Abstract
Background
While CD40L is typically a membrane glycoprotein expressed on activated T cells and platelets that binds and activates CD40 on the surface on antigen presenting cells, a soluble derivative (sCD40L) that appears to retain its biological activity after cleavage from cell membrane also exists. We recently reported that sCD40L is associated with clinical resolution of visceral leishmaniasis and protection against the disease. In the present study we investigated if this sCD40L is functional and exerts anti-parasitic effect in L. infantum-infected macrophages.
Methodology/Principal Findings
Macrophages from normal human donors were infected with L. infantum promastigotes and incubated with either sera from subjects exposed to L. infantum infection, monoclonal antibodies against human CD40L, or an isotype control antibody. We then evaluated infection by counting the number of infected cells and the number of parasites in each cell. We also measured a variety of immune modulatory cytokines in these macrophage culture supernatants by Luminex assay. The addition of sCD40L, either recombinant or from infected individuals’ serum, decreased both the number of infected macrophages and number of intracellular parasites. Moreover, this treatment increased the production of IL-12, IL-23, IL-27, IL-15, and IL1β such that negative correlations between the levels of these cytokines with both the infection ratio and number of intracellular parasites were observed.
Conclusions/Significance
sCD40L from sera of subjects exposed to L. infantum is functional and improves both the control of parasite and production of inflamatory cytokines of infected macrophages. Although the mechanisms involved in parasite killing are still unclear and require further exploration, these findings indicate a protective role of sCD40L in visceral leishmaniasis.
Introduction
Visceral leishmaniasis (VL) is a chronic systemic disease caused by infection with the protozoan parasite Leishmania infantum (chagasi). VL patients present with an intense parasitization of the spleen, liver and bone marrow, followed by symptoms that include fever, hepatosplenomegaly, anemia, leucopenia, and severe weight loss. VL is frequently fatal if not treated.
Control of leishmania infection is mediated by macrophages and is associated with production of inflammatory cytokines, such as IL-1β, TNF-α, IL-6 and IL-12 family members [1–5]. These cytokines stimulate microbicidal mechanisms, such as nitric oxide production, that are essential to parasite killing and clearance in experimental models, although in humans this is still controversial [1,5–7]. IL-12 also drives the differentiation of antigen-specific CD4+ T cells into IFN-γ and TNF producing Th1 cells which are critically required for protection [8], [9]. Conversely, establishment of Leishmania infection is associated with an impairment of specific Th1 responses to leishmania antigens [10] and high levels of IL-10 [11–13] that deactivates various signaling pathways [14] required for effective immune responses against the parasite.
The interaction of CD40 with its ligand CD40L represents an important costimulatory pathway required for the generation of effective T cell responses [15,16]. CD40 is present on surface on antigen presenting cells (APCs) such as B cells, monocytes, macrophages and dendritic cells, as well as on the membrane of various non-immune cells, such as endothelial and epithelial cells [16]. CD40L is primarily expressed on activated CD4+ T cells, but is also present on platelets and a small proportion of CD8+ T cells [16]. Stimulation through CD40 enhances the survival of APCs and promotes the secretion of IL-1, IL-6 IL-8, IL-10, IL-12, TNF-α, MIP-1α and enzymes such as matrix metalloproteinases, as well as synthesis of NO [17–20]. In numerous infectious diseases, the interaction of CD40 and CD40L can determine resistance or susceptibility to infection [21–23]. The role of costimulatory importance of CD40-CD40L signaling is well demonstrated in experimental models of leishmaniasis, [24–28], with strong CD40-CD40L signaling inducing IL-12 production by macrophages whereas weak signaling induces IL-10 production [29].
CD40L is also found as a soluble derivative (sCD40L) that is cleaved from activated T cells that appears to retain the ability to bind and activate CD40 on APC [30,31]. Some studies in cardiovascular disease and sepsis have described enhanced levels of sCD40L as an inflammatory mediator, and the presence of sCD40L is considered as a risk factor, and as an indicator of poor outcome, for these diseases [32,33]. However, we recently reported that sCD40L is associated with clinical resolution of VL. A gradual increase in the levels of serum sCD40L was observed during treatment and levels were negatively correlated with spleen size and parasite load. We also observed high levels of sCD40L in non-diseased individuals living in VL-endemic regions, suggesting that sCD40L may contribute to protection [34]. In the present study, we demonstrate that sCD40L in the serum of individuals exposed to L. infantum infection can bind to CD40 on L. infantum-infected macrophages and that it helps to control the infection.
Methods
Activity of serum sCD40L in L. infantum infected macrophages
Macrophages were derived from peripheral blood mononuclear cells (PBMC) isolated from the blood of healthy donors. Briefly, heparinized venous blood was obtained and PBMC separated by Ficoll Hypaque gradient (Sigma Aldrich). The cells were washed twice, counted and ressuspended in RPMI 1640 (Sigma Chemical) supplemented with 10% FBS and 1% penicillin then seeded in eight chamber Lab-Tek glass tissue culture slide (Nalge Nunc International) at 3x105 cells/well in a volume of 0.2 ml. After the cells were allowed to adhere for 2 h at 37°C in 5% CO2, non-adherent cells were removed by extensive washing. The adherent monocytes were incubated in supplemented medium at 37°C, 5% CO2 for 7 days to allow them to differentiate into macrophages. Macrophages were subsequently infected by adding stationary-phase L. infantum promastigotes (MHOM/BR/2009/LVHSE17) at a parasite to macrophage ratio of 10:1. Extracellular parasites were removed 2 hours later by extensive washing, and the infected cells incubated for a further 72 h.
To examine the role of sCD40L, infected cells were incubated with 20% human serum or 10 μg/ml monoclonal antibodies against human CD40L or an isotype control (both R&D Systems, Minneapolis, MN). The sera used were selected from DTH positive subjects and cured VL patients, with no signs of infection, with high levels of sCD40L (ranging from 30,618 to 145,551 pg/ml). Based on previous report [35], recombinant human CD40L (R&D Systems) was added at 2 μg/ml for both 2 h and 72 h incubation after infection. This concentration was also selected as being fully saturating as demonstrated in a dose-response curve (Fig 1). The supernatants were collected and stored at—80°C until cytokine measurement. To assess the infection ratio, cells were stained with Panotico fast (LaborClin, PR, BRA). The infection ratio was measured by counting the number of infected cells/100 macrophages and the number of parasites/100 macrophages, each of which was conducted in a blinded fashion and by three independent observers. All conditions were performed in duplicate. Ethical approval was obtained from the Hospital Universitário from Universidade Federal de Sergipe, Comissão Nacional de Ética em Pesquisa (CONEP), CAAE 0151.0.107.000–07 and CAAE 0123.0.107.000–11. All cured VL patients and normal donors signed an informed consent.
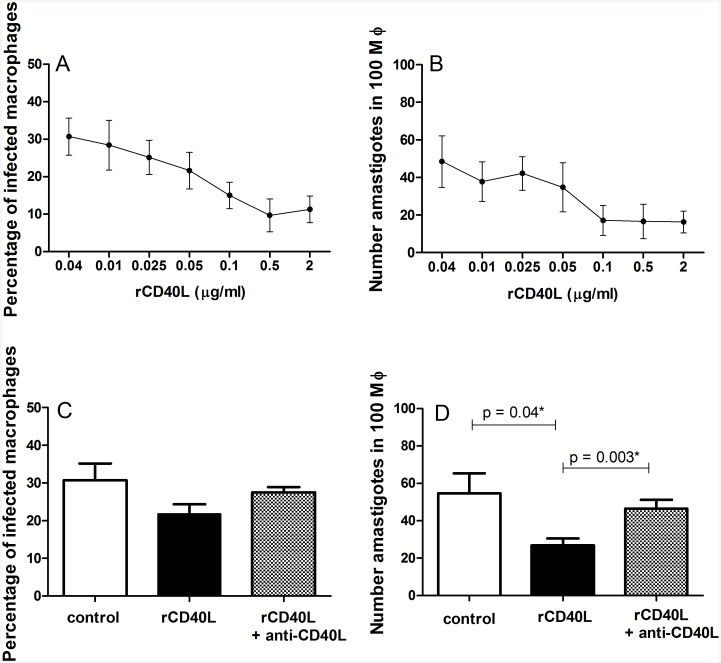
Fig 1. Treatment with recombinant CD40L (rCD40L) reduces L. infantum infection in macrophages.
L. infantum infection levels in macrophages were determined after 72 h incubation in complete RPMI media (control) or in the presence of rCD40L. A dose-response curve was evaluated across several concentrations of rCD40L, with (A) the number of infected macrophages/100 macrophages and (B) the number of amastigotes/100 macrophages determined. Secondly, rCD40L at 2 μg/ml, either alone or in combination with anti-CD40L (10 μg/ml) were added to the macrophage cultures. In (C) the number of infected macrophages/100 macrophages and in (D) the number of amastigotes/100 macrophages were measured. The results represent the mean and SD of 4 donors, with each experiment performed in duplicate. *Mann Whitney test.
Cytokine measurements
Concentrations of IL-1β, IL-6, IL-12p70, IL-15, IL-23, IL-27, and TNF-α in the culture supernatants of infected macrophages were determined by Luminex assay, according to the manufacturer’s instructions (Millipore, Massachusetts, USA).
Statistical analysis
D’Agostino-Pearson normality test was applied. Mann Whitney test was used for two groups comparisons. Paired analysis was performed by Wilcoxon signed rank test for non-Gaussian data or paired T test in data that fit Gaussian distribution, for comparison of cytokine levels between the experiments containing the sCD40L serum plus anti-CD40L or isotype control antibodies. Correlations between infection levels and cytokines released in culture were performed by Spearman or Pearson tests, according to the results of the normality test. All tests were performed using GraphPad Prism, version 5.03 (GraphPad Software, San Diego, USA). An α < 5% (p < 0.05) was considered statistically significant.
Results
Recombinant CD40L can control the parasite load of L. infantum-infected macrophages
Our previous report identified an inverse relationship between sCD40L levels in the sera of VL patients and cure in response to treatment, implicating a role for sCD40L in protection against L. infantum. To determine if sCD40L could have a protective role against L. infantum infection, we added recombinant human CD40L (rCD40L) in combination with anti-CD40L to human macrophages infected with L. infantum. Firstly, we demonstrated that a wide range of rCD40L concentrations could reduce the infection (Fig 1A and 1B) and determined to use 2 μg/ml of rCD40L in further experiments. Treatments did not interfere with parasite uptake by the macrophages (data not shown) and infection levels after 2 hours of incubation were comparable between the control without any stimulus (63.7% ± 12.61) and treatment with either rCD40L alone (74.3% ± 6.94) or treatment with rCD40L and anti CD40L together (66.0% ± 6.21). After 72 h of incubation, however, the presence of human rCD40L caused a significant decrease of the number of amastigotes per macrophages relative to control cells, and this could be reversed by the addition of anti-CD40L (Fig 1C and 1D). These data demonstrate that sCD40L has the potential to alter parasite growth in infected macrophages.
sCD40L in the sera of L. infantum infected individuals limits parasite growth in macrophages
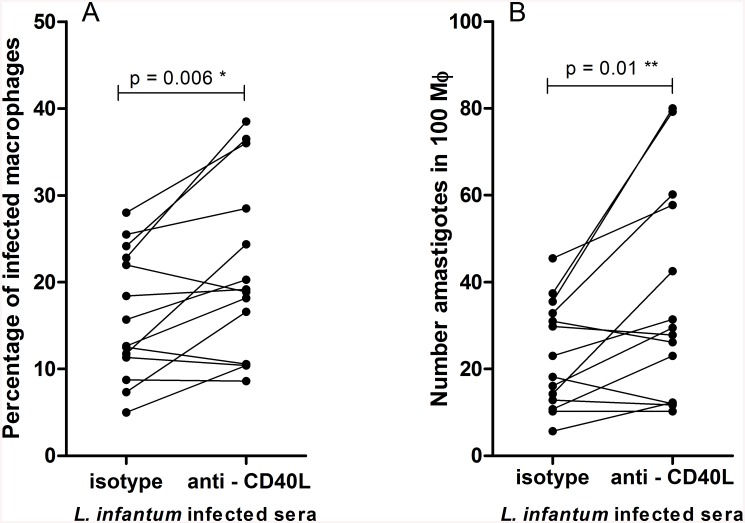
To determine if sCD40L in the serum of patients could afford some protection against L. infantum, we added serum from infected individuals to parasite infected macrophages in vitro. In the majority of cases the blockade of sCD40L increased both the number of infected macrophages and the number of parasites/ 100 macrophages over levels observed in macrophages incubated with control antibody (10 of 14 paired cultures in each case; Fig 2). In each case, the infection levels were increased by over fifty percent by anti-CD40L treatment. These data indicate that sCD40L in the sera of VL patients is functional and limits the ability of L. infantum to grow in macrophages.
Fig 2. Serum sCD40L improves the killing of L. infantum by infected macrophages.
L. infantum infection levels in macrophages were assessed after 72 h incubation with 20% serum from subjects infected with L. infantum in the presence of isotype control antibody (10 μg/ml) or anti-human CD40L (10 μg/ml). The (A) number of infected macrophages/100 macrophages and; (B) number of amastigotes/100 macrophages are shown. n = 14, *Paired t test and ** Wilcoxon signed rank test.
sCD40L enhances inflammatory cytokine production by L. infantum infected macrophages
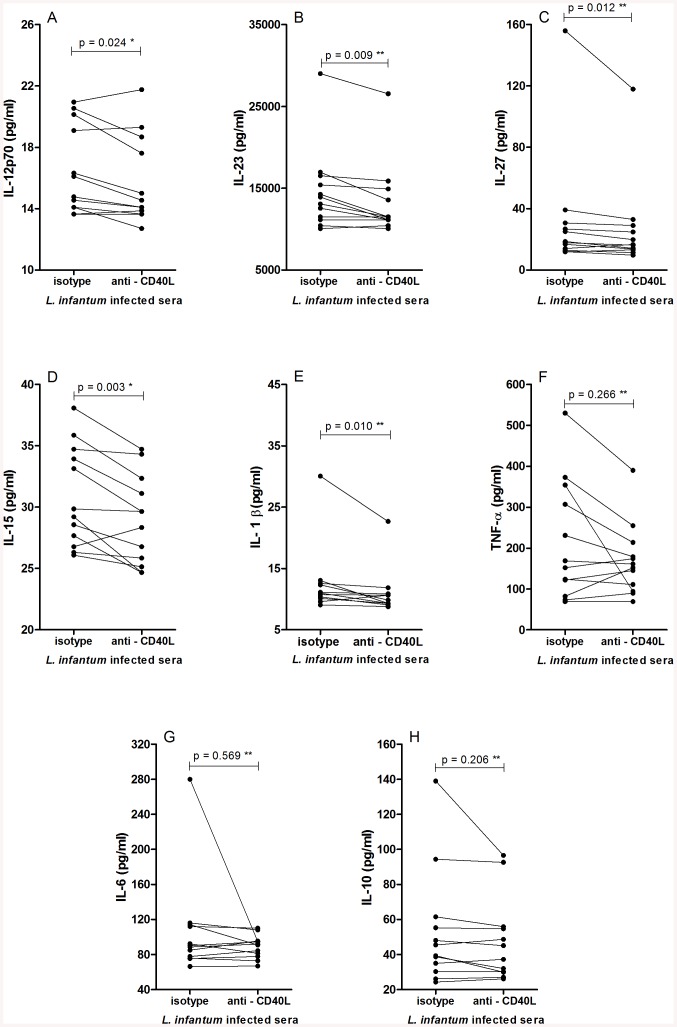
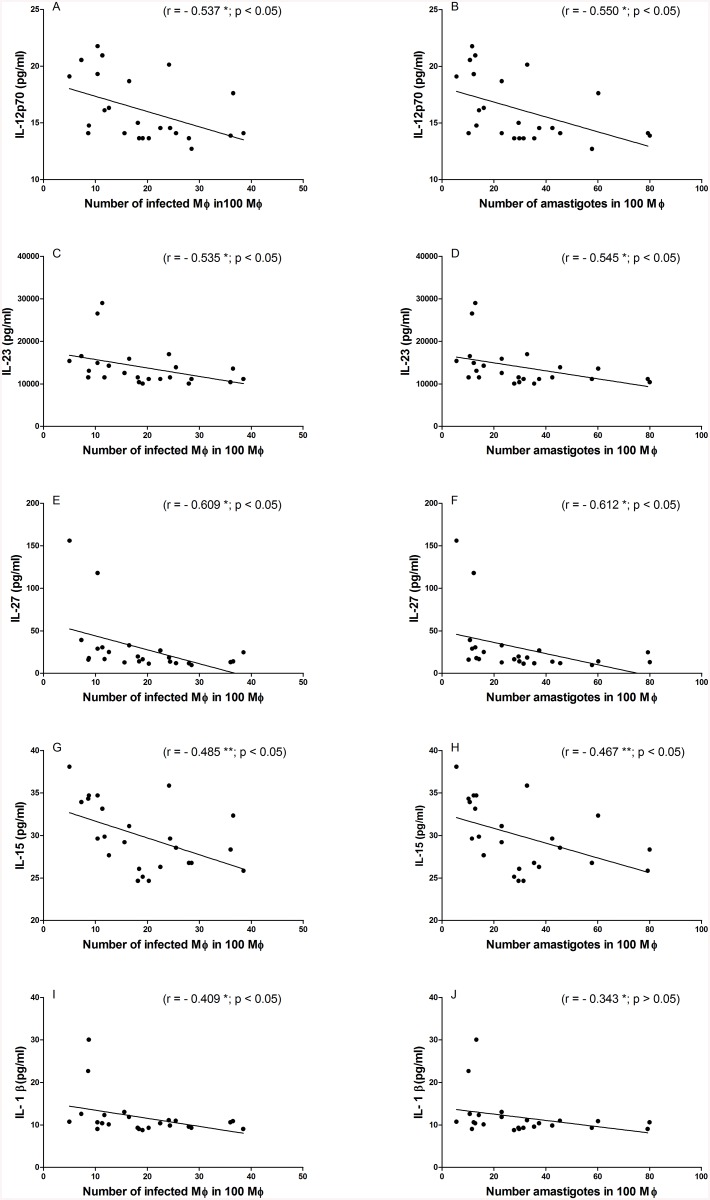
CD40L is known to influence various immune modulatory mechanisms, among them cytokine secretion. We therefore also evaluated how the addition of serum from L. infantum-infected individuals impacted the production of inflammatory and regulatory cytokines by infected macrophages. Incubations were conducted in the presence or absence of anti-human CD40L. The production of IL-12, IL-15, IL-23, IL-27 and IL1β were all significantly reduced by the blockade of sCD40L (Fig 3). In contrast, the production IL-6, IL-10 and TNF was not adversely affected (Fig 3). Interestingly, further examination revealed negative correlations between the levels of IL-12p70, IL-15, IL-23 and IL-27 measured in the culture supernatants and both the number of infected macrophages and the number of amastigotes (Fig 4). IL-1β levels correlated with the number of amastigotes (Fig 4). Thus, the blockade of sCD40L in the serum of infected individuals reduced cytokine production, implying that sCD40L retains its immune regulatory functions during L. infantum infection.
Fig 3. Blockade of serum sCD40L reduces inflammatory cytokine production by infected macrophages.
Levels of cytokines in culture supernatant of macrophages infected with L. infantum were measured after 72 hr incubation with 20% serum from subjects infected with L. infantum in presence of isotype control antibody (10 μg/ml) or anti human CD40L (10 μg/ml) (A) IL-12; (B) IL-23; (C) IL-27; (D) IL-15, E (IL-1β), F (TNF-α). G (IL-6) and H (IL-10). n = 12, *Paired t test, ** Wilcoxon signed rank test.
Fig 4. Cytokine levels in supernatants of L. infantum infected macrophages negatively correlate with infection levels.
Correlation between cytokine levels in culture supernatants incubated with serum sCD40L (n = 12) and with sCD40L plus anti-CD40L (n = 12) with the number of infected macrophages/100 macrophages and the number of amastigotes/100 macrophages from these experiments. (A) and (B) IL-12; (C) and (D) IL-23; (E) and (F) IL-27; (G) and (H) IL-15; (I) and (J) IL-1β; n = 24. *Spearman correlation test and ** Pearson test.
Discussion
We previously reported that higher levels of sCD40L are associated with clinical resolution of VL [34]. In this study, we demonstrate that sCD40L in the serum of infected subjects is active and can potentiate both the ability to control the infection, and inflamatory cytokine production, of L. infantum-infected macrophages. Although other molecules present in the sera may also have an effect, our demonstration that sCD40L neutralization by specific antibodies confirms the specificity of the effect of sCD40L on parasite killing.
CD40L is typically present in cell membrane, mainly of activated T cells. It has been demonstrated that stimulation through CD40 activates tumor necrosis factor receptor-associated factors (TRAF 1, 2, 3, 5 and 6), which in turn stimulate various kinases (p38 MAPK, ERKs, pI3K) and induce NF-κβ- and STAT-dependent gene expression. Together, this results in the production of antibodies, enzymes and a variety of cytokines including IL-1β, IL-6, IL-8, IL-10, IL-12 and TNF-α [16,36,37]. Additionally, CD40/CD40L signaling on monocytes/macrophages induces the production of NO [17,38], a major host defense mechanism against Leishmania [7]. The important role of CD40-CD40L signaling for protection in leishmaniasis has been demonstrated using recombinant proteins and knockout mice [25,27,28,39]. Furthermore, the role of CD40L in acquired resistance to leishmania infection is supported by the ability of this molecule to potentiate vaccine-induced immunity against L. major infection [40,41]. Despite the broad functional knowledge regarding the importance of the membrane form of CD40L, the role of sCD40L remains unclear. However, by demonstrating that sCD40L levels progressively increase during clinical resolution of the disease and that levels negatively correlate with parasite burden, our previous study presented circumstantial evidence of a protective role in VL for sCD40L [34]. Although sCD40L is a cleaved/shed form, it retains the ability to bind CD40 and activate macrophages, independently of T cells [31]. sCD40L has been described as a mediator of inflammation and a marker of poor prognosis in arterial coronary disease [32,42,43]. High sCD40L levels are detected in the serum of HIV-1 and sepsis patients, and are associated with poor prognosis in both diseases [33,44]. Considering that VL patients have impaired antigen-specific T cell responses during active disease [10], sCD40L could represent an important T cell independent regulator of macrophage function in VL. Indeed, our results demonstrate that the presence of sCD40L in serum helps to control infection levels, most likely by potentiating the microbicidal mechanisms of infected macrophages.
Our data also indicates that serum sCD40L increases the production of various cytokines (IL-12p70, IL-23, IL-27, IL-15 and IL-1β) by infected macrophages in vitro. Interestingly, these cytokines were negatively correlated with the number of infected macrophages. Several publications have demonstrated that CD40-CD40L signaling increases the production of IL-12, IL-23, IL-27 and IL-1β [45–48], which play a role on the activation of macrophage microbicidal activities [49–52]. L. major amastigotes can modulate the signaling pathway downstream of membrane CD40 engagement by inducing ERK1/2 and IL-10 production, which inhibits the p38MAPK/IL12 pathway resulting in replication of parasite and persistence of infection [35]. This down regulatory response can be overcome by stronger CD40-sCD40L signaling. Thus, the presence of sCD40L could be important for restoration of IL-12 production. Furthermore, the presence of IL-12 is crucial to induce a Th1 phenotype [53,54] during antigen presentation, required for an effective immune response in visceral leishmaniasis.
The other cytokines of IL-12 family, IL-23 and IL-27, can also stimulate a protective response against L. infantum [55,56]. Although some studies have associated IL-27with induction of IL-10 in vivo and suppression of immune response in VL [57], others have shown that it induces a STAT1/3- and NF-kappaB-dependent proinflammatory cytokine profile in human monocytes [49] and suppresses IL-10 production [50]. IL-1β has also been implicated in host resistance to Leishmania infection [2,5]. Signaling through IL-1R induces nitric oxide synthase (NOS2), which in turn mediates the production of NO [5]. Similar to our results, Ribbens et al showed that the addition of anti-CD40L to Th1/monocyte co-cultures can significantly reduce the production of IL-1β [58]. Confirming the effect of sCD40L present in sera on reduce the macrophage infection, we observed that recombinant CD40L (rCD40L) decrease the levels of infection and especially the number of intracellular parasites. Addition of rCD40L did not interfere with the phagocytosis, reinforcing that its action is in modulating the microbicidal response.
Further studies are required to more fully understand the effects of sCD40L in the presence of T cells or in vivo. However, the combination of our previous report [34], and the data presented here that demonstrate that engagement of, and signaling through, CD40 by sCD40L reduces parasite load in vitro, strongly indicate that sCD40L contributes to the control of infection.
In conclusion, our data suggest that sCD40L in the serum of L. infantum–infected individuals can enhance both the microbicidal response of infected macrophages and their production of inflamatory cytokines, each of which can promote killing of the parasites. Although datailed mechanistics studies are needed, this discovery suggests the potential use of sCD40L for future immunotherapy of human VL.
Acknowledgments
We thank the Pediatric and Infectious Disease Clinic Group from the University Hospital, Universidade Federal de Sergipe.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by: Fundação de Apoio à Pesquisa e à Inovação Tecnológica do Estado de Sergipe (FAPITEC)/SE/FUNTEC/Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grants: CNPq n°12/2009 Processo n° 019.203.02712/2009-8 (ARJ); CNPq n° 04/2011 Processo n. 019.203.01157/2011-9 (TRM). Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Grant: Programa Nacional de Incentivo à Pesquisa em Parasitologia Básica, Edital N° 032/2010 (ARJ). Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grants: PROCAD/CASADINHO- n°552721/2011-5 (RPA); Edital/Chamada: Universal 14/2011 Processo n. 483540/2011-0 (TRM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Liew FY, Li Y, Millott S. Tumour necrosis factor (TNF-alpha) in leishmaniasis. II. TNF-alpha-induced macrophage leishmanicidal activity is mediated by nitric oxide from L-arginine. Immunology. 1990; 71: 556–559. [PMC free article] [PubMed] [Google Scholar]
- 2. Ho JL, Badaro R, Schwartz A, Dinarello CA, Gelfand JA, Sobel J, et al. Diminished in vitro production of interleukin-1 and tumor necrosis factor-alpha during acute visceral leishmaniasis and recovery after therapy. J Infect Dis. 1992; 165: 1094–1102. [DOI] [PubMed] [Google Scholar]
- 3. Bacellar O, Brodskyn C, Guerreiro J, Barral-Netto M, Costa CH, Coffman RL, et al. Interleukin-12 restores interferon-gamma production and cytotoxic responses in visceral leishmaniasis. J Infect Dis. 1996; 173: 1515–1518. [DOI] [PubMed] [Google Scholar]
- 4. Stager S, Maroof A, Zubairi S, Sanos SL, Kopf M, Kaye PM. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur J Immunol. 2006; 36: 1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva AL, Mineo TW, et al. Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013; 19: 909–915. 10.1038/nm.3221 [DOI] [PubMed] [Google Scholar]
- 6. Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med. 2011; 51: 337–351. 10.1016/j.freeradbiomed.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 7. Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004; 2: 820–832. [DOI] [PubMed] [Google Scholar]
- 8. Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, et al. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995; 154: 4623–4629. [PubMed] [Google Scholar]
- 9. McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev. 2004; 201: 206–224. [DOI] [PubMed] [Google Scholar]
- 10. Carvalho EM, Bacellar OA, Reed S, Barral A, Rocha H. Visceral leishmaniasis: a disease associated with inability of lymphocytes to activate macrophages to kill leishmania. Braz J Med Biol Res. 1988; 21: 85–92. [PubMed] [Google Scholar]
- 11. Ghalib HW, Piuvezam MR, Skeiky YA, Siddig M, Hashim FA, el-Hassan AM, et al. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993; 92: 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verma S, Kumar R, Katara GK, Singh LC, Negi NS, Ramesh V, et al. Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One. 2010; 5: e10107 10.1371/journal.pone.0010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007; 28: 378–384. [DOI] [PubMed] [Google Scholar]
- 14. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001; 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 15. Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998; 16: 111–135. [DOI] [PubMed] [Google Scholar]
- 16. van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000; 67: 2–17. [DOI] [PubMed] [Google Scholar]
- 17. Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996; 17: 487–492. [DOI] [PubMed] [Google Scholar]
- 18. Malik N, Greenfield BW, Wahl AF, Kiener PA. Activation of human monocytes through CD40 induces matrix metalloproteinases. J Immunol. 1996; 156: 3952–3960. [PubMed] [Google Scholar]
- 19. D'Aversa TG, Weidenheim KM, Berman JW. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am J Pathol. 2002; 160: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001; 276: 44527–44533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suttles J, Stout RD. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin Immunol. 2009; 21: 257–264. 10.1016/j.smim.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 22. Grewal IS, Borrow P, Pamer EG, Oldstone MB, Flavell RA. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997; 9: 491–497. [DOI] [PubMed] [Google Scholar]
- 23. Subauste CS. CD40 and the immune response to parasitic infections. Semin Immunol. 2009; 21: 273–282. 10.1016/j.smim.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heinzel FP, Rerko RM, Hujer AM. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell Immunol. 1998; 184: 129–142. [DOI] [PubMed] [Google Scholar]
- 25. Soong L, Xu JC, Grewal IS, Kima P, Sun J, Longley BJ Jr., et al. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996; 4: 263–273. [DOI] [PubMed] [Google Scholar]
- 26. McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect Immun. 2002; 70: 3994–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murray HW. Prevention of relapse after chemotherapy in a chronic intracellular infection: mechanisms in experimental visceral leishmaniasis. J Immunol. 2005; 174: 4916–4923. [DOI] [PubMed] [Google Scholar]
- 28. Murray HW, Lu CM, Brooks EB, Fichtl RE, DeVecchio JL, Heinzel FP. Modulation of T-cell costimulation as immunotherapy or immunochemotherapy in experimental visceral leishmaniasis. Infect Immun. 2003; 71: 6453–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathur RK, Awasthi A, Saha B. The conundrum of CD40 function: host protection or disease promotion? Trends Parasitol. 2006; 22: 117–122. [DOI] [PubMed] [Google Scholar]
- 30. Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek RA. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995; 25: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 31. Ludewig B, Henn V, Schroder JM, Graf D, Kroczek RA. Induction, regulation, and function of soluble TRAP (CD40 ligand) during interaction of primary CD4+ CD45RA+ T cells with dendritic cells. Eur J Immunol. 1996; 26: 3137–3143. [DOI] [PubMed] [Google Scholar]
- 32. Tousoulis D, Androulakis E, Papageorgiou N, Briasoulis A, Siasos G, Antoniades C, et al. From atherosclerosis to acute coronary syndromes: the role of soluble CD40 ligand. Trends Cardiovasc Med. 2010; 20: 153–164. 10.1016/j.tcm.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 33. Lorente L, Martin MM, Varo N, Borreguero-Leon JM, Sole-Violan J, Blanquer J, et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit Care. 2011; 15: R97 10.1186/cc10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Oliveira FA, Vanessa Oliveira Silva C, Damascena NP, Passos RO, Duthie MS, Guderian JA, et al. High levels of soluble CD40 ligand and matrix metalloproteinase-9 in serum are associated with favorable clinical evolution in human visceral leishmaniasis. BMC Infect Dis. 2013; 13: 331 10.1186/1471-2334-13-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat Med. 2004; 10: 540–544. [DOI] [PubMed] [Google Scholar]
- 36. Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J Immunol. 2006; 176: 5388–5400. [DOI] [PubMed] [Google Scholar]
- 37. Andrade RM, Wessendarp M, Portillo JA, Yang JQ, Gomez FJ, Durbin JE, et al. TNF receptor-associated factor 6-dependent CD40 signaling primes macrophages to acquire antimicrobial activity in response to TNF-alpha. J Immunol. 2005; 175: 6014–6021. [DOI] [PubMed] [Google Scholar]
- 38. Tian L, Noelle RJ, Lawrence DA. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995; 25: 306–309. [DOI] [PubMed] [Google Scholar]
- 39. Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996; 4: 283–289. [DOI] [PubMed] [Google Scholar]
- 40. Gurunathan S, Irvine KR, Wu CY, Cohen JI, Thomas E, Prussin C, et al. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998; 161: 4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 41. Chen G, Darrah PA, Mosser DM. Vaccination against the intracellular pathogens Leishmania major and L. amazonensis by directing CD40 ligand to macrophages. Infect Immun. 2001; 69: 3255–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, et al. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003; 348: 1104–1111. [DOI] [PubMed] [Google Scholar]
- 43. Yan JC, Zhu J, Gao L, Wu ZG, Kong XT, Zong RQ, et al. The effect of elevated serum soluble CD40 ligand on the prognostic value in patients with acute coronary syndromes. Clin Chim Acta. 2004; 343: 155–159. [DOI] [PubMed] [Google Scholar]
- 44. Sipsas NV, Sfikakis PP, Kontos A, Kordossis T. Levels of soluble CD40 ligand (CD154) in serum are increased in human immunodeficiency virus type 1-infected patients and correlate with CD4(+) T-cell counts. Clin Diagn Lab Immunol. 2002; 9: 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wesa A, Galy A. Increased production of pro-inflammatory cytokines and enhanced T cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol. 2002; 3: 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996; 184: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004; 202: 96–105. [DOI] [PubMed] [Google Scholar]
- 48. Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007; 204: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guzzo C, Che Mat NF, Gee K. Interleukin-27 induces a STAT1/3- and NF-kappaB-dependent proinflammatory cytokine profile in human monocytes. J Biol Chem. 2010; 285: 24404–24411. 10.1074/jbc.M110.112599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008; 180: 6325–6333. [DOI] [PubMed] [Google Scholar]
- 51. Indramohan M, Sieve AN, Break TJ, Berg RE. Inflammatory monocyte recruitment is regulated by interleukin-23 during systemic bacterial infection. Infect Immun. 2012; 80: 4099–4105. 10.1128/IAI.00589-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tan ZY, Bealgey KW, Fang Y, Gong YM, Bao S. Interleukin-23: immunological roles and clinical implications. Int J Biochem Cell Biol. 2009; 41: 733–735. 10.1016/j.biocel.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 53. Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004; 173: 1887–1893. [DOI] [PubMed] [Google Scholar]
- 54. Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004; 202: 139–156. [DOI] [PubMed] [Google Scholar]
- 55. Reinhard K, Huber M, Wostl C, Hellhund A, Toboldt A, Abass E, et al. c-Rel promotes type 1 and type 17 immune responses during Leishmania major infection. Eur J Immunol. 2011; 41: 1388–1398. 10.1002/eji.201041056 [DOI] [PubMed] [Google Scholar]
- 56. Shimizu M, Ogura K, Mizoguchi I, Chiba Y, Higuchi K, Ohtsuka H, et al. IL-27 promotes nitric oxide production induced by LPS through STAT1, NF-kappaB and MAPKs. Immunobiology. 2013; 218: 628–634. 10.1016/j.imbio.2012.07.028 [DOI] [PubMed] [Google Scholar]
- 57. Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, et al. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol. 2011; 186: 3977–3985. 10.4049/jimmunol.1003588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ribbens C, Dayer JM, Chizzolini C. CD40-CD40 ligand (CD154) engagement is required but may not be sufficient for human T helper 1 cell induction of interleukin-2- or interleukin-15-driven, contact-dependent, interleukin-1beta production by monocytes. Immunology. 2000; 99: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.