Abstract
Aims
A subset of colorectal carcinomas (CRCs) architecturally and cytologically resembles adenomatous change, making them difficult to diagnose on biopsy. This subset has not been well-characterized to date.
Methods and results
For 35 carcinomas with adenomatous-like areas (cytologic and surface architectural appearance that would be insufficient to warrant a diagnosis of adenocarcinoma if evaluated on biopsy), we recorded staging information, molecular data, clinical outcome, whether precursor adenoma was present, and whether prior biopsy had been diagnosed as malignant. Despite advanced T-category in 23 (66%) tumors, only 7 (20%) had nodal metastases, and only 5 patients (15%) developed distant metastases. Fifteen cases (43%) had been diagnosed as adenoma on biopsy. Twenty-one resections (60%) showed no residual associated adenoma, including 9 called adenoma on biopsy. Median follow-up was 44 months. Four patients (12%) died of disease; 22 were alive at last follow-up. KRAS mutation was seen in 14/24 (58%), and 4/17 (24%) were microsatellite-unstable. Patients had significantly improved survival compared to a cohort of patients with conventional well-differentiated CRC after controlling for age and stage (p=0.011).
Conclusions
Adenoma-like adenocarcinoma is an uncommon variant of CRC with a low rate of metastasis and good prognosis. Biopsy diagnosis of this lesion may be challenging.
Keywords: Colorectal Adenocarcinoma, Adenoma-Like Adenocarcinoma, Gene Mutation, Microsatellite Instability, Prognosis
INTRODUCTION
Screening for colorectal carcinoma (CRC) is widely accepted as a practical method of detecting premalignant lesions and early-stage carcinomas, both in average-risk patients and in those with increased risk due to family history or genetic syndromes.1 Frankly malignant lesions are grossly visible on colonoscopy, and tissue biopsy confirms the diagnosis in the vast majority of patients.
However, biopsy of a colorectal mass that appears malignant to the endoscopist may sometimes yield fragments of dysplastic tissue resembling an adenoma, without evidence of stromal invasion diagnostic of malignancy. In theory, this could be due to sampling of the precursor adenoma rather than the malignant component, and clinical management may proceed on the presumed diagnosis of CRC. We have observed that in some such cases, the adenocarcinoma present in the resection specimen demonstrates areas morphologically similar or identical to villous adenomas. The luminal surface in some of these tumors consists entirely of adenoma-like areas, suggesting that a diagnosis of malignancy would be difficult or impossible on biopsy without sampling deeper areas. This phenomenon has been reported previously in case series,2–6 but these reports have not yet prompted widespread recognition of tumors with this morphology as a unique subtype of well-differentiated CRC.
To this end, we have analyzed several cases of well-differentiated CRC in order to identify the unique clinical, morphologic, and molecular properties of this particular subtype, which we have termed “adenoma-like adenocarcinoma” of the colorectum.
MATERIALS AND METHODS
With approval from the Institutional Review Board at Vanderbilt University, slides from 1,003 CRC resections in the institution-restricted files of one of the authors were reviewed for adenoma-like features. These were defined as areas demonstrating villiform architecture, no more than rare foci of high-grade nuclear atypia, and either the absence of desmoplasia or limited zones of desmoplasia closely outlining infiltrating glands, rather than forming expansive regions of fibrous tissue. These areas were considered truly “adenoma-like” upon concluding that, had they been observed in a biopsy specimen rather than as part of a resection specimen, the diagnosis of villous adenoma would have been rendered rather than adenocarcinoma. For inclusion, a tumor had to display such features along the luminal surface, though areas deeper within the colonic wall often also demonstrated such architecture. Tumors with associated intraluminal “dirty necrosis” or tumor budding were excluded, as were cases of conventional well-differentiated CRC that, while (by definition) composed almost entirely of glandular structures, demonstrated unambiguous mural infiltration diagnostic of malignancy.
Twenty-nine cases with at least focal adenoma-like features were identified, representing 2.9% of the cases examined. Six additional cases were identified either through review of CRC cases for other research projects or prospectively during routine practice, bringing the total number to 35 carcinomas from 34 patients. The presence of adenoma-like features was confirmed by other authors.
For each case, all available slides were reviewed, along with the original pathology report, to determine tumor size, depth of invasion, and spread to lymph nodes or distant sites. The tumors were staged using American Joint Committee on Cancer (AJCC) TNM 7th edition criteria.7 The percentage of the tumor demonstrating adenoma-like features was estimated, and the presence of other identifiable microscopic patterns was noted. The presence or absence of residual adenoma was also recorded. For each patient, sex, age, and date of resection were recorded, as were the date of and clinical status at last known follow-up. Thirty cases were biopsied prior to resection; the biopsy diagnoses were noted, and the biopsy slides were examined as well (although six biopsies were performed at referring institutions and were not available for review). The same clinicopathologic parameters, except for prior biopsy and precursor lesion details, were also available for a control group of 83 well-differentiated non-adenoma-like CRCs in our database.
Mutational analysis of KRAS, NRAS, BRAF, PIK3CA, AKT, PTEN, and SMAD4 was performed on the adenoma-like CRC cases using an in-house assay as previously described.8 Mutations analyzed included AKT1 (codon E17), BRAF (codons G466, G469, D594, G596, V600), KRAS (codons G12, G13, Q61, A146, K117), NRAS (codons G12, Q61), PIK3CA (codons H1047, E542, E545, Q546, D549), PTEN (codons R233, R159, R267), and SMAD4 (codons E330, D351, D355, R361). This panel was chosen based on mutation frequency and relevance to potential targeted therapy in CRC. Sanger sequencing was also performed by Vanderbilt Technologies for Advanced Genomics to examine the mutational status of GNAS codon 201. Results of microsatellite instability (MSI) testing were available for some tumors as well. For GNAS sequencing and MSI testing, tumor DNA was isolated from areas with a tumor cellularity of more than 30%.
The adenoma-like group and the control well-differentiated CRC group were compared using Cox proportional hazards regression analysis for tumor type and age at diagnosis with stratification by AJCC TNM stage. Correlations between T-category and N-category disease within each cohort were compared using the nonparametric test for trend across ordered groups.9 These were performed using Stata (version 13, College Station, TX).
RESULTS
Clinicopathologic findings for both groups are summarized in Table 1. Per gross reports, all 35 adenoma-like CRC were macroscopically identifiable as malignant and often described as polypoid or exophytic. Microscopically, in addition to the defining morphologic features discussed above, the lesions often had a pushing border, involving the wall of the colon either in a broad fashion (somewhat resembling an adenoma, but with the “surface” within the wall rather than along the mucosa) (Figs. 1A and B) or as several discrete foci demonstrating villous projections of malignant epithelium emanating from the lining of the spaces (Fig. 1C). The lumina of the adenoma-like glands were sometimes filled with inflammatory cells or wispy mucinous debris. Desmoplasia was either absent or at most focally present around isolated glands. On average, adenoma-like elements comprised approximately 50% of tumor area; only one case was entirely adenoma-like. In twenty-eight cases (80%), the non-adenoma-like areas displayed dilated glands filled with mucin at the leading edge of the tumor (Fig. 1D). Otherwise, the non-adenoma-like areas showed well-differentiated CRC of no particular subtype.
TABLE 1.
Clinicopathologic features of 35 adenoma-like and 83 well-differentiated CRC
| Patient demographics | Adenoma-like | Well-differentiated |
| Number of patients | 34 | 83 |
| Age at diagnosis in years (mean; range) | 69; 52–89 | 67; 27–89 |
| Male:female ratio | 14:20 | 45:38 |
| Tumor characteristics | Adenoma-like | Well-differentiated |
| Number of CRC | 35 | 83 |
| Size of tumors in cm (mean; range) | 4.8; 1.0–15.0 | 3.8; 0.1–14.8 |
| Location of tumors | ||
| Cecum | 5 (14%) | 12 (15%) |
| Ascending colon | 13 (37%) | 18 (22%) |
| Hepatic flexure | 2 (6%) | 1 (1%) |
| Transverse colon | 2 (6%) | 5 (6%) |
| Splenic flexure | 2 (6%) | 1 (1%) |
| Descending colon | 0 | 11 (13%) |
| Rectosigmoid | 11 (31%) | 31 (37%) |
| Other/not specified | 0 | 4 (5%) |
| Pathologic T-category | ||
| pT1 | 5 (14%) | 16 (19%) |
| pT2 | 6 (17%) | 20 (24%) |
| pT3 | 20 (57%) | 30 (36%) |
| pT4 | 4 (11%) | 16 (19%) |
| Not specified | 0 | 1 (1%) |
| Pathologic N-category | ||
| pN0 | 28 (80%) | 59 (71%) |
| pN1 | 6 (17%) | 11 (13%) |
| pN2 | 1 (3%) | 7 (8%) |
| Not specified | 0 | 6 (7%) |
| Pathologic M-category | ||
| pM0 | 30 (86%) | 69 (83%) |
| pM1 | 5 (14%) | 14 (17%) |
| Lymphovascular invasion present | 7 (20%) | 13/76 (17%) |
| Perineural invasion present | 1 (3%) | 0/77 (0%) |
| Mucinous features present | 28 (80%) | 41/83 (49%) |
| Precursor lesion present | 14 (40%) | N/A |
| Prior biopsy called adenoma | 15/30 (50%) | N/A |
| No residual adenoma on resection | 9/15 (60%) | N/A |
| Follow-up length in months (median; range) | 44; 2–185 | 48; 0–214 |
| Patients alive at last follow-up | 22/34 (65%) | 45/83 (54%) |
| Patients dead of disease | 4/34 (12%) | 16/83 (19%) |
| Patients dead of other/unknown causes | 8/34 (24%) | 22/83 (27%) |
Figure 1.
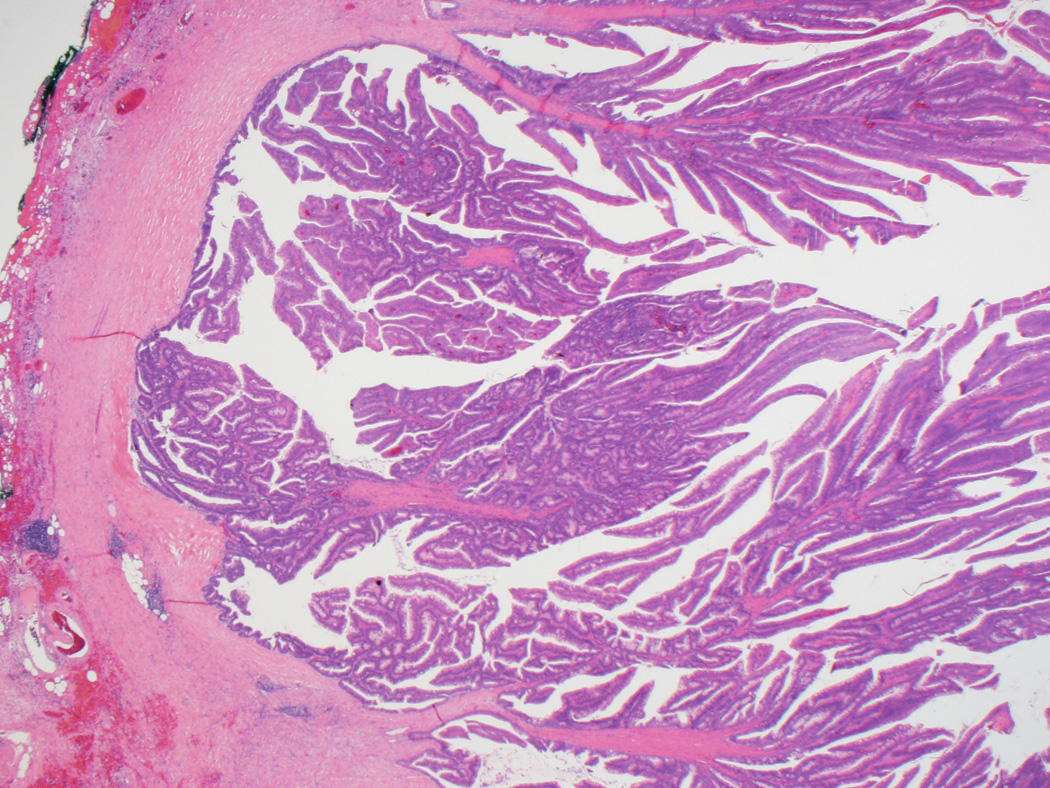
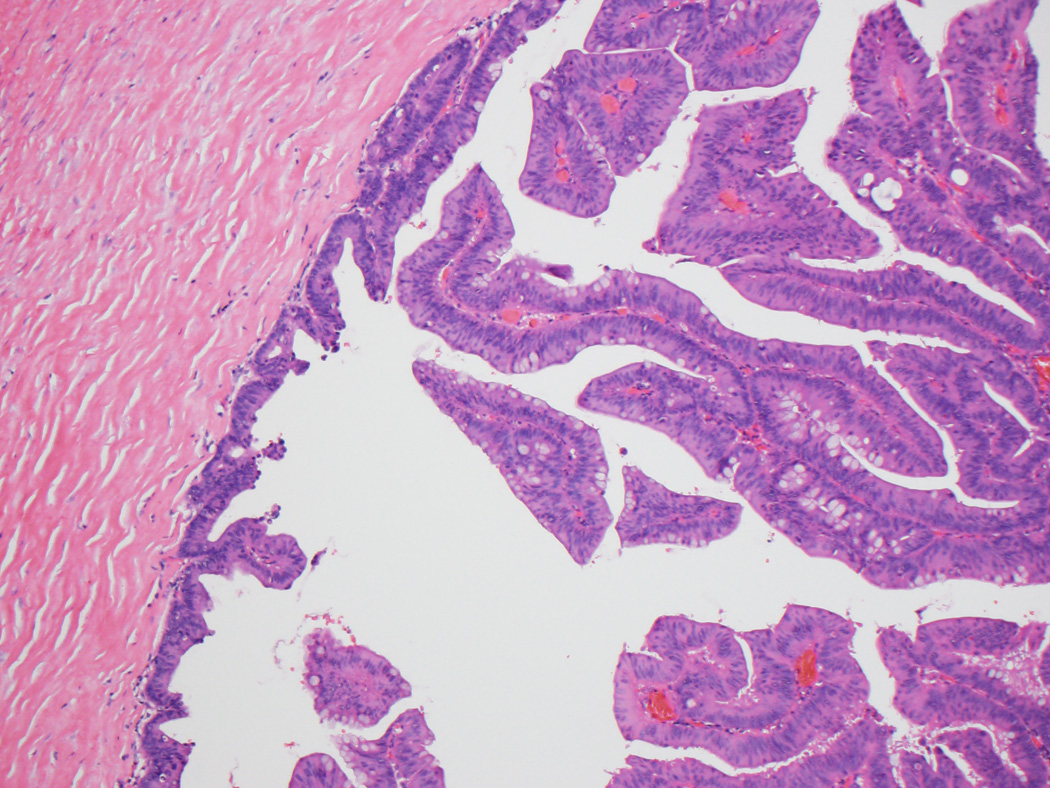
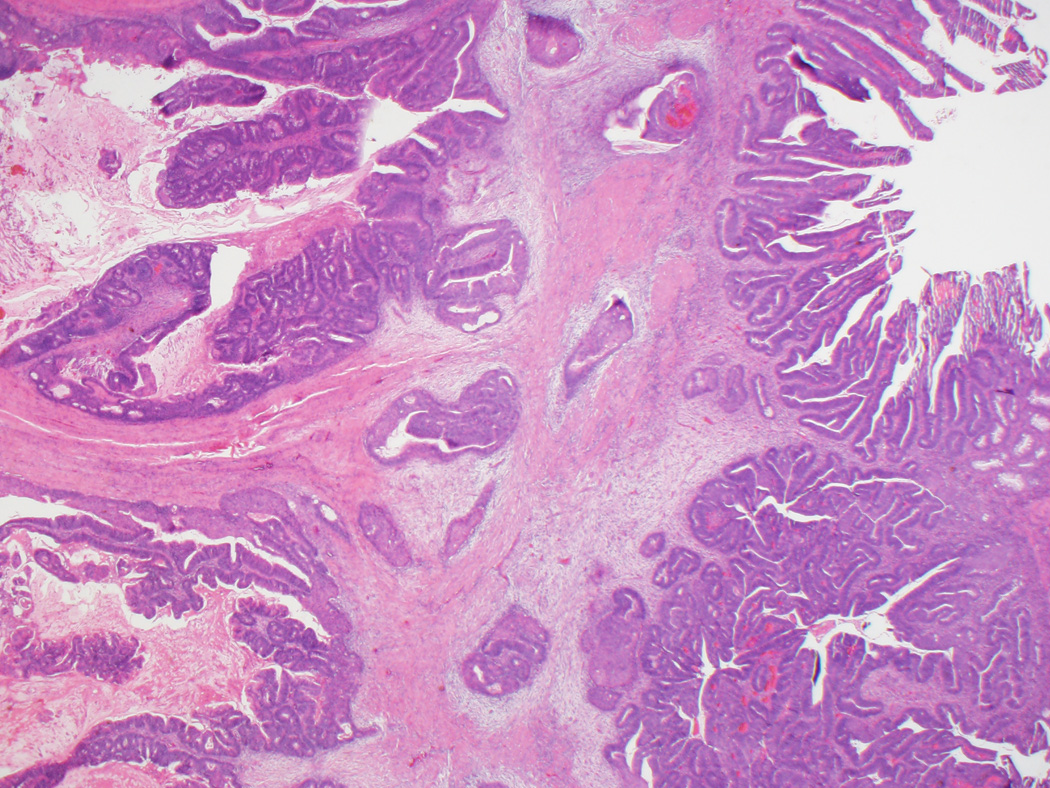
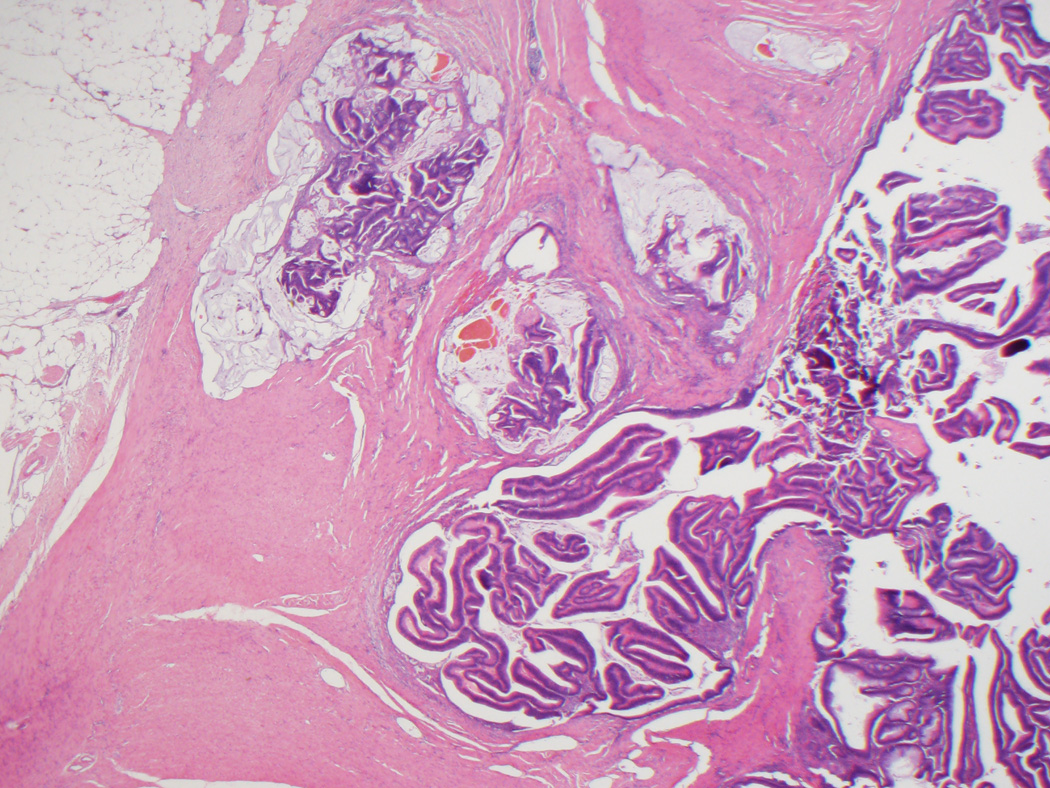
A, Typical appearance of an adenoma-like CRC, with a pushing border and villiform architecture. These tumors demonstrate low-grade nuclear features and often lack stromal desmoplasia. B, True invasion is indicated by the absence of surrounding lamina propria. C, A synchronous adenoma-like adenocarcinoma in the same patient as in Figures 1A and 1B has a slightly different pattern of infiltration, forming cystic areas in the colonic wall. Some malignant glands demonstrate peripheral desmoplasia, while others do not. D, Adenoma-like adenocarcinomas also often showed dilated glandular structures at the leading edge, filled with blue-gray mucin but not dirty necrosis.
Fourteen adenoma-like CRC (40%) had an identifiable precursor adenoma (tubular, tubulovillous, or villous). This component was sometimes difficult to distinguish from invasive adenoma-like CRC. Clues to the diagnosis of adenoma included lack of definitive basement membrane penetration and tubular, rather than villous, architecture, as tubular features were not seen in adenoma-like CRCs.
Prior biopsy had been performed on 30 of the 35 adenoma-like CRC, half of which were diagnosed as an adenoma or were otherwise not diagnostic of malignancy; the other 15 were called malignant. Slides from 24 biopsies were available for review. We generally agreed with the interpretation on all of them, though in rare instances, subtle findings potentially suggestive of adenocarcinoma were present (such as glands with distorted architecture, or non-desmoplastic fibroblastic hyperplasia replacing lamina propria) (Figs. 2A–C). Nine of the 15 cases called adenoma on biopsy showed no evidence of adenoma on resection.
Figure 2.
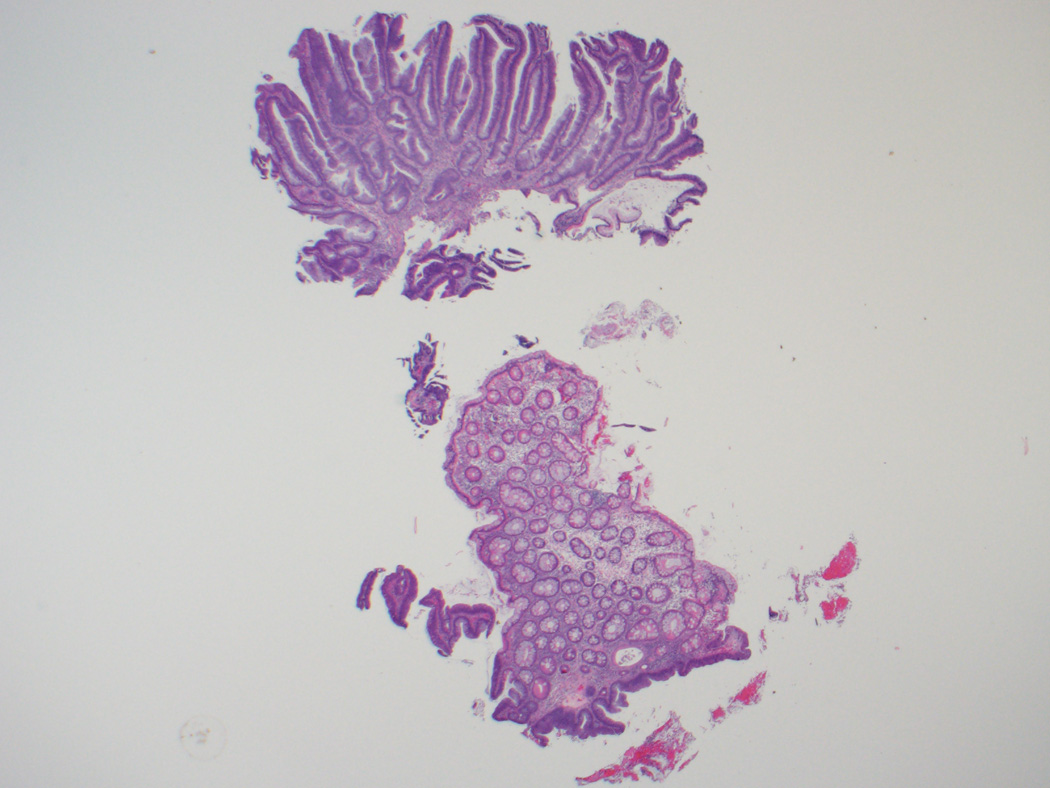
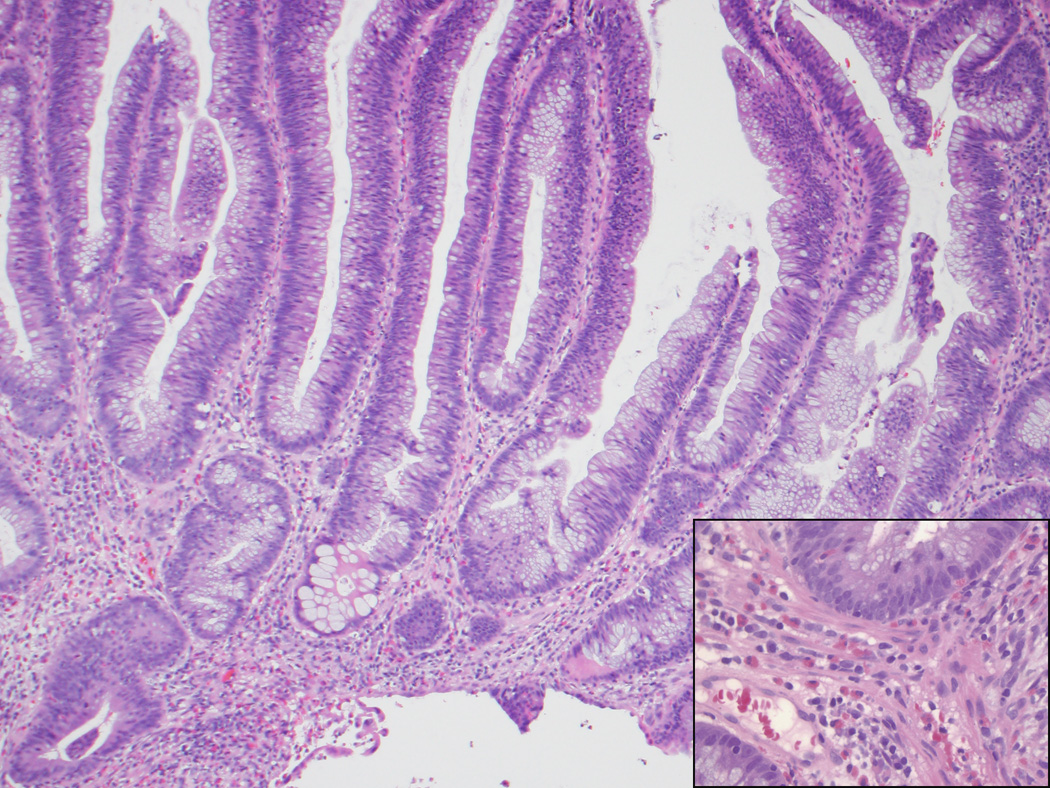
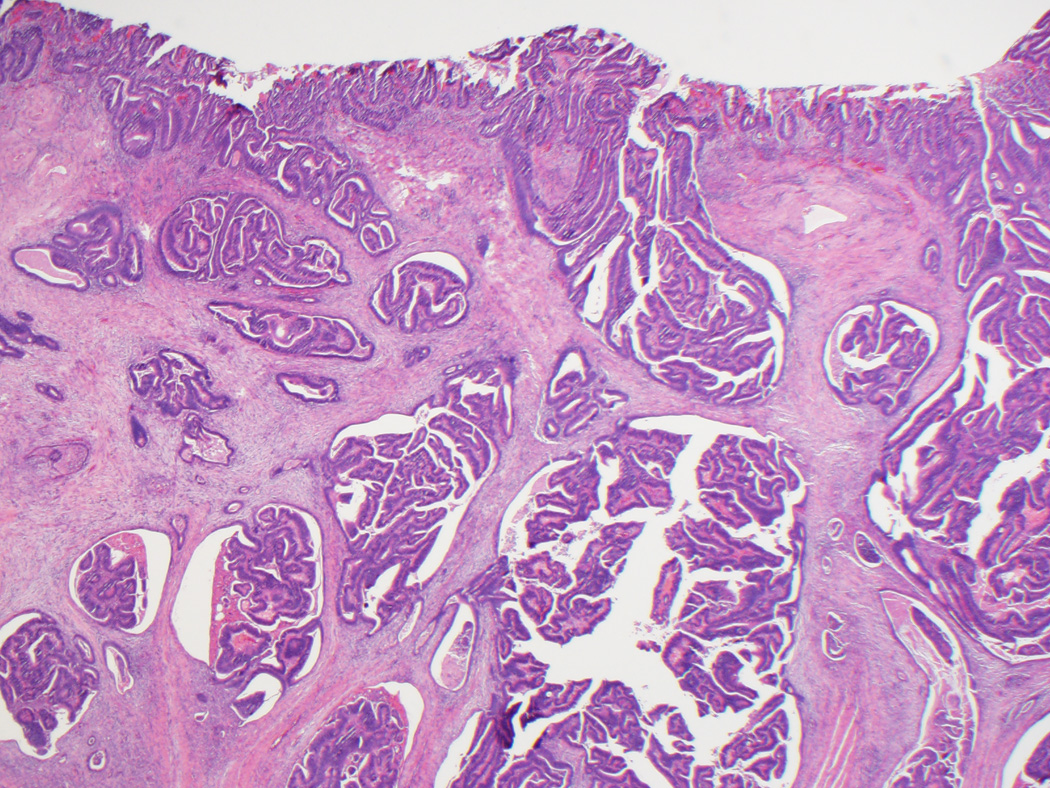
A, B, This biopsy sample of the surface of an adenoma-like CRC was diagnosed as an adenoma, given its villous architecture, low-grade nuclei, and lack of desmoplasia or necrosis. While there are no unequivocal features of malignancy, the gland in the lower left gland shows architectural distortion that could be interpreted as indicative of malignancy, and the tissue surrounding it (inset, B) resembles lamina propria but shows a mild degree of fibroblastic hyperplasia. C, On resection, the carcinoma was almost entirely adenoma-like, and no adjacent adenoma was present.
Most adenoma-like CRC were pT3 or pT4, but only seven (20%) displayed nodal metastases. Four of these involved 1 node; the highest involved node count was 4. All but one case with nodal disease were pT3 or pT4. T-category correlated with risk of nodal metastasis for well-differentiated conventional CRC (p=0.031) but not adenoma-like CRC (p=0.178). Five patients (15%) developed distant metastases (2 pulmonary, 1 uterine, 1 hepatic, 1 omental). The metastases all resembled conventional CRC, save for those in one node and in the omentum.
Molecular analysis performed on 24 of the 35 adenoma-like cases is summarized in Table 2. Seventeen (71%) showed mutations in at least one analyzed gene. The most frequently altered gene was KRAS, with mutations in 14 tumors (58%). Mutations in PIK3CA and BRAF were also observed. Microsatellite instability testing was performed on 17 cases, of which 4 (24%) were MSI-high. All four had mucinous features, and two showed an adjacent “Crohn’s like” reaction;10 all four patients were alive at last follow-up.
TABLE 2.
| Molecular features of adenoma-like adenocarcinoma | |
| KRAS mutation | 14/24 (58%) |
| G12V – 5 tumors | |
| G12D – 4 tumors | |
| G13D – 2 tumors | |
| G12C – 1 tumor | |
| G12S – 1 tumor | |
| K117N – 1 tumor | |
| PIK3CA mutation in addition to KRAS mutation | 4/24 (17%) |
| H1047R – 2 tumors | |
| E545K – 2 tumors | |
| BRAF V600E mutation | 3/24 (13%) |
| NRAS, AKT, PTEN, or SMAD4 mutations | 0/24 (0%) |
| GNAS codon 201 mutation | 0/26 (0%) |
| High microsatellite instability | 4/17 (24%) |
| Additional BRAF V600E mutation, included above – 1 tumor | |
| Additional KRAS G13D mutation, included above – 1 tumor | |
| MLH1 promoter methylation – 1 tumor | |
| No additional molecular findings – 1 tumor | |
Median follow-up for the adenoma-like patients was 44 months (range: 2–185). Twelve patients had fewer than 24 months of follow-up; of these, nine were alive and had only recently undergone resection, and three died of other disease.
Only four adenoma-like patients died of disease. Two had distant metastases at time of surgery and survived for 18 and 40 months after resection; the other two developed distant metastases later and survived for 108 and 140 months after resection. In all four, the CRC showed at most 50% adenoma-like areas. Available molecular data showed no mutation in one case and a KRAS mutation in another.
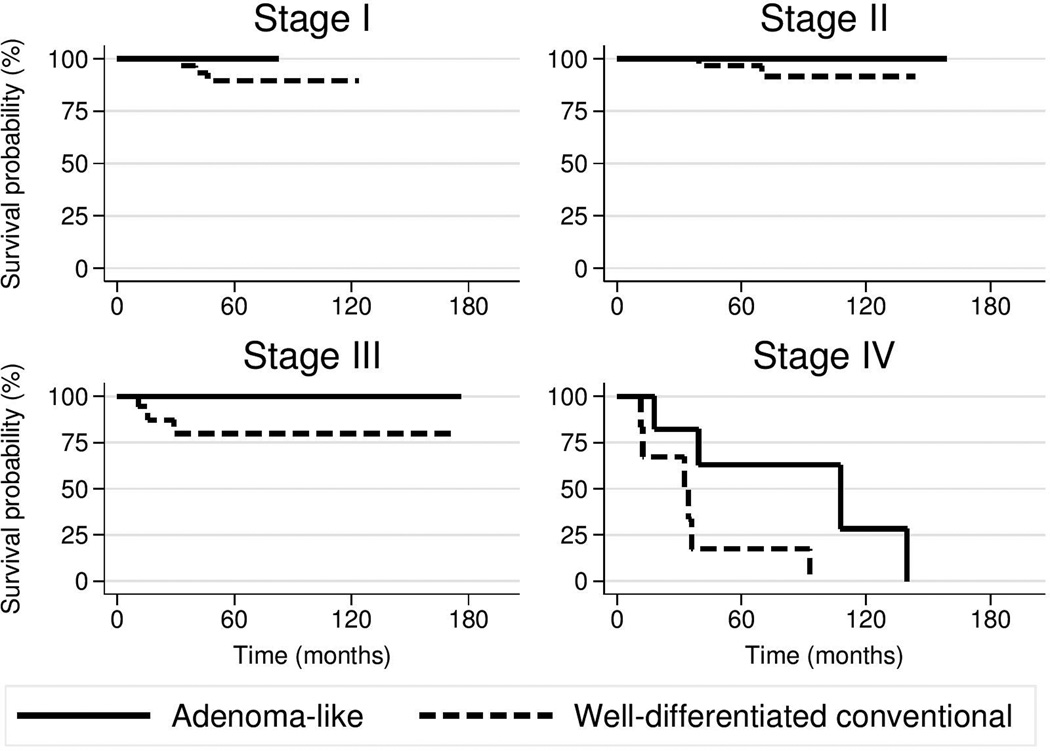
Patient age (p=0.33) and AJCC stage (p=0.37) did not significantly different between the adenoma-like and control groups. For adenoma-like CRC, disease-specific survival rates at 1, 3, and 5 years were 100%, 95%, and 89%; for well-differentiated conventional CRC, these rates were 96%, 86%, and 81% (Fig. 3). The adenoma-like subtype showed a hazard ratio of 0.17 (95% confidence interval, 0.04–0.67) for disease-specific survival compared to well-differentiated conventional CRC, after controlling for patient age and stratifying by AJCC stage (p=0.011).
Figure 3.
Kaplan–Meier age-adjusted survival curves for patients with adenoma-like CRC and well-differentiated CRC, by AJCC TNM stage.
DISCUSSION
A limited number of reports exist on the distinctive subtype of well-differentiated CRC we refer to here as adenoma-like adenocarcinoma.2–6 A 1998 report of 20 cases2 described tumors “composed of clusters having straight, finger-like villous structures.” They exhibited a low rate of lymph node metastasis, but survival data was not included. Subsequent reports included similar CRCs arising in 17 of 50 villous adenomas,3 two cases of “papillary adenocarcinoma” (with one appearing similar to adenoma-like CRC),4 and 13 cases of “pure villous carcinoma.”5
The largest series to date (n=36) was reported by Loy and Kaplan in 2004.6 They reported findings similar to ours, with a low rate of lymph node or distal metastasis, and only one patient potentially dying of disease (though this patient had five synchronous CRCs). Thirteen (36%) of their cases were diagnosed as adenoma on biopsy, similar to our proportion of 15/30 (50%). The unfortunate fact that diagnosing these lesions as malignant on biopsy can be difficult or occasionally impossible therefore appears to be a defining characteristic of adenoma-like adenocarcinoma. One criterion Loy and Kaplan used to distinguish between adenoma and invasive adenocarcinoma was the presence of “epithelial islands in desmoplastic stroma,” which they noted in 24/36 cases (67%). We also observed focal stromal desmoplasia in some of our tumors and agree that such a finding can be useful on biopsy to suggest malignancy, though this finding is often subtle or absent. In resection specimens, the overall architecture of these tumors also indicates mural invasion. While a diagnosis of pseudoinvasion might be considered in cases with a predominantly polypoid configuration, the lack of intermixed hemosiderin or lamina propria surrounding the deeper glands argues against this interpretation.11
While the morphologic features of these tumors have been characterized previously, their molecular alterations have not been reported. The predominant finding was mutation of KRAS, usually in codon 12 or 13, which was present in 58% of our cases with sufficient DNA for analysis. This proportion is higher than for CRC in general (35–45%),12 but this may simply reflect the limited sample size in our study. Although KRAS mutations have been suggested to impart a worse prognosis in CRC patients,13–14 only four of the 34 patients in this series died of disease, including only one of the 14 with a known KRAS mutation. Therefore, the deleterious effect of KRAS mutation for conventional CRC does not appear to impact the clinical behavior of adenoma-like CRC.
Similarly, BRAF V600E mutations, seen in 3/24 of our cases (12.5%), may predict a poor prognosis in MSI-stable CRC; this mutation occurs in approximately 9% of CRC.15 MSI-high status, which is found in approximately 15% of CRC overall,16 was present in a slightly higher number of cases in our cohort (4/17; 24%). This finding is linked to improved outcome and mucinous features (as seen in our cohort), as well as decreased response to 5-fluorouracil therapy.16 Considering the unique morphology of this type of adenocarcinoma, the potential role of other novel molecular alterations cannot be discounted. As an example, Fecteau et al17 reported GNAS mutations in 10 of 428 colorectal carcinomas; all 10 cases were right-sided, nine additionally harbored a KRAS or BRAF mutation, and 7 of 8 displayed villous morphology on histologic review. Similar molecular findings have been reported in non-malignant villous adenomas.18 However, none of our 26 cases tested for a GNAS mutation demonstrated one.
As the majority of our cases were pulled from a database that, while large and representative, does not contain every single colorectal carcinoma resected at our institution during the time frame of the study, our reported prevalence of 3% among colorectal adenocarcinomas is only an estimate. Indeed, Rubio2 reported an incidence of 5%, and Loy and Kaplan6 found an incidence of around 9%. These slight discrepancies may also be due to differences in diagnostic criteria; for example, we required the presence of adenoma-like areas along the surface of a lesion, as this component is the most amenable to sampling on biopsy.
As reported previously, patients with adenoma-like adenocarcinoma appear to do well relative to patients with more conventional CRC, though a statistical analysis comparing outcome data has not been previously performed, and existing reports only mentioned one case with distant metastases, and no patients who died from disease without potential other causes.6 Four patients in our study died of disease, two of whom survived for more than 100 months postoperatively. Seven cases spread to lymph nodes (20%), and five patients developed distant metastases (15%); in comparison, CRC overall spreads to lymph nodes in approximately 36% of cases, and to distant sites in approximately 20% of cases.19
Direct comparison between our adenoma-like cohort and a cohort of patients with well-differentiated CRC demonstrated significantly better disease-specific survival among the former group, with a hazard ratio of 0.17. The improved outcome of patients with adenoma-like adenocarcinoma is further noteworthy because, in most cases, the histologic subtype of a colorectal carcinoma is believed to either have no impact on prognosis or to worsen prognosis. The World Health Organization currently recognizes six subtypes of colorectal adenocarcinoma, of which only one (medullary) is considered to impart an improved prognosis.20 Additionally, the superficial adenoma-like component distinguishes this entity from other well-differentiated CRC that lack this finding, including distinct subtypes such as low-grade tubuloglandular adenocarcinoma (a bland malignancy seen in patients with inflammatory bowel disease that often arises from flat dysplasia).21
While some authors have referred to this subtype of adenocarcinoma as “villous adenocarcinoma”2–3,5–6 or “papillary adenocarcinoma,”4 we chose the name “adenoma-like adenocarcinoma” because that name can help explain to clinicians why such lesions may be diagnosed as adenomas on biopsy. Furthermore, the term “villous carcinoma” was used in earlier literature to refer to a typical CRC arising from a villous adenoma and hence may be misinterpreted.6
Regardless of the specific terminology used or minor variances in diagnostic criteria, it should be recognized that there exists an unusual adenoma-like subtype of CRC with particular clinicopathologic features: It can mimic adenoma on biopsy, it spreads beyond the colon uncommonly, and it rarely causes patient death. Its recognition may therefore influence treatment decisions (such as whether to offer adjuvant therapy given a low risk of metastasis), though additional studies, with emphasis on patient management, would be necessary to confirm this hypothesis.
ACKNOWLEDGEMENTS
GNAS sequencing was performed with assistance from the Vanderbilt Innovative Translational Research Shared Resource supported by the Vanderbilt-Ingram Cancer Center, the TJ Martell Foundation, and the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation.
FUNDING SUPPORT
This project is funded by NIH/NCI P50CA095103.
Footnotes
AUTHOR CONTRIBUTIONS
R. S. Gonzalez reviewed all biopsy and resection slides, collected data, and wrote the manuscript. J. M. M. Cates provided statistical analysis and expert advice. M. K. Washington provided case files, reviewed slides, and provided expert advice. R. D. Beauchamp and R. J. Coffey assisted with molecular analysis. C. Shi designed the study, reviewed slides, assisted with molecular analysis, and edited the manuscript.
REFERENCES
- 1.U.S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. [Accessed 13/02/2015]; http://www.uspreventiveservicestaskforce.org/uspstf08/colocancer/colors.htm. [Google Scholar]
- 2.Rubio CA, Yanagisawa A, Kato Y. Histologic phenotypes of colonic carcinoma in Sweden and in Japan. Anticancer Res. 1998;18:2649–2656. [PubMed] [Google Scholar]
- 3.Yao T, Kajiwara M, Kouzuki T, Iwashita A, Tsuneyoshi M. Villous tumor of the colon and rectum with special reference to roles of p53 and bcl-2 in adenoma-carcinoma sequence. Pathol Int. 1999;49:374–382. doi: 10.1046/j.1440-1827.1999.00881.x. [DOI] [PubMed] [Google Scholar]
- 4.Palazzo JP, Edmonston TB, Chaille-Arnold LM, Burkholder S. Invasive papillary adenocarcinoma of the colon. Hum Pathol. 2002;33:372–375. doi: 10.1053/hupa.2002.32228. [DOI] [PubMed] [Google Scholar]
- 5.Takata M, Yao T, Nishiyama KI, Nawata H, Tsuneyoshi M. Phenotypic alteration in malignant transformation of colonic villous tumours: with special reference to a comparison with tubular tumours. Histopathology. 2003;43:332–339. doi: 10.1046/j.1365-2559.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 6.Loy TS, Kaplan PA. Villous adenocarcinoma of the colon and rectum: a clinicopathologic study of 36 cases. Am J Surg Pathol. 2004;28:1460–1465. doi: 10.1097/01.pas.0000141394.64707.02. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Carducci MA, Compton CC, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. [Google Scholar]
- 8.Mikhitarian K, Pollen M, Zhao Z, et al. Epidermal growth factor receptor signaling pathway is frequently altered in ampullary carcinoma at protein and genetic levels. Mod Pathol. 2014;27:665–674. doi: 10.1038/modpathol.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985 Jan-Mar;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 10.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001 Feb;158(2):527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muto T, Bussey HJ, Morson BC. Pseudo-carcinomatous invasion in adenomatous polyps of the colon and rectum. J Clin Pathol. 1973;26:25–31. doi: 10.1136/jcp.26.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol. 2012;18:5171–5180. doi: 10.3748/wjg.v18.i37.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst. 1998;90:675–684. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 14.Phipps AI, Buchanan DD, Makar KW, et al. KRAS-mutation status in relation to colorectal cancer survival: the joint impact of correlated tumour markers. Br J Cancer. 2013;108:1757–1764. doi: 10.1038/bjc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao C, Liao RY, Qiu LX, Wang XW, Ding H, Chen Q. BRAF V600E mutation and resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer: a meta-analysis. Mol Biol Rep. 2011;38:2219–2223. doi: 10.1007/s11033-010-0351-4. [DOI] [PubMed] [Google Scholar]
- 16.Ng K, Schrag D. Microsatellite instability and adjuvant fluorouracil chemotherapy: a mismatch? J Clin Oncol. 2010;28:3207–3210. doi: 10.1200/JCO.2010.28.9314. [DOI] [PubMed] [Google Scholar]
- 17.Fecteau RE, Lutterbaugh J, Markowitz SD, Willis J, Guda K. GNAS Mutations Identify a Set of Right-Sided, RAS Mutant, Villous Colon Cancers. PLoS One. 2014 Jan 30;9(1):e87966. doi: 10.1371/journal.pone.0087966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada M, Sekine S, Ogawa R, et al. Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol. 2012 Sep;228(1):113–118. doi: 10.1002/path.4012. [DOI] [PubMed] [Google Scholar]
- 19.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. Apr, [Accessed 13/02/2015]. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site. [Google Scholar]
- 20.Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum. In: Bosman FT, Carniero F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010. [Google Scholar]
- 21.Levi GS, Harpaz N. Intestinal low-grade tubuloglandular adenocarcinoma in inflammatory bowel disease. Am J Surg Pathol. 2006 Aug;30(8):1022–1029. doi: 10.1097/00000478-200608000-00014. [DOI] [PubMed] [Google Scholar]