Abstract
Background
The Lung Allocation Score (LAS) has changed organ allocation for lung transplantation in the United States. Previous investigations of transplant recipients have reported an association between high LAS scores and an increased risk of death after lung transplantation. We hypothesize that a high LAS predicts survival in cystic fibrosis (CF) lung transplant recipients in the United Network for Organ Sharing (UNOS) Scientific Registry of Transplant Recipients (SRTR) database.
Methods
A cohort study of 1,437 U.S. adult CF lung-transplant recipients from May 1, 2005 through December 31, 2012. The cohort was divided into a high-risk group and a low-risk group based on lung allocation score. Survival data were examined using Kaplan-Meier estimates and Cox proportional hazard models to compare survival. The primary outcome was adjusted survival at 1-year after lung transplantation.
Results
The high-risk group of 318 patients with a median lung allocation score of 69.6 (interquartile range 56.3-87.2) was compared to a low-risk group of 1,119 patients with a median lung allocation score of 38.8 (interquartile range 36.3-42.3). Patients in the high-risk group had a 41% increased relative risk of cumulative mortality at 1 year after transplantation compared to the low-risk group (16.1% vs. 12.0%). After adjustment for known predictors of mortality, the risk of death at 1-year post-transplantation remained elevated (hazard ratio, 1.41; 95% confidence interval 1.00 to 2.01). The high-risk group had worse survival at 90-days, and 2-years after lung transplantation.
Conclusions
High lung allocation scores are associated with worse survival in CF lung transplant recipients.
INTRODUCTION
Lung transplantation is a widely accepted therapeutic option for individuals with cystic fibrosis (CF) and advanced pulmonary disease. 1 Appropriately chosen lung transplant candidates can benefit from prolonged survival and improved health-related quality of life. 2,3 However, donor lung availability remains limited and long-term post-transplant outcomes are not optimal with a median 5-year survival of 54 percent. 4
In May 2005, the United Network for Organ Sharing (UNOS) implemented the lung allocation scoring system to prioritize patients awaiting lung transplantation in the United States. The lung allocation score (LAS) utilizes validated demographic and clinical data (table 1) to prioritize candidates by “net-transplant benefit” based on wait-list urgency and predicted post-transplant survival. 5 Wait-list urgency is defined as predicted 1-year survival without lung transplantation and post-transplant survival is defined as predicted 1-year survival with lung transplantation. 6 Scores range from 0 to 100 with a higher score reflecting individuals with most urgent need and greatest chance of success after transplantation. The LAS has been associated with shorter wait-list times and improved survival.6-10
Table 1.
Lung Allocation Score Components
| Waiting List Urgency Parameters | |
| Age | O2 requirement at rest |
| Body mass index | Diabetes mellitus |
| Diagnosis | Six-minute walk distance < 150 feet |
| Functional status | Continuous mechanical ventilation |
| FVC (% predicted) | Partial pressure of CO2 |
| Pulmonary artery systolic pressure | |
| Post-transplant Survival Variables | |
| Age | Continuous mechanical ventilation |
| Functional status | Diagnosis |
| FVC (% predicted) | Pulmonary capillary wedge pressure |
The UNOS Scientific Registry of Transplant Recipients (SRTR) database has been used to examine post-transplant survival in lung transplant recipients with high LAS. In a previous publication, our group investigated all lung transplant recipients three years after institution of the LAS, and found an 8 percent absolute increase (75% vs 83%) in 1-year, post-transplant mortality for patients in the highest LAS quintile (LAS>46) compared to those in the lower four quintiles (LAS≤46). 6 Studies investigating the impact of the LAS on survival in patients with chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and pulmonary hypertension have reported a 5 to 15 percent survival difference in high-risk LAS groups when compared to the low-risk groups. 10-13 However, these studies did not adjust their findings for other identified survival predictors including donor cytomegalovirus (CMV) status, donor lung ischemic time, and patient insurance status.14-17
A rigorous investigation of the strength of association between LAS and survival is particularly important in the CF population who represent a unique subset of the transplant population. Their relative youth compared to other lung transplant recipients, variable adherence to medications, and pre-transplant history of chronic infection may differentially impact the LAS-survival relationship. Thus, we hypothesize that high LAS scores may be associated with worse survival in CF lung transplant recipients
METHODS
The current study used de-identified data from the UNOS SRTR database of the thoracic organ transplant registry.4
Study Design
Adult (≥18 years of age) patients with CF, undergoing first-time lung transplantation in the United States were entered into the study between May 1, 2005 (date of LAS implementation) and December 31, 2012. Patients undergoing combined heart-lung transplantation or repeat lung transplantation were not included.
Our analysis examined the following independent variables: age, sex, body mass index (BMI), creatinine, diabetic status, forced expiratory volume at 1 second (FEV1), forced vital capacity (FVC), pre-transplant hemodynamic measures, six-minute walk test, supplemental oxygen use, serum partial pressure of carbon dioxide (pCO2), pre-transplant mechanical ventilation, educational attainment (high school diploma or less v. any degree of post-secondary education), insurance status (private v. non-private), center volume, donor CMV status, graft ischemic time, wait-list time, and LAS. Donor CMV status was characterized as high-risk if positive, and low-risk if negative, based on previously reported data. 14,18
Our primary end-point was 1-year post-transplant survival. Secondary end-points included 90-day, 2-year, and 5-year post-transplant survival. Furthermore, we examined survival for lung transplant recipients who survived to 1-year, known as conditional 1-year survival. Study subjects were censored if they were lost to follow-up or the study period ended.
Statistical Analysis
Descriptive analysis was performed with calculation of means, standard deviations and medians for continuous variables, and proportions for categorical variables. Bivariate analyses were conducted using t-tests, or Wilcoxon’s rank-sum test for continuous variables and chi-square or Fisher’s exact tests for categorical variables. The top quintile of the cohort by LAS corresponded to a LAS near 50; for clarity this cut-off was chosen to describe a high-risk cohort (LAS ≥ 50), and a low-risk comparison group (LAS < 50) as modeled in previous studies. 6,19 We also examined LAS as a continuous variable, and as a variable re-scaled in increments of 10, in order to provide clarity of hazard estimates. Survival was modeled using the Kaplan-Meier product limit estimator with statistical differences between survival curves assessed using the Mantel-Cox log-rank test. 20 Cox proportional hazard regression models were used to account for possible differences in survival patterns that may be due to imbalances in severity of illness between the two groups. 21 Covariates in multivariable analyses were chosen based upon biologic significance and clinical relevance. To address bias from missing data (assumed missing at random), the multivariate imputation by chained equations method of multiple imputation in Stata (5 data sets were imputed and analyzed) was performed. Conditional survival at 1 year was examined in a similar fashion.
Statistical significance was defined as a 2-tailed <0.05. There was greater than 80% power to detect a relative risk difference of 1.3 between groups, assuming a sample size of 1,000 patients and overall mortality rate of 15%. All statistical analyses were performed using STATA software (version 11.0, StataCorp LP, College Station, TX). Patients in the UNOS database give informed consent permitting their de-identified records to be used for research purposes. The study was reviewed and approved by the institutional review board at Johns Hopkins School of Medicine.
RESULTS
Cohort Characteristics
A total of 1,437 adult CF patients underwent lung transplantation between May 1, 2005 and December 31, 2012. The mean (±SD) age of the cohort was 31 (9.5) years and 47.2% were female. Patients spent a median time of 104 days on the waiting list (IQR, 27 to 304 days). The median follow-up time after lung transplantation was 732 days (IQR, 334 to 1,433 days). During the study there were 416 deaths. All patients had a LAS recorded prior to transplantation with a median score of 40.6 (IQR, 37.0 to 48.2). The LAS ranged from 29.2 to 95.2.
As previously described, patients with a LAS ≥50 were classified as high-risk (N=318) and compared to patients with a LAS <50 (N=1,119). Those in the high-risk group had a median follow-up of 631 days (IQR 200 to 1,103 days) and the comparison group had a median follow-up of 749 days (IQR 351 to 1,471 days). There were 98 deaths in the high-risk group, and 318 deaths in the comparison group. Of deaths occurring within the first year, 49% (23/47) occurred within the first 90 days in the high-risk group compared to 37% (46/124) within the comparison group (p-value=0.02).
The baseline characteristics for the patients are shown in table 2. Notably, patients in the high-risk group were more likely to be female, have a lower BMI and reduced lung function than patients in the comparison group. High-risk patients were also more likely to have respiratory failure requiring mechanical ventilation (35.3% vs. 1.6%). High-risk patients had worse pulmonary hemodynamics as measured by pre-transplant right heart catheterization, and were more likely to have a pulmonary capillary wedge pressure >20 mm Hg. Furthermore, at the time of transplantation, high-risk patients were more likely to require higher levels of oxygen supplementation, to have higher serum partial pressures of carbon dioxide, and to perform worse on six-minute walk testing. Median wait time in the high-risk group was significantly less at 59 days compared to 115 days for the comparison group. Of note, 94% of CF lung transplantations were performed at high volume transplant centers (≥20 lung transplants/year).
Table 2.
Demographics and Clinical Characteristics of Adult Cystic Fibrosis Lung Transplant Recipients in the Low-risk (LAS <50) and High-risk (LAS ≥50) Groups
| LAS<50 (N=1119) | LAS≥50 (N=318) | p-value | |
|---|---|---|---|
| Age (years) | 31.1 ± 9.4 | 30.6 ± 9.6 | 0.44 |
| Gender (% female) | 45.1 | 54.4 | 0.003 |
| BMI (kg/m2) | 19.8 ± 5.7 | 19.1 ± 2.8 | 0.02 |
| FEV1 (% predicted) | 25.7 ± 13.9 | 23.5 ± 13.7 | 0.01 |
| FVC (% predicted) | 40.4 ± 13.0 | 35.0 ± 13.5 | <0.001 |
| Six-minute walk (%<150 feet)a | 8.5 | 26.7 | 0.003 |
| Creatinine (mg/dL) | 0.76 ± 0.45 | 0.70 ± 0.27 | 0.05 |
| Diabetic (%) | 45.3 | 50.5 | 0.10 |
| PA systolic pressure (mm Hg) | 37.8 ± 9.9 | 43.8 ± 13.2 | <0.001 |
| PA mean pressure (mm Hg) | 25.5 ± 7.1 | 30.0 ± 9.2 | <0.001 |
| PCWP (% >20) | 3.7 | 7.7 | 0.01 |
| Oxygen at rest (liters/min) | 3.0 ± 2.1 | 6.2 ± 4.4 | <0.001 |
| pCO2 (mm Hg) | 49.6 ± 13.0 | 64.7 ± 22.3 | <0.001 |
| Ventilator (% yes) | 1.6 | 36.2 | <0.001 |
| Ischemic time (hours) | 5.7 ± 1.6 | 5.8 ± 1.6 | 0.39 |
| Waiting time (days) | 115 (35, 330)† | 59 (13, 226)† | <0.001† |
| Donor/recipient CMV mismatch (%) | 63.8 | 64.5 | 0.83 |
| Non-private insurance (%) | 40.8 | 39.9 | 0.79 |
| Graduate from Secondary Education (%) | 64.0 | 61.7 | 0.47 |
| Center volume (% ≥20 tx/year) | 93.0 | 96.5 | 0.15 |
| LAS | 39.5 ± 4.3 | 71.2 ± 15.4 | <0.001 |
16.8% of cohort with recorded 6-MWT data (212: LAS<50, 30 LAS≥50)
median (IQR): Wilcoxon rank sum
LAS, Lung Allocation Score; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; pCO2, serum partial pressure of carbon dioxide; tx, transplants
Survival Analysis
In unadjusted analyses, a high-risk LAS was associated with worse cumulative survival at 1 year post-transplantation. Figure 1 demonstrates the differences in the Kaplan-Meier failure estimates between the high-risk and comparison groups at 1-year post-transplantation. The cumulative percentage of patients dying at one-year was significantly higher in the high-risk group (16.1% vs. 12%, p=0.04 by log-rank test) when compared to the comparison group. Similarly, using Cox proportional hazards modeling, the risk of death was increased in the high-risk group (hazard ratio [HR] 1.41, 95% confidence interval [CI] 1.01 to 1.97) versus the comparison group. Univariate analyses of key clinical characteristics are summarized in table 3.
Figure 1.
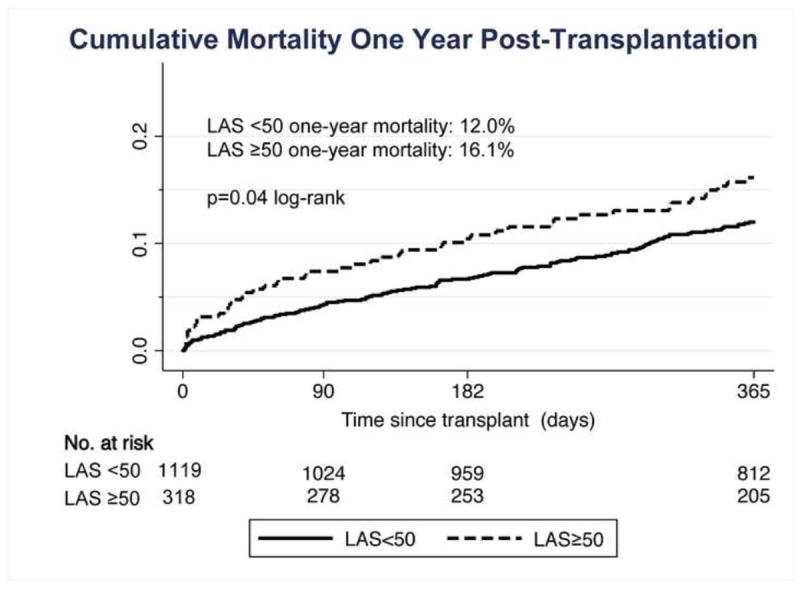
Kaplan-Meier Survival Estimates of time to death at 1-Year Post lung transplantation
Table 3.
Unadjusted Cox Hazard Regression of Mortality at 1-Year Post-Transplantation
| Variables of Interest | HR (95% CI) | p-value |
|---|---|---|
| High-risk LAS (LAS≥50) | 1.41 (1.01-1.97) | 0.045 |
| Age | 0.98 (0.96-1.00) | 0.11 |
| Female Gender | 0.85 (0.63-1.14) | 0.28 |
| Body Mass Index | 0.98 (0.92-1.03) | 0.36 |
| Creatinine | 1.16 (0.97-1.39) | 0.11 |
| Diabetic status | 1.27 (0.94-1.72) | 0.12 |
| Supplemental Oxygen requirement | 1.10 (0.99-1.22) | 0.07 |
| Serum partial pressure of carbon dioxide (pCO2) | 1.01 (1.00, 1.02) | 0.03 |
| Forced expiratory volume in 1 second (FEV1) | 0.99 (0.97-1.00) | 0.10 |
| Forced Vital Capacity (FVC) | 0.99 (0.98-1.01) | 0.28 |
| Pulmonary Artery Systolic Pressure | 1.00 (0.99-1.01) | 0.76 |
| Pulmonary Artery Mean Pressure | 1.00 (0.98-1.03) | 0.79 |
| Pulmonary Capillary Wedge Pressure ≥20mm Hg | 1.37 (0.64-2.94) | 0.42 |
| Mechanical Ventilation | 2.28 (1.53-3.40) | <0.001 |
| Ischemic time | 1.04 (0.95-1.15) | 0.40 |
| Donor CMV antibody positive | 1.34 (0.96-1.89) | 0.09 |
| Graduate from Secondary Education (high school) | 0.80 (0.58-1.11) | 0.18 |
| Non-private insurance | 1.41 (1.04-1.90) | 0.03 |
HR = hazard ratio; CI = confidence interval; LAS = lung allocation score
Multivariable analysis Cox proportional hazard modeling was performed to examine the association of LAS on survival as shown in Table 4. The LAS-survival relationship was adjusted for age, sex, donor CMV status, graft ischemic time, and insurance status. Patients in the high-risk LAS group had a hazard risk of death of 1.41 (95% CI 1.00 to 2.01, p=0.05) at 1 year post-transplantation when compared to the comparison group. We also examined this model with LAS as a continuous variable (HR 1.02; 95% CI, 1.00 to 1.03, p<0.001), and re-scaled in 10-point increments (HR 1.18; 95% CI, 1.08 to 1.29, p=0.01), which revealed an associated risk of death within the first year after lung transplantation. Thus, a 10-point increase in LAS corresponds to an 18% increase in the relative hazard of mortality at 1 year. This same multivariable model was used to examine secondary outcomes: risk of death at 90 days, 2 years, and 5 years post-transplantation. (Table 4) Patients in the high-risk LAS group had a hazard risk of death of 1.98 (95% CI, 1.27 to 3.12), 1.38 (1.04 to 1.83), and 1.26 (0.99 to 1.60) at 90 days, 2 years, and 5 years post-transplantation, respectively. The hazard ratio at 5 years did not meet statistical significance with a p value of 0.07. Thirty-eight percent of the cohort (547/1,437) had the opportunity to provide survival data beyond 5 years given the cohort’s time at risk. Data were complete for all covariates used in our multivariable analysis except for ischemic time (6.7% missing) and donor CMV status (7% missing).
Table 4.
Multivariable Adjusted Cox Proportional Hazard Regression Analysis
| Covariates | Relative Adjusted Hazard for Deatha | |||||||
|---|---|---|---|---|---|---|---|---|
| 90 days | 1 Yearb | 2 Years | 5 Years | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | P | |
| LAS≥50 | 1.98 (1.27, 3.12) | 0.003 | 1.41 (1.00, 2.01) | 0.05 | 1.38 (1.04, 1.83) | 0.03 | 1.26 (0.99, 1.60) | 0.07 |
| Age | 1.01 (0.99, 1.04) | 0.27 | 1.00 (0.98, 1.01) | 0.58 | 0.98 (0.96, 0.99) | 0.002 | 0.97 (0.96, 0.98) | >0.001 |
| Gender (% F) | 0.86 (0.56, 1.34) | 0.51 | 0.89 (0.61, 1.29) | 0.58 | 1.13 (0.88, 1.46) | 0.34 | 1.07 (0.87, 1.33) | 0.48 |
| Ischemic time | 1.17 (1.03, 1.33) | 0.02 | 1.10 (0.99, 1.24) | 0.18 | 1.04 (0.96, 1.13) | 0.30 | 0.99 (0.93, 1.06) | 0.80 |
| Donor CMV+ | 1.48 (0.91, 2.40) | 0.11 | 1.62 (1.07, 2.46) | 0.14 | 1.22 (0.93, 1.59) | 0.15 | 1.15 (0.92, 1.43) | 0.21 |
| N-P Insurance | 1.40 (0.91, 2.16) | 0.13 | 1.49 (1.02, 2.17) | 0.03 | 1.29 (1.00, 1.66) | 0.05 | 1.34 (1.09, 1.66) | 0.01 |
HR = hazard ratio; CI = confidence interval; CMV+ = positive CMV antibody; N-P = non-private, LAS = lung allocation score
Adjusted for all covariates listed in the table.
Primary Outcome
CF patient survival in the subgroup of patients having already survived one year is referred to as 1-year conditional survival and is displayed in table 5. Among the subgroup of CF patients who survived to year one, we found no association of LAS on survival between those in the high-risk LAS group and the comparison group (HR 1.09, 95% CI 0.78 to 1.51). Of the multivariable covariates, only older age is associated with improved survival (HR 0.95, 95% CI 0.92 to 0.97). Notably, graft ischemic time and donor CMV status did not impact overall survival for those who achieved one-year survival.
Table 5.
Multivariable Adjusted Cox Proportional Hazard Regression for Conditional Survival to 1-yeara
| Covariates | HR (95% CI) | p |
|---|---|---|
| LAS≥50 | 1.09 (0.78, 1.51) | 0.62 |
| LAS(10) | 1.00 (0.91, 1.10) | 0.99 |
| Age | 0.95 (0.93, 0.97) | <0.001 |
| Gender (% F) | 1.31 (1.00, 1.72) | 0.05 |
| Donor CMV+ | 1.04 (0.79, 1.37) | 0.79 |
| N-P Insurance | 1.34 (1.02, 1.76) | 0.03 |
| Ischemic time | 0.94 (0.86, 1.02) | 0.16 |
HR = hazard ratio; CI = confidence interval; CMV+ = positive CMV antibody; N-P = non-private, LAS = lung allocation score
Analysis of patients who survive to 1-year
DISCUSSION
The major finding of our study was that a high-risk LAS (LAS≥ 50) was associated with a 41% relative increase in mortality at 1-year post transplantation. Furthermore, a 10-point increase in LAS incrementally increased the risk of death at 1-year post-transplantation by 18%. For example, a CF patient transplanted with a LAS of 60 has a 36% higher risk of death at one year compared to a CF patient transplanted with a LAS of 40. Secondary analysis revealed a similar negative association between high-risk LAS and risk of death at 90-days, and 2-years post-transplantation. Risk of death at 5-years was not statistically significant (p=0.07); however the trend was in a similar direction and a minority of the cohort (38%) had the opportunity to provide person-time at 5 years.
Close examination of the high-risk and low-risk LAS groups’ Kaplan-Meier estimates reveal that the difference in the slopes of the survival curves is most pronounced in the first 90-days post transplantation; thereafter, the survival curves more closely parallel each other. These findings support the notion that a high LAS prior to transplant may correlate with an increased risk of early post-operative complications. As death within 90 days was uncommon in our cohort, affecting less than 3% of transplant recipients, we did not have statistical power to characterize differences between the two risk groups (table 6). Furthermore, the UNOS SRTR data-file does not capture the discrete timing and types of infection in the post-transplant period; therefore, we are unable to ascertain if the presence of, and subsequent death from, infection is differentially distributed between high-risk and low-risk LAS groups in the early post-transplant period.
Table 6.
Causes of Death in first 90-days after transplantation
| Cause of Death | Percent |
|---|---|
| Bacterial septicemia | 23.2 |
| Multi-system organ failure | 13.0 |
| Primary graft failure | 7.2 |
| Bacterial pneumonia | 4.3 |
| Aspergillus sp. infection | 4.3 |
| Bacterial infection: other | 2.8 |
| Cardiac arrest | 2.8 |
| Unknown or Other | 42.4 |
As expected, patients in the high-risk LAS group were more likely to require mechanical ventilation since pre-transplant mechanical ventilation is heavily weighted in the lung allocation scoring system. However, it was unexpected that in a univariate Cox model of mechanical ventilation and survival, the risk of death at 1-year was increased almost three-fold compared to patients without pre-transplant respiratory failure. In fact, of the component variables of the LAS, the need for mechanical ventilation is the strongest predictor of 1-year post-transplant death. Despite this increased risk of death, 1-year survival was still 83.8%, which is similar to the 1-year survival seen in lung transplant recipients with other forms of end-stage-lung disease. 18 Moreover, for patients who survived to 1-year, there was no difference between patients requiring mechanical ventilation. Thus, our study supports the recommendations that mechanical ventilation should not be considered an absolute contraindication, but rather remain a relative contraindication in CF lung transplant candidates.22
In our adjusted survival model, increased age at time of transplant was associated with a decrease in the risk of death at 2-years and 5-years post-transplantation. These data support previous findings that have shown that older CF patients have improved survival outcomes with lung transplantation. 19,23 We, as others, speculate that the improved survival in older CF lung transplant recipients may be related to improved treatment adherence secondary to behavioral independence and emotional maturation in the older adult transplant recipient.24
In our multivariable model, survival was also found to be negatively associated with non-private medical insurance status. Insurance status is a crude marker for socioeconomic status, and in our adjusted model, non-private insurance was associated with worse survival at one, two, and five years after transplantation. Finally, organ ischemic time, and donor CMV status have previously been shown to impact short-term survival. 14-17 We did see an association with organ ischemic time an 90-day post-transplant survival, but not at 1, 2, or 5 years. Since our cohort examined patients after May of 2005, it is possible that improvements in organ preservation and intraoperative techniques have diminished the impact of organ ischemic time. Furthermore, we did not see an association with donor-recipient mismatch CMV status. It is possible that post-transplant CMV prophylaxis and treatment protocols have led to improvements in survival in patients with a CMV status mismatch.
There are several limitations to our study. First, the UNOS database provides limited follow-up and in some cases missing data; therefore, we examined short-term survival from 90-days through 5-years using covariates with less than 7% missing data. Second, survival is a strong response outcome; however, other clinically relevant endpoints such as quality of life and the development of chronic lung allograft dysfunction (CLAD) cannot be characterized in our analysis. Finally, although the data were collected prospectively and adjusted for potential confounders, residual confounding may be present.
Current guidelines recommend that a patient with CF be considered for lung transplantation when the FEV1 falls below 30 percent-predicted, there is a rapid decline in FEV1 or there is an increased frequency of pulmonary exacerbation requiring hospitalization. 22 The decision when to list a CF patient for lung transplantation is made by the transplant center after a medical and psychosocial evaluation, and it is not until after transplant listing that the LAS is subsequently calculated. Our findings implicate that utilization of the LAS earlier in the assessment for transplant may help in ascertaining net transplant benefit since the LAS is a predictor of 1-year of post-transplant survival. Modifiable risk factors that increase the LAS, such as BMI could be a focus of the medical transplant team prior to listing.
In conclusion, CF lung transplant recipients with a high LAS have decreased post-transplant survival. It is important to note that the high-risk LAS group has overall survival rates comparable to other non-CF lung transplant recipients. 4 Therefore, an elevated LAS should not prohibit lung transplantation, rather it may be helpful to identify and optimize modifiable factors to reduce risk. Given the association of a high LAS with decreased survival 1-year post-transplant, its use should be considered in guiding appropriateness of patient candidacy.
Acknowledgments
Dr. Braun was funded by the National Institute of Health KL25RR025006-5 during the preparation of this manuscript. Dr. Dasenbrook receives funding from the Cystic Fibrosis Foundation: DASENB10A0.
Footnotes
Presented at the North American Cystic Fibrosis Conference’s 25th Annual Meeting; Anaheim, California, November 3-5, 2011.
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egan TM, Detterbeck FC, Mill MR, et al. Long term results of lung transplantation for cystic fibrosis. Eur J Cardiothorac Surg. 2002;22(4):602–609. doi: 10.1016/s1010-7940(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 2.Charman SC, Sharples LD, McNeil KD, Wallwork J. Assessment of survival benefit after lung transplantation by patient diagnosis. J Heart Lung Transplant. 2002;21(2):226–232. doi: 10.1016/s1053-2498(01)00352-7. [DOI] [PubMed] [Google Scholar]
- 3.Gerbase MW, Spiliopoulos A, Rochat T, Archinard M, Nicod LP. Health-related quality of life following single or bilateral lung transplantation: A 7-year comparison to functional outcome. Chest. 2005;128(3):1371–1378. doi: 10.1378/chest.128.3.1371. [DOI] [PubMed] [Google Scholar]
- 4.2009 annual report of the US organ procurement and transplantation network and the scientific registry of transplant recipients: Transplant data 1999–2008. department of health and human services, health resources and services administration, healthcare systems bureau, division of transplantation, rockville, MD; united network for organ sharing, richmond, VA; arbor research collaborative for health, ann arbor, MI available from: Http://Www.ustransplant.org/annual_reports/current/default.htm
- 5.2009 OPTN/SRTR annual report 1999-2008. HHS/HRSA/HSB/DOT.
- 6.Merlo CA, Weiss ES, Orens JB, et al. Impact of U.S. lung allocation score on survival after lung transplantation. J Heart Lung Transplant. 2009;28(8):769–775. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Gries CJ, Mulligan MS, Edelman JD, Raghu G, Curtis JR, Goss CH. Lung allocation score for lung transplantation: Impact on disease severity and survival. Chest. 2007;132(6):1954–1961. doi: 10.1378/chest.07-1160. [DOI] [PubMed] [Google Scholar]
- 8.Gries CJ, Rue TC, Heagerty PJ, Edelman JD, Mulligan MS, Goss CH. Development of a predictive model for long-term survival after lung transplantation and implications for the lung allocation score. J Heart Lung Transplant. 2010 doi: 10.1016/j.healun.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: A multicenter study. J Thorac Cardiovasc Surg. 2008;135(1):166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Lung allocation score predicts survival in lung transplantation patients with pulmonary fibrosis. Ann Thorac Surg. 2009;88(6):1757–1764. doi: 10.1016/j.athoracsur.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Benza RL, Miller DP, Frost A, Barst RJ, Krichman AM, McGoon MD. Analysis of the lung allocation score estimation of risk of death in patients with pulmonary arterial hypertension using data from the REVEAL registry. Transplantation. 2010;90(3):298–305. doi: 10.1097/TP.0b013e3181e49b83. [DOI] [PubMed] [Google Scholar]
- 12.Nunley DR, Bauldoff GS, Holloman CH, Pope-Harman A. The lung allocation score and survival in lung transplant candidates with chronic obstructive pulmonary disease. Lung. 2009;187(6):383–387. doi: 10.1007/s00408-009-9180-4. [DOI] [PubMed] [Google Scholar]
- 13.Russo MJ, Iribarne A, Hong KN, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest. 2010;137(3):651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan AJ, Dummer JS, Paradis IL, et al. Cytomegalovirus infection and survival in lung transplant recipients. J Heart Lung Transplant. 1991;10(5 Pt 1):638–44. discussion 645-6. [PubMed] [Google Scholar]
- 15.Novick RJ, Bennett LE, Meyer DM, Hosenpud JD. Influence of graft ischemic time and donor age on survival after lung transplantation. J Heart Lung Transplant. 1999;18(5):425–431. doi: 10.1016/s1053-2498(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 16.Weiss ES, Allen JG, Meguid RA, et al. The impact of center volume on survival in lung transplantation: An analysis of more than 10,000 cases. Ann Thorac Surg. 2009;88(4):1062–1070. doi: 10.1016/j.athoracsur.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Allen JG, Arnaoutakis GJ, Orens JB, et al. Insurance status is an independent predictor of long-term survival after lung transplantation in the united states. J Heart Lung Transplant. 2011;30(1):45–53. doi: 10.1016/j.healun.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: Twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010;29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Weiss ES, Allen JG, Modi MN, Merlo CA, Conte JV, Shah AS. Lung transplantation in older patients with cystic fibrosis: Analysis of UNOS data. J Heart Lung Transplant. 2009;28(2):135–140. doi: 10.1016/j.healun.2008.11.903. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JASA. 1958;53:457–481. [Google Scholar]
- 21.Cox DR. Regression models and life-tables (with discussion) Journal of the Royal Statistical Society (Series B) 1972;74:187–200. [Google Scholar]
- 22.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the pulmonary scientific council of the international society for heart and lung transplantation. J Heart Lung Transplant. 2006;25(7):745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Liou TG, Adler FR, Cox DR, Cahill BC. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med. 2007;357(21):2143–2152. doi: 10.1056/NEJMoa066359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wray J, Radley-Smith R. Cognitive and behavioral functioning of children listed for heart and/or lung transplantation. Am J Transplant. 2010;10(11):2527–2535. doi: 10.1111/j.1600-6143.2010.03282.x. [DOI] [PubMed] [Google Scholar]


