Abstract
Objective
To determine the prognostic significance of histologic type/subtype in a large series of patients with primary resected retroperitoneal sarcoma.
Summary Background Data
The histologic diversity and rarity of retroperitoneal sarcoma has hampered the ability to predict patient outcome.
Methods
From a single-institution, prospective database, 675 patients treated surgically for primary, non-metastatic retroperitoneal sarcoma during 1982–2010 were identified and histologic type/subtype was reviewed. Clinicopathologic variables were analyzed for association with disease-specific death (DSD), local recurrence (LR), and distant recurrence (DR).
Results
Median follow-up for survivors was 7.5 years. The predominant histologies were well-differentiated liposarcoma, dedifferentiated liposarcoma, and leiomyosarcoma. Five-year cumulative incidence of DSD was 31%, and factors independently associated with DSD were R2 resection, resection of ≥3 contiguous organs, and histologic type. Five-year cumulative incidence for LR was 39% and for DR was 24%. R1 resection, age, tumor size, and histologic type were independently associated with LR; size, resection of ≥3 organs, and histologic type were independently associated with DR. Liposarcoma and leiomyosarcoma were associated with late recurrence and DSD (as long as 15 years from diagnosis). For solitary fibrous tumor, local recurrence was uncommon (<10%), but early distant recurrence was common (36% at 5 years). Nomograms were developed to predict DSD, LR, and DR.
Conclusions
Histologic type/subtype is the most important independent predictor of DSD, LR, and DR in primary retroperitoneal sarcoma. Histology predicts the pattern and incidence of LR and DR and will aid in more accurate patient counseling and selection of patients for adjuvant therapy trials.
INTRODUCTION
Retroperitoneal soft tissue sarcomas (RPSs), excluding visceral sarcomas, account for 0.15% of all malignancies1 and about 15% of soft tissue sarcomas. Surgery remains the primary treatment modality, with complete resection the only chance for cure. The roles of radiation therapy and chemotherapy remain controversial.2 In prior studies of primary RPS, the most important prognostic factors for disease-specific survival were grade and completeness of resection, with incompletely resected patients having similar survival from initial presentation to that of unresected patients.3
Retroperitoneal sarcomas comprise a spectrum of histologic types/subtypes; the most common are liposarcoma and leiomyosarcoma. For many of these RPSs, the histologic type/subtype defines the grade.4, 5 However, although grade is commonly incorporated into staging systems and prognostic models, histologic subtype has been little investigated in terms of patterns of local and distant recurrence and their effect on prognosis. Such investigations have been hampered by the rarity of these tumors and by differences in surgical technique and use of non-surgical therapies, which confound multi-institutional analysis.6–9
The purpose of this study was to delineate the influence of histologic type/subtype on recurrence, metastasis, and survival. To undertake this, we analyzed a large, single-institution cohort of patients with primary, resected RPS who had been carefully assessed for histologic type/subtype and followed prospectively.
METHODS
Patients
The 675-patient cohort used in this study comprised all adult patients (>16 years of age) who underwent surgical exploration for primary retroperitoneal sarcoma at Memorial Sloan Kettering Cancer Center (MSKCC) from July 1982 through July 2010. Data for these patients were entered in the prospectively-maintained institutional database. Patients with desmoids or gastrointestinal stromal tumors were excluded. Approval for this study was obtained from the institutional review board, and all patients gave informed consent.
A tumor was considered a primary lesion if it had previously been untreated and there was no evidence of metastatic disease. Overall tumor size was defined as the sum of the perpendicular maximum diameters of the primary tumors as reported at the time of initial surgical resection, and tumors were grouped into three overall size categories: <10 cm, 10–20 cm, and ≥20 cm. Tumor grade was classified as low or high on the basis of established criteria such as degree of differentiation, nuclear pleomorphism, and number of mitoses per high-powered field. Histology was reviewed by a sarcoma pathologist (NPA) and, based on the World Health Organization criteria,10 tumors were classified into 6 histologic groups: liposarcoma, leiomyosarcoma, malignant peripheral nerve sheath tumor, sarcoma not otherwise specified (NOS), solitary fibrous tumor, translocation-associated (synovial sarcoma, rhabdomyosarcoma, Ewing sarcoma, fibrosarcoma, inflammatory myofibroblastic tumor), or other (malignant granular cell tumor, malignant mesenchymoma, myosarcoma). Liposarcomas were subdivided into 5 subtypes: well-differentiated, dedifferentiated, myxoid (<5% round cell component), round cell (≥5% round cell), or pleomorphic. For most analyses, the liposarcomas were divided into a high-grade group (dedifferentiated, round cell, and pleomorphic) and a low-grade group (well differentiated and myxoid.) Where sufficient archived tissue was available, immunohistochemistry for MDM2 and CDK4 was performed to retrospectively identify tumors originally designated as malignant fibrous histiocytoma, undifferentiated sarcoma, or sarcoma not otherwise specified. Those tumors staining positive for CDK4 or MDM2 were then reclassified as dedifferentiated liposarcoma.
Total gross excision was defined as complete resection by the operating surgeon. Margins were evaluated both grossly and microscopically in 6 dimensions (superior, inferior, medial, lateral, anterior, and posterior). Margins were categorized as clear, microscopically positive, or grossly positive. A clear margin indicated that there was no tumor within 1 mm from the edge of the inked specimen; a microscopically positive margin indicated microscopically discernible extension of tumor to within <1 mm of the edge of the inked specimen.
The variables considered for univariate and multivariate analysis were sex, age at diagnosis, overall tumor size, histologic grade (high or low), histologic type/subtype, location within the retroperitoneum (abdominal/retroperitoneal versus pelvic), etiologic association with radiation, number of organs resected, need for vascular resection, and margins/completeness of resection (negative, microscopically positive, grossly positive).
Some patients received chemotherapy or radiation as neoadjuvant (13%) or adjuvant (12%) treatment at the discretion of the multidisciplinary soft tissue sarcoma group or as part of clinical trials (see Table 1). Because such treatment was relatively uncommon and performed in highly selected patients, the details of adjuvant treatment were not included in the statistical analysis.
Table 1.
Demographics of the 675 patients
| Variable | Value (% or range) |
|---|---|
| Gender | |
| Male | 299 (44%) |
| Female | 376 (56%) |
| Median age, years (range) | 60 (16–91) |
| Retroperitoneal location | |
| Abdomen | 556 (82%) |
| Pelvis | 108 (16%) |
| Unknown | 11 (2%) |
| Median tumor size, cm (range) | 17 (2–139) |
| Radiation-associated | 14 (2%) |
| Grade | |
| Low | 242 (36%) |
| High | 431 (64%) |
| Number of organs resected | |
| 0 | 284 (42%) |
| 1 | 207 (31%) |
| 2 | 112 (16%) |
| 3 | 39 (6%) |
| 4 | 18 (3%) |
| ≥5 | 15 (2%) |
| Histology* | |
| Liposarcoma: well-differentiated & myxoid | 186 (28%) |
| Liposarcoma: dedifferentiated, round cell & pleomorphic | 213 (32%) |
| Low-grade leiomyosarcoma | 18 (3%) |
| High-grade leiomyosarcoma | 132 (20%) |
| Solitary fibrous tumor | 33 (5%) |
| Malignant peripheral nerve sheath tumor | 23 (3%) |
| Translocation-associated and other | 34 (5%) |
| Not otherwise specified (NOS) | 35 (5%) |
| Completeness of resection | |
| Exploratory laparotomy only (no resection) | 43 (6%) |
| R0 | 337 (50%) |
| R1 | 237 (35%) |
| R2 | 58 (9%) |
| Vascular resection performed | 67 (10%) |
| Pre-operative radiation** | 28 (4%) |
| Post-operative radiation | 26 (4%) |
| Pre-operative chemotherapy** | 73 (11%) |
| Post-operative chemotherapy | 48 (7%) |
MSKCC, Memorial Sloan Kettering Cancer Center;
One patient had an unresectable leiomyosarcoma of unknown grade.
Of the 89 patients (13%) who received pre-operative therapy, 16 (2%) received radiation only, 61 (9%) received chemotherapy only, and 12 (2%) received both.
Statistical Methods
The time to disease-specific death was defined as the time from operation until death caused by disease or to last follow-up. Times to local and distant recurrence were defined as the time from operation until date of local or distant recurrence, respectively, or to last follow-up. We estimated the cumulative incidence for DSD, LR, and DR. Estimation, testing, and regression modeling of the cumulative incidences were performed in a competing risks framework, using Gray’s k-test and the Fine & Gray regression model.11, 12 For analysis of sarcoma-specific death, deaths due to conditions unrelated to sarcoma were treated as competing events. The competing events for the LR analysis were deaths without LR, and competing events for DR analysis were deaths without DR.
All competing risk analyses excluded patients who had exploratory surgery but no resection (n = 43); the LR analysis also excluded patients who had incomplete resection (n = 58). A total of 632 patients were used in the DSD and DR analyses and 574 total patients were used in the LR analysis.
Nomograms were developed for each of the three outcomes. Multivariate models included covariates with p values <0.2 from univariate competing risks regression. Nomograms were evaluated by calibration plot, which compares nomogram-predicated probability with observed outcome, and by concordance index. The concordance index is a measure of how well the model discriminates between those who are high and low risk for the event of interest. In absence of censored data, a concordance index of 1 indicates perfect concordance, i.e. (1) all patients experiencing the competing event have lower risk predictions than patients experiencing the event of interest and (2) event times of patients experiencing the event of interest are perfectly ordered according to the predicted risks.13 A concordance index of 0.5 indicates that the model has no discriminative ability and is essentially random.
All analyses were done using R version 2.12.1, with the cmprsk, Design, and QHScrnomo packages.
RESULTS
Table 1 lists the descriptive statistics for the 675 patients with primary retroperitoneal sarcoma comprising the study population. The median follow-up was 3.3 years for all patients and 7.5 years for survivors; maximum follow-up was 24 years. The most frequent histologic type/subtypes were dedifferentiated, round cell, and pleomorphic liposarcomas (high-grade liposarcomas; 32% of patients), well differentiated and myxoid liposarcomas (low-grade liposarcomas; 28%), and high-grade leiomyosarcoma (20%). Overall, 64% of tumors were high grade, and median size was 17 cm (range, 2–139 cm). Local treatment consisted of surgical resection for all patients with a total gross excision accomplished in 574 patients (85%), whereas 58 patients (9%) had grossly positive margins and 43 patients (6%) underwent exploratory laparotomy with no attempt at resection. At least one contiguous organ was resected in 391 patients (58%), most commonly the kidney (192 patients, 28%). Three or more contiguous organs were resected in 72 patients (11%).
Disease-specific survival
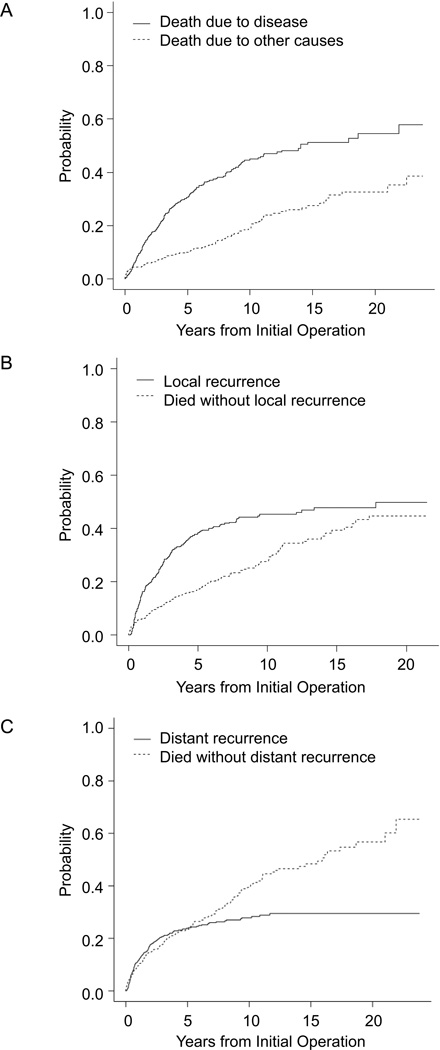
There were 335 deaths, of which 228 (68%) were attributed to sarcoma. The median time to sarcoma-specific death was 14.1 years (95% CI, 10.5 years to not reached). Disease-specific survival was 69% at 5 years and 55% at 10 years. The cumulative incidence of disease-specific death, calculated in a competing-risk analysis, was 31% at 5 years and 51% at 15 years (Figure 1A).
Figure 1.
Cumulative incidence of (A) sarcoma-specific death, (B) local recurrence, and (C) distant recurrence.
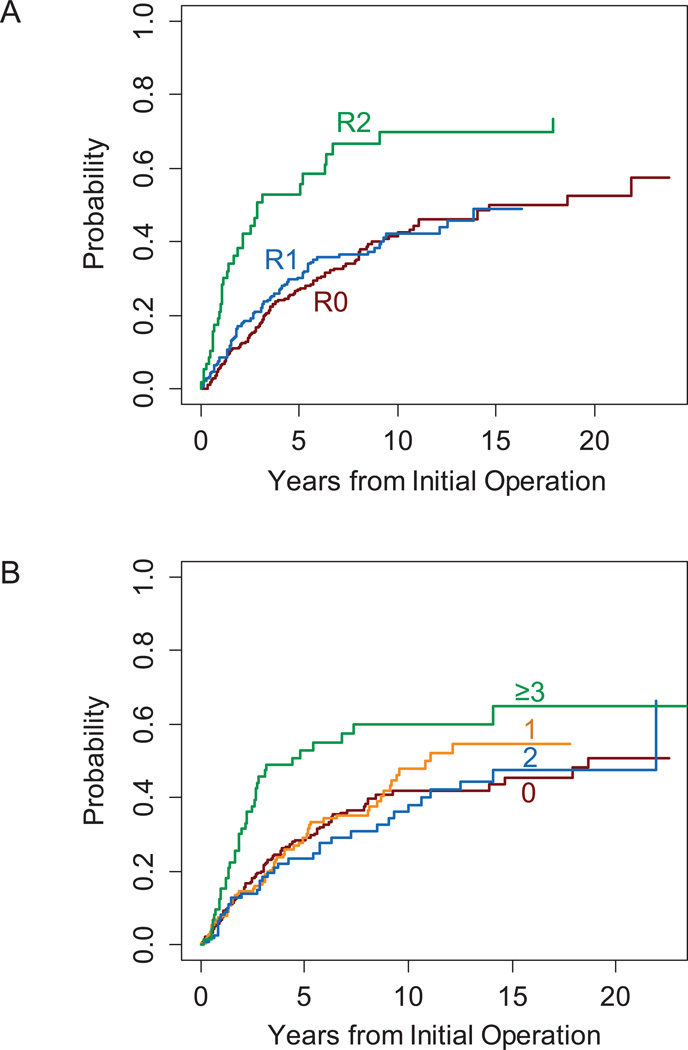
The univariate analysis of prognostic factors of importance to DSD for all 632 patients with resection of their primary retroperitoneal sarcoma is shown in Table 2. Histologic type (p<0.001), R2 resection (p<0.001), and 3 or more contiguous organs resected (p<0.001) were all significantly associated with DSD on univariate analysis. On multivariate analysis, these associations were confirmed. The influence of histologic subtype, as measured by the hazard ratio (HR), was in general greater than for other variables, with hazard ratios of 3.9 for high-grade leiomyosarcoma and 4.3 for MPNST (compared to well-differentiated and myxoid liposarcomas).
Table 2.
Factors associated with disease-specific death
| Factor | Univariate P | Multivariate P | Multivariate HR (95% CI) |
|---|---|---|---|
| Age (>60 vs ≤60 years) | 0.51 | – | – |
| Sex (male vs female) | 0.22 | – | – |
| Location (pelvis vs other) | 0.75 | – | – |
| Size | |||
| 10–20 cm vs <10 cm | 0.51 | 0.44 | 1.2 (0.8–1.8) |
| ≥20 cm vs <10 cm | 0.59 | 0.07 | 1.5 (1.0–2.3) |
| Radiation-associated (yes vs no) | 0.14 | 0.37 | 1.5 (0.6–3.7) |
| Histology* | |||
| Dedifferentiated, round cell & pleomorphic LPS, sarcoma NOS | <0.001 | <0.001 | 3.4 (2.3–5.1) |
| Low-grade leiomyosarcoma | 0.98 | 0.78 | 1.2 (0.3–4.9) |
| High-grade leiomyosarcoma | <0.001 | <0.001 | 3.9 (2.5–6.3) |
| Solitary fibrous tumor | 0.04 | 0.02 | 2.2 (1.1–4.5) |
| Malignant peripheral nerve sheath tumor | <0.001 | <0.001 | 4.3 (2.0–9.2) |
| Translocation-associated sarcoma | <0.001 | <0.001 | 3.8 (2.0–7.3) |
| Number of organs resected (≥3 vs <3) | <0.001 | 0.002 | 1.9 (1.3–2.8) |
| Vascular resection (yes vs no) | 0.88 | – | – |
| Completeness of resection (R2 vs R0/R1) | <0.001 | <0.001 | 2.5 (1.6–3.8) |
compared to well-differentiated and myxoid liposarcoma
CI, confidence interval; LPS, liposarcoma; NOS, not otherwise specified
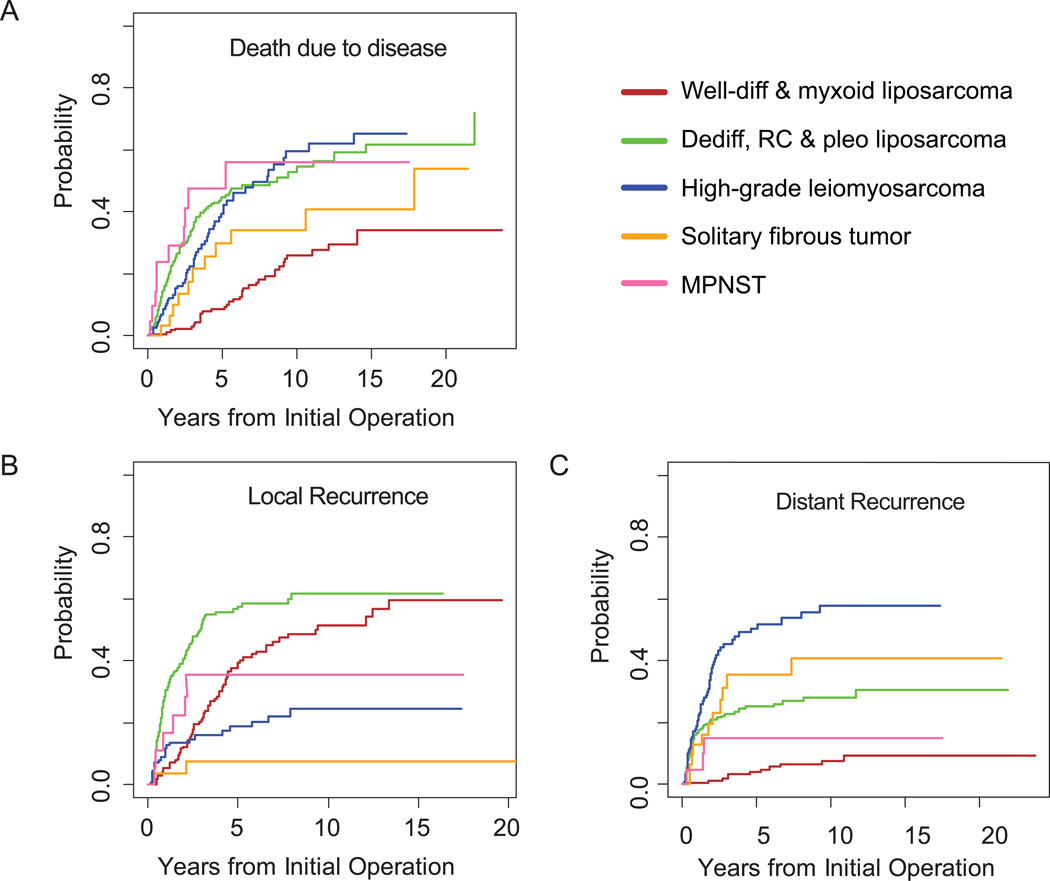
Disease-specific death is shown according to margin status and number of organs resected in Figure 2 and according to histologic subtype in Figure 3A. DSD varied widely according to histologic subtype. The cumulative incidence of DSD was lowest for patients with well-differentiated or myxoid liposarcomas (25% at 10 years; 95% CI, 18% to 34%), in contrast to those patients with dedifferentiated, round cell or pleomorphic liposarcomas, (53%; 95% CI, 44% to 61%), high-grade leiomyosarcoma (60%; 95% CI, 48% to 71%), or MPNST (56%; 95% CI, 30% to 82%). Patients with SFT had an intermediate risk of DSD (34%; 95% CI, 15% to 53%) at 10 years.
Figure 2.
Cumulative incidence of sarcoma-specific death by (A) margin status and (B) number of organs resected.
Figure 3.
Cumulative incidence by histologic type for (A) sarcoma-specific death, (B) local recurrence, and (C) distant recurrence. Well-diff, well-differentiated; dediff, dedifferentiated; RC, round cell; pleo, pleomorphic; MPNST, malignant peripheral nerve sheath tumor.
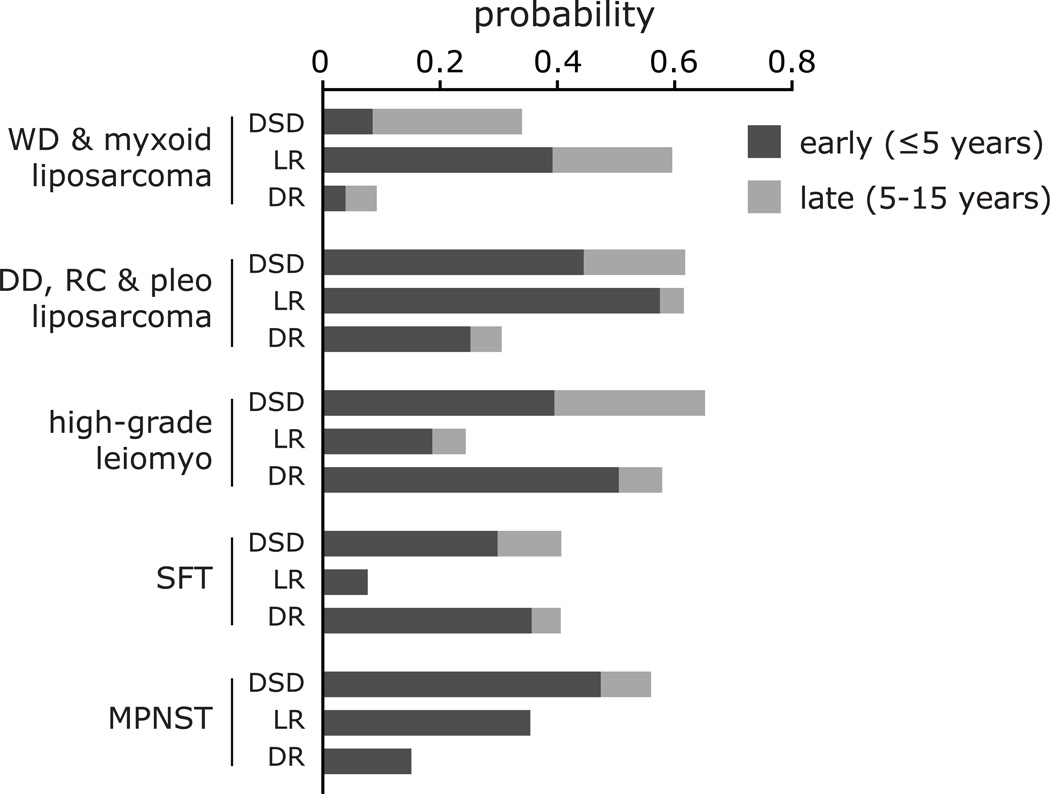
The risk of late (>5 year) disease-specific death also varied by histologic subtype (Figure 4). For patients with liposarcoma and leiomyosarcoma, the absolute incidence of late DSD was 17–26%. In relative terms, this amounted to almost one-third of disease-specific deaths in those with dedifferentiated, round cell, or pleomorphic liposarcoma or high-grade leiomyosarcoma, but three-quarters of those with well-differentiated or myxoid liposarcoma. In contrast, for MPNST (n=21), the cumulative incidence of DSD rapidly reached 47% by year 5, but only one disease-specific death (5%) occurred beyond 5 years.
Figure 4.
Patterns of disease-specific death (DSD), local recurrence (LR), and distant recurrence (DR) across histologic types and subtypes. The graph shows the probability of early events (within 5 years of initial surgery) and late events (between 5 and 15 years after initial surgery). DD, dedifferentiated; leiomyo, leiomyosarcoma; lipo, liposarcoma; MPNST, malignant fibrous histiocytoma; pleo, pleomorphic; RC, round cell; SFT, solitary fibrous tumor; WD, well-differentiated.
Local recurrence
For the 574 patients who had complete gross resections, 218 (38%) had developed local recurrence by the time of last follow-up. Cumulative incidence of LR was 39% at 5 years and 45% at 10 years (Figure 1B). In univariate analysis, the factors significantly associated with local recurrence were histologic type, location, tumor size, number of contiguous organs resected, R1 vs R0 resection, and vascular resection. On a multivariate analysis, the only factors independently associated with LR were histologic type, tumor size, R1 vs R0 resection, and age (Table 3).
Table 3.
Factors associated with local recurrence
| Factor | Univariate P | Multivariate P | Multivariate HR (95% CI) |
|---|---|---|---|
| Age (>60 vs ≤60 years) | 0.07 | 0.002 | 0.63 (0.5–0.8) |
| Sex (male vs female) | 0.47 | – | – |
| Location (pelvis vs other) | 0.002 | 0.34 | 0.8 (0.5–1.3) |
| Size | |||
| 10–20 cm vs <10 cm | 0.01 | 0.08 | 1.6 (0.9–2.8) |
| ≥20 cm vs <10 cm | <0.001 | 0.01 | 2.1 (1.2–3.8) |
| Radiation-associated (yes vs no) | 0.77 | – | – |
| Histology* | |||
| Dedifferentiated, round cell & pleomorphic LPS, sarcoma NOS | <0.001 | <0.001 | 2.3 (1.7–3.1) |
| Low-grade leiomyosarcoma | 0.1 | 0.31 | 0.5 (0.1–2.0) |
| High-grade leiomyosarcoma | 0.003 | 0.55 | 0.8 (0.5–1.5) |
| Solitary fibrous tumor | 0.008 | 0.03 | 0.1 (0.02–0.8) |
| Malignant peripheral nerve sheath tumor | 0.81 | 0.92 | 1.0 (0.4–2.6) |
| Translocation-associated sarcoma | 0.32 | 0.91 | 1.1 (0.4–2.9) |
| Number of organs resected (≥3 vs <3) | 0.003 | 0.86 | 1.0 (0.7–1.6) |
| Vascular resection (yes vs no) | 0.03 | 0.54 | 0.8 (0.5–1.5) |
| Completeness of resection (R1 vs R0) | <0.001 | <0.01 | 1.5 (1.1–1.9) |
compared to well-differentiated and myxoid liposarcoma
CI, confidence interval; LPS, liposarcoma; NOS, not otherwise specified
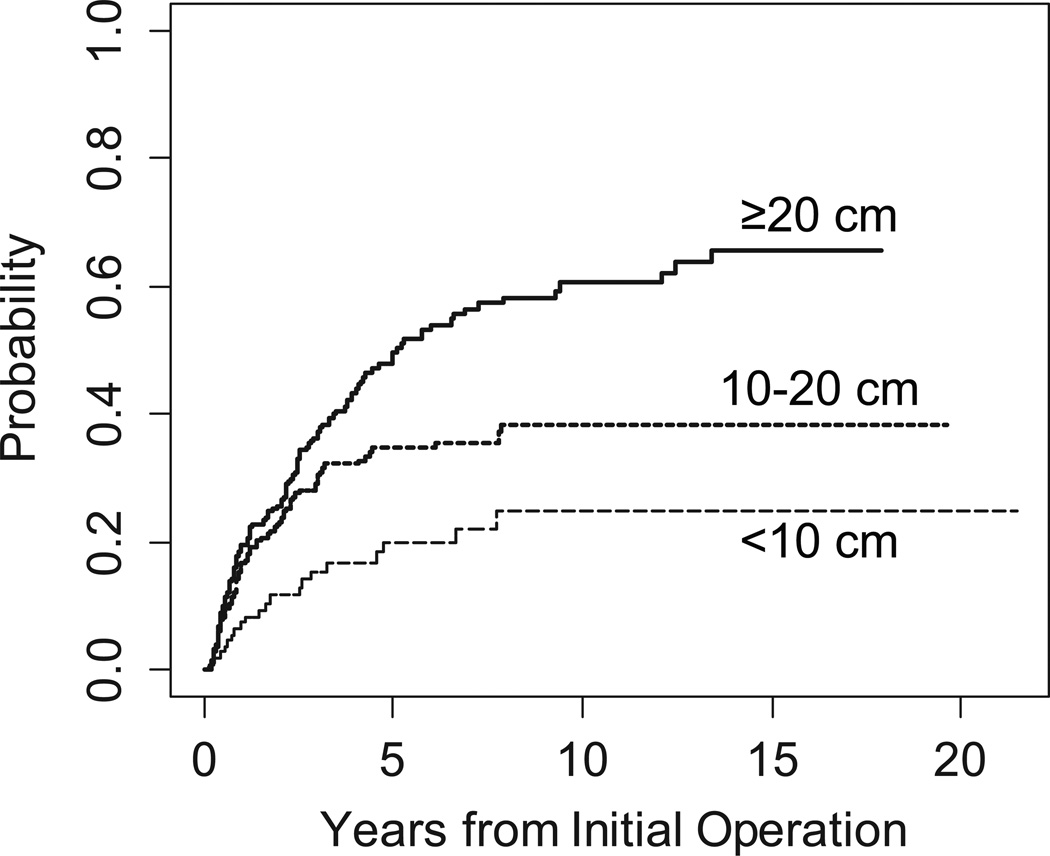
Cumulative incidence curves of LR by tumor size are illustrated in Figure 5 and curves by histologic type are illustrated in Figure 3B. As with DSD, the incidence and pattern of local recurrence varied widely among histologic subtypes. Local recurrence was rarest for patients with SFT (8%), and all of these patients' local recurrences occurred within 3 years of diagnosis. The high-grade liposarcomas had the highest incidence of early LR (58% by 5 years; 95% CI, 50% to 65%); later, the incidence rose little, to 62% by 15 years (95% CI, 53% to 70%). MPNST also had a high rate of early local recurrence: 35% at 3 years (95% CI, 11% to 60%), but local recurrence then plateaued with none occurring after 3 years. In contrast, well-differentiated and myxoid liposarcoma showed a 39% incidence of LR at 5 years (95% CI, 31% to 47%), which continued to slowly climb to 60% by 15 years (95% CI, 48% to 71%). This is nearly identical to the 62% 15-year rate for the high-grade liposarcomas. The incidence for high-grade leiomyosarcoma was 16% (95% CI, 9% to 23%) at 3 years and reached a plateau of 24% (95% CI, 15% to 34%) at 8 years.
Figure 5.
Cumulative incidence of local recurrence by tumor size.
Distant recurrence
There were 153 patients who had distant recurrence. The cumulative incidence of distant recurrence from a competing risk analysis is illustrated in Figure 1C, with 24% of patients developing distant recurrence at 5 years and 29% at 15 years.
The univariate analysis of prognostic factors associated with DR was performed on all 632 patients with primary retroperitoneal sarcoma (Table 4). The factors significantly associated with DR were histologic type, tumor size (p=0.04), vascular resection (p<0.001), and 3 or more contiguous organs resected (p=0.004). On multivariate analysis, significant associations were found for all these variables except vascular resection. The influence of histologic subtype on distant recurrence, as measured by HR, was greater than for non-histologic factors. The HRs for histologic subtype (with low-grade liposarcomas as the comparator) spanned a large range of hazard ratios: from 2.4 for MPNST to 11.6 for high-grade leiomyosarcoma.
Table 4.
Factors associated with distant recurrence
| Factor | Univariate P | Multivariate P | Multivariate HR (95% CI) |
|---|---|---|---|
| Age (>60 vs ≤60 years) | 0.65 | – | – |
| Sex (male vs female) | 0.07 | – | – |
| Location (pelvis vs other) | 0.09 | – | – |
| Size | |||
| 10–20 cm vs <10 cm | 0.18 | 0.01 | 1.8 (1.1–2.8) |
| ≥20 cm vs <10 cm | 0.04 | 0.51 | 1.2 (0.7–2.0) |
| Radiation-associated (yes vs no) | 0.09 | 0.63 | 1.3 (0.5–3.8) |
| Histology* | |||
| Dedifferentiated, round cell & pleomorphic LPS, sarcoma NOS | <0.001 | <0.001 | 4.7 (2.4–9.0) |
| Low-grade leiomyosarcoma | 0.04 | 0.01 | 5.3 (1.5–19) |
| High-grade leiomyosarcoma | <0.001 | <0.001 | 11.6 (5.7–23) |
| Solitary fibrous tumor | <0.001 | <0.001 | 6.3 (2.6–15) |
| Malignant peripheral nerve sheath tumor | 0.13 | 0.21 | 2.4 (0.6–9.2) |
| Translocation-associated sarcoma | <0.001 | <0.001 | 6.5 (2.7–16) |
| Number of organs resected | |||
| 1 or 2 vs 0 | 0.2 | 0.10 | 1.4 (0.9–2.0) |
| ≥3 | 0.004 | 0.002 | 2.4 (1.4–4.4) |
| Vascular resection (yes vs no) | <0.001 | 0.43 | 1.2 (0.8–2.0) |
| Completeness of resection (R2 vs R0/R1) | 0.65 | – | – |
compared to well-differentiated and myxoid liposarcoma
CI, confidence interval; LPS, liposarcoma; NOS, not otherwise specified
Cumulative incidences of DR by histologic type are illustrated in Figures 3C and 4. High-grade leiomyosarcoma had the highest cumulative incidence of distant recurrence (58% at 10 years; 95% CI, 47% to 69%), followed by solitary fibrous tumor (41%; 95% CI, 21% to 60%) and high-grade liposarcoma (28%; 95% CI, 22% to 35%). MPNST and low-grade liposarcoma had 10-year incidences of only 15% (95% CI, 0% to 32%) and 8% (95% CI, 3% to 12%), respectively.
Nomograms for prediction of DSD, LR, and DR
Nomograms were constructed to predict DSD, LR, and DR at 3, 5, and 15 years (see nomogram figures, Supplemental Digital Content 1–3). The calibration plots (Supplemental Digital Content 4) demonstrate good agreement between the predictions made by the nomogram and the actual outcomes. The concordance indices and bootstrapped 95% confidence intervals were 0.71 (0.66 – 0.74) for the DSD nomogram, 0.71 (0.67 – 0.75) for the LR nomogram, and 0.74 (0.69 – 0.77) for the DR nomogram. In contrast, when the patient cohort was analyzed using the post-operative sarcoma nomogram14 and the current AJCC staging system,15 the concordance indices were 0.60 and 0.62, respectively.
DISCUSSION
This study addresses the impact of individual histologic subtypes on the natural history of resected, non-metastatic primary retroperitoneal sarcomas. Rather than focusing on overall or disease-specific survival, we attempted to separate patterns of local and distant recurrence. We found that patterns of early and late recurrence and death differed among histologic subtypes in complex ways (Figure 4). As a tool for estimating prognosis, nomograms were developed for disease-specific death, local recurrence, and distant recurrence. These nomograms, along with an understanding of the patterns of recurrence and disease-specific death, will assist with patient counseling and follow-up.
Among the histologic types, low-grade liposarcoma (well-differentiated and myxoid) was unique in having a high rate of local recurrence—approaching 60%—but a very low rate of distant recurrence. Low-grade liposarcoma was also notable for the high proportion of late recurrence and late mortality, with 34% of the risk of local recurrence and 75% of the risk of disease-specific death occurring more than 5 years after surgery. Previous work has shown that, compared with well-differentiated liposarcoma, both dedifferentiated liposarcoma and high-grade leiomyosarcoma have higher rates of distant metastasis.5 However, we found that patients with high-grade leiomyosarcoma have a disease-specific survival almost identical to those with dedifferentiated liposarcoma, but have a much lower local recurrence rate and a higher distant recurrence rate. For solitary fibrous tumor, while local recurrence was extremely rare, the long-term distant recurrence rate was 41%—a surprisingly high rate of metastasis for what has traditionally been thought to be a low-grade tumor of unknown malignant potential. MPNST was notable for having almost no late recurrences or disease-specific deaths, despite having substantial rates of early local recurrences and deaths.
Radical resection remains the cornerstone of treatment for retroperitoneal sarcomas. There is, however, considerable controversy over the appropriate extent of surgical resection. The issue is contentious16–19 and has spurred vigorous point and counterpoint discussion.20–23 Set against this context, the work presented here represents 30 years of a uniform operative approach to resection of retroperitoneal sarcomas, namely resection with the intent of obtaining negative microscopic margins, without resection of adjacent but uninvolved organs. This approach has been combined with low rates of radiation and chemotherapy. We note in passing that without using the more aggressive "compartmental resection” philosophy and with only 27% of patients receiving additional non-surgical therapies, our 5-year disease-specific survival was 69%. This is similar to the 5-year overall survival rates of 60–66% in recent European series that used both compartmental resections and much higher (60–70%) rates of radiation and chemotherapy.17, 18, 24
Multiple nomograms for prognostication of soft tissue sarcoma exist.4, 8, 25, 26 Until now, all have focused on overall or disease-specific survival as endpoints and many of these did not incorporate all of the most common histologic types and subtypes found in the retroperitoneum. The distinct patterns of local and distant recurrence of the various subtypes of RPS found in our study suggest that separate nomograms predicting local versus distant disease, in addition to disease-specific survival, will be useful.
A persistent motivation for development and refinement of nomograms for soft tissue sarcoma is the limited utility of the current AJCC staging system,9, 27, 28 particularly for retroperitoneal sarcomas. Where the AJCC staging system has but a few variables, nomograms effectively integrate numerous prognostic variables, thereby improving their ability to predict the probability of death and recurrence. This principle is demonstrated once again by the concordance index of 0.62 for the AJCC compared with 0.71 for our revised nomogram. More accurate prognostication allows better post-operative counseling of patients and, potentially, more appropriate surveillance of high-risk patients.
The original post-operative soft tissue sarcoma nomogram generated disease-specific mortality probabilities after resection of all soft tissue sarcomas.14 This prognostic tool relies on age, size, depth, site, and histology, and has been externally validated.29, 30 However, despite the large number of patients in this series (n=2,136), only 288 (13%) had retroperitoneal tumors, limiting the ability to discriminate outcome among specific histologic types. Since its publication in 2002, further study of retroperitoneal sarcomas has provided insight into the different biologic behaviors of liposarcoma subtypes.5, 31 With the use of MDM2 and CDK4 immunohistochemistry, many tumors termed malignant fibrous histiocytoma have been re-classified as high-grade liposarcomas.32 Thus, the improved concordance index of our retroperitoneal-only sarcoma nomogram reflects better site-specific calibration, inclusion of histologic types and their subtypes, and re-classification of tumors previously labeled as malignant fibrous histiocytoma.
These nomograms apply only to patients with primary (rather than recurrent) RPS. We chose to include only those with primary tumors because at MSKCC a large proportion of patients with recurrent tumors were initially managed outside of our institution, with the heterogeneity in diagnosis and treatment (especially operative) that this entails.
This work has several limitations. Firstly, the advantages of this single-institution study (prospective data collection, uniformity of work-up and management, uniform pathologic review) come with the disadvantage that patients referred to MSKCC may not be representative of the patients treated at other institutions, which may limit the generalizability of our findings. Secondly, validation of our findings using patient cohorts from other institutions may prove difficult due to differences in surgical approach (see above) and treatment philosophy (which influences the use of radiation and chemotherapy). In our series, only a small minority of patients (13%) received pre-operative therapy, including just 4% receiving pre-operative radiation. This is in stark contrast to other recently published series,8, 24, 33 where >50% of patients received some form of pre-operative therapy, with 14–30% receiving pre-operative radiation therapy. Additionally, pre-operative therapy relies on core biopsy, which has been shown to be inaccurate for histology in 25% of cases and for grade in 12% of cases.34 This may lead to imprecision in the delineation of histologic type and subtype. Finally, the nomograms have not been externally validated, though they have good concordance and calibration internally. Indeed, external validation of these nomograms may prove difficult in the absence of a population of patients treated with similarly low rates of non-surgical therapies. On the other hand, our nomograms may provide an “adjuvant-free” standard to test adjuvant therapies against in future trials of loco-regional or systemic therapies. For instance, one might design a randomized trial of surgery alone versus surgery with pre-operative radiotherapy (a modality specifically designed to improve local control), with the endpoint of local recurrence. It would be essential to stratify such a trial by histologic type and estimated risk of LR due to the likely differences in radiation sensitivity among subtypes and the large differences in local control rates (5-year rates ranging from 42% for high-grade liposarcoma to 92% for SFT). The need to stratify by histology would apply even if the outcome for such a trial were disease-specific survival, because certain retroperitoneal histologies such as solitary fibrous tumor and leiomyosarcoma have a relatively low (10 to 20%) incidence of local recurrence but a relatively high (40 to 50%) incidence of distant recurrence. Thus, the potential efficacy of a local modality such as pre-operative radiation would be significantly reduced for solitary fibrous tumor and leiomyosarcoma histology, where death largely occurs from distant metastasis.
To further enhance the clinical/pathological nomograms developed in this manuscript, we hope to develop combined models that will incorporate important molecular variables such as changes in mRNA and microRNA expression, copy number alterations, and mutations. For example, we have developed a multigene predictor of distant recurrence–free survival across the five liposarcoma subtypes, including well-differentiated and dedifferentiated liposarcoma.35 The predictor was developed from U133A mRNA expression data from 95 liposarcoma samples, and predictions were based on the expression of 588 genes. We found, after validating the predictor in a test set of 45 cases, that patients with low risk score from the model had a 3-year distant recurrence–free survival of 83% vs. 45% for patients with high risk score (P=0.001). The concordance probability for risk score was 0.73. In contrast, the concordance probability for histologic subtype was 0.67. Similar results were obtained with a second predictor based on only 11 genes. These predictors were highly enriched for genes involved in adipogenesis, DNA replication, mitosis, and the spindle assembly checkpoint, and these results demonstrate that gene expression data can improve outcome prediction in liposarcoma patients. We have also found that copy number alterations in well-differentiated and dedifferentiated liposarcoma are predictive of outcome.36 After profiling tumor samples using Agilent 244K comparative genomic hybridization arrays, we identified 9 regions of copy number alteration as recurrent in dedifferentiated liposarcoma but not well-differentiated liposarcoma. Among patients with primary retroperitoneal dedifferentiated liposarcoma, loss of 19q13 was associated with poor recurrence-free survival (HR, 2.9; P=0.01) and with poor disease-specific survival (HR, 7.4; P=0.002). This chromosome region includes the CEBP gene, and copy number loss of 19q correlated with downregulation of CEBP mRNA expression. We had previously found that CEBP mRNA expression independently predicts distant recurrence–free survival for patients with primary liposarcoma.35 Taken together, these results suggest that 19q loss and decreased CEBP expression may serve as important biomarkers of poor outcome in primary retroperitoneal dedifferentiated liposarcoma. In addition, CEBP downregulation may result from epigenetic defects, as 24% of dedifferentiated liposarcoma had CEBP promoter methylation that was associated with decrease in CEBP expression.37 These results suggest that primary retroperitoneal dedifferentiated liposarcoma patients harboring CEBP promoter methylation are at high risk of recurrence. Such patients would form the ideal cohort for a clinical trial of methylation inhibitors alone or in combination with HDAC inhibitors.
In conclusion, in patients with RPS, histologic subtype drives not only disease-specific survival, but also patterns of local and distant recurrence. Future work should focus on identifying genetic and molecular markers of prognosis and recurrence.
Supplementary Material
Acknowledgment
We thank Janet Novak of MSKCC for editing the manuscript.
Supported by NIH SPORE in Soft Tissue Sarcoma P50 CA 140146-01 (SS, LXQ), NIH Soft Tissue Sarcoma program project grant P01 CA 047179 (SS, MFB, LXQ), NIH/NCI Cancer Center Support Grant P30 CA008748 (SS, LXQ), the David and Monica Gorin Sarcoma Fund (MT).
Footnotes
Financial disclosures: none
Presented in part at the 18th Annual Meeting of the Connective Tissue Oncology Society, New York, NY, October, 2013.
REFERENCES
- 1.Singer S, Maki RG, O'Sullivan B. Soft Tissue Sarcoma. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 1533–1576. [Google Scholar]
- 2.Thomas DM, O'Sullivan B, Gronchi A. Current concepts and future perspectives in retroperitoneal soft-tissue sarcoma management. Expert Rev Anticancer Ther. 2009;9:1145–1157. doi: 10.1586/era.09.77. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. doi: 10.1097/01.sla.0000086542.11899.38. discussion 370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 7.Brown RE, St Hill CR, Greene QJ, et al. Impact of histology on survival in retroperitoneal sarcomas. Am J Surg. 2011;202:748–752. doi: 10.1016/j.amjsurg.2011.09.001. discussion 752–3. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31:1649–1655. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 9.Nathan H, Raut CP, Thornton K, et al. Predictors of survival after resection of retroperitoneal sarcoma: a population-based analysis and critical appraisal of the AJCC staging system. Ann Surg. 2009;250:970–976. doi: 10.1097/SLA.0b013e3181b25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher CDM, Bridge JA, Hogendoorn P, et al. WHO classification of tumours. Lyon, France: IARC Press; 2013. WHO classification of tumours of soft tissue and bone. [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 12.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics. 1988:1141–1154. [Google Scholar]
- 13.Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 14.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, et al. The AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 16.Gronchi A. Extended surgery for retroperitoneal sarcoma: the key to maximizing the potential for cure and survival. Pro. Oncology (Williston Park) 2013;27:640–642. [PubMed] [Google Scholar]
- 17.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 18.Gronchi A, Miceli R, Colombo C, et al. Frontline extended surgery is associated with improved survival in retroperitoneal low- to intermediate-grade soft tissue sarcomas. Ann Oncol. 2012;23:1067–1073. doi: 10.1093/annonc/mdr323. [DOI] [PubMed] [Google Scholar]
- 19.Mussi C, Colombo P, Bertuzzi A, et al. Retroperitoneal sarcoma: is it time to change the surgical policy? Ann Surg Oncol. 2011;18:2136–2142. doi: 10.1245/s10434-011-1742-z. [DOI] [PubMed] [Google Scholar]
- 20.Gronchi A, Pollock R. Surgery in retroperitoneal soft tissue sarcoma: a call for a consensus between Europe and North America. Ann Surg Oncol. 2011;18:2107–2110. doi: 10.1245/s10434-011-1746-8. [DOI] [PubMed] [Google Scholar]
- 21.Gronchi A, Pollock RE. Quality of local treatment or biology of the tumor: which are the trump cards for loco-regional control of retroperitoneal sarcoma? Ann Surg Oncol. 2013;20:2111–2113. doi: 10.1245/s10434-013-2971-0. [DOI] [PubMed] [Google Scholar]
- 22.Pollock RE. Extended surgery for retroperitoneal sarcoma: too much surgery for some and not enough for others? Con. Oncology (Williston Park) 2013;27:641–642. [PubMed] [Google Scholar]
- 23.Raut CP, Swallow CJ. Are radical compartmental resections for retroperitoneal sarcomas justified? Ann Surg Oncol. 2010;17:1481–1484. doi: 10.1245/s10434-010-1061-9. [DOI] [PubMed] [Google Scholar]
- 24.Bonvalot S, Miceli R, Berselli M, et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17:1507–1514. doi: 10.1245/s10434-010-1057-5. [DOI] [PubMed] [Google Scholar]
- 25.Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Stat Med. 2003;22:3515–3525. doi: 10.1002/sim.1574. [DOI] [PubMed] [Google Scholar]
- 26.Anaya DA, Lahat G, Wang X, et al. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann Oncol. 2010;21:397–402. doi: 10.1093/annonc/mdp298. [DOI] [PubMed] [Google Scholar]
- 27.Abbott AM, Habermann EB, Parsons HM, et al. Prognosis for primary retroperitoneal sarcoma survivors: a conditional survival analysis. Cancer. 2012;118:3321–3329. doi: 10.1002/cncr.26665. [DOI] [PubMed] [Google Scholar]
- 28.Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American Joint Committee on Cancer Staging Systems versions 6 and 7. Ann Surg Oncol. 2013;20:3377–3383. doi: 10.1245/s10434-013-3052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eilber FC, Brennan MF, Eilber FR, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101:2270–2275. doi: 10.1002/cncr.20570. [DOI] [PubMed] [Google Scholar]
- 30.Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103:402–408. doi: 10.1002/cncr.20778. [DOI] [PubMed] [Google Scholar]
- 31.Singer S, Socci ND, Ambrosini G, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res. 2007;67:6626–6636. doi: 10.1158/0008-5472.CAN-07-0584. [DOI] [PubMed] [Google Scholar]
- 32.Coindre JM, Mariani O, Chibon F, et al. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol. 2003;16:256–262. doi: 10.1097/01.MP.0000056983.78547.77. [DOI] [PubMed] [Google Scholar]
- 33.Bremjit PJ, Jones RL, Chai X, et al. A contemporary large single-institution evaluation of resected retroperitoneal sarcoma. Ann Surg Oncol. 2014;21:2150–2158. doi: 10.1245/s10434-014-3616-7. [DOI] [PubMed] [Google Scholar]
- 34.Heslin MJ, Lewis JJ, Woodruff JM, et al. Core needle biopsy for diagnosis of extremity soft tissue sarcoma. Ann Surg Oncol. 1997;4:425–431. doi: 10.1007/BF02305557. [DOI] [PubMed] [Google Scholar]
- 35.Gobble RM, Qin LX, Brill ER, et al. Expression profiling of liposarcoma yields a multigene predictor of patient outcome and identifies genes that contribute to liposarcomagenesis. Cancer Res. 2011;71:2697–2705. doi: 10.1158/0008-5472.CAN-10-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crago AM, Socci ND, DeCarolis P, et al. Copy number losses define subgroups of dedifferentiated liposarcoma with poor prognosis and genomic instability. Clin Cancer Res. 2012;18:1334–1340. doi: 10.1158/1078-0432.CCR-11-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor BS, DeCarolis PL, Angeles CV, et al. Frequent alterations and epigenetic silencing of differentiation pathway genes in structurally rearranged liposarcomas. Cancer Discov. 2011;1:587–597. doi: 10.1158/2159-8290.CD-11-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.