Abstract
Background
Brooke-Spiegler syndrome (BSS) is probably an underdiagnosed genodermatosis that predisposes for the development of cylindromas, spiradenomas and trichoepitheliomas mainly of the head and neck. Wide phenotypic variability regarding the number and type of lesions can be observed within a family. Mutations of the CYLD gene are identified in the vast majority of cases and play a key role in BSS pathogenesis.
Main observations
Two first degree relatives with numerous erythematous telangiectatic nodules of the scalp present for decades, with recurring tendency regardless the multiple previous excisions. Histopathological review of the lesions revealed predominantly "spiradenocylindromas" in the proband and cylindromas in her sister. The suspicion of BSS was confirmed after detection of a new nonsense germline mutation of CYLD (c.1783C>T pGln 595*) in the proband.
Conclusions
BSS diagnosis can be challenging and is based on clinical-pathological correlation, positive familial association and identification of CYLD mutations. CYLD exerts antineoplastic effects by downregulating intracellular NF-κB signalling pathways. The reported mutation affecting the ubiquitin-specific protease domain leads to a truncated and catalytically inactive enzyme. Despite the expanding list of CYLD mutations no firm genotype-phenotype correlation is known so far. Early recognition and treatment of BSS avoid disfiguring changes like "turban tumor".
Keywords: CYLD protein, cylindroma, dermoscopy, gene, mutation, spiradenoma
Introduction
Brooke-Spiegler syndrome (BSS; OMIM 605041) is a rare hereditary disorder characterized by predisposition for multiple skin adnexal tumors derived from the folliculo-sebaceous-apocrine unit, specifically cylindromas, spiradenomas and trichoepitheliomas.[1,2]
These tumors that are uncommon in the general population, begin during adolescence, typically involving the scalp, face and neck, and gradually increase in number and size throughout life.[3,4]
The two ends within the phenotypic spectrum of BSS are familial cylindromatosis (FC; OMIM 132700) and multiple familial trichoepithelioma (MFT; MIM 601606), allelic disorders that share germline mutations of the cylindromatosis (CYLD) tumor-suppressor gene. Different manifestations of each can be present to a variable degreewithin a single family.[1-3]
Apart from skin adnexal tumors, an increased incidence of salivary gland, parathyroid and ovarian neoplasms has been found in patients with BSS, as well as inflammatory disorders such as ulcerative colitis. This probably reflects the physiological importance and ubiquity of CYLD protein expression.[1,5,6]
Despite the growing number of mutations reported over the last decade, there is no defined genotype-phenotype correlation in BSS.[1,3,7]
We describe two members of a family affected by multiple nodular scalp lesions, present for decades and with clinical and histological features consistent with BSS and confirmed after identification of a new germline mutation of CYLD gene.
Case reports
Proband:
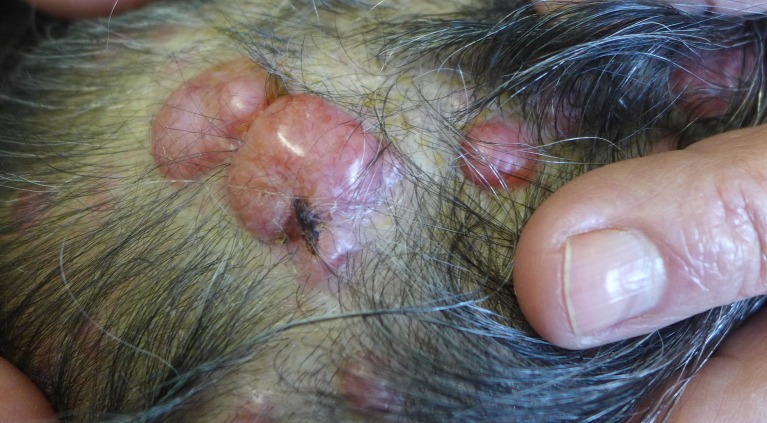
An 80-year-old Portuguese Caucasian woman presented with multiple slowly growing papules and nodules of the scalp, some episodically painful, which started during adolescence. Thirteen lesions were excised in a general surgery department, due to pain and aesthetic discomfort, but a dermatology evaluation was requested due to the emergence of new scalp lesions. Clinical examination showed multiple aggregated erythematous telangiectatic papules and nodules on the scalp, sometimes lobulated, with elastic consistency ranging in size from 1x1 to 2x3 cm [Fig. 1]. Most lesions shared dermoscopic features of cylindromas with arborizing telangiectasia and scattered white globules on a milky to salmon-pink background [Fig. 2]. Excisional biopsy of three nodules revealed, in all of them, a predominant spiradenoma pattern, with multiple inter-anastomosing trabeculae composed of a biphasic cell population (small cells with hyperchromatic nuclei at the periphery and clear larger cells at the centre) surrounded by a highly vascularized hyaline stroma and with a sparse lymphocytic infiltrate [Fig. 3]. Areas of cylindroma were also seen, with nests of basaloid cells in close apposition in a jigsaw puzzle-like pattern surrounded by a thick hyaline membrane [Fig. 4]. Histopathological review of previously excised lesions confirmed the presence of mainly "spiradenocylindromas", and less often cylindromas and spiradenomas, raising the suspicion of BSS.
Figure 1.
Scalp lesions. Confluent erythematous telangiectatic nodules and papules on the scalp of the proband, consistent with spiradenocylindromas and cylindromas.
Figure 2.
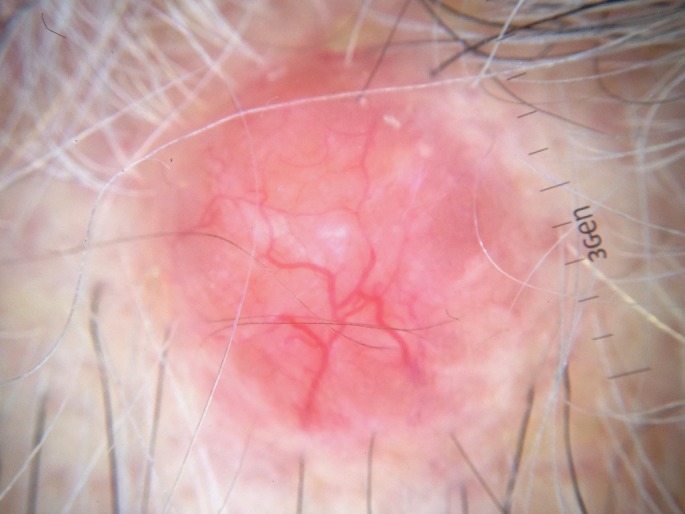
Dermatoscopic features of cylindroma within a scalp nodule. Arborizing telangiectasia more pronounced at the periphery, scattered white globules at the centre and a salmon-pink background.
Figure 3.
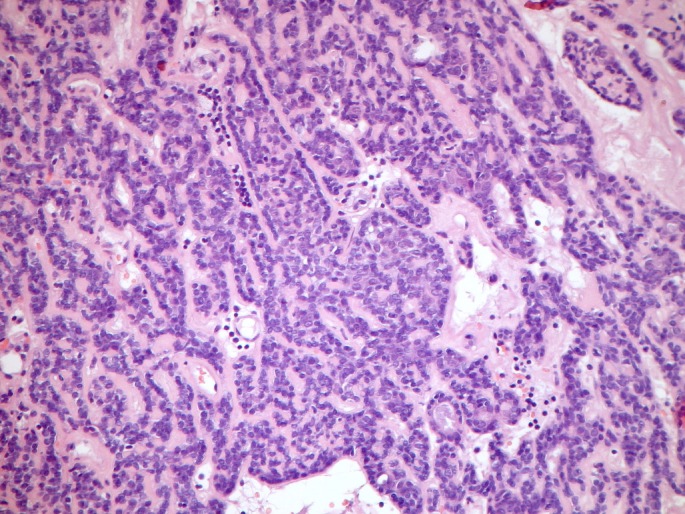
Spiradenoma pattern. Multiple inter-anastomosing trabeculae composed of small cells with hyperchromatic nuclei at the periphery and clear larger cells at the centre. Sparse lymphocytic infiltrate.
Figure 4.
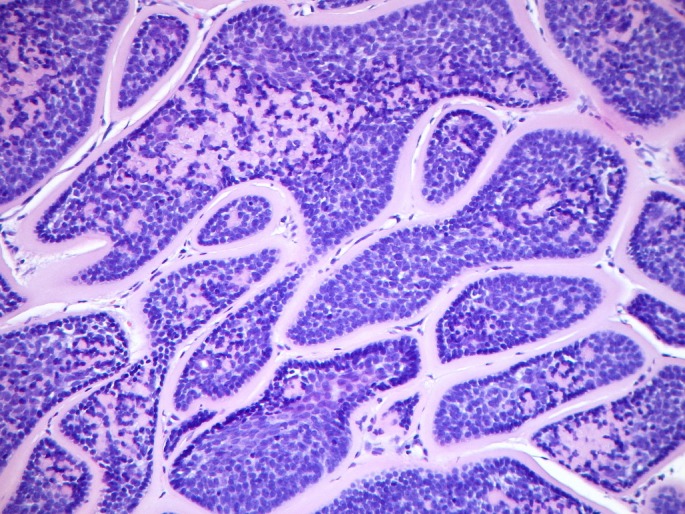
Cylindroma pattern. Nests of basaloid cells in a "jigsaw" puzzle pattern surrounded by a thick hyaline membrane. Tendency for palisading of epithelial darker cells at the periphery.
Case 2:
The 77-year-old sister of the proband also presented similar scalp lesions for decades: aggregated erythematous nodules with 2 to 3 cm in diameter, with smooth telangiectatic surface. Lesions began in the second decade of life and five of them had already been removed in another hospital for esthetical reasons, with no histological evaluation known. Two further symptomatic nodules that were excised corresponded to typical cylindromas histologically.
Within the family, four of ten uncles had a history of multiple scalp lesions. Patients’ offspring were, so far, not affected by similar lesions.
In the presence of multiple adnexal scalp tumors with histological features of cylindroma and spiradenoma in two first-degree relatives we considered the diagnosis of BSS.
The proband was assessed for CYLD gene germline mutation in peripheral blood sample. A heterozygous nonsense mutation was detected in exon 11 of CYLD gene, responsible for the introduction of a premature stop codon at position 595 amino acid (c.1783C>T pGln 595*).
Patients referred no other complaints and clinical and ultrasound evaluation of salivary glands as well as abdominal ultrasound were normal.
Discussion
BSS is probably an underdiagnosed autosomal dominant genodermatosis, with variable but increasing penetrance with age (60-100%), resulting from germline mutations in the CYLD gene located on chromosome 16q12-q13.[1,3,8] These mutations are found roughly in 80-85% of BSS cases.[7] However, in the remaining cases one should take into account that de novo mutations of other germline genes, alterations in noncoding regions, large chromosomal rearrangements or deletions, or other changes not detected by conventional sequencing can be present.[2,7]
CYLD gene consists of 20 exons, 17 of which translate an evolutionarily conserved 120 kDa cytoplasmic protein belonging to the family of deubiquitinating enzymes.[1,7] It exerts a tumor suppressor effect by negative regulation of the intracellular signalling pathways of NF-κB, JNK and Wnt.[4,5] CYLD mutations ultimately increase NF-κB expression, leading to resistance to apoptosis and facilitating tumor development.[4,5] There are currently at least 93 known germline mutations of CYLD, most of them involving its 3‘ terminal, which translates the catalytic domain of the enzyme (exons 8-20).[3] About 90% of the tumor-eliciting mutations result in a truncated CYLD protein and are mainly of frameshift or nonsense type.[1,3,7]
In our proband we detected a new germline heterozygous mutation of CYLD, (c.1783C>T p.Gln595*), responsible for the introduction of a premature stop codon. This mutation in exon 11 (aa 595), at the beginning of ubiquitin-specific protease (USP) domain (aa 583-956) leads to the production of a truncated and catalytically inactive enzyme. The subsequent loss of heterozygosity of wild-type allele at CYLD locus, or the (more frequent) occurrence of other somatic mutations of CYLD may be responsible for the development of the lesions described.[1,7]
As in our cases, the lesions more frequently found in BSS cases are cylindromas and spiradenomas. These closely-related tumors share the same anatomical distribution, have controversial histogenesis and can occur close together in the same patient. They are usually poorly differentiated, and show overlapping cytomorphological and immunohistochemical features of apocrine and eccrine lineage.[8,9]
Nevertheless, Sellheyer recently proposed that spiradenomas and cylindromas are not sudoriparous but poorly differentiated follicular tumors derived from the hair follicle bulge. He based his proposal on the increased expression of CD200 in both types of lesions whereas, in his extensive study, he had shown that tumors classified as eccrine in lineage (hidradenoma, poroma, dermal duct tumor, and hidroacanthoma simplex) were CD200 negative.[9]
Cylindromas are benign basaloid painless tumors distributed preferentially over the areas that are rich in hair follicles and sebaceous glands, typically begin during puberty although sometimes earlier, grow slowly and affect mainly women, with a ratio 6-9:1.[1,3,8,10] Clinically they consist of nodular erythematous lesions with overlying telangiectasia and variable size distributed over the scalp, face and neck, whereas the trunk, extremities and the pubic region are less affected.[3,10] Multiple cylindromas may coalesce in the scalp and form the so-called turban tumor.[1,7,10] Malignant transformation of cylindromas may occur with an aggressive behaviour in the context of BSS, characterized by rapid growth, pink-blue colour, ulceration and haemorrhage.[3] The reported dermatoscopic features of cylindroma include arborizing vessels more pronounced at the periphery on a whitish-pinkish background, blue dots and globules and ulceration.[11] These findings are similar to those observed in the excised spiradenocylindromas of the proband. Histologically, cylindromas are non-encapsulated dermal nodules composed of islands and cords of basaloid cells, apposed in a jigsaw-puzzle pattern and surrounded by a thick, hyaline, PAS-positive basement membrane. Epithelial cells at the periphery of the islands have a tendency for palisading and are darker than those located in the centre of the nodules.[1,12]
Spiradenomas typically present as purple to bluish nodules of variable size in nearly any location, and can be associated with paroxystic pain. They commonly occur together with cylindromas, giving rise to spiradenocylindromas denoting the possible common histogenesis.[1,7] Histologically spiradenomas consist of one or more sharply delineated large basophilic nodules within the dermis, sometimes extending into the subcutis, composed of trabeculae, sheets, cords or islands with two main cell types: small epithelial basaloid cells with hyperchromatic nuclei at the periphery and larger cells with pale nuclei in the centre. Duct-like structures can be observed within the lesions. Larger spiradenomas may have a hyaline stroma and vascular ectasia. Unlike cylindromas, they may be peppered with lymphocytes.[1,7,12]
Although not observed in our patients, trichoepitheliomas may also integrate the clinical spectrum of BSS. They are benign neoplasms with follicular germinative differentiation. Trichoepitheliomas classically occur as skin coloured papules of the midface, mainly on the nose and nasolabial folds.[1,2,8] Microscopically they consist of uniform basaloid cells with peripheral palisading arranged in variably sized nodules or nests, frequently in a cribriform pattern and with central keratinization of the infundibular type keratinization. These cells are surrounded by a dense stroma containing papillary mesenchymal bodies.[12]
Since BSS is an autosomal dominant genodermatosis, if we assume that these sisters share the same germline mutation, it becomes clear that there is a lack of genotype-phenotype correlation with mainly spiradenocylindromas in one case and only cylindromas in the other. In fact, it has been difficult to establish these correlations due to intra- and interfamilial variability of the clinicopathologic manifestations of BSS, highlighting the putative role of other genetic (e.g. interaction of loss of heterozygosity or other somatic mutations with germline CYLD mutations) or still unrecognized epigenetic events.[3,7]
The clinical spectrum of BSS extends beyond the skin with major and minor salivary glands being the most frequent location of extracutaneous neoplasms, namely basal cell adenomas and adenocarcinomas. Although this risk is low, their evaluation is imperative.[2,4] Ponti and colleagues recently reported an ovarian Brenner tumor positive for a CYLD mutation in a BSS patient.[6]
So far there are no curative therapies for the skin lesions of BSS. Various ablative and non-ablative techniques have been used with variable results. Ideally they should be attempted early to avoid disfiguring changes such as the turban tumor. The most common ablative therapies consist of classical excision, dermoabrasion, CO2 or erbium-YAG lasers, or cryotherapy.[5,8] In the case of cylindromas, enucleation has been successfully used for simultaneous but minimally aggressive removal of multiple lesions.[13]
Total scalp excision followed by combined reconstruction with artificial dermis and split skin graft has been used in turban tumor cases, with good results.[10,14]
Topical formulations containing salicylic acid or prostaglandin A1, which act by inhibiting NF-kB, are a possible nonablative treatment for solitary or small sized lesions.[4,14,15]
Conclusion
Early recognition of BSS is imperative, because of the potential multiplicity of disfiguring lesions within members of a family, the risk of malignant transformation, as well as the association with other neoplasms. Genetic counselling and dermatological evaluation should be provided in these cases.
References
- Blake PW, Toro JR. Update of cylindromatosis gene (CYLD) mutations in Brooke-Spiegler syndrome: novel insights into the role of deubiquitination in cell signaling. Hum Mutat. 2009;30:1025–1036. doi: 10.1002/humu.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Nasti S, Losi L, Pastorino L, Pollio A, Benassi L, Giudice S, Bertazzoni G, Veratti E, Azzoni P, Bianchi Scarrà G, Seidenari S. Brooke-Spiegler syndrome: report of two cases not associated with a mutation in the CYLD and PTCH tumor-suppressor genes. J Cutan Pathol. 2012;39:366–371. doi: 10.1111/j.1600-0560.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- Guardoli D, Argenziano G, Ponti G, Nasti S, Zalaudek I, Moscarella E, Lallas A, Piana S, Specchio F, Martinuzzi C, Raucci M, Pellacani G, Longo C. A novel CYLD germline mutation in Brooke-Spiegler syndrome. J Eur Acad Dermatol Venereol. 2015;29:457–462. doi: 10.1111/jdv.12578. [DOI] [PubMed] [Google Scholar]
- Ponti G, Pellacani G, Seidenari S, Pollio A, Muscatello U, Tomasi A. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239–256. doi: 10.1016/j.critrevonc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Peltonen S, Kankuri-Tammilehto M. Brooke-Spiegler syndrome associated with ulcerative rectosigmoiditis. Acta Derm Venereol. 2013;93:112–113. doi: 10.2340/00015555-1391. [DOI] [PubMed] [Google Scholar]
- Ponti G, Ruini C, Girolomoni G, Pellacani G, Farnetani F, Pastorino L, Ghiorzo P, Witkowski AM, Bianchi-Scarrà G, Tomasi A, Loschi P, Nasti S. Brooke-Spiegler syndrome tumor spectrum beyond the skin: a patient carrying germline R936X CYLD mutation and a somatic CYLD mutation in Brenner tumor. Future Oncol. 2014;10:345–350. doi: 10.2217/fon.13.198. [DOI] [PubMed] [Google Scholar]
- Grossmann P, Vanecek T, Steiner P, Kacerovska D, Spagnolo DV, Cribier B, Rose C, Vazmitel M, Carlson JA, Emberger M, Martinek P, Pearce RL, Pearn J, Michal M, Kazakov DV. Novel and recurrent germline and somatic mutations in a cohort of 67 patients from 48 families with Brooke-Spiegler syndrome including the phenotypic variant of multiple familial trichoepitheliomas and correlation with the histopathologic findings in 379 biopsy specimens. Am J Dermatopathol. 2013;35:34–44. doi: 10.1097/DAD.0b013e31824e7658. [DOI] [PubMed] [Google Scholar]
- Rathi M, Awasthi S, Budania SK, Ahmad F, Dutta S, Kumar A. Brooke-spiegler syndrome: a rare entity. Case Rep Pathol. 2014;2014:231895. doi: 10.1155/2014/231895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellheyer K. Spiradenoma and cylindroma originate from the hair follicle bulge and not from the eccrine sweat gland: an immunohistochemical study with CD200 and other stem cell markers. J Cutan Pathol. 2015;42:90–101. doi: 10.1111/cup.12406. [DOI] [PubMed] [Google Scholar]
- Singh DD, Naujoks C, Depprich R, Schulte KW, Jankowiak F, Kübler NR, Handschel J. Cylindroma of head and neck: review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516–521. doi: 10.1016/j.jcms.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Cohen YK, Elpern DK. Dermatoscopic pattern of a cylindroma. Dermatol Pract Concept. 2014;4:67–68. doi: 10.5826/dpc.0401a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon D. 33 - Tumors of cutaneous appendages. In: Weedon's Skin Pathology (Weedon D, ed), 3rd edn. Edinburgh: Churchill Livingstone; 2010. pp. 757–807. [Google Scholar]
- Brass D, Rajan N, Langtry J. Enucleation of cylindromas in Brooke-Spiegler syndrome: a novel surgical technique. Dermatol Surg. 2014;40:1438–1439. doi: 10.1097/DSS.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren LJ, Ferdinandus P, van der Hulst R, Frank J, Tuinder S. A novel therapeutic strategy for turban tumor: scalp excision and combined reconstruction with artificial dermis and split skin graft. Int J Dermatol. 2014;53:246–249. doi: 10.1111/ijd.12199. [DOI] [PubMed] [Google Scholar]
- Oosterkamp HM, Neering H, Nijman SM, Dirac AM, Mooi WJ, Bernards R, Brummelkamp TR. An evaluation of the efficacy of topical application of salicylic acid for the treatment of familial cylindromatosis. Br J Dermatol. 2006;155:182–185. doi: 10.1111/j.1365-2133.2006.07224.x. [DOI] [PubMed] [Google Scholar]