Abstract
Background
Salvia libanotica (S. Libanotica) is a commonly used herb in folk medicine in Lebanon and the Middle East. The present study aimed to assess the scientific basis for the therapeutic use of S. libanotica in glycemia and to evaluate its effects on lipemia and abdominal fat.
Methods
Animals were fed a high-fat diet and allocated into a control and three experimental groups (GI, GII and GIII) receiving incremental doses of the plant water extract in drinking water (50, 150 and 450 mg/Kg body weight respectively) for six weeks.
Results
The intake of S. libanotica extract was associated with a significant decrease in fasting serum glucose (102.9 ± 10.8 in GII and 87.5 ± 6.4 in GIII vs. 152.1 ± 7.9 mg/dl in controls) and a two fold increase in fasting serum insulin (GIII) and liver glycogen content (GII and GIII). Group III also had better glucose tolerance following intraperitoneal glucose challenges. Additionally, the plant extract intake produced a significant improvement in serum HDL (34.4 ± 2.4 in GIII vs. 27.2 ± 1.9 mg/dl in controls) and HDL/LDL cholesterol ratio (2.79 ± 0.32 in GII and 3.02 ± 0.31 in GIII vs. 1.74 ± 0.18 in controls), as well as a decrease in abdominal fat.
Conclusion
The current study is the first to demonstrate that the chronic intake of S. libanotica infusion helps in the prevention of high fat-induced hyperglycemia and dyslipidemia. This supports the plant use as a remedy for the prevention of type 2 diabetes and cardiovascular diseases.
Keywords: Salvia libanotica, Glycemia, Blood glucose, Blood lipid, Blood cholesterol
Background
Diabetes and cardiovascular diseases are established as serious public health problems around the globe and are among the top five causes of death in most Western and Arab countries [1, 2]. Insulin resistance, hyperglycemia and dyslipidemia are common metabolic abnormalities that could potentially lead to type 2 diabetes and cardiovascular diseases [3]. A very palatable diet that is widely available nowadays is the high fat diet, rich in saturated fats, which is known to contribute to insulin resistance especially when it induces abdominal obesity [4]. This is characterized by impaired insulin-stimulated glucose uptake by peripheral tissues and subsequent hyperglycemia, in addition to abnormalities in hepatic insulin signaling leading to elevated blood lipids and gluconeogenesis and lower glycogen and HDL levels [5]. While pharmacological treatments are widely prescribed to manage these abnormalities, most are costly and have adverse side effects [6]. Thus, many patients and healthcare professionals are seeking alternative treatments by using herbal remedies due to their potential effectiveness and limited cost and toxicity [7].
Salvia libanotica (also named S. Fruiticosa and S. triloba) of the “Lamiaceae” family is an aromatic medicinal plant commonly known as East Mediterranean sage or Lebanese sage [8]. In Lebanon, Syria and Jordan, the plant is commercially available and widely used by the elderly and folk medicine practitioners [9]. Its leaves are usually used as infusion for the relief of headaches, stomachaches, abdominal pain, hyperglycemia and many other ailments [10–13].
In Lebanese folk medicine, S. libanotica is widely used for its anti-diabetic properties in spite of the scarcity of scientific evidence of its efficacy in the literature [9]. To our knowledge, only one study described the hypoglycemic effects S. libanotica when using a 10 % infusion of the plant leaves at an oral dose of 250 mg of dry leaves/kg body weight in rabbits [14]. The proposed mechanisms that were hypothesized by the authors included a role of the plant in carbohydrate intestinal absorption and/or modification of hepatic metabolic processes, since no effect was observed on plasma insulin [14]. On the other hand, many studies have established the hypoglycemic effect of leaves of the closely related to Salvia officinalis (S. officinalis) species. Indeed, S. officinalis methanolic extract was shown to significantly decrease serum glucose in diabetic but not healthy rats, with no effect on pancreatic insulin release [15, 16]. Another study showed that water-ethanol extract of S. officinalis reduced blood glucose in normoglycemic and mildly alloxan-diabetic, but not in severely alloxan-diabetic mice [17]. Additionally, replacing water with S. officinalis infusion for 14 days resulted in lower fasting plasma glucose with no effect on glucose clearance in normal mice [18].
The effects of S. libanotica on blood lipemia have not previously been studied. Yet, S. officinalis was shown to have potential cardio-protective effect by increasing blood HDL cholesterol levels and decreasing total cholesterol, triglyceride and LDL cholesterol levels in hyperlipidemic patients [19]. While the health benefits of S. officinalis plant extract are relatively well established, the effects of S. libanotica on glycemia and lipidemia have not been fully elucidated. Moreover, most studies on S. libanotica are short term studies [12], and have been conducted on its essential oil rather than on water extract [20–22]. In order to simulate human consumption and folk medicine protocols which rely on herbal infusions [14, 20–22], the present study was carried out to assess the effect of chronic intake (6 weeks) of S. libanotica water extract on glycemia and lipemia and explore the associated mechanisms of action in healthy rats fed a high-fat diet.
Methods
Plant material
S. libanotica plant was collected from different areas throughout Lebanon between July and September 2013. The plant was identified according to the characteristics described in “Handbook of Medicinal Herbs” book [23]. Also, a voucher specimen of the plant material used has been deposited at the herbarium of the Department of Natural Sciences, Lebanese American University (reference number: SL-2-2013). Fresh plant material was collected from wild bushes; leaves were separated and dried in the shade at room temperature. The dried leaves (130 g) were then chopped into small pieces and soaked in 2 L pre-boiled water for 30 min for extraction. The water extract was filtered and subjected to freeze drying yielding 8.3 % w/w of dry material that was stored in refrigerated amber glass containers. This procedure was repeated weekly throughout the study.
Animals
Adults Spargue-Dawley rats (Lebanese American University Stock) weighing 180–240 g were housed in a temperature and humidity-controlled room under a 12:12 light/dark cycle (lights on at 0800 h). Rats were randomly allocated into four weight-matched groups of ten rats each and were studied for 6 weeks. Animals were fed ad libitum a standard chow diet to which 5 % of coconut oil was added in order to increase the diet’s atherogenicity (Table 1) [24]. The control group was given water, and the other groups were given three different doses of S. libanotica in drinking water (GI: 50 mg/Kg body weight; GII: 150 mg/Kg body weight; GIII: 450 mg/Kg body weight). All experimental protocols were approved by the Animal Ethical subcommittee of the Lebanese American University, which complies with Guide for the Care and Use of Laboratory Animals [25].
Table 1.
Nutrient composition of the high fat diet compared to standard high carbohydrate (HC) diet
| Standard HCa | High Fatb | |
|---|---|---|
| Protein (% wt) | 19 | 18.1 |
| Carbohydrates (% wt) | 65.3 | 62.2 |
| Sugars | 9.2 | 8.8 |
| Fat (% wt) | 9.6 | 13.9 |
| Fat breakdown | ||
| Saturated fat | 18 % | 21 % |
| Mono-unsaturated fat | 29 % | 28 % |
| Poly-unsaturated fat | 47 % | 45 % |
| Fiber (% wt) | 4.3 | 4.1 |
| Metabolizable energy (kJ/g) | 17.7 | 18.7 |
| Energy (Protein) | 18 % | 16 % |
| Energy (Carbohydrate) | 62 % | 56 % |
| Energy (Fat) | 20 % | 28 % |
aLaboratory rodent starter diet no. 1, Hawa Chicken Co. (Safra, Lebanon)
bDiet derived from the standard HC diet enriched with coconut oil
Glucose tolerance test
On days 17 and 36 of the experiment, an ipGTT (intraperitoneal glucose tolerance test using intraperitoneal injection of 300 g glucose/L in physiologic saline in a dose of 5.83 mL/Kg boy weight) was performed on rats that have been fasted for 3 h. After 45 min, blood was collected from the animals’ tail vein and assayed for glucose level.
Metabolic profile
After 6 weeks, fasted rats (18 h) were anesthetized and sacrificed after 18 h. Serum samples were isolated from 4 ml of venous blood which were drawn from the inferior vena cava and stored at −80 °C for subsequent analysis. Serum glucose, lipids (total cholesterol, HDL cholesterol, triglycerides) and liver enzyme activities of aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP) were determined using the relevant Spinreact kits (Spinreact, Spain). LDL cholesterol was calculated using the Friedwald equation (LDL = total cholesterol – HDL – (triglycerides/5)) [26]. Serum insulin was determined using Rat-insulin ELISA kit (Merck Millipore,Germany). All serum samples were run in duplicate and analyzed within the same assay. In addition, following sacrifice, the intra-abdominal fat (epididymal, mesenteric and retroperitoneal) was removed from the animals, after which it was cleaned, blotted on a filter paper and weighed.
Liver glycogen content
Rats’ livers were isolated, weighed, and then preserved at −45 °C until testing. Hepatic glycogen was assayed using 1 g of tissue according to the extraction method mentioned by Hassid and Abraham [27]. Briefly, 1 g of tissue was homogenized with 10 % trichloroacetic acid solution and glycogen was precipitated using 95 % ethanol. After diluting it with distilled water, glycogen was then determined colorimetrically by the anthrone method.
In-vitro digestive enzymes inhibition assays
The effects of S. libanotica on the intestinal enzymes alpha-amylase and alpha-glucosidase were determined in-vitro [28, 29]. Solutions of plant extracts were prepared at concentrations of 10, 20, 40, 60, 80 and 100 mg/dl. Controls were prepared by replacing the plant extract with distilled water, and acarbose was used as the positive control.
In-vitro alpha-amylase inhibition assay
For each concentration, 1 ml of the plant extract solution was mixed with 1 ml of 0.5 % starch solution and 1 ml of enzyme solution (0.5–1 unit/ml in 20 mM sodium phosphate buffer, pH:6.9), and the mixture was incubated at 25 °C for 3 min. Afterwards, 1 ml of color reagent was added. The latter was prepared by mixing 96 nM of 3,5 dinitrosalicylic acid (20 ml) with 5.31 M sodium potassium tartrate in 2 M sodium hydroxide (8 ml), all made up to 12 ml volume with deionized water. The mixture was placed in closed tubes in a water bath at 85 °C for 15 min. Tubes were then removed from water bath, cooled and diluted with 9 ml of distilled water and absorbance was read at 540 nm in a spectrophotometer.
Where ΔAControl = Atest − ABlank and ΔAsample = Atest − ABlank
In-vitro alpha-glucosidase inhibition assay
Alpha-glucosidase reaction mixture was prepared by adding 2.9 mM p-nitrophenyl- α-D-glucopyranoside (pNPG) to 0.25 mL of each plant extract concentrations and 0.6 U/ml α-glucosidase in sodium phosphate buffer (pH 6.9). The reaction mixture was incubated at 25 °C for 5 min and then the reaction was stopped by boiling for 2 min. Absorbance of the resulting p-nitrophenol (pNP) was determined at 405 nm using spectrophotometer.
Statistical analysis
Data are reported as Mean ± SEM. Results were analyzed by one-way analysis of variance (ANOVA). Significant main effect differences were tested using Tukey–Kramer’s post hoc test for multiple comparison. All data were analyzed with the statistical package SPSS 18 and statistical significance was defined as p < 0.05.
Results and discussion
Results
Chronic intake of S. libanotica extract showed a trend of weight loss in the majority of animals, with a significant decrease in abdominal fat percent in GIII (Table 2). Fasting serum glucose concentrations on day 42 of the study were lower in the treated groups compared to controls with the difference reaching statistical significance at the two highest doses used (Fig. 1a). Similarly, ipGTT on day 17 and day 36 of the study showed significantly (p < 0.05) lower venous blood glucose levels in GIII versus control group, 45 min after intrapertitoneal injection of glucose solution (Fig. 2). Additionally, the fasting serum insulin concentrations were higher in the treated groups compared to controls with significance (p < 0.05) reached at the highest dose (Fig. 1b). Furthermore, there was a dose dependent increase in liver glycogen with significance (p < 0.05) reached in GII and GIII (Fig. 1c).
Table 2.
Body weight gain and abdominal fat after 6 weeks of S. libanotica intake
| Control | GI | GII | GIII | |
|---|---|---|---|---|
| Body weight gain (g) | 96.71 ± 5.31 | 76.33 ± 10.03 | 82.00 ± 5.84 | 71.20 ± 14.63 |
| Abdominal fat (g) | 2.96 ± 0.34 | 2.91 ± 0.37 | 2.61 ± 0.51 | 2.53 ± 0.41 |
| Abdominal fat (% body weight) | 0.78 ± 0.08 | 0.79 ± 0.10 | 0.74 ± 0.14 | 0.57 ± 0.06* |
Data are presented as mean ± SEM, (n = 10). *corrected p-value ≤ 0.05 vs. control using ANOVA and Tukey’s post-hoc test
Fig. 1.
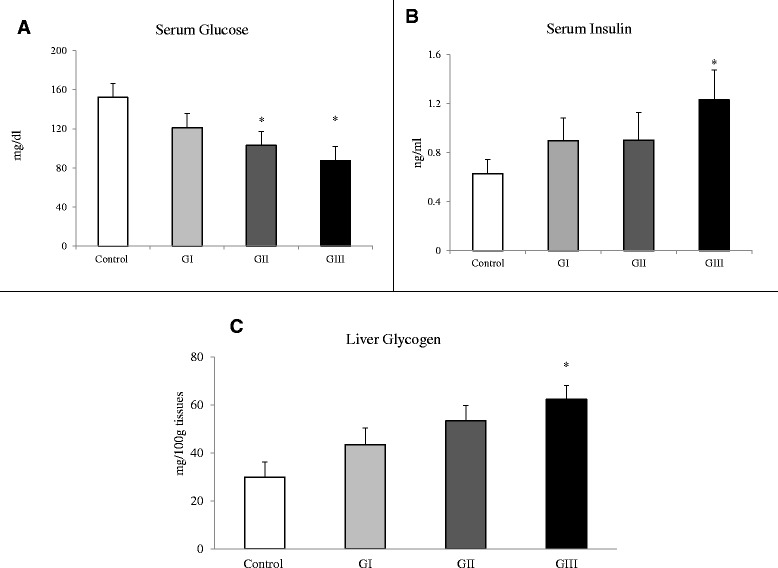
Fasting serum glucose and insulin, and liver glycogen content. Fasting serum glucose (a), insulin (b) and liver glycogen (c) after 6 weeks of S. libanotica intake. Data are presented as mean ± SEM, (n = 10). *corrected p-value ≤ 0.05 vs. control using ANOVA and Tukey’s post-hoc test
Fig. 2.
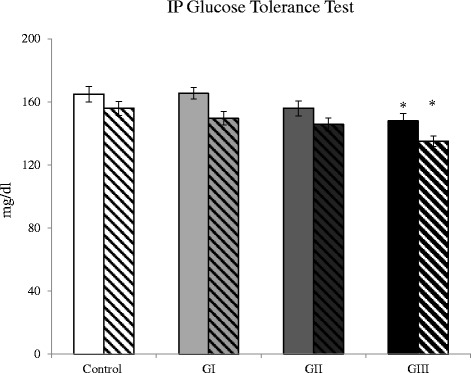
Glucose tolerance test. Serum glucose, 45 min after intraperitoneal injection of glucose solution (ipGTT) on days 17 (clear bars) and 36 (dashed bars) of the experiment. Data are presented as mean ± SEM, (n = 10). *corrected p-value ≤ 0.05 vs. control using ANOVA and Tukey’s post-hoc test
As for blood lipids, there was no significant difference in serum total cholesterol, LDL-cholesterol (LDL) and triglycerides among groups.
Conversely, plant treatment was associated with higher fasting serum HDL-cholesterol (HDL) and HDL/LDL ratio but the differences were only significant in GIII in the former and in GII and GIII in the latter compared to control group (Table 3).
Table 3.
Fasting serum triglycerides, total cholesterol, LDL-cholesterol (LDL), HDL-cholesterol (HDL) and HDL/LDL ratio after 6 weeks of S. libanotica intake
| Control | GI | GII | GIII | |
|---|---|---|---|---|
| Triglycerides (mg/dl) | 37.9 ± 1.7 | 39.8 ± 2.1 | 35.4 ± 4.8 | 38.3 ± 4.2 |
| Total Cholesterol (mg/dl) | 49.8 ± 2.8 | 50.2 ± 3.3 | 51.4 ± 4.8 | 54.5 ± 3.1 |
| LDL Cholesterol (mg/dl) | 15.6 ± 2.3 | 15.1 ± 3.0 | 11.2 ± 3.3 | 11.4 ± 3.2 |
| HDL Cholesterol (mg/dl) | 27.2 ± 1.9 | 27.4 ± 2.5 | 31.2 ± 2.6 | 34.4 ± 2.4* |
| HDL/LDL ratio | 1.74 ± 0.18 | 1.81 ± 0.25 | 2.79 ± 0.32* | 3.02 ± 0.31* |
Data are presented as mean ± SEM, (n = 10). *corrected p-value ≤ 0.05 vs. control using ANOVA and Tukey’s post-hoc test
Serum levels of AST and ALT in the different experimental groups were similar to those of the control. On the other hand, serum LDH levels in GII and GIII, but not GI, were significantly lower than that of the control group (Table 4). The dose dependent in vitro assay did not show any effect of the different doses of the plant extract, ranging from 10 to 100 mg/dl, on the intestinal enzymes alpha-glucosidase and alpha-amylase.
Table 4.
Liver enzymes (AST, ALT and LDH) activities after 6 weeks of S. libanotica intake
| Control | GI | GII | GIII | |
|---|---|---|---|---|
| AST | 41.5 ± 1.84 | 37.4 ± 2.93 | 39.9 ± 3.23 | 35.10 ± 2.47 |
| ALT | 23.1 ± 1.60 | 21.8 ± 1.41 | 18.0 ± 1.34 | 20.4 ± 1.51 |
| LDH | 382 ± 15.5 | 360 ± 23.6 | 241 ± 27.3* | 220 ± 25.3* |
Values are expressed as mean ± SEM (n = 10)
Data are presented as mean ± SEM, (n = 10). *corrected p-value ≤ 0.05 vs. control using ANOVA and Tukey’s post-hoc test
Discussion
The objective of the current study was to investigate the effect of chronic administration of S. libanotica water extract on glycemia, serum lipid profile, food intake, and abdominal fat of rats fed a high fat diet, as well as to explore potential mechanisms of action. The present work is the first to simulate human consumption of S. libanotica by mimicking its usual consumption as herbal infusion in the Middle East. Results showed a significant decrease in fasting blood glucose and an increase in serum insulin and liver glycogen. Moreover, treatment with plant extract was associated with improved lipid profiles characterized by higher HDL-cholesterol and HDL/LDL ratio, as well a decrease in abdominal fat percent.
Although not significant, chronic intake of the plant extract caused 15.2 to 26.4 % decrease in body weight gain in animals receiving the extract compared to control group. A similar decrease in abdominal fat ranging between 1.6 to 14.5 % of the animals was also observed. Moreover, calculation of the abdominal fat/body weight ratio showed a significant decrease in group III compared to control animals, suggesting a potential benefit of plant intake on abdominal fat deposition.
High fat diet administration for 6 weeks induced fasting hyperglycemia (152.1 ± 7.9 mg/dl) in the control group, while treatment with S. libanotica normalized fasting glucose at the two higher doses used (150 and 450 mg/Kg body weight). This appeared to be mediated by an insulinotropic effect of the plant water extract, which was demonstrated through the dose dependent increase in fasting serum insulin level. Therefore, we suggest that the plant treatment triggered more insulin secretion which might have increased glucose uptake by the tissues and thus lowered serum glycemia. This is in line with the higher liver glycogen content observed, which is also an indication of improved hepatic insulin sensitivity [30]. Such mechanism is supported by the in-vitro assessment of alpha-glucosidase and alpha-amylase activities, which did not reveal any inhibitory effect of S. libanotica water extract on these carbohydrate-digesting enzymes.
The plant hypoglycemic effect was also evident in the glucose tolerance test (ipGTT), whereby plant treatment at the dose of 450 mg/kg body weight (GIII) was associated with significantly lower glycemia, 45 min following an intrapertitoneal injection of glucose solution. This effect was even significant only after 17 days of plant treatment. This improved glucose tolerance could be the result of higher insulin response or increased insulin sensitivity, however none of these parameters was measured and thus further investigations are needed. Previous work by Perfumi et al. [14] showed lower blood glucose levels after an oral glucose load in normoglycemic and alloxan-induced hyperglycemic rabbits that were given a single oral dose of S. fruticosa infusion (at 250 mg dry leaves/kg body weight); however, no hypoglycemic effect was found after repeated administration of the infusion for seven days in normoglycemic rabbits. Moreover, the latter study did not find an effect of the plant extract on fasting or glucose-stimulated insulin levels. The discrepancy between the present results and those of Perfumi et al. [14] could be due to the differences in animal models used (rabbits vs. rats), mode of plant extract administration (single daily oral dose vs. drinking water) and study duration (7d vs. 42d). Moreover, based on the extract yield obtained from the current study, the dose used in Perfumi’s study would be about 21 mg water extract/kg bodyweight, which is substantially lower than the doses used in the present work.
The chronic intake of S. libanotica water extract significantly increased HDL cholesterol (group III) levels and HDL/LDL ratio (group II and III). The latter was found to be a strong marker of atherosclerosis compared to LDL and HDL alone [31], thereby suggesting a cardioprotective potential of the plant. To the best of our knowledge, this study is the first to demonstrate the dual hypolipidemic and hypoglycemic effect of the chronic intake of the plant extract. Both effects were not associated with toxicity since there was no elevation in serum activities of ALT and AST after 6 weeks of supplementation with the extract. Results also showed that LDH levels were significantly reduced in Group II and III, indicating the absence of pathological damage, as LDH is an enzyme that is found in almost all body cells and is released upon cell damage and destruction. Additionally, plant safety was further confirmed in our lab when a separate group of rats survived incremental oral doses of the plant extract reaching 8000 mg/Kg body weight.
Conclusions
The present study demonstrated the first evidence of the hypoglycemic and hypolipidemic effect of the chronic intake of S. Libanotica water extract in healthy rats, and proved to be safe throughout the study. Therefore, the results suggest a promising role of S. Libanotica water extract in the prevention of chronic diseases such as diabetes, and cardiovascular diseases, with low cost and without compromising safety. Future studies are warranted to further explore mechanisms of action and to fractionate and purify the plant extract in order to identify the active ingredient(s) responsible for the observed beneficial effects.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- Groups I, II & III
GI, GII & GIII
- HDL
High-density lipoprotein
- ipGTT
Intraperitoneal glucose tolerance test
- LDL
Low density lipoprotein
- Salvia libanotica
S. libanotica
- Salvia officinalis
S. officinalis
- pNPG
p-nitrophenyl- α-D-glucopyranoside
- ANOVA
One-way analysis of variance
- SPSS
Statistical Package for the Social Sciences
Footnotes
Maya Bassil and Nadine Zeeni contributed equally to this work.
Competing interests
The author(s) declare that they have no competing interests.
Authors’ contributions
NZ and CD carried out serum analysis for glucose, triglyceride, total, LDL and HDL cholesterol as well as determination of liver enzymes and liver glycogen. MB carried out the serum insulin assay. MB, MM and CD carried out in-vitro alpha-amylase and alpha-glucosidase inhibition assays. The manuscript was drafted by MB and NZ and reviewed by CD and MM. All authors read and approved the final manuscript.
Contributor Information
Maya Bassil, Email: mbassil@lau.edu.lb.
Costantine F Daher, Email: cdaher@lau.edu.lb.
Mohammad Mroueh, Email: mmroueh@lau.edu.lb.
Nadine Zeeni, Email: nadine.zeeni@lau.edu.lb.
References
- 1.World Health Organization . World Health Statistics 2014. 2014. [Google Scholar]
- 2.Abdul Rahim HF, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H, Mataria A, Mendis S, Mokdad A, Husseini AH. Health in the Arab world A view from within two non-communicable diseases in the Arab world. Lancet. 2014;383:356–67. doi: 10.1016/S0140-6736(13)62383-1. [DOI] [PubMed] [Google Scholar]
- 3.Karpe F, Dickmann RJ, Frayn NK. Fatty acids, obesity, and insulin resistance, time for a reevaluation. Diabetes. 2011;60:2441–9. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riccardi G, Giacco RA, Rivellese A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–56. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Samuel VT, Shulman GI. Mechanisms for insulin resistance, common threads and missing links. Cell. 2012;148(5):852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inzucchi SE. Oral antihyperglycemic therapy for type two diabetes scientific review. J Am Med Assoc. 2002;287:360–72. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 7.Saad B, Azaizeh H, Said O. Tradition and perspectives of Arab herbal medicine: a review. Evid Based Complement Alternat Med. 2005;2:475–9. doi: 10.1093/ecam/neh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouterde P. Nouvelle Flore du Liban et de la Syrie. Beyrouth (Lebanon) Lebanon: Dar El Machrek; 1970. [Google Scholar]
- 9.Gali-Muhtasib H, Hilan C, Khater C. Traditional uses of Salvia Libanotica (east meditteranean sage) and the effects of its essential oils. J Ethnopharmacol. 2000;71:513–20. doi: 10.1016/S0378-8741(99)00190-7. [DOI] [PubMed] [Google Scholar]
- 10.Hilan C, Khazzaka K, Sfeir R. Antimicrobial effect of essential oil of Salvia libanotica (Sage) Br J Phytother. 1997;4:1–3. [Google Scholar]
- 11.Todorov S, Philianos S, Petkov V, Harvala C, Zamfirova R, Olimpiou H. Experimental pharmacologicalstudy of three species from genus Salvia. Acta Physiol Pharmacol Bulg. 1984;10:13–20. [PubMed] [Google Scholar]
- 12.Dapkevicius A, Venskutonis R, Beek T, Linssen J. Antioxidant activity of extracts obtained by different isola-tion procedures from some aromatic herbs grown in Lithuania. J Sci Food Agric. 1998;77:140–6. doi: 10.1002/(SICI)1097-0010(199805)77:1<140::AID-JSFA18>3.0.CO;2-K. [DOI] [Google Scholar]
- 13.Schilcher H. Effects and side-effects of essential oils. In: Baerheim A, Svendsen JJ, Scheffer C, editors. Essential oils and aromatic plants. Netherlands: Springer; 1985. pp. 217–31. [Google Scholar]
- 14.Perfumi M, Arnold N, Tacconi R. Hypoglycemic activity of Salvia Fructicosa mill from Cyprus. J Ethnopharmacol. 1991;34:135–40. doi: 10.1016/0378-8741(91)90030-H. [DOI] [PubMed] [Google Scholar]
- 15.Eidi M, Eidi A, Zamanizadeh H. Effects of Salvia Officinalis on serum glucose and insulin in healthy and Sterptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;100:310–3. doi: 10.1016/j.jep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Eidi A, Eidi M, Darzi R. Antidiabetic effect of Olea Europaea L. in normal and diabetic rats. Phytother Res. 2009;23:347–50. doi: 10.1002/ptr.2629. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon-Aguilar FJ, Roman-Ramos R, Flores-Saenz JL, Aguirre-Garcia F. Investigation on the hypoglycemic effects of extracts of four Mexican medicinal plants in normal and alloxan-diabetic mice. Phytother Res. 2002;16:383–6. doi: 10.1002/ptr.914. [DOI] [PubMed] [Google Scholar]
- 18.Lima CF, Azevedo MF, Araujo R, Fernandes-Ferreira M, Pereira-Wilson C. Metformin like effect of Salvia Officinalis (common sage): is it useful in diabetes prevention? Br J Nutr. 2006;96:326–33. doi: 10.1079/BJN20061832. [DOI] [PubMed] [Google Scholar]
- 19.Kianbakht S, Abasi B, Perham M, Hashem Dabaghian F. Antihyperlipidemic effects of Salvia officinalis L. leaf extract in patients with hyperlipidemia: a randomized double-blind placebo-controlled clinical trial. Phytother Res. 2011;25:1849–53. doi: 10.1002/ptr.3506. [DOI] [PubMed] [Google Scholar]
- 20.Laude EA, Morice AH, Grattan TJ. The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulm Pharmacol. 1994;7:179–84. doi: 10.1006/pulp.1994.1021. [DOI] [PubMed] [Google Scholar]
- 21.Pattnaik S, Subramanyam VR, Bapaji M, Kole CR. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. [PubMed] [Google Scholar]
- 22.Lachenmeier DW, Walch SG. The choice of Thujone as drug for diabetes. Nat Prod Res. 2011;25:1890–2. doi: 10.1080/14786419.2011.622279. [DOI] [PubMed] [Google Scholar]
- 23.Duke JA, Bogenschtg-Godwin MJ. Ducellier J. Duke PAK: Handbook of medicinal herbs. CRC Press; 2002. [Google Scholar]
- 24.Daher CF, Koulajian KB, Haddad N, Baroody GM. Effect of hydroxycut intake on fasted and postprandial lipemia in rats. J Toxicol Environ Health A. 2006;69:1587–601. doi: 10.1080/15287390500468688. [DOI] [PubMed] [Google Scholar]
- 25.National research Council. Guide for the care and use of laboratory animals. Washington (DC), USA: The National Academies Press 2011.
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Hassid WF, Abraham S. Chemical procedures for analysis of polysaccharide. Acad Press New York. 1957;3:34–6. [Google Scholar]
- 28.Nickavar B, Abolhasani L, Izadpanah H. α-amylase inhibitory activities of six Salvia species. Iran J Pharm Res. 2008;7:297–303. [Google Scholar]
- 29.Matsui T, Yoshimoto C, Osajima K, Oki T, Osajima Y. In vitro survey of α-lucosidase inhibitory food components. Biosci Biotechnol Biochem. 1996;60:2019–22. doi: 10.1271/bbb.60.2019. [DOI] [PubMed] [Google Scholar]
- 30.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713–23. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enomoto M, Adachi H, Hirai Y, Fukami A, Satoh A, Otsuka M et al. LDL-C/HDL-C ratio predicts carotid intima-media thickness progression better than HDL-C or LDL-C alone. J Lipids 2011, doi:10.1155/2011/549137 [DOI] [PMC free article] [PubMed]


