Abstract
Background
Studies report conflicting evidence regarding the existence of a DCIS-associated premalignant pathway in BRCA mutation carriers. We aimed to examine the prevalence, phenotype, and expression of oncodrivers in pure DCIS (pDCIS) and invasive breast cancer with concurrent DCIS (IBC + DCIS) in mutation carriers.
Methods
A cohort of BRCA1 and BRCA2 mutation carriers >18 years old who underwent surgery for breast cancer at an academic hospital (1992–2011) and had pathology available for review were included for study. Invasive breast cancer (IBC) and DCIS were stained for ER, PR, HER1, HER2, and HER3, and C-MET. DCIS prevalence was evaluated. Correlation of IBC and DCIS phenotypes was evaluated in patients with IBC + DCIS. DCIS and IBC expression of tumor markers were examined by BRCA mutation.
Results
We identified 114 breast tumors. Of all BRCA1-associated tumors, 21.1 % were pDCIS and 63.4 % were IBC + DCIS. Of all BRCA2-associated tumors, 23.3 % were pDCIS and 60.5 % were IBC + DCIS. In BRCA1 and BRCA2 mutation carriers with IBC + DCIS, there was a significant correlation in ER, PR, and HER3 expression between the DCIS and IBC components. Most BRCA1-associated DCIS did not express ER, PR or HER2, while most BRCA2-associated DCIS did express ER and PR. BRCA1− as well as BRCA2-associated DCIS had expression of HER3 and C-MET.
Conclusions
The majority of BRCA-associated tumors had DCIS present. Concordance of DCIS and IBC phenotypes was high, arguing for the existence of a DCIS-associated premalignant pathway. Oncodrivers HER3 and C-MET were expressed in the DCIS of mutation carriers, suggesting an opportunity for prevention strategies.
Keywords: Breast cancer, Ductal carcinoma in situ, BRCA, Biomarkers, Prevention, Oncodrivers
Background
For individuals who carry a germline mutation in either the BRCA1 or BRCA2 gene, risk of developing breast and/or ovarian cancer is much greater than among the general population [1, 2]. However, mutation carriers only account for 7–10 % of breast cancers cases and 10–15 % of ovarian cancers cases [3–7]. Several studies have shown that the morphological and immunohistochemical phenotypes of BRCA1- and BRCA2-related breast cancers differ from that of sporadic breast cancers [8–10]. Compared to sporadic breast cancers, both BRCA1- and BRCA2-related breast cancers are more likely to be high grade and poorly differentiated [11–14]. It has been shown that BRCA1-related breast cancers have low expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor (HER2/neu) as compared with sporadic breast cancers [15]. However, the ER and PR expression of BRCA2-related breast cancers does not seem to differ from that of sporadic cancers [16].
Much is known about the phenotypic differences between BRCA-associated and sporadic breast cancers, yet little is known about the differences in their pre-invasive progression pathways. Because more BRCA-related breast cancers are discovered between screening mammograms and with no prior pathologic findings [17, 18], it has previously been thought that the DCIS-associated premalignant pathway that exists among sporadic breast cancers is not present within the BRCA-associated disease spectrum. Studies have reported that DCIS is less often found near BRCA1- and BRCA2-associated invasive tumors when compared to sporadic tumors [19, 20]. However, more recent studies have found that high-risk pre-invasive lesions such as DCIS are more frequently found in prophylactic mastectomy specimens of BRCA mutation carriers than in control mammoplasty specimens [21–24].
Within the general population, DCIS has become a target for therapies aimed to prevent the development of invasive breast cancer. The relatively long period of latency between the onset of DCIS and development of sporadic invasive breast cancer has offered an opportunity to develop neoadjuvant interventions. Several neoadjuvant trials are underway for patients with DCIS, including anti-estrogen therapies and vaccines targeting HER2, both which have shown promise for patients with DCIS [25–27]. This hints at the possibility for new prevention options for patients with phenotypes underserved by currently available therapies, such as BRCA mutation carriers.
Today, the prevention of breast cancer among BRCA1 and BRCA2 mutation carriers has focused on surgical options such as risk-reducing bilateral mastectomy and bilateral salpingo-oophorectomy. Fortunately, these strategies have been shown to dramatically decrease the risk of breast cancer development [28, 29]. Nonetheless, there exist significant long-term consequences as well as effects on quality-of-life as a result of these surgical prevention strategies [30]. For this reason, efforts have been made to identify non-surgical prevention techniques for this patient population. Importantly, there is evidence that tamoxifen decreases risk of primary breast cancer as well as contralateral breast cancer for BRCA1 and BRCA2 mutation carriers [31, 32]. While studies are underway investigating additional options for chemoprevention in mutation carriers, there currently exist a paucity of non-surgical prevention strategies for this high-risk patient population.
Due to the substantial risk of breast cancer conferred by the BRCA1 and BRCA2 mutations, development of prevention strategies for mutation carriers is imperative. Chemoprevention strategies have been developed for the general population based on the known phenotypes of spontaneous DCIS and breast cancer, suggesting the importance of evaluating the phenotypes of hereditary breast tumors in order to develop targeted therapies. A growing body of literature supports targeting the HER family for prevention of ER-negative and possibly ER-positive breast tumors [33]. As such, investigating the phenotypes of BRCA-associated DCIS, specifically the HER tumor antigens, could elucidate possible targets to exploit in DCIS as a means of preventing invasive tumors in mutation carriers.
In this study we first aimed to identify the prevalence of pure DCIS and DCIS associated with invasive tumors among BRCA mutation carriers. We then aimed to assess the correlation between the phenotypes of invasive tumors and their corresponding DCIS in order to evaluate the role of DCIS in BRCA-associated tumor progression. Finally, we aimed to determine the unique immunophenotypes of BRCA1- and BRCA2-associated DCIS to investigate oncodriver expression that may be applicable to future prevention strategies.
Methods
Study population
After receiving approval from the Institutional Review Board at the University of Pennsylvania (protocol #814211), we obtained a list of patients seen in the High Risk Screening Clinic who were found to have a BRCA1 or BRCA2 mutation and were enlisted in the database for research purposes. We then restricted to mutation carriers who underwent a mastectomy or lumpectomy at our institution during the years of 1992–2011. We excluded all patients before 1992, because this was the year that the electronic pathology record system was first introduced at our institution. We then included only those patients who had pathology specimens available for review and who had specimens with sufficient tissue available for staining purposes (Fig. 1).
Fig. 1.

Criteria for study inclusion. BRCA1 and BRCA2 mutation carriers who were seen in the High Risk Screening Clinic before 2013 and were enlisted in the database for research were included for review. Patients who did not have pathology available, who were treated prior to 1992 (the year our electronic health record was introduced), or who treated at an outside hospital were excluded from the study. Patients who had tumor specimens without adequate tissue for staining were excluded from the study
Pathology review and staining
All breast cancer specimens were reviewed with a surgical pathologist for presence of DCIS and for tumor characteristics, including morphology of DCIS, distance of the DCIS from invasive tumor, and invasive and in situ nuclear grade.
All available pathology blocks for both invasive tumor and DCIS were cut and stained for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 1 (HER1), human epidermal growth factor receptor 2 (HER2), human epidermal growth factor receptor 3 (HER3), and hepatocyte growth factor receptor (C-MET). In patients who had both invasive tumor and concurrent DCIS, we stained both the DCIS and the invasive components. All stains were interpreted by a single surgical pathologist. Both the percentage of positively stained nuclei and the intensity of staining (0–3) was recorded. An H-score was calculated for HER1, HER3, and C-MET by multiplying the percentage of positive nuclei by the stain intensity. Patients for whom one or more of the stains were unsuccessful or not interpretable were excluded from the study.
Statistical methods
We examined patient characteristics by BRCA status using a Chi square test or t test, as appropriate. Associations between DCIS characteristics and DCIS prevalence, including pure DCIS and invasive breast cancer-associated DCIS, and mutation status were assessed by the Chi square test. In patients with invasive breast cancer with concurrent DCIS, Pearson correlation coefficients were calculated to determine correlation between HER1, HER2, and C-MET score in DCIS and invasive tumor, while a linear trend test was used to determine correlation between ER, PR, and HER2 intensity in DCIS and invasive tumor. Magnitude of DCIS and invasive tumor HER1, HER3 and C-MET score were compared by mutation status using the Student’s t test, while the Wilcoxon rank sum test was used to compare ER, PR and HER2 intensity.
Data management was performed using SAS Version 9.2 (SAS Institute Inc. 2009, Cary, NC, USA) and statistical analyses were performed using SPSS Version 21 (IBM Corp) or Stata/SE Version 11.1 (StataCorp, College Station, TX, USA). A p-value of <0.05 was considered significant for all statistical analyses.
Results
We identified 114 breast tumors, of which 71 (62.3 %) were BRCA1-associated and 43 (37.7 %) were BRCA2-associated. Of all IBC, 80.2 % had concurrent DCIS. Of all BRCA1-associated tumors, 11 (15.5 %) were pure invasive tumors, 15 (21.1 %) were pure DCIS, and 45 (63.4 %) were invasive tumors with concurrent DCIS. Of all BRCA2-associated tumors, 7 (16.3 %) were pure invasive tumors, 10 (23.3 %) were pure DCIS, and 26 (60.5 %) were invasive tumors with concurrent DCIS. Prevalence of these three tumor types did not differ by mutation status (p = 0.95).
When we examined the DCIS in tumors that had both invasive and in situ components, we found that the characteristics of the DCIS did not differ by mutation status (Table 1). For the majority of BRCA1- and BRCA2-associated tumors, the percentage of DCIS was less than 50 %, the DCIS morphology was comedo or cribriform, and the DCIS grade was high. For both BRCA1- and BRCA2-associated tumors, the majority of DCIS was intermixed with the invasive tumor or just on the periphery (<2 mm from the invasive tumor) (Fig. 2).
Table 1.
Characteristics of DCIS found in BRCA mutation carriers with invasive tumors and concurrent DCIS
| BRCA1 | BRCA2 | P-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| % DCIS | 0.14 | ||
| <15 % | 15 (53.6 %) | 8 (32.0 %) | |
| 15–49 % | 9 (32.1 %) | 8 (32.0 %) | |
| 50+% | 4 (14.3 %) | 9 (36.0 %) | |
| DCIS morphology | 0.10 | ||
| Solid | 4 (11.4 %) | 3 (10.7 %) | |
| Cribriform | 9 (25.7 %) | 14 (50.0 %) | |
| Comedo | 19 (52.3 %) | 7 (25.0 %) | |
| Complex | 3 (8.6 %) | 4 (14.3 %) | |
| DCIS Distance from IBC | 0.31 | ||
| Intermixed | 12 (42.9 %) | 14 (56.0 %) | |
| Peripheral (<2 mm from IBC) | 14 (50.0 %) | 11 (44.0 %) | |
| Distant (>2 mm from IBC) | 2 (7.1 %) | 0 (0 %) | |
| DCIS Grade | 0.63 | ||
| Low | 2 (5.7 %) | 1 (3.6 %) | |
| Intermediate | 11 (31.4 %) | 12 (42.9 %) | |
| High | 22 (68.9 %) | 15 (53.6 %) | |
Fig. 2.

Appearance of tumors with both invasive and in situ components. The majority of DCIS was located on the periphery of the invasive tumor (<2 mm from invasion) or intermixed with it, not distant from the invasive tumor
When examining tumors that had both invasion and concurrent DCIS, we found the correlation between the invasive and in situ components to be high for most immunophenotypes. In BRCA1 mutation carriers with IBC + DCIS, the correlation between the DCIS and IBC (Tables 2, 3) was highly significant for ER, PR, HER1, HER3 (Fig. 3), and C-MET (Fig. 4). In BRCA2 mutation carriers with IBC + DCIS, the correlation between the DCIS and IBC was highly significant for ER, PR, HER2, and HER3.
Table 2.
Correlation of IBC and DCIS expression of ER, PR, and HER2 in mutation carriers with IBC with concurrent DCIS, stratified by BRCA mutation
| IBC ER intensity | BRCA1 | BRCA2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DCIS ER intensity | DCIS ER intensity | |||||||||
| 0 | 1 | 2 | 3 | P value* | 0 | 1 | 2 | 3 | P value* | |
| 0 | 23 | 0 | 2 | 1 | <0.001 | 4 | 1 | 0 | 0 | <0.001 |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 0 | 0 | 1 | 3 | 0 | 0 | 4 | 3 | ||
| 3 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 8 | ||
| IBC PR intensity | DCIS PR intensity | DCIS PR intensity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | P value* | 0 | 1 | 2 | 3 | P value* | |
| 0 | 23 | 0 | 3 | 2 | <0.001 | 5 | 0 | 0 | 0 | 0.003 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | ||
| 3 | 0 | 0 | 0 | 4 | 0 | 1 | 1 | 1 | ||
| IBC HER2 intensity | DCIS HER2 intensity | DCIS HER2 intensity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | P value* | 0 | 1 | 2 | 3 | P value* | |
| 16 | 5 | 0 | 0 | 0.46 | 7 | 5 | 0 | 0 | <0.001 | |
| 6 | 4 | 0 | 0 | 1 | 6 | 0 | 0 | |||
| 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |||
* Linear trend test for a table with ordered rows and columns
Table 3.
Correlation of IBC and DCIS expression of HER1, HER3, and C-MET in mutation carriers with IBC with concurrent DCIS, stratified by BRCA mutation
| BRCA1 | BRCA2 | |||||
|---|---|---|---|---|---|---|
| Pearson Correlation, r | N | P value | Pearson Correlation, r | N | P value | |
| HER1 score | 0.43 | 42 | 0.005 | −0.09 | 23 | 0.69 |
| HER3 score | 0.87 | 25 | <0.001 | 0.95 | 19 | <0.001 |
| C-MET score | 0.47 | 29 | 0.01 | 0.34 | 17 | 0.19 |
Fig. 3.
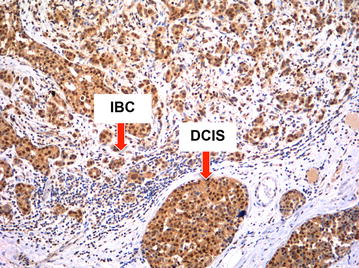
BRCA1 tumor with high correlation of DCIS expression of HER3 and adjacent invasive tumor expression of HER3
Fig. 4.

BRCA1 tumor with high correlation of DCIS expression of CMET and adjacent invasive tumor expression of CMET
Most BRCA1-associated DCIS and IBC had 0/3 staining intensity for ER, PR and HER2, while most BRCA2-associated DCIS and IBC had 3/3 staining intensity for ER and PR (Tables 4, 5). DCIS expression of ER, PR, and HER2 intensity was significantly higher in BRCA-2 tumors compared to BRCA-1 tumors (Table 4). IBC expression of ER and PR intensity were significantly higher in BRCA-2 tumors compared to BRCA-1 tumors (Table 5).
Table 4.
Comparison of DCIS expression of ER, PR, and HER2 between BRCA1- and BRCA2-associated tumors
| BRCA1 | BRCA2 | Wilcoxon rank sum P value | |
|---|---|---|---|
| ER intensity | <0.001 | ||
| 0 | 32 | 7 | |
| 1 | 1 | 1 | |
| 2 | 4 | 6 | |
| 3 | 7 | 14 | |
| PR intensity | 0.001 | ||
| 0 | 31 | 9 | |
| 1 | 2 | 1 | |
| 2 | 4 | 6 | |
| 3 | 7 | 13 | |
| HER2 intensity | 0.04 | ||
| 0 | 31 | 14 | |
| 1 | 14 | 14 | |
| 2 | 1 | 1 | |
| 3 | 0 | 2 |
Table 5.
Comparison of IBC expression of ER, PR, and HER2 between BRCA1- and BRCA2-associated tumors
| BRCA1 | BRCA2 | Wilcoxon rank sum P value | |
|---|---|---|---|
| ER intensity | <0.001 | ||
| 0 | 38 | 8 | |
| 1 | 1 | 0 | |
| 2 | 4 | 7 | |
| 3 | 2 | 11 | |
| PR intensity | <0.001 | ||
| 0 | 40 | 10 | |
| 1 | 0 | 1 | |
| 2 | 1 | 5 | |
| 3 | 4 | 10 | |
| HER2 intensity | 0.56 | ||
| 0 | 33 | 19 | |
| 1 | 13 | 7 | |
| 2 | 3 | 2 | |
| 3 | 0 | 2 |
BRCA1-associated DCIS had expression of HER3 and C-MET (H-Score 99.5 and 101.9, respectively), but lower expression of HER1 (H-Score 6.5), (Table 6). BRCA-2 associated DCIS also had expression of HER3 and C-MET (H-Score 84.3 and 124.8, respectively), but lower expression of HER1 (H-Score 16.5), (Table 6). DCIS expression of HER3, C-MET and HER1 did not differ significantly by mutation status. Similarly, BRCA1-associated IBC and BRCA2-associated IBC had expression of HER3 and C-MET, but lower expression of HER1 (Table 7), which did not differ significantly by mutation status.
Table 6.
Comparison of DCIS expression of HER1, HER3, and C-MET between BRCA1- and BRCA2-associated tumors
| BRCA1 | BRCA2 | Student’s t test P value | |||
|---|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | ||
| HER1 score | 56 | 6.43 ± 1.81 | 31 | 15.48 ± 10.43 | 0.40 |
| HER3 score | 38 | 99.47 ± 9.88 | 28 | 84.29 ± 11.60 | 0.32 |
| C-MET score | 39 | 101.92 ± 10.74 | 30 | 124.83 ± 9.31 | 0.12 |
Table 7.
Comparison of IBC expression of HER1, HER3, and C-MET between BRCA1- and BRCA2-associated tumors
| BRCA1 | BRCA2 | Student’s t test | |||
|---|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | P value | |
| HER1 score | 50 | 20.30 ± 5.59 | 29 | 11.38 ± 8.36 | 0.50 |
| HER3 score | 41 | 81.46 ± 7.84 | 22 | 72.95 ± 15.86 | 0.41 |
| C-MET score | 49 | 131.29 ± 9.35 | 21 | 131.67 ± 16.72 | 0.49 |
Discussion
In sporadic breast cancers the molecular profile of DCIS and the genetic progression pathway from in situ to invasive cancer have been well characterized [34–40]. The aims of the current study were to determine the prevalence of DCIS, investigate the unique immunophenotypes of DCIS, and assess the relationship between the phenotypes of invasive tumors and their in situ counterparts, among BRCA mutation carriers. By means of this investigation we aimed to better understand BRCA-associated DCIS and its role in the hereditary tumor progression pathway.
We were able to analyze the pathology from 104 BRCA-associated breast tumors. As expected, only 23 tumors were pure in situ lesions, while the remainder were invasive tumors with or without concurrent DCIS. Over 80 % of all invasive tumors had concurrent DCIS. This is a relatively high rate of DCIS among BRCA-associated invasive tumors, compared with reports from prior studies that range from 20 to 56 % [41, 42]. Studies of sporadic invasive breast tumors have found rates of concurrent DCIS ranging from 56 to 71 % [20, 41]. Our study suggests that DCIS occurs within hereditary invasive tumors at a rate similar to that of sporadic tumors, further supporting the hypothesis that DCIS is a precursor to invasive carcinoma in BRCA mutation carriers. Additionally, our finding that most of the DCIS was high-grade suggests that the entrance point for BRCA-associated DCIS in the tumor progression pathway may be at the high-grade stage, unlike the progression pathway of sporadic breast tumors which is thought to begin with low-grade in situ disease [40, 43].
We found that among patients with invasive cancer with concurrent DCIS, the concordance of expression between the DCIS and invasive tumor was remarkably high for most biomarkers. Additionally, the majority of DCIS was found intermixed with the invasive tumor or in close proximity of it. These findings further support the existence of a DCIS-associated premalignant pathway among patients with BRCA mutations. Studies of sporadic breast tumors have similarly shown that the molecular profile and immunophenotype of DCIS usually parallels that of its invasive counterpart [38–40], supporting the popular believe that sporadic tumors progress through DCIS before developing into invasive tumors.
We found that most BRCA1-associated invasive and in situ tumors were triple negative, while most BRCA2-associated tumors expressed ER and PR but did not express HER2. Prior studies have similarly shown that BRCA1-associated tumors tend to be ER, PR and HER2 negative, while BRCA2-associated tumors are more often ER and PR positive [15, 16]. Our finding that ER, PR, and HER2 expression in mutation carriers mimics what has been demonstrated in many other studies, serves to validate and strengthen the other results of this current study. However, while most BRCA1-associated tumors were triple negative, still a substantial number of BRCA1-associated DCIS had expression of ER (20.8 %) or PR (20.8 %), which supports previous studies that have demonstrated the benefit of anti-estrogen therapy and oophorectomy in BRCA1 patients [28, 29, 32].
Few investigators have examined other oncodriver expression in DCIS of mutation carriers, and thus our study evaluating HER1, HER3, and C-MET adds to the current body of literature regarding immunophenotypes of hereditary breast cancer. We found that DCIS expressed HER3 and C-MET for both BRCA1 and BRCA2 mutation carriers. This finding begs the consideration of how to implement strategies to target these oncodriver signaling pathways in BRCA mutation carriers so to prevent DCIS and invasive tumors. As the current treatment options for mutation carriers with DCIS are quite limited, there is undoubtedly a need for the development of new techniques geared to halt the development of DCIS in this patient population. Given the results of our study, possible options for such an effort might include the development of vaccines to target HER3 and C-MET oncodriver signaling pathways, or the utilization of kinase inhibitors for prevention. Nonetheless, it is clear that BRCA mutation carriers are a unique patient population deserving further investigation, particularly in regards to prevention strategies that might one day be utilized to prevent the development of pre-invasive or invasive tumors.
There are several limitations to the present study. First, while our study was able to directly assess the prevalence and immunophenotypes of DCIS in patients with known BRCA mutations, our dataset did not include patients with sporadic breast tumors for comparison. As such, we could only speculate how the characteristics of DCIS among our patients might compare to that of non-mutation carriers examined in other studies. Secondly, women with BRCA mutations are a small patient population and thus the number of subjects available for study inclusion was quite limited. Furthermore, some of our patients were excluded from the study because their pathology was no longer available for review or the slides that were available did not have sufficient tissue for all stains to be completed. While we do not have any reason to believe that this group of excluded patients was inherently different from the group maintained for study inclusion, we cannot be certain that there was no skewing of our data as a result of those patients lost.
Conclusions
In conclusion, we found that the majority of BRCA-associated tumors had DCIS present. Among tumors with both invasive and in situ components, the concordance of DCIS and IBC phenotypes was remarkably high, arguing for the existence of a DCIS-associated premalignant pathway. HER3 and C-MET were expressed in the DCIS of mutation carriers, suggesting an opportunity to target these oncodriver pathways as a means to prevent DCIS and invasive breast cancer. We hope future efforts will aim at investigating and implementing DCIS prevention strategies in mutation carriers.
Authors’ contributions
RY participated in study design, database management, review of pathology, statistical analysis, drafting of the manuscript, and revisions of the manuscript. RM participated in statistical analysis and revisions of the manuscript. KL participated in review of pathology and statistical analysis. HG participated in review of pathology. KN participated in revisions of the manuscript. SD participated in database management and revisions of the manuscript. RK participated in study design, statistical analysis and revisions of the manuscript. PZ participated in review of pathology. BC conceived of the study, participated in study design, and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was supported in part by the Abramson Cancer Center Support Grant P30-CA316520 (BJC), Komen Foundation for the Cure (SMD), Breast Cancer Research Foundation (SMD, KN), and Pennies in Action® (http://www.pennies-in-action.org) (BJC).
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- ER
estrogen receptor
- PR
progesterone receptor
- HER1
human epidermal growth factor receptor 1
- HER2
human epidermal growth factor receptor 2
- HER3
human epidermal growth factor receptor 3
- C-MET
hepatocyte growth factor receptor (C-MET)
- DCIS
ductal carcinoma in situ
- IBC
invasive breast cancer
- pDCIS
pure ductal carcinoma in situ
- IBC + DCIS
invasive breast cancer with concurrent ductal carcinoma in situ
Contributor Information
Rachel L. Yang, Email: rlyang@stanford.edu
Rosemarie Mick, Email: rmick@mail.med.upenn.edu.
Kathreen Lee, Email: Kathreen.Lee@uphs.upenn.edu.
Holly L. Graves, Email: holly.graves@uphs.upenn.edu
Katherine L. Nathanson, Email: knathans@exchange.upenn.edu
Susan M. Domchek, Email: susan.domchek@uphs.upenn.edu
Rachel R. Kelz, Email: rachel.kelz@uphs.upenn.edu
Paul J. Zhang, Email: pjz@mail.med.upenn.edu
Brian J. Czerniecki, Email: brian.Czerniecki@uphs.upenn.edu
References
- 1.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318–2324. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2318::AID-CNCR21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999;91(11):943–949. doi: 10.1093/jnci/91.11.943. [DOI] [PubMed] [Google Scholar]
- 5.Malone KE, Daling JR, Neal C, Suter NM, O’Brien C, Cushing-Haugen K, et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer. 2000;88(6):1393–1402. doi: 10.1002/(SICI)1097-0142(20000315)88:6<1393::AID-CNCR17>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 8.Armes JE, Venter DJ. The pathology of inherited breast cancer. Pathology. 2002;34(4):309–314. doi: 10.1080/00313020220147113. [DOI] [PubMed] [Google Scholar]
- 9.Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(9):2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Marcus JN, Watson P, Page DL, Narod SA, Lenoir GM, Tonin P, et al. Hereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer. 1996;77(4):697–709. doi: 10.1002/(SICI)1097-0142(19960215)77:4<697::AID-CNCR16>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Palacios J, Honrado E, Osorio A, Cazorla A, Sarrio D, Barroso A, et al. Immunohistochemical characteristics defined by tissue microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations: differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin Cancer Res Off J Am Assoc Cancer Res. 2003;9(10 Pt 1):3606–3614. [PubMed] [Google Scholar]
- 12.Eerola H, Heikkila P, Tamminen A, Aittomaki K, Blomqvist C, Nevanlinna H. Histopathological features of breast tumours in BRCA1, BRCA2 and mutation-negative breast cancer families. Breast Cancer Res BCR. 2005;7(1):R93–R100. doi: 10.1186/bcr953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakhani SR, Gusterson BA, Jacquemier J, Sloane JP, Anderson TJ, van de Vijver MJ, et al. The pathology of familial breast cancer: histological features of cancers in families not attributable to mutations in BRCA1 or BRCA2. Clin Cancer Res Off J Am Assoc Cancer Res. 2000;6(3):782–789. [PubMed] [Google Scholar]
- 14.Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomarker Prevent Publ Am Assoc Cancer Res Cosponsor Am Soc Prevent Oncol. 2012;21(1):134–147. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chappuis PO, Nethercot V, Foulkes WD. Clinico-pathological characteristics of BRCA1- and BRCA2-related breast cancer. Semin Surg Oncol. 2000;18(4):287–295. doi: 10.1002/(SICI)1098-2388(200006)18:4<287::AID-SSU3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Bane AL, Beck JC, Bleiweiss I, Buys SS, Catalano E, Daly MB, et al. BRCA2 mutation-associated breast cancers exhibit a distinguishing phenotype based on morphology and molecular profiles from tissue microarrays. Am J Surg Pathol. 2007;31(1):121–128. doi: 10.1097/01.pas.0000213351.49767.0f. [DOI] [PubMed] [Google Scholar]
- 17.Scheuer L, Kauff N, Robson M, Kelly B, Barakat R, Satagopan J, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(5):1260–1268. doi: 10.1200/JCO.20.5.1260. [DOI] [PubMed] [Google Scholar]
- 18.Komenaka IK, Ditkoff BA, Joseph KA, Russo D, Gorroochurn P, Ward M, et al. The development of interval breast malignancies in patients with BRCA mutations. Cancer. 2004;100(10):2079–2083. doi: 10.1002/cncr.20221. [DOI] [PubMed] [Google Scholar]
- 19.Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Vijver MJ, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90(15):1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 20.Pathology of familial breast cancer differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases, Breast Cancer Linkage Consortium. Lancet. 1997;349(9064):1505–1510. doi: 10.1016/S0140-6736(96)10109-4. [DOI] [PubMed] [Google Scholar]
- 21.Kauff ND, Brogi E, Scheuer L, Pathak DR, Borgen PI, Hudis CA, et al. Epithelial lesions in prophylactic mastectomy specimens from women with BRCA mutations. Cancer. 2003;97(7):1601–1608. doi: 10.1002/cncr.11225. [DOI] [PubMed] [Google Scholar]
- 22.Hoogerbrugge N, Bult P, Bonenkamp JJ, Ligtenberg MJ, Kiemeney LA, de Hullu JA, et al. Numerous high-risk epithelial lesions in familial breast cancer. Eur J Cancer. 2006;42(15):2492–2498. doi: 10.1016/j.ejca.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Kroiss R, Winkler V, Kalteis K, Bikas D, Rudas M, Tea M, et al. Prevalence of pre-malignant and malignant lesions in prophylactic mastectomy specimens of BRCA1 mutation carriers: comparison with a control group. J Cancer Res Clin Oncol. 2008;134(10):1113–1121. doi: 10.1007/s00432-008-0383-5. [DOI] [PubMed] [Google Scholar]
- 24.Hoogerbrugge N, Bult P, de Widt-Levert LM, Beex LV, Kiemeney LA, Ligtenberg MJ, et al. High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21(1):41–45. doi: 10.1200/JCO.2003.02.137. [DOI] [PubMed] [Google Scholar]
- 25.Hwang ES, Esserman L. Neoadjuvant hormonal therapy for ductal carcinoma in situ: trial design and preliminary results. Ann Surg Oncol. 2004;11(1 Suppl):37S–43S. doi: 10.1007/BF02524794. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez RJ, Buzdar AU, Fraser Symmans W, Yen TW, Broglio KR, Lucci A, et al. Novel clinical trial designs for treatment of ductal carcinoma in situ of the breast with trastuzumab (herceptin) Breast J. 2007;13(1):72–75. doi: 10.1111/j.1524-4741.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 27.Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 28.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA J Am Med Assoc. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finch AP, Lubinski J, Moller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(15):1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stan DL, Shuster LT, Wick MJ, Swanson CL, Pruthi S, Bakkum-Gamez JN. Challenging and complex decisions in the management of the BRCA mutation carrier. J Womens Health (Larchmt). 2013;22(10):825–834. doi: 10.1089/jwh.2013.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King MC, Wieand S, Hale K, Lee M, Walsh T, Owens K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA J Am Med Assoc. 2001;286(18):2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 32.Phillips KA, Milne RL, Rookus MA, Daly MB, Antoniou AC, Peock S, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(25):3091–3099. doi: 10.1200/JCO.2012.47.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howe LR, Brown PH. Targeting the HER/EGFR/ErbB family to prevent breast cancer. Cancer Prev Res (Phila). 2011;4(8):1149–1157. doi: 10.1158/1940-6207.CAPR-11-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada S, Mick R, Roses RE, Graves H, Niu H, Sharma A, et al. The significance of HER-2/neu receptor positivity and immunophenotype in ductal carcinoma in situ with early invasive disease. J Surg Oncol. 2011;104(5):458–465. doi: 10.1002/jso.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabbs DJ, Chivukula M, Carter G, Bhargava R. Basal phenotype of ductal carcinoma in situ: recognition and immunohistologic profile. Modern Pathol Off J US Can Acad Pathol Inc. 2006;19(11):1506–1511. doi: 10.1038/modpathol.3800678. [DOI] [PubMed] [Google Scholar]
- 36.Meijnen P, Peterse JL, Antonini N, Rutgers EJ, van de Vijver MJ. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer. 2008;98(1):137–142. doi: 10.1038/sj.bjc.6604112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res BCR. 2008;10(4):R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mommers EC, Leonhart AM, Falix F, Michalides R, Meijer CJ, Baak JP, et al. Similarity in expression of cell cycle proteins between in situ and invasive ductal breast lesions of same differentiation grade. J Pathol. 2001;194(3):327–333. doi: 10.1002/path.910. [DOI] [PubMed] [Google Scholar]
- 39.Castro NP, Osorio CA, Torres C, Bastos EP, Mourao-Neto M, Soares FA, et al. Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res BCR. 2008;10(5):R87. doi: 10.1186/bcr2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Diest PJ. Ductal carcinoma in situ in breast carcinogenesis. J Pathol. 1999;187(4):383–384. doi: 10.1002/(SICI)1096-9896(199903)187:4<383::AID-PATH299>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Arun B, Vogel KJ, Lopez A, Hernandez M, Atchley D, Broglio KR, et al. High prevalence of preinvasive lesions adjacent to BRCA1/2-associated breast cancers. Cancer Prev Res (Phila) 2009;2(2):122–127. doi: 10.1158/1940-6207.CAPR-08-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Groep P, van Diest PJ, Menko FH, Bart J, de Vries EG, van der Wall E. Molecular profile of ductal carcinoma in situ of the breast in BRCA1 and BRCA2 germline mutation carriers. J Clin Pathol. 2009;62(10):926–930. doi: 10.1136/jcp.2009.065524. [DOI] [PubMed] [Google Scholar]
- 43.Fujii H, Szumel R, Marsh C, Zhou W, Gabrielson E. Genetic progression, histological grade, and allelic loss in ductal carcinoma in situ of the breast. Cancer Res. 1996;56(22):5260–5265. [PubMed] [Google Scholar]


