Abstract
Background
Inteleukin (IL)12 and IL23 are two main cytokines involved in the pathogenesis of immune-mediated disease. IL12 is produced by macrophages and B lymphocytes and mediates differentiation of Th1 lymphocytes, while IL23 is a pro-inflammatory cytokine essential for the differentiation of Th17 cells. Ustekinumab is a human monoclonal antibody directed against the p40 protein subunit shared by IL12 and IL23, therefore it blocks the signal transmission of both cytokines.
Main observations
We present two cases and discuss the long-term efficacy of ustekinumab as a treatment of psoriasis in patients affected by autoimmune diseases, rheumatoid arthritis and Sjögren's syndrome, who presented with severe psoriasis after anti-TNF treatment.
Conclusions
To the best of our knowledge, these are the first cases reported in the literature describing the long-term good efficacy of ustekinumab not only on paradoxical forms of psoriasis induced by anti-TNF-α drugs, but also on the articular involvement in a patient affected by RA and in a patient affected by Sjögren syndrome.
Keywords: psoriasis, autoimmune diseases, Ustekinumab, rheumatoid arthritis, Sjögren's syndrome
Introduction
Th17 cells represent a subset of T cells able to produce IL-17, a potent inflammatory cytokine involved in the pathogenesis of several autoimmune diseases, such as psoriasis, psoriatic arthritis, systemic lupus erythematosus and rheumatoid arthritis.[1,2]
It has been proposed that in systemic autoimmune diseases both type I INF and IL-17/Th17 responses contribute to disease pathogenesis and that both systems may sustain each other.[3] In particular, IL-17 has been proposed to act in synergy with TNF-α. Different cytokines are involved in the differentiation of Th17 cells from naïve T cells, for instance IL23, which is a pro-inflammatory cytokine, composed of two subunits, p19 and p40. The p40 subunit is shared with IL12. IL23 and IL12 have different receptors and different effects: IL12, secreted by macrophages and B lymphocytes, induces development of INF-γ producing Th1 cells; IL23 is involved in differentiation of Th17 cells in a proinflammatory context and especially in the presence of TGF-β and IL-6. IL-17, acting in combination with other pro-inflammatory cytokines, induces osteoclastogenesis, cartilage collagen breakdown and has a regulatory role on synovial cells (FLS).[4,5] High serum concentrations of interleukin-23 (IL23) and polymorphisms in the IL23 receptor are associated also with ankylosing spondylitis and enthesitis.[6] The role of IL23 in the induction of psoriasis has been confirmed in a mouse model by injecting IL23 in the skin of the ears; this resulted in epidermal hyperplasia and inflammatory cellular infiltration similar to psoriasis, which was mediated by TNF-α, IL-22, IL-17A and IL-17F.[7]
Ustekinumab is a fully human monoclonal antibody directed against the p40 protein subunit shared by IL12 and IL23, it prevents the interaction of IL12 and IL23 with their cell surface receptors, blocking Th-1/IL12 and Th-17/IL23 inflammatory pathways.[8] Ustekinumab is currently approved for the treatment of moderate to severe psoriasis and for the treatment of active psoriatic arthritis, leading to a rapid and durable improvement of both diseases.[9]
Case description
We present two cases of patients suffering from psoriasis, associated with autoimmune diseases as rheumatoid arthritis and Sjögren's syndrome.
Case 1
A 60-year-old woman suffering from rheumatoid arthritis, arterial hypertension and previous tuberculosis, was referred to our combined dermatology and rheumatology outpatients clinic because she developed diffuse cutaneous psoriasis during treatment with anti-TNF (adalimumab). The diagnosis of rheumatoid arthritis was made 6 years ago when she developed symmetrical polyarthritis of proximal interphalangeal (IFP) and metacarpophalangeal (MCP) joints, wrists, ankles and knees. At the time of diagnosis erythrocyte sedimentation rate (ESR) was 48 mm/h (normal value <20 mm/h), C-reactive protein (CRP) was 13 mg/L (normal value <3 mg/L), rheumatoid factor (FR) was 313 IU/mL (nv <15 IU/mL) and anti-citrullinated protein antibodies (ACPA) were 165 IU/mL (nv <20 IU/mL).
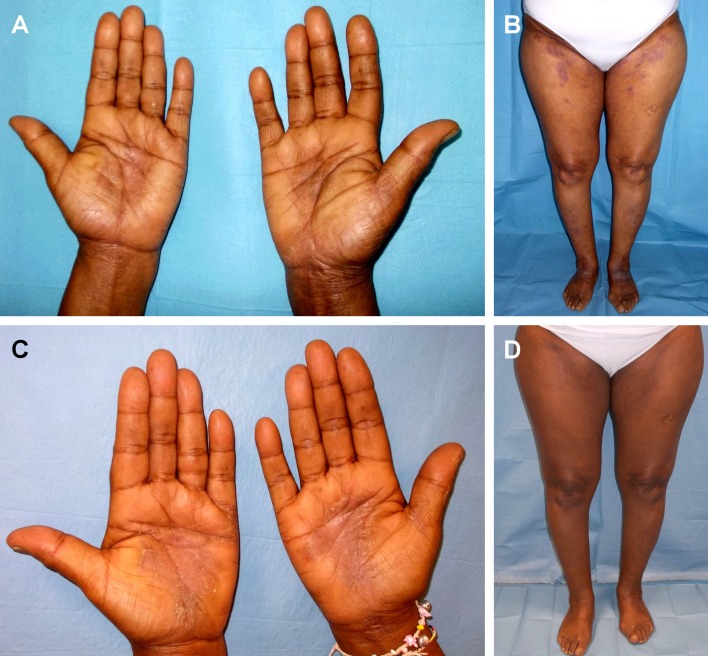
Throughout the years, she had been treated with several disease modifying anti-rheumatic drugs (sDMARDs), such as as methotrexate, sulfasalazine, leflunomide, hydroxychlorochine and glucocorticosteroids, and with etanercept. These treatments were ineffective or not well tolerated. After 12 months from the diagnosis, adalimumab 40 mg subcutaneous every 2 weeks in combination with methotrexate 15 mg weekly was started with improvement, and Disease Activity Score (DAS28) — ESR of 2.1 was reached in 9 months of treatment. After 12 months of treatment, diffuse psoriatic plaques with palmoplantar involvement was observed with a Psoriasis Area and Severity Index Score (PASI) of 28. [Fig. 1A]. Laboratory evaluation showed: ESR 18 mm/h, CRP 3 mg/L, FR 38 IU/mL, ACPA 112 IU/mL.
Figure 1.
Diffuse psoriasis with palmoplantar involvement (PASI 12.8) in a patient with rheumatoid arthritis before treatment (A, B) and after 24 weeks of treatment (PASI 2.1) (C, D).
Treatment with ustekinumab, 45 mg subcutaneous injection, was started. After 12 weeks, the patient showed a cutaneous improvement (PASI 10). The articular disease was stable, no swelling or/and tenderness in joints was observed. After 24 weeks of treatment, psoriasis persisted exclusively at the hands and feet (PASI 6.1). At 28 weeks, a complete resolution of the skin involvement (PASI 0) and stability of the joints (DAS28-ESR 1.9) were noticed [Fig. 1B] and was stable at follow-up after 3 years of treatment. Laboratory tests revealed ESR 16, CRP 0 mg/L, FR 32,7 IU/mL and ACPA 123 IU/mL.
Case 2
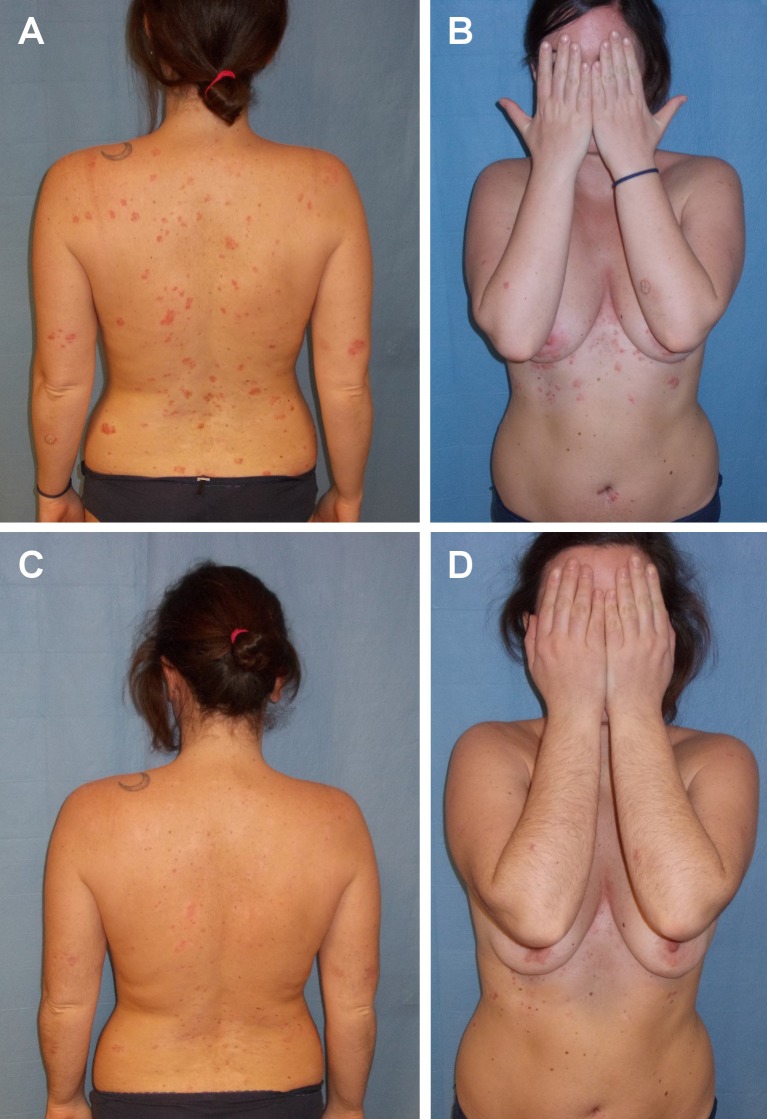
A 43-year-old woman affected by Sjögren syndrome and diffuse psoriatic plaques was referred to our combined rheumatology — dermatology out-patient clinic. Onset of psoriasis was 2 years ago, 6 months after delivery of her third child. She was previously treated with methotrexate, cyclosporine, infliximab and etanercept, all discontinued because of ineffectiveness. After 18 months she developed arthritis of knees, ankles, both wrists and MCP of the II and III fingers. The patient also developed xerostomia and xerophatlmia [Fig. 2A]. Laboratory tests revealed anti-nuclear antibodies (ANA) 1:160 speckled, anti-Sjögren’s-syndrome-related antigen A antibodies (Ro/anti-SSA) positive at high titer, FR and ACPA negative, ESR 28 mm/h, and CRP 7 mg/L (<3 mg/L). Schirmer’s test and break-up time (BUT) were positive and articular MRI showed synovitis and bone erosions of the left wrist. The patient was diagnosed with Sjögren's syndrome. Therapy with methylprednisolone 40 mg and hydroxychloroquine was started with initial benefit on the articular involvement, on the skin lesions and on the xerosis- related symptoms. After one month however, hydroxychloroquine was suspended because of itching and worsening of psoriasis. Therapy with azathioprine 100 mg/d and prednisone 25 mg/d (to be progressively tapered) was begun, with control of clinical symptoms. These treatments were however discontinued after 2 months because of an increase in transaminase levels. Therefore, treatment with ustekinumab 45 mg associated with prednisone 25 mg/day (progressively tapered) was started, with drastic improvement of psoriasis (from PASI 10.2 to PASI 2 in 24 weeks), and improvement in joint pain [Fig. 2B]. MRI of the wrist at 24 weeks of therapy with ustekinumab showed remission of synovitis and stability of bone erosions. After 3 years of ustekinumab treatment no signs or symptoms related to joint involvement nor cutaneous disease are present.
Figure 2.
Diffuse psoriasis (PASI 8) in a patient with Sjögren syndrome before treatment (A, B) and after 24 weeks of treatment (PASI 2.4) (C, D).
Discussion
Skin lesions developing after anti-TNFα therapy were first reported as case reports of patients with inflammatory bowel disease (IBD);[10] they also have been described in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondilytis. A French study from 2010, to date the largest one on paradoxical effect of anti-TNF-α therapy, reviewed the clinical history of 85 patients affected by IBD (69 with Crohn’s disease, 15 with ulcerative colitis, 1 with indeterminate colitis) who developed inflammatory skin lesions after anti-TNF- treatment. The majority of these lesions (73%) were classified as psoriasiform, the remaining ones (27%) were classified as eczematiform lesions.[11] The skin symptoms did not correlate with intestinal symptoms, and women, especially those with a familial history of skin diseases, were more likely to develop these symptoms than men.
According to the results for the British Society for Rheumatology Biologics Register, in patients with rheumatoid arthritis treated with anti-TNF, the incidence rate for the development of new-onset psoriasis is 1.04 per 1,000 patientyears (95% CI, 0.97-1.54).[12] Patients treated with adalimumab, naïve to any other anti-TNF-α drugs, had a higher incidence rate of psoriasis than patients treated with infliximab or etanercept.[13] Besides skin manifestations, some patients receiving anti-TNF-α treatment, have been reported to develop also autoimmune diseases, sarcoidosis, uveitis and scleritis, de novo IBD and de novo joint symptoms. The first case series reporting paradoxical joint symptoms after anti-TNF-α treatment, is an unpublished study from Van Moerkercke. This study included a cohort of 1300 patients receiving adalimumab or infliximab for IBD. Twenty-one of these patients developed polyarthralgia, among them 19 had never reported arthralgia or arthritis in their medical history; only 2 patients had a known spondylarthropathy with axial localization but developed supplementary complaints of peripheral polyarthralgia. In most patients, these symptoms corresponded to morning stiffness and pain, involving the hands and wrists, but no signs of arthritis with synovitis were found. Interestingly, high titers of ANAs (>1:280) were found in 11 patients.[14] Paradoxical inflammation should occur during anti-TNF-α treatment. There are probably multiple pathogenic mechanisms involved. In fact, the neutralization of TNF-α sustains IFNα production by dendritic cells (pDCs) and enhances the expression of other pro-inflammatory cytokines IL-1β, IL-6, IL-17, Il-21 and IL-22, also reducing the number of circulating Treg cells.[15,16] Clearly, the triangular interplay between TNF-α, type I IFNs and IL-17 of great importance and targeting one of these cytokines may affect the others.[17]
The IL23/IL-17 pathway inhibitors are a new group of biologic drugs for the treatment of both psoriasis and psoriatic arthritis. Therapies that modulate IL23/IL12 directly (such as ustekinumab) affect both Th1 and Th17 differentiation.[18] Considering its modulating activity on different cytokines from TNF-α, described cases show that the use of Ustekinumab may be considered in those paradoxical refractory forms of skin psoriasis induced by treatment with anti-TNF-α and in the course of autoimmune diseases. To the best of our knowledge, these are the first cases reported in the literature describing the long-term efficacy of Ustekinumab not only on paradoxical forms of psoriasis induced by anti-TNF-α drugs, but also on the articular involvement in a patient affected by RA and in a patient affected by Sjögren syndrome.
References
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi A, Espinosa A, Wahren-Herlenius M. IL-17: a new actor in IFN-driven systemic autoimmune diseases. Eur J Immunol. 2012;42:2274–2284. doi: 10.1002/eji.201242653. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- Koshy PJ, Henderson N, Logan C, Life PF, Cawston TE, Rowan AD. Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokines. Ann Rheum Dis. 2002;61:704–713. doi: 10.1136/ard.61.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, Eberl G, Buckley CD, Kastelein RA, Pierce RH, Laface DM, Cua DJ. IL23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, Gorman DM, Smith K, de Waal Malefyt R, Kastelein RA, McClanahan TK, Bowman EP. IL23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett BL, Tyring SK. Ustekinumab for chronic plaque psoriasis. Lancet. 2008;371:1639–1640. doi: 10.1016/S0140-6736(08)60702-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, Fretzin S, Kunynetz R, Kavanaugh A. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–640. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- Adams DR, Buckel T, Sceppa JA. Infliximab associated new-onset psoriasis. J Drugs Dermatol. 2006;5:178–179. [PubMed] [Google Scholar]
- Rahier JF, Buche S, Peyrin-Biroulet L, Bouhnik Y, Duclos B, Louis E, Papay P, Allez M, Cosnes J, Cortot A, Laharie D, Reimund JM, Lémann M, Delaporte E, Colombel JF. et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol. 2010;8:1048–1055. doi: 10.1016/j.cgh.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol. 2012;9:496–503. doi: 10.1038/nrgastro.2012.125. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Dixon WG, Watson KD, King Y, Groves R, Hyrich KL, Symmons DP. et al. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2009;68:209–215. doi: 10.1136/ard.2007.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino G, Danese S, Pariente B, Allez M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-α agents. Autoimmun Rev. 2014;13:15–19. doi: 10.1016/j.autrev.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF-IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HL, Napierata L, Stedman N, Benoit S, Collins M, Nickerson-Nutter C, Young DA. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. 2010;62:430–440. doi: 10.1002/art.27203. [DOI] [PubMed] [Google Scholar]
- Grine L, Dejager L, Libert C, Vandenbroucke RE. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25–33. doi: 10.1016/j.cytogfr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Novelli L, Chimenti MS, Chiricozzi A, Perricone R. The new era for the treatment of psoriasis and psoriatic arthritis: perspectives and validated strategies. Autoimmun Rev. 2014;13:64–69. doi: 10.1016/j.autrev.2013.08.006. [DOI] [PubMed] [Google Scholar]