Summary
Bilaterally symmetric motor patterns—those in which left-right pairs of muscles contract synchronously and with equal amplitude (such as breathing, smiling, whisking, locomotion)—are widespread throughout the animal kingdom. Yet surprisingly little is known about the underlying neural circuits. We performed a thermogenetic screen to identify neurons required for bilaterally symmetric locomotion in Drosophila larvae, and identified the evolutionarily-conserved Even-skipped+ interneurons (Eve/Evx). Activation or ablation of Eve+ interneurons disrupted bilaterally symmetric muscle contraction amplitude, without affecting the timing of motor output. Eve+ interneurons are not rhythmically active, and thus function independently of the locomotor CPG. GCaMP6 calcium imaging of Eve+ interneurons in freely-moving larvae showed left-right asymmetric activation that correlated with larval behavior. TEM reconstruction of Eve+ interneuron inputs and outputs showed that the Eve+ interneurons are at the core of a sensorimotor circuit capable of detecting and modifying body wall muscle contraction.
Introduction
Bilaterally symmetric motor patterns—those in which muscle contractions on the left and right sides of the body occur synchronously and with equal amplitude—are widespread throughout the animal kingdom. They regulate respiration, speech, smiling, whisking, flight, and various locomotor gaits. Surgical manipulations in both vertebrates and invertebrates have shown that contralaterally-projecting commissural interneurons are required for bilaterally symmetric motor output, demonstrating that symmetric motor output is not merely a default state (Dubayle and Viala, 1996; Jahan-Parwar and Fredman, 1980; Lanuza et al., 2004; Murchison et al., 1993; von der Porten et al., 1982). In the mouse, genetic deletion of the Dbx1+ transcription factor from V0 interneurons disrupted left-right synchronous motor output during respiration and caused perinatal lethality (Bouvier et al., 2010). Loss of dbx1 locus affects both ventral Evx1+ interneurons and dorsal Evx1− interneurons, whereas a more specific loss of just the dorsal Dbx1+ interneurons had no effect on breathing. Taken together these data implicate Evx1+ interneurons in regulating respiratory motor rhythms (Bouvier et al., 2010). However, this interpretation is clouded by the observation that mice lacking Evx1 protein appear to breathe normally, despite any detectable Evx1 or Evx2 protein in V0 interneurons (Moran-Rivard et al., 2001). These findings demonstrate how little we understand about the molecules and neural circuitry underlying bilaterally symmetric motor output, despite its broad and essential functions.
Drosophila larval crawling is a genetically tractable model system for investigating the molecular and neuronal underpinnings of symmetric motor output. Larval crawling is a simple, robust motor behavior that involves waves of rhythmic, bilaterally symmetric body wall muscle contractions (Heckscher et al., 2012). The segmented larva has ~30 bilateral body wall muscles per segment and a similar number of motor neurons, and their role during larval locomotion has been characterized (Berni et al., 2012; Crisp et al., 2008; Crisp et al., 2011; Dixit et al., 2008; Heckscher et al., 2012; Hughes and Thomas, 2007; Lahiri et al., 2011; Pulver and Griffith, 2010; Schaefer et al., 2010). In contrast, there are ~270 bilateral interneurons per segment (Heckscher et al., 2014; Rickert et al., 2011) and their role in locomotion is almost completely unknown (Kohsaka et al., 2014). Recently, we identified several hundred Gal4 lines that express in a sparse pattern of neurons in the late embryonic CNS, and determined their expression pattern at single neuron resolution for 75 of these lines (Heckscher et al., 2014; Manning et al., 2012). We used this collection of sparsely-expressed Gal4 lines to express the warmth-activated TRPA1 cation channel and screen for locomotor defects in newly hatched larvae. We identified a small pool of interneurons (“ELs”) that express the evolutionarily-conserved transcription factor Even-skipped (Eve; Evx1/2 in mammals) that are required to maintain bilaterally symmetric motor output.
Eve/Evx+ interneurons are found in the nerve cord of almost all bilateral animals examined to date, including annelids, chordates, insects, fish, birds and mammals, as well as a proposed common ancestor between invertebrates and vertebrates, Platynereis dumerilii (Avaron et al., 2003; Copf et al., 2003; Denes et al., 2007; Ferrier et al., 2001; Holland, 2013; Ikuta et al., 2004; Moran-Rivard et al., 2001; Sordino et al., 1996; Takatori et al., 2008; Thaeron et al., 2000). In all cases where the morphology of Eve/Evx+ interneurons has been examined, they have contralateral ascending projections, such as the zebrafish CoSA and mouse V0v interneurons (Figure 1A)(Moran-Rivard et al., 2001; Suster et al., 2009). In flies, Eve is expressed in segmentally-reiterated subsets of interneurons and motor neurons, but not in the brain (Figure S1)(Frasch et al., 1987). The Eve/Evx transcription factor is well known to specify neuronal identity and regulate axon pathfinding in fly and worm motor neurons as well as in mammalian interneurons (Broihier and Skeath, 2002; Doe et al., 1988; Esmaeili et al., 2002; Fujioka et al., 2003; Landgraf et al., 1999; Moran-Rivard et al., 2001; Zarin et al., 2014). However, despite years of intense study the behavioral role of the Eve/Evx+ interneurons remains poorly defined. Our results show that the Eve+ interneurons are part of a sensorimotor circuit that maintains left-right symmetric of muscle contraction amplitude in Drosophila larvae.
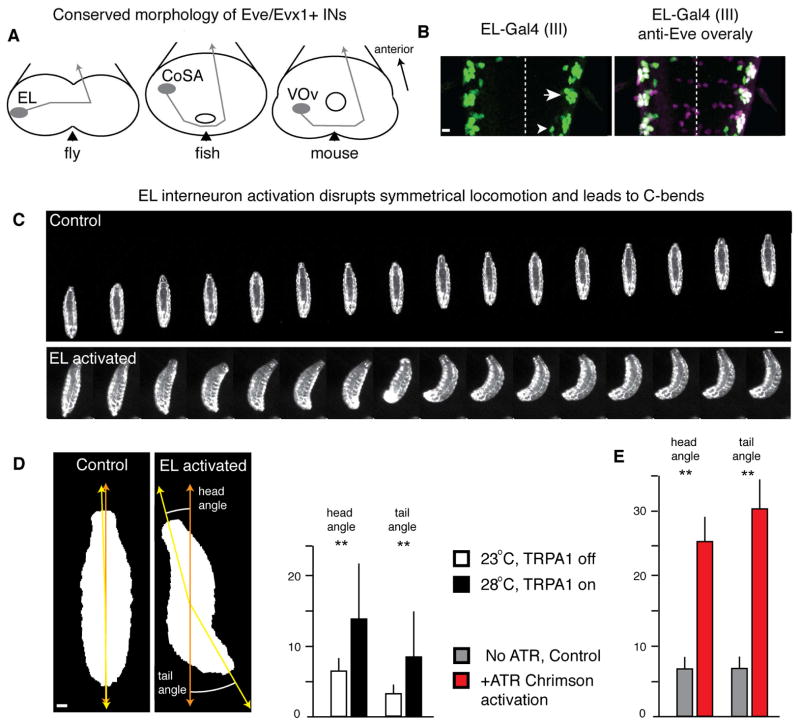
Figure 1. Activation of EL interneurons causes larval crawling defects.
(A) Eve/Evx1+ interneurons have commissural ascending axons in flies, fish and mouse. Midline, black arrowheads; anterior, up in all figures unless noted.
(B) EL-gal4 (green) is consistently in nine ELs (arrow) and stochastically in few Eve-negative non-ELs (arrowhead). Eve protein, magenta (colocalization with EL-gal4, white); midline, dashed. Scale bar, 10 μm. Genotype: EL-gal4/UAS-nls-GFP.
(C) Activation of ELs reduces larval crawling speed and induces C-bends. Frames shown at 0.5 sec. intervals. Scale bar, 150 μm. Genotype: UAS-dTRPA1/+; EL-gal4(III)/EL-gal4(III). Control: 23°C, TRPA1 off; EL activated: 28°C, TRPA1 on.
(D) TRPA1 activation of ELs results in larval C-bends with laterally displaced head and tail; genotype as in C. See Movies S1–S2. Scale bar, 40 μm. Average and SEM shown, ** p<0.05, t-test.
(E) Chrimson activation of ELs results in larval C-bends with laterally displaced head and tail. Genotype: UAS-Chrimson.mVenus/+; EL-gal4(III)/+. Control: larvae raised on food without all-trans-retinal (ATR), EL activated: raised on food with ATR. See Movies S3–S4. Average and SEM shown, ** p<0.05, t-test.
Results
The EL interneurons maintain left-right symmetric larval locomotion
To identify interneurons required for larval locomotion we used a collection of Gal4 lines that sparsely label neurons in the late embryonic CNS (Heckscher et al., 2014; Manning et al., 2012) to express the warmth-activated cation channel TRPA1 (Pulver et al., 2009) and screened for defects in larval locomotion. We screened newly hatched larvae for locomotor defects following activation of TRPA1 (28°C) that were reversed following inactivation of TRPA1 (23°C). Here we focus on the evolutionarily-conserved Eve+ lateral (EL) interneurons that are specifically targeted by the EL-gal4 line (Figure 1B)(Fujioka et al., 1999).
Wild type first instar larvae crawl with a linear posture at both 23°C and 28°C (data not shown), as do larvae expressing TRPA1 in the ELs at 23°C (Figure 1C top; Movie S1, Table S1). In contrast, raising the temperature to 28°C to induce TRPA1 stimulation of the ELs resulted in slower crawling and abnormal left-right asymmetric body posture, which we call “C-bends” (Figure 1C,D; Movie S2, Table S1). Similarly, Chrimson optogenetic stimulation of ELs resulted in pronounced C-bends (Figure 1E, Movies S3–4). C-bends are different from normal larval turning because they can occur in posterior segments, whereas larval turning is performed by anterior segments (Berni, 2015; Lahiri et al., 2011). We conclude that bilateral activation of EL interneurons is sufficient to disrupt left-right symmetric body posture.
We tested next whether the ELs were required for left-right symmetric locomotion. We used EL-gal4 to express the pro-apoptotic Hid/Reaper proteins, which typically removed all but 1–2 EL per hemisegment (Figure 2A). Similar to EL activation, ablation of the ELs led to slow crawling speeds and “wavy” body posture, including C-bends (Figure 2B–E, Movie S5). Because ablation removes statistically similar numbers of ELs from the left and right sides of the nerve cord (Figure 2A), and because C-bends can occur in both directions within the same animal (Figure 2C), we conclude that bilateral ablation leads to a randomized left-right asymmetric body posture.
Figure 2. Ablation of EL interneurons causes larval crawling defects.
(A) L1 CNS stained for Eve protein, with the focal plane showing a subset of Eve+ motor neurons (pseudocolored magenta) and the lateral cluster of Eve+ EL interneurons (pseudocolored green). EL ablation reduces EL number from ~10 to 1.63 ± 0.21 (left) and 1.54 ± 0.19 (right). The left-right difference is not significant (t-test, n = 4 larvae). Scale bar, 10 μm. Control genotype: UAS-reaper, UAS-hid/Y. EL ablated genotype: UAS-reaper, UAS-hid/Y;;EL-gal4/+.
(B–D) Ablation of ELs decreases larval crawling speed and induces C-bends. Genotypes as in A. (B,C) Frames are shown at 0.5 sec intervals. Scale bar, 150 μm. (D) Scale bar, 40 μm. Average and SEM shown, ** p<0.05, t-test. See Movie S5.
(E) EL-gal4+ brain neurons are not required for normal locomotion. Genotypes from left: (1) y w; (2) UAS-reaper, UAS-hid/Y; (3) EL-gal4 (III)/+; (4) UAS-reaper, UAS-hid/Y;;EL-gal4/+; (5) tsh-Gal80/+; EL-gal4/+; (6) UAS-reaper, UAS-hid/Y; tsh-Gal80/+; EL-gal4/+ (in this genotype only EL-gal4+ neurons in the brain are ablated). (B,D,E) Average and SEM shown, ** p<0.05, t-test.
Although EL interneurons are present only in the nerve cord, the EL-gal4 line is stochastically expressed in a few cells in the brain (Figure S1). To test whether ablation of these neurons caused locomotor defects, we used tsh-gal80 (Clyne and Miesenbock, 2008) to inhibit EL-gal4 in the nerve cord but not in the brain. We found that ablation of the EL-gal4 neurons in only the brain had no defects in locomotion (Figure 2E). We conclude that the Eve+ ELs within the nerve cord are required for bilaterally symmetric crawling in Drosophila larvae, and that the normal function of EL interneurons is to maintain left-right symmetric muscle contractions during linear locomotion.
EL interneurons maintain left-right symmetric muscle contraction amplitude without affecting contraction timing
To determine how the EL interneurons regulate motor output, we quantified muscle contraction timing in wild type, EL ablated, and EL activated larvae. We found that all genotypes showed left-right synchronous muscle contractions (Figure 3A–C, Table S2). The lack of effect on muscle contraction timing suggests that the ELs are not part of the central pattern generator (CPG), addressed in more detail below. We conclude that EL interneurons are not required for left-right synchronous timing of muscle contraction.
Figure 3. Ablation or activation of EL interneurons results in failure to maintain symmetrical left-right muscle length without affecting left-right timing in L1 larvae.
(A–C) Control (A), EL ablated (B), and EL activated (C) larvae quantified for resting muscle length, contraction amplitude, and contraction timing. Left: Muscle marker MHC:GFP. Center: schematic of raw data. Right: plot of A5 muscle length on the left (blue) or right (red) over two cycles of relaxation and contraction. Scale bar, 100 μm. Genotypes are (A,B) UAS-dTRPA1, MHC:GFP/UAS-dTRPA1 (A; control at 23°C, n=8) or (B; activated at 30°C, n= 6). (C) UAS-reaper, UAS-hid/+; MHC:GFP/+; EL-gal4/+ (n = 9). See Figure S1 and Movies S6–S8.
We next measured left-right muscle resting length and maximum contraction amplitude. Control larvae showed bilateral symmetry in resting muscle length and maximum contraction amplitude (Figure 3A, Movie S6, Table S2). In contrast, both EL ablated and EL activated larvae showed significant left-right differences in resting muscle length and maximum muscle contraction amplitude during forward locomotion (Figure 3B–C, Movie S7–8, Table S2). The resting muscle phenotype is consistent with our observations that EL disruption can create left-right asymmetry in larvae at rest (data not shown). We conclude that the EL interneurons are required for maintaining bilaterally symmetric muscle contraction amplitude, both at rest and during active muscle contraction.
Calcium imaging reveals functional interactions among EL interneurons
To better understand the neural circuit containing the EL interneurons, we asked if the ELs could be part of the central pattern generator (CPG) for locomotion. We performed calcium imaging in the isolated CNS, which lacks all sensory input, and asked if ELs showed locomotion-like patterns of activity. As a positive control, we confirmed that motor neurons show organized locomotion-like posterior to anterior waves activity (Figure S2, Movie S9) as has been previously reported (Pulver and Griffith, 2010; Schaefer et al., 2010). In contrast, the ELs showed only spontaneous activity in individual neurons (Figure S2, Movie S10). We conclude that the EL interneurons are neither part of the locomotor CPG, nor receive input from the locomotor CPG.
Next, to understand how TRPA1-induced stimulation of EL interneurons could lead to a behavioral phenotype, we asked how the EL interneurons themselves responded to bilateral activation. We used TRPA1 to chronically stimulate EL interneurons, similar to our behavioral experiments, and monitored EL activity using the calcium sensor GCaMP6m. Imaging was done in the isolated CNS to reduce movement artifacts and eliminate sensory input (Figure 4A). We observed three types of response. Most commonly, the EL interneurons were strongly activated on one side of the CNS and weakly activated on the other side; at stimulus offset the response reliably switched sides (Figure 4A–C, group 1, n = 10, Movie S11). This left-right asymmetric response to presumably bilaterally symmetric TRPA1 activation suggests that left-right EL interneurons exhibit functional interactions. Less commonly we observed bilaterally symmetrical activity that was low during stimulation and increased at stimulus offset (Figure 4B–C, group 2, n = 6) or EL activity mirroring TRPA1 activity (Figure 4B–C, group 3, n = 6), the response expected if the ELs had no functional interactions. For all groups, once the pattern of EL activity was established it remained constant for the duration of the chronic TRPA1 stimulation interval; this is in contrast to EL activity within intact larvae (see next section). We conclude that there can be functional interactions between left-right EL interneurons.
Figure 4. Calcium imaging reveals functional interactions between left-right EL interneurons.
Isolated L1 CNS preparations expressing GCaMP6m and TRPA1 in the ELs. In this experiment, TRPA1 activity cycles from “off” (23°C) to “on” (28°C) and back “off” (23°C) with the TRPA1 “on” interval at least one minute long. There are three classes of response to this experiment (groups 1–3).
(A) Left: schematic of preparation and GCaMP6m/TRPA1 expression in ELs. Right: example from group 1 (Movie S11). Note that both sides start at similar levels, but the left side is more active during the chronic TRPA1 “on” interval, and the right side becomes more active after TRPA1 stimulus offset. Scale bar, 25 μm.
(B) Representative individual plots of GCaMP6m fluorescence ( F/F) for group 1-group 3.
(C) Data from B replotted as average plots with standard error. Genotype: UAS-dTRPA1/UAS-GCaMP6m; EL-gal4/EL-gal4.
(D–E) Controls for isolated CNS preparation experiments. (D) Preparations expressing GCaMP6m and TRPA1 in ELs held at baseline temperature (23°C). Genotype: UAS-dTRPA1/UAS-GCaMP6m; EL-gal4/EL-gal4 (E) Preparations expressing only GCaMP6m in ELs with temperature shifts as in B-C. Genotype: UAS-GCaMP6m/UAS-GCaMP6m; EL-gal4/EL-gal4.
Calcium imaging of EL activity within intact, freely-moving larvae provide functional evidence that the EL interneurons are part of a sensorimotor circuit
We wanted to understand how EL interneurons respond to stimulation in vivo, and whether EL response is correlated with larval behavior. We expressed both TRPA1 and GCaMP6 in ELs, induced chronic TRPA1 activation, and imaged EL activity in intact, freely crawling larvae. We observed epochs of left-right asymmetric EL activity in every case (n=5) (Figure 5A). Interestingly, EL interneurons could undergo repeated left-right switches in activity despite chronic TRPA1 activation; in contrast, similar experiments using isolated CNS preparations never showed left-right switching (Figure 4). We propose that left-right activity switching within the intact larvae is due to sensory input.
Figure 5. EL interneuron activity is correlated with contralateral muscle contractions within freely crawling larvae.
All data are from intact larvae during forward locomotion with chronic TRPA1 activation of EL interneurons.
(A) Top left: schematic of intact larval preparation and GCaMP6m/TRPA1 expression in EL interneurons. Top right: left-right (L-R) asymmetric GCaMP6m fluorescence in EL interneurons taken from indicated times during plot below (grey arrows). Bottom: Intact L1 larvae expressing GCaMP6m and TRPA1 in EL interneurons were held at 32°C and mean fluorescence intensity was measured in left (blue) and right (red) EL interneurons. Note the blue line is interrupted when fluorescent intensity dropped to levels indistinguishable from background fluorescence. Genotype: UAS-dTRPA1/UAS-GCaMP6m; EL-gal4/EL-gal4.
(B–D) Representative single larva data from Movie S12. (B) The larva was moving forward, so frames were manually aligned. The top row: EL GCaMP6m fluorescence (L, left and R, right); bottom row: body angle (arrows). Scale bar, 50 μm. (C) Plot of left and right EL fluorescence intensity over the time interval shown in B. (D) Plot of fluorescence index (bright side fluorescence - dim side fluorescence/total fluorescence) and body angle for the same time interval shown in B. Genotype as in A.
(E) Averages from 10 epochs of left-right EL activity switching in 3 larvae, aligned to the time of switching (t = 0). EL activity (green) is correlated with contralateral body bending (orange). Average and standard deviation shown. Genotype as in A.
Next, we asked whether left-right asymmetrical EL interneuron activity is correlated with a specific larval behavior. We repeated the experiment above using a low-power objective to measure the calcium signal within left and right ELs while simultaneously monitoring body position using intrinsic autofluorescence of the larvae. We focused our analysis on epochs where EL activation switched from high on one side to high on the other. We selected the ten epochs showing the largest switches in left-right EL activity (without attention to the behavioral data) and aligned the traces to the moment EL activity switched sides (Figure 5B–D, Movie S12). We found that a switch in EL activity was correlated with body bending on the side contralateral to the side with high EL activity (100%, n = 3 larvae, 10 switches; Figure 5E). The strong correlation between EL activity and subsequent contralateral motor activity (inferred from body bending) is consistent with EL interneuron activation of contralateral motor neurons.
Identification of individual EL interneurons by light and electron microscopy
Our behavioral and functional imaging data support the hypothesis that EL interneurons are part of a sensorimotor circuit that regulates muscle contraction amplitude. To characterize the network context in which the ELs operate, we identified their pre- and post-synaptic partners using transmission electron microscopy (TEM) reconstructions. We analyzed multiple hemisegments of two different first instar larvae: one a full CNS reconstruction from a 6 h old larva, and the other a 1.5 segment reconstruction of A2/A3 segments from a 12–24 h old larva (Ohyama et al., 2015). Because TEM reconstruction of neural circuits is laborious, we identified a smaller subset of functionally important ELs using the split Gal4 approach (Luan et al., 2006). The R11F02-gal4 line is expressed in a subset of ELs plus other neurons (Heckscher et al., 2014), so we generated R11F02-gal4AD and EL-gal4DBD lines and crossed them together to label only the R11F02+ EL+ co-expressing neurons (hereafter called 11F02 ∩ ELs). This restricted labeling to just five ELs per hemisegment (Figure 6A). Activation of these five neurons produced a phenotype similar to that seen when activating all ELs with EL-gal4 (Figure 6B–C, Movie S13–14). We conclude that the 11F02 ∩ ELs are a functionally relevant subset of the full EL interneuron population.
Figure 6. Identification of individual EL interneurons by light and electron microscopy.
(A–C) Activation of a subset of ELs is sufficient to cause C-bends. (A) 11F02 ∩ EL-gal4 driving membrane-bound GFP (green) co-stained for Eve protein (magenta). Anterior up. Scale bar 20 μm. (B–C) Chrimson optogenetic activation of 11F02 ∩ ELs results in larval C-bends. Average and SEM shown, ** p<0.05, t-test. Genotype: UAS-Chrimson.mVenus/EL-gal4AD; R11F02-gal4DBD/+. Control: larvae raised without ATR. 11F02 ∩ EL activated: raised with ATR. Scale bar 100 μm. See Movies S13–S14.
(D) Individual 11F02 ∩ ELs detected using MCFO. The two projection interneurons (A08c, A08s) and three local interneurons (A08e1-e3) all have contralateral projections. Anterior, up; midline, arrowhead. Scale bar 5 μm.
(E–H) Individual 11F02 ∩ ELs reconstructed from serial section TEM volume of the younger Larva 1 except where noted. Anterior up, midline arrowhead. (E) Individual 11F02 ∩ ELs are shown below their cognate neurons from MCFO analysis. (F) A08e1-3 local ELs from the older Larva 2 volume. Upper left schematic shows posterior/cross section view, with landmark Fasciclin II bundles shown in grey. (G) All 11F02 ∩ ELs reconstructed in segment A1 and A2; A1L neurons colored yellow. Note the clustered soma and common proximal axon fascicle. (H) Segmentally homologous neurons are highly similar (A08e3 shown in A1, A2, A3 left hemisegments).
(I) Bilaterally homologous 11F02 ∩ ELs are more similar to each other than to other ELs (lines show the shortest total path for indicated neurons). Y axis: ratio of input-output/input+output synapse number; X axis: neurite branch length (total neurite length – principle branch in nm).
To determine the unique morphology of the five 11F02 ∩ EL interneurons, which is a prerequisite for finding the matching neuron in the TEM reconstructions, we used multicolor flip-out (MCFO) (Nern et al., 2015). We found that two ELs had contralateral projections ascending to the brain (A08c, A08s) and three had contralateral projections that remained local (A08e1-A08e3) (Figure 6D). Both projection and local 11F02 ∩ ELs can be distinguished from each other based on their unique 3-dimensional pattern of neural arbors (Figure 6D; Table S3). We conclude that each 11F02 ∩ EL interneuron has a distinctive morphology, allowing us to identify the morphologically identical interneurons within the TEM reconstructions.
To identify individual 11F02 ∩ EL interneurons using TEM, we used their shared and distinct features to identify and categorize the neurons (see Methods). We use the term ‘reconstructed’ to indicate tracing of all neuronal processes, and the term ‘annotate’ for identifying pre- and post-synaptic partners. We reconstructed and annotated all five 11F02 ∩ ELs in the younger “Larva 1” TEM volume which includes the entire CNS (Figure 6E–I), and the three local 11F02 ∩ ELs in the older “Larva 2” TEM volume which contains only segment A3 (Figure S3). For each 11F02 ∩ EL interneuron we observed a stereotyped morphology in multiple segments (Figure 6H), in left and right hemisegments (Figure 6I), and in multiple larvae (Figure S3). No other adjacent neurons in the TEM volumes shared common features with the 11F02 ∩ ELs and matched the MCFO morphology. We conclude that we have identified the 11F02 ∩ EL interneurons in the TEM reconstructions.
The EL interneurons receive direct proprioceptor input and generate direct motor neuron output
Our first goal was to determine whether the 11F02 ∩ EL interneurons had direct sensory input or direct motor output within the TEM volumes. We benefited from prior annotation of many sensory and motor neurons (Ohyama et al., 2015), but we also reconstructed additional sensory and motor neurons to ensure that each sensory neuron class was represented (chordotonal, external sensory, proprioceptors) and each motor neuron class was represented (dorsal-, ventral-, and lateral-projecting motor neurons) (Kohsaka et al., 2012; Singhania and Grueber, 2014). We discovered that multiple proprioceptive sensory neurons – but few or no external sensory or chordotonal neurons – formed direct presynaptic contacts with both local and projection EL interneurons (Figure 7A,B). The proprioceptors always formed their presynaptic contacts on ipsilateral arbors of the local EL interneurons; that is, left body wall proprioceptors synapse with EL interneurons whose cell bodies are on the left side of the CNS (Figure 7A,C). We found that the proprioceptor-EL contacts were highly specific and reproducible across sides of the CNS, multiple segments, and multiple larvae (Figure 7A, Figure S3, S4). For example, the ventral bipolar dendrite (vbd) proprioceptor always formed presynaptic contacts with the A08e3 arbor, but not the intermingled A08e1 or A08e2 arbors, and the number of vbd contacts was always greater on the A08e2 lateral arbor and fewer on its medial arbor (Figure 7A,C,D). The functional significance of different proprioceptors targeting different ELs remains to be determined (see Discussion), however the specificity and reproducibility of synapse positions and numbers confirms the accuracy of our reconstruction and annotations. The function of proprioceptive neurons in Drosophila larvae has not been tested, but proprioceptive neurons monitor muscle length in many insects (Simon and Trimmer, 2009; Tamarkin and Levine, 1996), and thus we propose that the proprioceptor-EL connectivity we observe is used to convey body wall muscle contraction amplitude information to the EL interneurons.
Figure 7. Local EL interneurons have monosynaptic proprioceptive inputs and monosynaptic motor outputs.
Anatomical reconstruction of sensory-EL-motor neuron pathway in A3 of the older Larva 2.
(A) Summary of the pathway showing the indicated number of synapses between proprioceptive sensory neurons (purple), local ELs (A08e1-A08e3; black), and motor neurons (green). For clarity, the connectivity between local ELs is shown separately (inset). Neurons with unilateral connections were excluded.
(B) Proprioceptive neurons are the sensory class with the most presynaptic contacts on ELs.
(C) The vbd-A08e3-RP2 pathway is bilaterally symmetric at the level of arbor morphology, synapse number, and synapse location. Top: the A3 left vbd has two zones of pre-synaptic contacts with A08e3, which forms synapses with the ventral-most region of the RP2 motor neuron dendritic arbor. Bottom: the A3 right vbd-A08e3-RP2 pathway has the similar location and number of synaptic contacts. Posterior view; dorsal up, midline, dashed line.
(D–E) Examples of synapse morphology in the TEM reconstruction for vbd-A08e3 (left) and A08e3-RP2 (right). Note the pre-synaptic vesicle accumulation and electron density at the synapse. Synapses were identified as in (Ohyama et al., 2015).
(F) Stimulation of ELs with Chrimson activates dorsal motor neurons. 488 nm laser illuminated the neuropil, which simultaneously activated Chrimson in ELs (red) and allowed for visualization of GCaMP6m fluorescence in CQ2-labeled dorsal motor neurons (green). Each line shows GCaMP6m signal in a different isolated brain preparation. Horizontal lines show baseline fluorescence. Response is significantly different between EL activation and controls, p<0.05, Chi-Square. The top, middle, and bottom datasets are: (top) the indicated genotype + ATR (n =11); (middle) the indicated genotype without ATR (n=6); (bottom) the indicated genotype without UAS-Chrimson and +ATR (n=7).
Next, we determined whether EL interneurons formed presynaptic contacts with motor neuron dendrites. We found that the ELs formed direct presynaptic contacts from the ELs to dorsal-projecting motor neurons RP2, U1, and U2, but not to ventral- or lateral-projecting motor neurons (Figure 7A,C,E). The EL interneurons always formed their presynaptic contacts on the contralateral motor neurons; that is, EL interneurons on the left side of the CNS formed presynaptic contacts with motor neurons projecting to the right body wall (Figure 7A,C). Thus, if the ELs were to provide excitatory drive to their target motor neurons, it would explain why EL activation correlates with contralateral motor neuron output within intact crawling larvae (see above). Consistent with this hypothesis, we found that ELs are cholinergic (Figure S5), and therefore could provide excitatory drive to motor neurons, similar to previously described cholinergic excitatory pre-motor neurons (Baines et al., 2001; Pym et al., 2006). Consistent with this conclusion, bilateral Chrimson stimulation of ELs resulted in motor neuron activation (Figure 7F). We conclude that local ELs are functionally pre-synaptic to contralaterally-projecting motor neurons.
Jaam interneurons: a link between proprioceptive neurons and EL interneurons
The proprioceptor-EL-motor neuron anatomical circuit described above is unlikely to be functioning in isolation. Thus, we searched for additional neurons that had a similar or greater number of presynaptic contacts with the ELs compared to proprioceptors (see Methods). We discovered two interneurons with 8–18 presynaptic contacts per EL interneuron, called Jaam1 and Jaam3 (Figure 8A). Jaam2 had morphology similar to Jaam1/Jaam3 but connected to the ELs via Jaam1 (Figure 8A, inset; Figure S5). Over 7% of all Jaam1/Jaam3 presynaptic contacts were on the ELs, similar to the combined number of dorsal and ventral proprioceptor neuron inputs to the ELs (Figure 8B, top). Interestingly, over 30% of the Jaam1-3 neurons inputs were from the dorsal and ventral proprioceptors (Figure 8B, bottom). Thus, the Jaam neurons provide a link from proprioceptors to EL interneurons. Similar to proprioceptor-EL connectivity, Jaam neurons formed highly specific contacts with their input and output neurons. For example, the dorsal bipolar dendrite (dbd) proprioceptive neuron provides input to Jaam1 but not Jaam2/Jaam3, and the Jaam1 neuron provides input to the A08e2 but not A08e1/3, despite their intermingled arbors (Figure 8A,C). In addition, there was specific, reproducible ipsilateral and contralateral connectivity between Jaam1-3 neurons (Figure 8A, inset). Although the functional role of the Jaam neurons in presenting proprioceptive activity to the EL interneurons is currently unknown, it is clear that the Jaam interneurons provide an anatomical link between proprioceptors and EL interneurons.
Figure 8. EL interneurons have disynaptic proprioceptive inputs.
Disynaptic input from proprioceptors to local ELs via the Jaam neurons. Data from Larva 1, segment A1.
(A) Disynaptic connectivity from proprioceptive sensory neurons (purple) to Jaams (magenta) to local ELs (gray); monosynaptic proprioceptor-EL connectivity shown with light gray lines. For clarity, the connectivity between Jaams is shown separately (inset). Neurons with unilateral connections were excluded.
(B) Top: Jaam1,3 neurons provide major inputs into the local EL interneurons (A08e1-e3). Bottom: Proprioceptive neurons provide major inputs into the Jaam1-3 neurons. For both top and bottom, the left graph shows % of total inputs (includes neurons that have not yet been fully reconstructed) and right graph shows % of known inputs (only fully reconstructed and annotated neurons).
(C) Synaptic specificity: Jaam1 (dark magenta) and Jaam3 (light magenta) reproducibly target distinct, stereotyped regions of the different EL interneuronal arbors (light grey, A08e1; dark grey, A08e2), as seen in the inset (right). Posterior view; dorsal up; midline, dashed line.
Saaghi interneurons: a link between EL interneurons and motor neurons
We showed above that local EL interneurons formed direct presynaptic contacts with motor neurons. However, the number of synapses between each EL-motor neuron was relatively few (range: 1–7), and were reliably detected with only 3–4 motor neurons of the ~30 per segment. We therefore searched for neurons that had a comparable number of EL presynaptic contacts (see Methods). We discovered two interneurons with a range of 2–9 EL presynaptic contacts, which we call Saaghi neurons 1 and 3 (SA1, SA3; Figure 9A; Figure S6). SA1/SA3 received 10% of all EL presynaptic contacts, far greater than the number EL presynaptic contacts to dorsal-projecting motor neurons (Figure 9B, top). In contrast to the EL interneurons which had outputs to only the dorsal-projecting motor neurons, the SA1/SA3 neurons had outputs to all classes of motor neurons (Figure 9A). For example, SA1 formed over 33–37 presynaptic contacts with dorsal-projecting motor neurons, 15–33 to ventral-projecting motor neurons, and 2–8 to lateral-projecting motor neurons (Figure 9A). Moreover, the SA1/SA3 neurons allocated 20% of their total presynaptic contacts to motor neurons (Figure 9B, bottom). Thus, the SA1/SA3 premotor neurons provide a link from EL interneurons to all classes of motor neurons. Interestingly, the disynaptic EL-SA-motor neuron pathway connects the ELs with ipsilateral motor neurons (Figure 9A, black lines), whereas the monosynaptic EL-motor neuron pathway connects ELs to contralateral motor neurons (Figure 9A, grey lines). These two pathways could generate synergistic output if the SA neurons are inhibitory (see Discussion).
Figure 9. EL interneurons have disynaptic motor neuron outputs.
Anatomical circuit reconstruction of EL-SA-motor neuron pathway from Larva 1 segment A1 reveals the 11F02 ∩ ELs have disynaptic motor neuron output via the SA interneurons.
(A) Synaptic connections between local ELs (grey), pre-motor SAs (cyan), and motor neurons (green). Monosynaptic EL-MN connectivity shown with light gray lines. Only bilateral connections between specific neurons (ELs and SAs) or motor neuron groups (dorsal, ventral, lateral) are shown. Number of motor neurons in each class shown in parentheses.
(B) Top: the major output of the 11F02 ∩ ELs are the SAs. Bottom: the major output of the SAs are motor neurons.
(C) The three local ELs A08e1-e3 (from light to dark grey) project to a common region of the SA1 dendritic arbor; (C′) enlargement of boxed region in C. Posterior view; dorsal up, midline, dashed line.
In contrast to the specificity of proprioceptor-EL connectivity, the EL-SA-motor neuron connectivity is distributed; each EL synapses with both SA neurons, and each SA neuron synapses with all motor neuron classes (Figure 9C). This shows that the EL interneurons have the potential to regulate the activity of all body wall muscles, and suggests that different mechanisms of circuit formation may be used by proprioceptor-Jaam-ELs and by EL-SA-motor neurons. Although the role of the SA1/SA3 neurons in translating EL activity into motor output is currently unknown, our data shows EL interneurons are positioned at the heart of an anatomical sensorimotor circuit that is well suited for detecting and modifying body wall muscle contraction and body posture.
Discussion
Drosophila larvae: a model system for investigating left-right symmetric motor output
Bilaterally symmetric motor patterns—those with muscle contractions on the left and right sides of the body occurring synchronously and with equal amplitude—have broad and essential functions. Despite the nearly ubiquitous use of bilaterally symmetric motor patterns throughout the animal kingdom we understand surprisingly little about the relevant neural circuitry. Here we identify an anatomical sensorimotor circuit containing an evolutionarily-conserved population of Eve/Evx+ interneurons that is required to maintain left-right symmetric muscle contraction amplitude both during active muscle contraction and at rest. To our knowledge, these interneurons are the first known to regulate bilaterally symmetric muscle contraction amplitude. In mouse, Sim1+ V3 interneurons have a related function during alternating gait (Zhang et al., 2008). In the future, it will be interesting to directly examine muscle contraction amplitude in “V3 defective” mice to determine whether this class of interneuron is responsible for balancing amplitude of left-right muscle contraction during alternating motor patterns. Similarly, it will be interesting to determine the role of Drosophila interneurons expressing the Sim1 homolog, Single-minded, during left-right symmetric motor output.
EL interneurons are part of a sensorimotor circuit
We show that EL interneurons act in a sensorimotor circuit independent of the central pattern generator that generates locomotion. First, in the absence of sensory input ELs do not show locomotion-like patterns of activity (Figure S2). Second, EL perturbation does not alter left-right timing of muscle contraction (Figure 3). Third, EL perturbation alters muscle contraction amplitude during locomotion and at rest (Figure 3).
Our data suggest that EL interneurons receive sensory input that is primarily proprioceptive. Because proprioceptive neurons can detect muscle length and movement (Simon and Trimmer, 2009; Tamarkin and Levine, 1996), they are well suited to convey muscle amplitude information to the ELs. Closer inspection of the proprioceptor to EL connectivity generates interesting hypotheses. First, proprioceptors are presynaptic to both projection and local EL interneurons; the former may send body posture information to the brain, while the latter may act locally to maintain left-right symmetric muscle length in each segment. Second, the Jaam interneurons are well positioned to process sensory information (e.g. from dorsal or ventral regions of the body wall) prior to transmitting information to the ELs. Although we currently know little about Jaam neurotransmitter expression or function, their position in the circuit raises the question of whether EL interneurons show state-dependent responses to proprioceptive inputs.
Our data demonstrate that EL interneurons are presynaptic to motor neurons and can modify motor output. EL perturbation results in slow crawling and asymmetric left-right muscle contraction amplitude, while optogenetic stimulation of ELs induces motor neuron activity. The majority of ELs are cholinergic and likely excitatory, they provide direct input to contralateral motor neurons, and motor neurons are glutamatergic and excitatory (Kohsaka et al., 2012). Thus, EL activity on one side of the body should result in increased contralateral motor neuron activity and contralateral muscle contraction. This may be reinforced by the disynaptic (EL-SA-MN) pathway, in which EL activity would prevent ipsilateral motor neuron activity if the SA neurons are inhibitory. This model awaits future characterization of SA neurotransmitter expression and function. We propose the hypothesis that ipsilateral muscle relaxation (via the EL-SA-MN pathway) together with contralateral muscle contraction (via the direct EL-MN pathway) is used for dynamic adjustment of body posture.
How do EL interneurons maintain left-right symmetric muscle contraction amplitude?
Left-right differences in muscle contraction amplitude inevitably arise due to stochastic external (environmental) or internal (CNS/muscle) asymmetries. Without proper compensation, these perturbations would result in mismatched muscle contraction amplitude on left-right sides of the body. We hypothesize that sensory input generates a representation of body wall curvature that is delivered to the EL interneurons. Left-right interactions among ELs would allow them to compare left versus the right sides of the body, followed by EL stimulation of motor output to restore left-right symmetric muscle length.
How does EL interneuron ablation and activation generate the same phenotype? We favor a model in which ELs are part of a “perturbation-compensation” circuit. A larva that experiences an asymmetrical perturbation from an external or internal source would generate left-right mismatched muscle contraction amplitudes in the absence of any compensation. We propose that the EL circuit detects and compensates for these asymmetries. When the ELs are absent or constitutively active, they lose the ability to perform the left-right comparison and the asymmetries persist. In this way two “opposite” manipulations yield the “same” phenotype.
A conserved function of Eve/Evx+ interneurons in neuronal circuitry and behavior?
There is deep conservation of genetic programs that specify neuronal fate. This is particularly true for the Even-skipped+ (Eve or Evx+ in vertebrates) interneurons, which have been found in all bilateral animals examined to date except C. elegans. Annelids, chordates, insects, fish, birds, and mammals—as well as the presumed last common ancestor between invertebrates and vertebrates, Platynereis dumerilii —all contain Eve/Evx+ interneurons (Avaron et al., 2003; Copf et al., 2003; Denes et al., 2007; Ferrier et al., 2001; Fujioka et al., 2003; Holland, 2013; Ikuta et al., 2004; Landgraf et al., 1999; Moran-Rivard et al., 2001; Sordino et al., 1996; Suster et al., 2009; Takatori et al., 2008; Thaeron et al., 2000). Evx+ neurons in mice are commissural, excitatory, and directly contact motor neurons (Lanuza et al., 2004; Moran-Rivard et al., 2001); here we show that fly Eve+ interneurons are commissural, likely excitatory, and directly contact motor neurons. One hypothesis to explain the remarkable parallels between Eve/Evx+ interneurons is that the last common ancestor between vertebrates and invertebrates was segmented and motile; and thus the genetic programs used to create locomotor circuitry may be evolutionarily ancient.
We have shown that the Drosophila Eve+ lateral interneurons are required to maintain left-right symmetrical motor output in the larva. Do Evx+ interneurons have a similar function in other organisms? Genetic removal of Evx1+ interneurons in mice did not reveal any specific function in either gross motor patterns or in the timing of left-right alternating motor neuronal activity as assayed by nerve root recordings (Lanuza et al., 2004; Moran-Rivard et al., 2001). Subsequently, a broader genetic manipulation which reduced the number of Evx1+ interneurons to 25% of wild type levels, as well as ablating a large but unspecified number of Evx1− neurons, resulted in a hind limb hopping phenotype during fast locomotion (Talpalar et al., 2013). This study raised the possibility that Evx1+ interneurons regulate locomotion in mice. In our study we show that highly specific ablation or activation of Eve+ lateral interneurons disrupts larval crawling. It will be interesting to determine whether Evx1+ interneurons regulate bilaterally symmetric or alternating gait in other organisms, as well as whether Eve+ interneurons regulate alternating gait or symmetric flight in adult flies.
Experimental Procedures
Fly genetics
For complete list of fly stocks see Supplemental Experimental Procedures. For EL-AD and CQ2-lexA molecular constructs and transgenic flies were generated using standard methods as previously described (Pfeiffer et al., 2008; Pfeiffer et al., 2010).
Embryo immunostaining
We used standard methods to stain Drosophila embryos and larvae (Manning et al., 2012). For list of primary antibodies see Supplemental Experimental Procedures. Secondary antibodies were from Invitrogen/Molecular Probes (Eugene, OR) and were used according to manufacturer’s instructions. Images were acquired on a Zeiss 700 or 710 confocal microscope with a 40X objective. Images were cropped in ImageJ (NIH) and assembled in Illustrator and Photoshop (Adobe).
Larval behavior
We recorded behavior in 0–4 h first instar larvae, except late first instar to second larvae were recorded for experiments using Chrimson. Brightfield whole larval recordings. Behavior arenas were made of 6% agar in grape or apple juice, 2 mm thick. Behavior was recorded at 23°C, unless otherwise noted. Temperature was measured using Omega HH508 thermometer, and controlled with a custom-built thermoelectric controller and peltier device. Arenas were placed under a Leica S8APO dissecting microscope and a red light (700 nm, Metaphase Technologies) illuminated a single larva. The microscope was equipped with a Scion 1394 Camera, using Scion VisiCapture software. Images were acquired at either 4 HZ or 7.5 HZ. All larvae were fed yeast paste lacking all-trans-retinal (ATR) except where noted. Also see Supplemental Experimental Methods. Fluorescent whole larval recordings (muscle kinematics). Behavior arenas were placed on sapphire slides. Larva were allowed to cross the field of view then the stage was manually moved to keep the larvae in view, resulting in several recordings per larva. Images were acquired at 10 HZ with a 10x objective on a McBain spinning disc confocal microscope equipped with a Hamamatsu EM-CCD camera, and Volocity software (PerkinElmer). For image analysis see Supplemental Experimental Methods.
Calcium imaging
For Figure 4, a freshly dissected CNS from a newly-hatched larva was placed directly on sapphire slides in HL3.1 saline. Note there were fine manual adjustments for small changes in focal plane upon temperature shift. For Figure 5 intact larval recordings see muscle kinematics section above. The relationship between the EL calcium signal and body position was complex, so we focused our analysis on epochs where EL activation switched from high on one side to high on the other. For Figure 7C, a freshly dissected CNS from a newly-hatch larva was placed on a slide in HL3.1 saline. A region of interest encompassing the nerve cord neuropil, with motor neuron dendrites in focus was illuminated with 488 nm light at 10% laser power to simultaneously activate Chrimson and monitor GCaMP6m fluorescence. For Figure S2 we used the protocol as described above except we used Baines’ saline (Marley and Baines, 2011), and maintained a constant temperature between 26–28°C. Temperature was controlled as described above. Imaging was done with a 40x objective on the McBain spinning disc, as described above. For details of image analysis see Supplemental Experimental Methods.
Multicolor flip out (MCFO) to label and name single EL interneurons
We used published methods to label single EL interneurons in first instar larvae (Nern et al., 2015). The stock MCFO-3 was crossed to EL-gal4 (Supplemental Experimental Procedures). The progeny first instar larvae were dissected, stained for the MCFO epitopes and Eve protein, and imaged on a Zeiss 700 or 710 confocal microscope. Segments containing single MCFO+ Eve+ neurons were analyzed in dorsal view and posterior view, which allowed each neuron to be classified as one of the five 11F02 ∩ ELs. The name of each 11F02 ∩ EL interneuron was chosen to match its name in the third instar abdominal CNS. Jaam is Persian for ‘wineglass’ (reflecting the strong association with sensory input) and saaghi (SA neurons) is Persian for ‘one who brings a gift’ (reflecting their role in presenting information to the motor neurons).
Reconstructing single EL interneurons and determining their synaptic partners within the serial section TEM volumes
We used two larval reconstructions: one a full CNS reconstruction from a 6h old first instar larva, and the other a 1.5 segment reconstruction of A2/A3 segments from a 12–24h old first instar larva (Ohyama et al., 2015). We reconstructed neurons in CATMAID using a Google Chrome browser as previously described (Ohyama et al., 2015). To identify single EL interneurons within the TEM volume we used the following features observed in the MCFO “ground truth” data set: (1) All 11F02 ∩ ELs share a common ventro-anterior cell body position; (2) all 11F02 ∩ ELs share a common proximal axon fascicle; (3) all 11F02 ∩ ELs have contralateral projections; (4) each 11F02 ∩ ELs has a characteristic morphology when viewed dorsally and posteriorly (Table S3). Using these criteria, we reconstructed neurons with ventro-anterior soma until we found one that matched the morphology of an individual 11F02 ∩ EL interneuron; we then reconstructed adjacent neurons projecting in a common proximal axon fascicle to “enrich” for the remaining 11F02 ∩ ELs. Note only bilaterally symmetric connections are shown in Figures.
To identify direct sensory inputs and motor outputs we relied on previously reconstructed sensory and motor neurons, supplemented by reconstruction of under-represented classes such as lateral projecting motor neurons and proprioceptive sensory neurons. To identify interneurons with direct presynaptic connections to EL interneurons, we reconstructed neurites that contacted clusters of post-synaptic sites on EL arbors. If a reconstructed neuron accumulated several (3+) presynaptic contacts with an EL interneuron, we continued reconstruction. In this way, we could rapidly focus on the neurons with the greatest number of presynaptic contacts with an EL interneuron. Similar methods were used to identify neurons post-synaptic to each EL interneuron.
Supplementary Material
Highlights.
New model system for analysis of bilaterally symmetric motor output
Identify a role for the conserved Eve+ interneurons in locomotor behavior
Imaging of neural activity in an intact, freely-moving Drosophila larvae
Identify a multisynaptic sensorimotor circuit using TEM reconstruction
Acknowledgments
We would like to thank Tory Herman, Cris Neil, Matt Smear, and Chris Wreden for comments that improved this manuscript, and Jimmy Kelly and Taylor Kaser for technical assistance. This work was supported by NIH grant MH051383 (SRL), American Heart Association #0920025G postdoctoral fellowship (ESH), and the Howard Hughes Medical Institute, where CQD is an Investigator. ML was supported by a grant from the Wellcome Trust (092986/Z) and by an Isaac Newton Trust/Wellcome Trust ISSF Research Grant. We thank the Fly EM project team for providing the raw data of the whole CNS EM volume.
Footnotes
Author Contributions: ESH guided the project, co-wrote the manuscript, did behavioral and calcium imaging experiments as well as analysis except where noted below. SF and SRL contributed to the Ca2+ imaging experiments. MSC did initial behavioral screening. LM characterized EL-gal4 expression. LM and CQD generated and analyzed all L1 MCFO data. JWT identified and named the A08 neurons in L3 larvae. RDF generated the TEM volume. AAZ, AF, CQD, CSM, MZ, ML and AC reconstructed and annotated neurons in the TEM volumes. CQD guided the project and co-wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avaron F, Thaeron-Antono C, Beck CW, Borday-Birraux V, Geraudie J, Casane D, Laurenti P. Comparison of even-skipped related gene expression pattern in vertebrates shows an association between expression domain loss and modification of selective constraints on sequences. Evol Dev. 2003;5:145–156. doi: 10.1046/j.1525-142x.2003.03021.x. [DOI] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni J. Genetic dissection of a regionally differentiated network for exploratory behavior in Drosophila larvae. Curr Biol. 2015;25:1319–1326. doi: 10.1016/j.cub.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni J, Pulver SR, Griffith LC, Bate M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr Biol. 2012;22:1861–1870. doi: 10.1016/j.cub.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Broihier HT, Skeath JB. Drosophila homeodomain protein dHb9 directs neuronal fate via crossrepressive and cell-nonautonomous mechanisms. Neuron. 2002;35:39–50. doi: 10.1016/s0896-6273(02)00743-2. [DOI] [PubMed] [Google Scholar]
- Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Copf T, Rabet N, Celniker SE, Averof M. Posterior patterning genes and the identification of a unique body region in the brine shrimp Artemia franciscana. Development. 2003;130:5915–5927. doi: 10.1242/dev.00835. [DOI] [PubMed] [Google Scholar]
- Crisp S, Evers JF, Fiala A, Bate M. The development of motor coordination in Drosophila embryos. Development. 2008;135:3707–3717. doi: 10.1242/dev.026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp SJ, Evers JF, Bate M. Endogenous patterns of activity are required for the maturation of a motor network. J Neurosci. 2011;31:10445–10450. doi: 10.1523/JNEUROSCI.0346-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes AS, Jekely G, Steinmetz PR, Raible F, Snyman H, Prud’homme B, Ferrier DE, Balavoine G, Arendt D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–288. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Dixit R, Vijayraghavan K, Bate M. Hox genes and the regulation of movement in Drosophila. Dev Neurobiol. 2008;68:309–316. doi: 10.1002/dneu.20589. [DOI] [PubMed] [Google Scholar]
- Doe CQ, Smouse D, Goodman CS. Control of neuronal fate by the Drosophila segmentation gene even-skipped. Nature. 1988;333:376–378. doi: 10.1038/333376a0. [DOI] [PubMed] [Google Scholar]
- Dubayle D, Viala D. Localization of the spinal respiratory rhythm generator by an in vitro electrophysiological approach. Neuroreport. 1996;7:1175–1180. doi: 10.1097/00001756-199604260-00016. [DOI] [PubMed] [Google Scholar]
- Esmaeili B, Ross JM, Neades C, Miller DM, 3rd, Ahringer J. The C. elegans even-skipped homologue, vab-7, specifies DB motoneurone identity and axon trajectory. Development. 2002;129:853–862. doi: 10.1242/dev.129.4.853. [DOI] [PubMed] [Google Scholar]
- Ferrier DE, Minguillon C, Cebrian C, Garcia-Fernandez J. Amphioxus Evx genes: implications for the evolution of the Midbrain-Hindbrain Boundary and the chordate tailbud. Dev Biol. 2001;237:270–281. doi: 10.1006/dbio.2001.0375. [DOI] [PubMed] [Google Scholar]
- Frasch M, Hoey T, Rushlow C, Doyle H, Levine M. Characterization and localization of the even-skipped protein of Drosophila. Embo j. 1987;6:749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Analysis of an even-skipped rescue transgene reveals both composite and discrete neuronal and early blastoderm enhancers, and multi-stripe positioning by gap gene repressor gradients. Development. 1999;126:2527–2538. doi: 10.1242/dev.126.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Lear BC, Landgraf M, Yusibova GL, Zhou J, Riley KM, Patel NH, Jaynes JB. Even-skipped, acting as a repressor, regulates axonal projections in Drosophila. Development. 2003;130:5385–5400. doi: 10.1242/dev.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckscher ES, Lockery SR, Doe CQ. Characterization of Drosophila larval crawling at the level of organism, segment, and somatic body wall musculature. J Neurosci. 2012;32:12460–12471. doi: 10.1523/JNEUROSCI.0222-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckscher ES, Long F, Layden MJ, Chuang CH, Manning L, Richart J, Pearson JC, Crews ST, Peng H, Myers E, et al. Atlas-builder software and the eNeuro atlas: resources for developmental biology and neuroscience. Development. 2014;141:2524–2532. doi: 10.1242/dev.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PW. Evolution of homeobox genes. Wiley Interdiscip Rev Dev Biol. 2013;2:31–45. doi: 10.1002/wdev.78. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Yoshida N, Satoh N, Saiga H. Ciona intestinalis Hox gene cluster: Its dispersed structure and residual colinear expression in development. Proc Natl Acad Sci U S A. 2004;101:15118–15123. doi: 10.1073/pnas.0401389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan-Parwar B, Fredman SM. Motor program for pedal waves during Aplysia locomotion is generated in the pedal ganglia. Brain Res Bull. 1980;5:169–177. doi: 10.1016/0361-9230(80)90190-2. [DOI] [PubMed] [Google Scholar]
- Kohsaka H, Okusawa S, Itakura Y, Fushiki A, Nose A. Development of larval motor circuits in Drosophila. Dev Growth Differ. 2012;54:408–419. doi: 10.1111/j.1440-169X.2012.01347.x. [DOI] [PubMed] [Google Scholar]
- Kohsaka H, Takasu E, Morimoto T, Nose A. A Group of Segmental Premotor Interneurons Regulates the Speed of Axial Locomotion in Drosophila Larvae. Current Biology. 2014 doi: 10.1016/j.cub.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Shen K, Klein M, Tang A, Kane E, Gershow M, Garrity P, Samuel AD. Two alternating motor programs drive navigation in Drosophila larva. PLoS One. 2011;6:e23180. doi: 10.1371/journal.pone.0023180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Roy S, Prokop A, VijayRaghavan K, Bate M. even-skipped determines the dorsal growth of motor axons in Drosophila. Neuron. 1999;22:43–52. doi: 10.1016/s0896-6273(00)80677-7. [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning L, Heckscher ES, Purice MD, Roberts J, Bennett AL, Kroll JR, Pollard JL, Strader ME, Lupton JR, Dyukareva AV, et al. A Resource for Manipulating Gene Expression and Analyzing cis-Regulatory Modules in the Drosophila CNS. Cell reports. 2012;2:1002–1013. doi: 10.1016/j.celrep.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley R, Baines RA. Dissection of first- and second-instar Drosophila larvae for electrophysiological recording from neurons: the flat (or fillet) preparation. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.prot065649. [DOI] [PubMed] [Google Scholar]
- Moran-Rivard L, Kagawa T, Saueressig H, Gross MK, Burrill J, Goulding M. Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron. 2001;29:385–399. doi: 10.1016/s0896-6273(01)00213-6. [DOI] [PubMed] [Google Scholar]
- Murchison D, Chrachri A, Mulloney B. A separate local pattern-generating circuit controls the movements of each swimmeret in crayfish. J Neurophysiol. 1993;70:2620–2631. doi: 10.1152/jn.1993.70.6.2620. [DOI] [PubMed] [Google Scholar]
- Nern A, Pfeiffer BD, Rubin GM. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1506763112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, Mensh BD, Branson KM, Simpson JH, Truman JW, et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Griffith LC. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat Neurosci. 2010;13:53–59. doi: 10.1038/nn.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pym EC, Southall TD, Mee CJ, Brand AH, Baines RA. The homeobox transcription factor Even-skipped regulates acquisition of electrical properties in Drosophila neurons. Neural Dev. 2006;1:3. doi: 10.1186/1749-8104-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert C, Kunz T, Harris KL, Whitington PM, Technau GM. Morphological characterization of the entire interneuron population reveals principles of neuromere organization in the ventral nerve cord of Drosophila. J Neurosci. 2011;31:15870–15883. doi: 10.1523/JNEUROSCI.4009-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer JE, Worrell JW, Levine RB. Role of intrinsic properties in Drosophila motoneuron recruitment during fictive crawling. J Neurophysiol. 2010;104:1257–1266. doi: 10.1152/jn.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MA, Trimmer BA. Movement encoding by a stretch receptor in the soft-bodied caterpillar, Manduca sexta. J Exp Biol. 2009;212:1021–1031. doi: 10.1242/jeb.023507. [DOI] [PubMed] [Google Scholar]
- Singhania A, Grueber WB. Development of the embryonic and larval peripheral nervous system of Drosophila. Wiley Interdiscip Rev Dev Biol. 2014;3:193–210. doi: 10.1002/wdev.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordino P, Duboule D, Kondo T. Zebrafish Hoxa and Evx-2 genes: cloning, developmental expression and implications for the functional evolution of posterior Hox genes. Mech Dev. 1996;59:165–175. doi: 10.1016/0925-4773(96)00587-4. [DOI] [PubMed] [Google Scholar]
- Suster ML, Kania A, Liao M, Asakawa K, Charron F, Kawakami K, Drapeau P. A novel conserved evx1 enhancer links spinal interneuron morphology and cis-regulation from fish to mammals. Dev Biol. 2009;325:422–433. doi: 10.1016/j.ydbio.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Takatori N, Butts T, Candiani S, Pestarino M, Ferrier DE, Saiga H, Holland PW. Comprehensive survey and classification of homeobox genes in the genome of amphioxus, Branchiostoma floridae. Dev Genes Evol. 2008;218:579–590. doi: 10.1007/s00427-008-0245-9. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature. 2013;500:85–88. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- Tamarkin DA, Levine RB. Synaptic interactions between a muscle-associated proprioceptor and body wall muscle motor neurons in larval and Adult manduca sexta. J Neurophysiol. 1996;76:1597–1610. doi: 10.1152/jn.1996.76.3.1597. [DOI] [PubMed] [Google Scholar]
- Thaeron C, Avaron F, Casane D, Borday V, Thisse B, Thisse C, Boulekbache H, Laurenti P. Zebrafish evx1 is dynamically expressed during embryogenesis in subsets of interneurones, posterior gut and urogenital system. Mech Dev. 2000;99:167–172. doi: 10.1016/s0925-4773(00)00473-1. [DOI] [PubMed] [Google Scholar]
- von der Porten K, Parsons DW, Rothman BS, Pinsker H. Swimming in Aplysia brasiliana: analysis of behavior and neuronal pathways. Behav Neural Biol. 1982;36:1–23. doi: 10.1016/s0163-1047(82)90201-1. [DOI] [PubMed] [Google Scholar]
- Zarin AA, Asadzadeh J, Hokamp K, McCartney D, Yang L, Bashaw GJ, Labrador JP. A transcription factor network coordinates attraction, repulsion, and adhesion combinatorially to control motor axon pathway selection. Neuron. 2014;81:1297–1311. doi: 10.1016/j.neuron.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.