2006; Wittman et al., 2007; Park and Rugg, 2010; Yoo et al, 2012; Addante et al., 2015). A consistent finding from these studies is that ‘pre-stimulus subsequent memory effects’ are evident within the medial temporal lobe (MTL), especially the hippocampus (Adcock et al., 2006, Park and Rugg, 2010, Addante et al., 2015; see also Mackiewicz et al, 2006). With one exception (Yoo et al., 2012), to which we return in the discussion, MTL pre-stimulus subsequent memory effects have taken the form of greater activity for items that went on to be remembered (or recollected, see below) than for items that were forgotten (or judged as familiar only) on the subsequent memory test.
In several of the studies in which pre-stimulus subsequent memory effects were reported, encoding was intentional (Adcock et al., 2006; Mackiewicz et al, 2006; Wittman et al., 2007; Addante et al., 2015), raising the possibility that the effects depend upon the engagement of a deliberate encoding strategy. By contrast, Park and Rugg (2010) employed an incidental study task (pleasantness judgment) and, in addition, tested memory with the ‘Remember/Know’ procedure, permitting study items to be segregated according to whether they were later recollected or judged old solely on the basis of familiarity (Tulving, 1985; Gardiner and Richardson-Klavehn, 2000; Yonelinas, 2002). The critical experimental manipulation in Park and Rugg’s (2010) study was item modality; items (words) were presented in an unpredictable sequence either visually or auditorily, with the modality of each item signaled by a pre-stimulus cue. Recollection on the later memory test was markedly superior for the study items presented visually. Regardless of modality, however, it was reported that cue-related (pre-stimulus) activity in left anterior and right posterior hippocampus was greater for later recollected study items than it was for items that were endorsed as familiar only. In addition, pre-stimulus activity preceding auditory items was enhanced for items subsequently endorsed as recollected rather than familiar in right middle hippocampus.
On the basis of these findings, Park and Rugg (2010) concluded that pre-stimulus hippocampal subsequent memory effects can occur even when there is no intention or motivation to learn (cf. Adcock et al., 2006). They further suggested that the effects are associated with encoding processes that selectively support recollection- rather than familiarity-based recognition judgments, consistent with the wealth of other evidence indicating that recollection is more dependent upon the hippocampus than is familiarity (see Eichenbaum et al., 2007; Montaldi and Mayes, 2010, for reviews). Park and Rugg (2010) proposed that pre-stimulus hippocampal subsequent memory effects reflect the benefit to encoding that comes from the adoption of an optimal preparatory or ‘task’ set in anticipation of a study event, citing as support for this proposal their finding that study reaction times (RTs) were some 100 ms faster for items that were later recollected compared to items later judged familiar or missed. It should be noted that the authors left open the extent to which any such ‘set’ might be specific to each of the two study modalities, or might reflect a more general preparatory state. Park and Rugg (2010) further conjectured that the additional effects identified for auditory compared to visual study trials reflected the fact that optimal pre-stimulus preparation was particularly important in the auditory study condition, which was markedly the more difficult (as indexed by speed of the study judgments) of the two conditions.
The present experiment builds on that of Park and Rugg (2010) to further elucidate the functional significance of pre-stimulus hippocampal subsequent memory effects. Here, instead of varying the modality of the study items, we held modality constant and varied the study task, employing a pre-stimulus cue to inform participants of the task to be performed on each upcoming study item. As in Park and Rugg (2010) one of the tasks encouraged semantically oriented processing of the study item (animacy judgment). The alternate task, syllable judgment, required a non-semantic judgment. Importantly, in addition to giving rise to lower subsequent memory performance, syllable judgment is considerably more difficult than is judgment of animacy, as indexed by RT measures (e.g., Otten and Rugg, 2001; Otten et al., 2002; Park et al., 2008). Hence, according to Rugg and Park’s (2010) proposal (see above), syllable judgment should have more to gain from the adoption of an optimal task set. For this reason, we predicted that while pre-stimulus hippocampal subsequent memory effects would be evident for both tasks (replicating our prior findings), they would be larger, or evident in more regions of the hippocampus, in the syllable task. We also expected that this task would be associated with lower levels of subsequent recollection than in the animacy task, consistent with prior findings (e.g., Otten and Rugg, 2001; Otten et al., 2002; Park et al., 2008).
Eighteen young adults (nine male), aged between 18 and 21 years, participated in the experiment. Data collected from two additional participants were excluded – in one case because of insufficient trials in one of the critical conditions and in the other because of an abnormal structural scan. All participants were healthy, right-handed fluent English speakers with no self-reported history of neurological or psychiatric disease. The study was approved by the Institutional Review Board of the University of California, Irvine. Informed consent was obtained from each participant before proceeding with the experiment. Participants were remunerated.
Critical items comprised 378 words. The words denoted common objects (50% animate and 50% inanimate) and ranged in length from 2 to 12 letters. From the pool of stimulus words, 126 depicting animate objects and 126 depicting inanimate objects were randomly chosen for each participant to make up the study lists. The 252 words were divided into 4 lists of 63 words each, with an additional buffer word at the start of each list. Items within the study lists were pseudo-randomly ordered for each participant such that the same word type (animate or inanimate) or task type (animacy or syllable task – see below) did not occur more than three times in succession. The test list comprised the 252 critical words presented at study and the remaining 126 words from the initial word pool which served as new items. An additional buffer word was presented at the start of the test list. Experimental items within the test list were pseudo-randomly ordered for each participant such that the same class of test item (old items subjected to the syllable task at study, old items subjected to the animacy task at study and new items) did not occur more than three times in succession. Practice study and test lists were formed from items additional to those used to create the experimental lists.
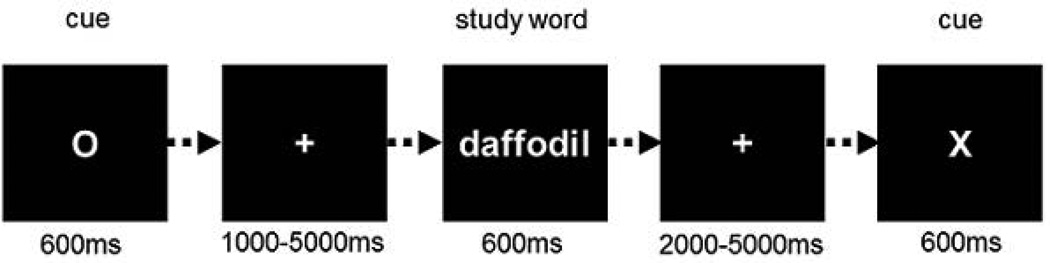
Participants were given instructions and a practice session on the study tasks prior to scanning. They were not informed that their memory for the study items would later be tested. Prior to functional scanning, a structural scan was acquired. During functional scanning, the 4 study lists were presented as 4 consecutive blocks that were separated by brief rest periods (approx. 1 min.). Cues presented prior to each word denoted whether the word should be subjected to an animacy (living or non-living) or a syllable (odd or even number) judgment – an ‘X’ cue denoted the upcoming syllable task and an ‘O’ cue the animacy task. To allow cue- and item-related activity to be deconvolved, the cue-stimulus interval varied randomly between 1s, 3s and 5s, and the interval between each item and the following cue varied between 2s, 3.5s and 5s. The mean across-participant correlations between the regressors modeling cue- and stimulus-related activity in each block (see below) were 0.47 for each condition (range = 0.14 – 0.59 for the syllable condition and 0.16 – 0.60 for the animacy condition). For both tasks, the mapping of left and right index fingers to response was counterbalanced across participants. Instructions emphasized the need for both speed and accuracy. Experimental items were viewed via a mirror located above the head coil. The order and timing of events for each trial during functional scanning were as follows: A red task cue (600 ms), a white fixation cross (1, 3 or 5 s), a white word (600 ms), and another white fixation cross (2, 3.5 or 5s). Words subtended a maximum vertical visual angle of 0.57° and a maximum horizontal visual angle of 3.78° at a 1-m (virtual) viewing distance. Words and cues were presented at fixation against a black background, words in a white lowercase Helvetica 30 point font and cues in red uppercase font. Figure 1 gives a schematic overview of the study procedure.
Figure 1.
Schematic overview of the study procedure.
Approximately 25 min after the scanning session, each participant undertook the memory test. Participants were first given instructions and a practice session on the test. Words were presented on a computer screen in the same format as at study. Each trial consisted of a red fixation cross for 500 ms, followed by the test word which stayed on the screen until a response was made. Participants were required to indicate whether the word had previously been presented using one of three response options: ‘remember’ (R), ‘know’ (K) or ‘new’ (N). An R judgment was required when recognition of a word was accompanied by retrieval of a specific detail or details from the study phase. A K judgment was required when a word was recognized as having been presented earlier but without recollection of any details from the study episode. An N response was to be given when the word was judged to have been unstudied, or if the participant was uncertain about a word’s study status. Participants responded R and K with the index and middle fingers of one hand, and N with the index finger of the opposite hand. Hand and finger assignments were counterbalanced across participants. Although the test was self-paced, participants were instructed to respond as quickly as possible without sacrificing accuracy. Words were replaced by a white fixation cross for 1 s once a response was made. Experimental control, including stimulus presentation, was implemented in the ‘Cogent’ software package (http://www.vislab.ucl.ac.uk/cogent.php).
Functional and anatomical images were acquired using a Philips Achieva 3T MR scanner equipped with an 8 channel parallel imaging head coil. Functional scans were acquired with a T2*–weighted echo-planar image (EPI) sequence using a sensitivity encoding (SENSE) reduction factor of 1.5 (TR 2 s, TE 30 ms, flip angle 70°, FOV 240×240, matrix size 80×78). Each EPI volume consisted of 30 slices (3mm thickness, 1 mm interslice gaps) acquired in ascending order, oriented parallel to the AC–PC line and positioned for full coverage of the cerebrum and most of the cerebellum. Functional data were acquired during each study block (252 volumes per block) and concatenated across the four blocks prior to model estimation. The first five volumes of each block were discarded to allow tissue magnetization to achieve a steady state. A T1-weighted anatomical image was acquired using a 3D magnetization-prepared rapid gradient echo (MP-RAGE) pulse sequence (FOV= 240×150, matrix size 320×320, voxel size 0.75 mm3, 200 slices, sagittal acquisition).
Functional images were preprocessed and analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk/spm). Volumes were motion and slice-time corrected, realigned and then spatially normalized to a standard EPI template (based on the Montreal Neurological Institute or ‘MNI’ reference brain; Cocosco et al., 1997). Normalized volumes were resampled into 3 mm isotropic voxels and smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel. The time series in each voxel were high-pass filtered to 1/128 Hz to remove low-frequency noise and scaled within-session to a grand mean of 100 across voxels and scans.
A single General Linear Model (GLM) was used to estimate both cue- and item-related activity segregated according to study task and subsequent memory judgments. Neural activity was modeled for each participant by delta functions (impulse event) at cue and stimulus onset (modeling cue- and stimulus-related activity respectively). These functions were convolved with a hemodynamic response function (HRF) along with its temporal and dispersion derivatives (Friston et al., 1998). This procedure yielded regressors in the GLM that modeled the BOLD response to the three item types of interest from each study task. These comprised studied words later correctly endorsed as ‘old’ and identified with an R or K response, and studied words later incorrectly identified as N (i.e., misses) on the subsequent memory test. In addition six regressors modeled movement-related variance, and session-specific constant terms were used to model differences in mean image intensity between sessions. The cue-related parameter estimates (related to subsequent R, K and N responses) were taken forward to a second stage of analysis in which participants were treated as a random effect.
The contrast of primary interest involved cue-related (pre-stimulus) activity elicited by study items later endorsed as R versus the activity elicited by items endorsed as K. Since we were also interested in the activity elicited by study items erroneously endorsed as N on the later memory test (misses), our primary second stage analysis was based on an ANOVA model. The ANOVA, implemented within SPM8, employed the factors of task (syllable, animacy) and response type (R,K,N). We restricted the outcome of the ANOVA contrasts to the hippocampus and surrounding MTL regions with a mask based on standard anatomical landmarks (Insausti et al., 1998). The mask was created by manually tracing (with MRIcro software; www.mricro.com) the MTL on coronal slices of the across-participants mean normalized anatomical image and smoothing the result with an 8mm FWHM Gaussian kernel. Contrasts were thresholded at p < .005 with a cluster extent of 17 contiguous voxels. The cluster extent threshold was determined by a Monte Carlo simulation implemented in the AlphaSim routine of the AFNI analysis package (NIMH, Bethesda, MD, USA; http://afni.nimh.nih.gov/afni) to give a corrected cluster-wise significance level of p < .05.
Task-invariant pre-stimulus subsequent memory effects were sought for with the main effect of the ANOVA after it had been exclusively masked by the F contrast of the interaction between task and subsequent memory (at a threshold of p < .05). To identify task-sensitive effects, we first identified MTL clusters showing a significant interaction between task and subsequent memory response (i.e. R, K and N). The clusters were then interrogated by extracting the mean parameter estimates for the BOLD responses elicited by cues to items that went on to be endorsed as R, K or N for the syllable and animacy tasks across all voxels within a 3 mm radius of the peak identified by the interaction effect. In addition, time courses of the BOLD responses in regions identified by the foregoing analyses were estimated from a separate finite impulse response (FIR) model. Time-courses were estimated across 7 time points (sampling interval of 2 s) starting 8 s prior to stimulus onset and continuing until 6 s post-stimulus onset. Time-courses, aligned at the onset of the study item, were estimated for the two item types of primary interest (studied words subsequently endorsed as R or K), collapsed across the three cue-stimulus intervals (1, 3 and 5s).
Mean study task accuracy was 0.91 (SD = 0.04) and 0.95 (SD = 0.03) for the syllable and animacy tasks, respectively; although high in both cases, these values differed significantly [t(17) = 3.05, p < 0.01]. Study RTs (see Table 1) were derived for items subsequently endorsed with R, K and N judgments in the recognition test and segregated according to study task (animacy or syllable). The data were analyzed with a two (study task) by three (subsequent memory: R, K, N) ANOVA. The analysis revealed a main effect of study task [F(1,17) = 25.72, p < 0.001], indicating faster responses for study items subjected to animacy judgments. There was also a main effect of subsequent memory [F(2,34) = 4.69, p < 0.025], but no significant interaction between study task and subsequent memory [F < 1.9]. Follow-up tests showed that, collapsed across study task, responses to study items later given an R judgment were slower than those to both subsequent K [t(17) = 2.48, p < 0.025] and N [t(17) = 2.64, p < 0.025] items. There was no significant difference in study RTs between items that were later given K and N responses [t < 1].
Table 1.
Mean reaction times (ms) (± SD) for correct syllable and animacy study task decisions segregated by subsequent memory.
| Remember | Know | New | |
|---|---|---|---|
| Sllable | 1790 (470) |
1681 (436) |
1710 (425) |
| Animacy | 1308 (220) |
1275 (197) |
1252 (184) |
Proportions of test responses in the different response categories are summarized in Table 2. Test performance was analyzed after transforming raw R and K rates according to the assumption that recollection and familiarity are independent (Yonelinas and Jacoby, 1995). Contrasts of the resulting recollection and familiarity estimates demonstrated a memory advantage for study items from the animacy task in both cases (recollection: 0.32 vs. 0.20, t(17) = 6.27; familiarity: 0.42 vs. 0.33, t(17) = 4.05, both ps < 0.001).
Table 2.
Mean proportions (± SD) of test responses according to study status and study task.
| Remember | Know | New | |
|---|---|---|---|
| Syllable - old | 0.22 (0.08) |
0.36 (0.10) |
0.42 (0.12) |
| Animacy - old | 0.34 (0.08) |
0.36 (0.09) |
0.29 (0.10) |
| New | 0.03 (0.03) |
0.13 (0.08) |
0.84 (0.10) |
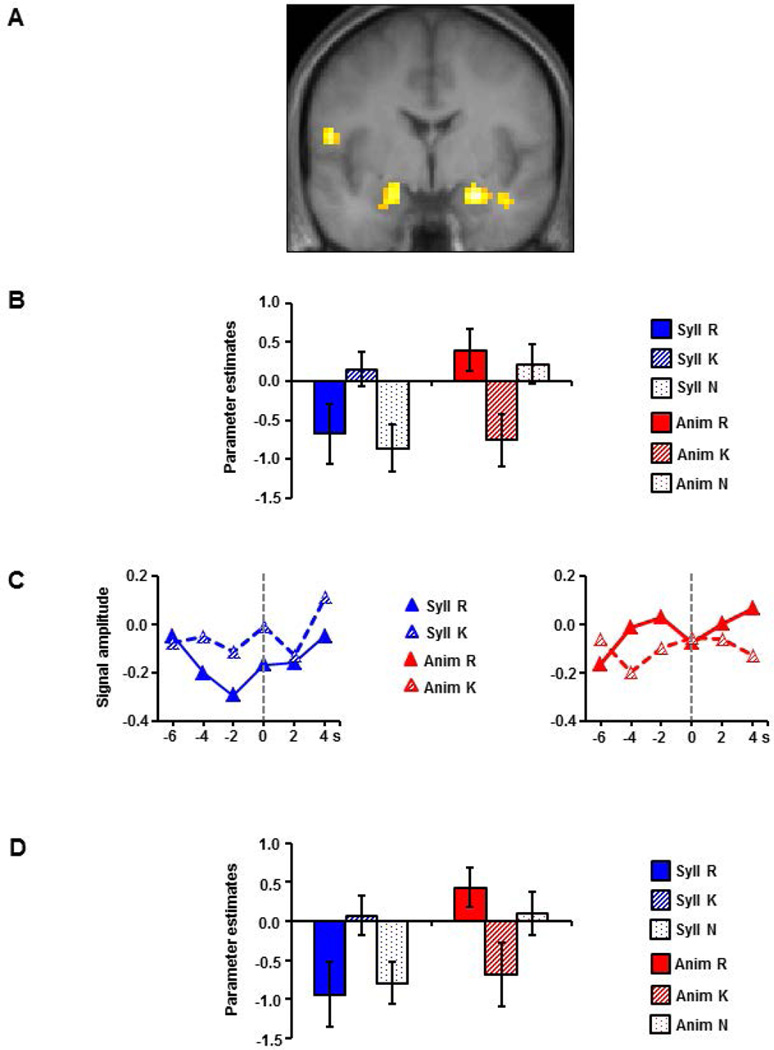
Turning to the fMRI data, we were unable to identify any MTL pre-stimulus subsequent memory effects that were common to the two study tasks. We did, however, identify significant interactions between task and subsequent memory in bilateral anterior hippocampus / amygdala (left peak: 18, −7, −14; Z = 3.27, 39 voxels, right peak: 27, −4, −20; Z = 3.61, 52 voxels) (see Figure 2A).
Figure 2.
(A) MTL regions demonstrating task selective pre-stimulus subsequent memory effects overlaid on sections of the across-participants mean T1-weighted structural image; (B) Bar graphs showing mean parameter estimates (arbitrary units) and standard errors within a 3mm radius of the peak voxels showing the effects; (C) Time courses of the effects aligned at the onset of the study item (0 s). Signal amplitude is in arbitrary units; (D) Bar graphs showing mean parameter estimates (arbitrary units) and standard errors within a 3mm radius of the peak voxels showing task-selective pre-stimulus effects from the subsidiary ANOVA that included only R and K items.
Mean parameter estimates are shown in Figure 2B for each cluster according to study task and subsequent memory judgment. The data were subjected to a three-way ANOVA, with factors of hemisphere, study task, and subsequent memory. Of necessity, given how the regions-of-interest were selected, the ANOVA revealed a two-way interaction between study task and subsequent memory (F(1,17) = 11.92, p < 0.001), while the three way interaction was not significant (F = 1.12). Follow-up tests on the data collapsed across the left and right clusters indicated that pre-stimulus activity associated with the syllable task was greater on trials associated with subsequent K than with subsequent R judgments [t(17) = 2.44, p < 0.05], whereas the reverse effect was evident for items studied in the animacy task [t(17) = 5.10, p < 0.001]. Analogously, while pre-stimulus activity was greater for trials associated with subsequent K than with subsequent N responses [t(17) = 2.82, p < 0.025] in the syllable task, activity was greater for N than for K trials [t(17) = 3.06, p < 0.01] in the animacy task. There were no reliable differences between R and N items in either task (ts < 0.1). The estimated time courses derived from the MTL clusters confirmed that the differences between R and K items in each task did indeed emerge pre-stimulus (see Figure 2C).
The foregoing findings suggest that the direction of pre-stimulus subsequent memory effects for R and K items reversed as a function of encoding task. These findings were derived, however, from an analysis model that also included a third category of study items (N items). Whereas this model is appropriate given that we wished to examine parameter items for all three item types (a model restricted only to R and K items would not allow unbiased assessment of parameter estimates for N items; Kriegeskorte et al., 2009), it does not allow the conclusion that the cross-over interaction for R and K items according to study task meets our pre-experimental criterion for a statistically reliable effect (i.e., p <.005 across at least 17 contiguous voxels). Accordingly, we re-ran the voxel-wise ANOVA after dropping the N items. The ANOVA identified two clusters in which the interaction effect was statistically significant, with peaks close to those identified by the previous analysis but extending slightly more posteriorly into the hippocampus (left peak: −21, −10, −14; Z = 3.12, 51 voxels, right peak: 21, −4, −20; Z = 3.88, 109 voxels). As is evident from Figure 2D, the interaction took the form of a cross-over that was qualitatively identical to the one identified in the original analyses (Figure 2B).
The behavioral findings from the study phase – reduced accuracy and slower RTs for items subjected to syllable than to animacy judgments – are consistent with previous findings that the syllable task is the more difficult (e.g., Otten and Rugg, 2001; Otten et al., 2002; Park et al., 2008). In contrast to the findings of Park and Rugg (2010), in the present study RTs to items later endorsed as R were longer than those to items that went on to receive K or N judgments, regardless of study task. It is unclear why the present findings differ from those reported in the prior experiment (although we note that, across subsequent memory studies in general, the patterning of RTs to study items receiving different judgments on the later memory test is highly inconsistent; compare, for example, Otten et al., 2001; Otten and Rugg, 2001; Gottlieb and Rugg, 2011). Importantly, in combination with the results reported by Park and Rugg (2010), the present findings indicate that pre-stimulus hippocampal subsequent memory effects are independent of the direction of any RT differences between study items that go on to attract different judgments on the subsequent memory test.
Based on the proposal of Park and Rugg (2010) that pre-stimulus hippocampal subsequent memory effects are sensitive to the difficulty of the encoding task (and, therefore, the value of optimal pre-stimulus preparation), we expected that the present effects would be greater or more numerous in the syllable task than in the animacy task. Our findings were in striking contrast to this prediction. Whereas pre-stimulus effects in the hippocampus/amygdala in the animacy task closely replicated those described by Park and Rugg (2010), effects identified in the syllable task, while evident in the same loci, were reversed in direction (Figure 2B).
The finding that a relative diminution in pre-stimulus activity can be predictive of enhanced memory performance is not unprecedented. Yoo et al. (2012) reported that visual scenes that went on to be correctly recognized on a later memory test were preceded by lower activity in parahippocampal cortex than were later forgotten scenes. Yoo et al. (2012) suggested that their findings might have reflected a trade-off between the allocation of neural resources to pre- and post-stimulus processing. By this argument, when fewer resources are allocated to task-irrelevant pre-stimulus processes, more resources are available to support encoding of the study item. Whereas a similar account might apply to the present finding of ‘reversed’ hippocampal pre-stimulus subsequent memory effects in the syllable task, the account obviously fails to accommodate the findings for the animacy task [or, indeed, for the ‘positive’ pre-stimulus effects reported in prior studies (e.g., Adcock et al., 2006; Park and Rugg, 2010; Addante et al., 2015)].
An alternative explanation for the present results stems from the well-attested finding – replicated here – that ‘shallow’ study tasks, such as the present syllable judgment task, are invariably associated with poorer subsequent memory performance than are ‘deep’ tasks such as animacy judgment (Craik and Lockhart, 1972). From this perspective, one might conjecture that the more effective the preparation for a shallow study task, and hence the more complete the pre-stimulus task set, the less accessible will the study item be on a later test of episodic memory. Thus, while an fMRI signature of effective preparatory processing, such as relative enhancement of hippocampal activity, will be predictive of successful memory when the study task is conducive to effective encoding, the opposite relationship will prevail when the task engages processes that promote ineffective encoding. Thus, it is on those ‘shallow’ study trials where preparatory processing is relatively ineffective, and hence where there is an increased likelihood of incidental semantic processing, that successful encoding is more likely to occur.
A complication for this account comes from the findings for study items that went on to be missed (N items). As is evident from Figure 2B, pre-stimulus activity associated with these items followed the same pattern as did the activity for R items, and did not differ significantly in magnitude from these items (Figure 2D shows that a similar pattern was evident in the parameter estimates for N items taken from the peaks of the interaction analysis restricted to R and K items). These findings are puzzling, in that they suggest that the same pre-stimulus state that is conducive to successful encoding of contextual information can also promote the failure to encode information supporting either recollection- or familiarity-based recognition. The present findings are not wholly without precedent, however. In three prior studies in which post-stimulus encoding-related activity was examined, a U-shaped profile similar to that observed for pre-stimulus activity in the present animacy task was observed (Davachi et al., 2003; Shrager et al., 2008; Staresina and Davachi, 2008). For instance, Shrager et al.(2008) reported that both study items later endorsed as ‘old’ with high confidence, and items later erroneously endorsed as new with high confidence, elicited greater hippocampal activity than did items that were later endorsed either as old or new with low confidence. Similarly, Davachi and Wagner (2003) and Staresina and Davachi (2008) each described U-shaped activity patterns across study items that later attracted, respectively, a correct source memory judgment, a correct recognition but incorrect source judgment, and an incorrect endorsement as new.
Shrager et al. (2008) proposed that, like the activity elicited by confident hits, the hippocampal activity elicited by high confidence misses in their study was a reflection of successful episodic encoding, but of information extraneous to the study item (cf. Uncapher and Wagner, 2009). We tentatively suggest that the present findings can be accommodated by an analogous account. By this account, the parallel pre-stimulus activity elicited by R and N items in the two tasks in both cases signifies a brain state that promotes encoding of episodic information, with the two item types differentiated by whether the encoded memory representation includes information about the study item that is accessible on the later memory test. We freely admit however that this account (like the proposal that inspired it) is highly speculative, and that other possibilities also deserve consideration.
To conclude, the present findings for the animacy task replicate those originally reported by Park and Rugg (2010), and add support to the proposal that hippocampal pre-stimulus subsequent memory effects in ‘deep’ (semantic) study tasks are associated with encoding processes that support later recollection, but not later familiarity-based recognition. The present findings go beyond these prior results, however, by demonstrating that the direction of hippocampal pre-stimulus subsequent memory effects is sensitive to the nature of the study task. As noted above, we conjecture that whether these effects take the form of relatively greater or relatively lower hippocampal activity for later recollected study items depends upon whether an optimal pre-stimulus task set facilitates the engagement of study processing that is conducive or unconducive for the formation of an episodic memory representation.
Acknowledgements
This research was supported by the National Institute of Mental Health (NIH 1R01MH074528). We thank the staff of the University of California, Irvine Research Imaging Center for their assistance in the collection of the data reported here. The authors also thank Ken Norman for his suggestion to include later missed study items in the fMRI analyses.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Addante RJ, de Chastelaine M, Rugg MD. Pre-stimulus neural activity predicts successful encoding of inter-item associations. NeuroImage. 2015;105:21–31. doi: 10.1016/j.neuroimage.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RS, Evans AC. Brainweb: Online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:S425. [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: A framework for memory research. J Verbal Learning Verbal Behav. 1972;11:671–684. [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Richardson-Klavehn A. Remembering and knowing. In: Tulving E, Craik FIM, editors. The Oxford Handbook of Memory. Oxford: Oxford University Press; 2000. pp. 229–244. [Google Scholar]
- Gottlieb LJ, Rugg MD. Effects of modality on the neural correlates of encoding processes supporting recollection and familiarity. Learn Memory. 2011;18:565–573. doi: 10.1101/lm.2197211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulous I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdale and hippocampus function in emotional memory. Proc Natl Acad Sci USA. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding Relationship between findings from across-and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex. 2001;11:1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. State-related and item-related neural correlates of successful memory encoding. Nat Neurosci. 2002;5:1339–1344. doi: 10.1038/nn967. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Park H, Uncapher MR, Rugg MD. Effects of study task on the neural correlates of source encoding. Learn Memory. 2008;15:417–425. doi: 10.1101/lm.878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Rugg MD. Pre-stimulus hippocampal activity predicts later recollection. Hippocampus. 2010;20:24–28. doi: 10.1002/hipo.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Uncapher MR. Encoding and retrieval in episodic memory: Insights from fMRI. In: Duarte A, Barense M, Addis DR, editors. Handbook on the Cognitive Neuroscience of Memory. Wiley-Blackwell; In press. [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequently remembered information. Neuron. 2008;59:547–553. doi: 10.1016/j.neuron.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cognitive Neurosci. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Düzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. Neuroimage. 2007;38:194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: Effects of size congruency. J Mem Lang. 1995;34:622–643. [Google Scholar]
- Yoo JJ, Hinds O, Ofen N, Thompson TW, Whitfield-Gabrieli S, Triantafyllou C, Gabrieli JD. When the brain is prepared to learn: enhancing human learning using real-time fMRI. NeuroImage. 2012;59:846–852. doi: 10.1016/j.neuroimage.2011.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]