Abstract
Background
Bacterial plasmids have a major impact on metabolic function and adaptation of their hosts. An indigenous plasmid was identified in a Chinese isolate (GX01) of the invasive phytopathogen Xanthomonas oryzae pv. oryzicola (Xoc), the causal agent of rice bacterial leaf streak (BLS). To elucidate the biological functions of the plasmid, we have sequenced and comprehensively annotated the plasmid.
Methods
The plasmid DNA was extracted from Xoc strain GX01 by alkaline lysis and digested with restriction enzymes. The cloned and subcloned DNA fragments in pUC19 were sequenced by Sanger sequencing. Sequences were assembled by using Sequencher software. Gaps were closed by primer walking and sequencing, and multi-PCRs were conducted through the whole plasmid sequence for verification. BLAST, phylogenetic analysis and dinucleotide calculation were performed for gene annotation and DNA structure analysis. Transformation, transconjugation and stress tolerance tests were carried out for plasmid function assays.
Results
The indigenous plasmid from Xoc strain GX01, designated pXOCgx01, is 53,206-bp long and has been annotated to possess 64 open reading frames (ORFs), including genes encoding type IV secretion system, heavy metal exporter, plasmid stability factors, and DNA mobile factors, i.e., the Tn3-like transposon. Bioinformatics analysis showed that pXOCgx01 has a mosaic structure containing different genome contexts with distinct genomic heterogeneities. Phylogenetic analysis indicated that the closest relative of pXOCgx01 is pXAC64 from Xanthomonas axonopodis pv. citri str. 306. It was estimated that there are four copies of pXOCgx01 per cell of Xoc GX01 by PCR assay and the calculation of whole genome shotgun sequencing data. We demonstrate that pXOCgx01 is a self-transmissible plasmid and can replicate in some Xanthomonas spp. strains, but not in Escherichia coli DH5α. It could significantly enhance the tolerance of Xanthomonas oryzae pv. oryzae PXO99A to the stresses of heavy metal ions. The plasmid survey indicated that nine out of 257 Xoc Chinese isolates contain plasmids.
Conclusions
pXOCgx01 is the first report of indigenous plasmid from Xanthomonas oryzae pv. oryzicola, and the first completely sequenced plasmid from Xanthomonas oryzae species. It is a self-transmissible plasmid and has a mosaic structure, containing genes for macromolecule secretion, heavy metal exportation, and DNA mobile factors, especially the Tn3-like transposon which may provide transposition function for mobile insertion cassette and play a major role in the spread of pathogenicity determinants. The results will be helpful to elucidate the biological significance of this cryptic plasmid and the adaptive evolution of Xoc.
Electronic supplementary material
The online version of this article (doi:10.1186/s12866-015-0562-x) contains supplementary material, which is available to authorized users.
Keywords: Xanthomonas oryzae pv. oryzicola, Indigenous plasmid, Self-transmissible, Tn3-like transposon, Heavy metal tolerance
Background
Xanthomonas oryzae pathovar oryzicola (here after, Xoc), one of the major pathogenic bacteria in rice, can cause rice bacterial leaf streak (BLS) resulting in significant reduction in yield and economic losses of rice production [1]. The pathogen invades rice mainly through leaf stomata or wounds and colonizes intercellular spaces in the mesophyll, resulting in water-soaked interveinal lesions that can develop into translucent streaks [1]. Rice resistance to BLS was found to be a quantitative trait and no major resistance genes were identified in rice up to now [2]. BLS is principally controlled by crop spraying with copper compounds and a few agricultural antibiotics. This seldom results in ideal control and often gives rise to environmental concerns. As a result, BLS is gradually becoming one of the major limiting factors to rice production in the tropical and sub-tropical areas in Asia and Africa [1, 3, 4].
Plasmids are extrachromosomal autonomously replicating DNA molecules, which often carry genes that may benefit the survival of the host organism, such as antibiotic resistance, heavy metal tolerance, and toxin production [5]. They are also known as a type of mobile genetic element which plays very important roles in horizontal gene transfer and gene exchange in nature [6]. Substantial numbers of animal and plant pathogenic bacteria were found harboring plasmids which effected their adaptation, pathogenicity and evolution [5, 7].
Plasmids have been reported from many xanthomonads. X. albilineans, X. arboricola pv. pruni, X. axonopodis pathovars cyamopsidis, dieffenbachiae, glycines, manihotis, phaseoli, vignicola, and vitians, and X. citri pv. citri, X. campestris pathovars campetris, malvacearum and vesicatoria, and X. hortorum pathovars hederae and pelargonii, and X. fuscans subsp. fuscans and X. oryzae pv. oryzae possess at least one type of plasmid in each bacterium [8–17]. The complete DNA sequences of some plasmids have been published from X. albilineans, X. arboricola pv. pruni, X. axonopodis pv. citri, X. axonopodis pv. glycines, X. fuscans subsp. fuscans, X. campestris pv. campestris and X. campestris pv. vesicatoria [12–19]. Plasmids from Xanthomonas are significantly diverse in size and gene composition. Some of them carry genes encoding macromolecule secretion systems, effectors, heavy metal exporters, plasmid stability factors, and DNA mobile elements. A range of plasmid-mediated phenotypes, including virulence, toxin and hormone production, and resistance to bactericides, have been reported in many other phytopathogenic bacteria [11]. However, the plasmid biology of Xanthomonas is still not well understood [11, 12].
To date, hundreds of Xoc strains have been isolated and identified from Asia and Africa [20–23], and complete genomes of two Xoc strains BLS256 from the Philippines [24] and CFBP7342 from Burkina Faso (GenBank Accession: CP007221) have been determined. However, there are no reports about plasmids in any Xoc strains, or a complete plasmid DNA sequence from X. oryzae species.
In our previous study, a rifampicin-resistance spontaneous mutant, named GX01 [25], was selected from the wild type strain LT4, which was isolated from the rice leaf with typical BLS symptoms in Liantang Town of Hezhou City of Guangxi, in the central area of South China rice growing regions, where a specific population of the wild rice (Oryza rufipogon) is thought to be the most recent ancestor of Oryza sativa japonica [26]. A cryptic plasmid was found by chance in this high virulent strain GX01. To our knowledge, this is the first report about indigenous plasmids in X. oryzae pv. oryzicola. In this study, we sequenced and comprehensively annotated this conjugative plasmid.
Methods
Bacterial strains, plasmids, and general culture conditions
Bacterial strains and plasmids used in this study are listed in Table 1.
Table 1.
Bacterial strains and plasmids
| Strains or plasmids | Relevant traitsa | Source or reference |
|---|---|---|
| Strains | ||
| Xanthomonas spp. | ||
| X. oryzae pv. oryzicola GX01 | Harboring plasmid pXOCgx01; Rifr | This study |
| X. oryzae pv. oryzicola isolates | Isolates from China; some harboring plasmids | |
| X. campestris pv. campestris 8004 | plasmidless; Rifr | [57] |
| X. oryzae pv. oryzae PXO99A | plasmidless; Rifr (a rifampicin resistant mutant selected in our lab) | [58] |
| Xoo PXO99A/pXOCgx01::Tn5 | PXO99A harboring plasmid pXOCgx01 insertion with Tn5; Rifr, Kanr | This study |
| Enterobacteriaceae | ||
| EC100D pir + | F−, Ф80d/lacZM15, lacX74, recA1, endA1, galU, mcrA, galK, λ−, rpsL, araD139, nupG, pir+ | Eppicentre Biotechnologies |
| DH5α | F−, Ф80d/lacZΔM15, deoR, endA1, hsdR17, phoA, supE44, λ−, thi-1, gyrA96, relA1 | Our library |
| Plasmid | ||
| pUC19 | Cloing vector; Ampr | Our library |
aRifr, Kanr and Ampr indicated resistance to rifampin, kanamycin and ampicillin, respectively
Xoc strains and Xoo PXO99A were cultured in Nutrient Broth (NB) medium (per liter: 5.0 g hipolypeptone, 1.0 g yeast extract, 3.0 g beef extract, 10.0 g sucrose, pH 7.0) at 28 °C and Xcc 8004 was cultured in NYGB medium (per liter: 5.0 g peptone, 3.0 g yeast extract and 20.0 g glycerol) [27] at 28 °C. Escherichia coli strain DH5α and EC100D™ pir + were cultured in Luria–Bertani (LB) broth (per liter: trytone 10.0 g, yeast extract 5.0 g, NaCl 10.0 g) at 37 °C. When required, media were supplemented with antibiotics as follows: 50 μg/mL rifampicin (Rif), 25 μg/mL kanamycin (Kan) or 100 μg/mL ampicillin (Amp).
Plasmid isolation and identification
Plasmid DNA from E. coli cells and Xoc/Xoo/Xcc strains were extracted by the alkaline lysis method as described by O’Sullivan and Klaenhammer [28] with some modifications. To estimate the size and profile polymorphisms of plasmids from different Xoc strains, digestion reactions with different restriction endonucleases were done after plasmid harvest, and all the samples were checked by 0.8 % agarose gel electrophoresis.
Plasmid DNA sequencing
Good quality plasmid DNA fragments of pXOCgx01 were isolated and selected by digestion with BamHI, PstI, SphI and EcoRI, respectively, and were cloned into vector pUC19 with corresponding sites. 15 pUCBamHI subclones, 22 pUCEcoRI subclones, 39 pUCPstI subclones and 48 pUCSphI subclones were gotten, and sequenced by Sanger sequencing using vector specific M13 primer pair. Sequences were assembled by using Sequencher software. Meanwhile, whole-genome shotgun sequencing of Xoc strain GX01 was performed using the Illumina platform, and sequences were assembled by using SOAPdenovo Packages. Gaps were closed by primer walking and sequencing, and at last multi-PCR were done through the whole plasmid sequence for verification.
Annotation and bioinformatics analysis
Open reading frames (ORFs) containing more than 30 amino acid residues were predicted using Glimmer V3.02 [29] and GeneMarkS V4.30 [30], and verified by manually analysis. Potential protein-coding sequences were subsequently analyzed manually using BLAST suite of programs, including BLASTN, BLASTP, BLASTX, clusters of orthologous groups (COG) and conserved domain database (CDD). The protein motifs and domains of all ORFs were characterized based on intensive searches against public databases using Interproscan tools. tRNA genes were identified by using tRNAscan-SE. GC skew analysis and the circular-genome-map drawing were performed using BRIG software [31].
Phylogenetic analyses of gene clusters were performed with BLASTN search and multiple alignments were developed with MEGA 6 [32] and PHYLIP. The phylogenetic tree of the whole sequences of plasmid pXOCgx01 was drew by using the online tool EvolView described by Zhang [33].
The method of the Delta similarity (δ*) calculation is based on the method described by Karlin method [34]:
Here f and g is the FASTA sequence of the two compared genome DNA sequence and XY is the combination of the 4 nucleotide base ATG, function ρ is the odds ratio of the two nucleotide base X and Y:
Here function fx is the frequency of the nucleotide base X occurs in the fasta sequence f or g, and function fXY is the frequency of the dinucleotide XY in the sequence under study.
PCR assays
For screening for the presence of plasmid pXOCgx01, single clones were selected and transferred to a new NA medium plate, and pXOCgx01-specific primers targeting genes involved in pXOCgx01 T4SS genes, resolvase genes and transposase genes were designed using the program Vector NTI V11.5. Single bacterial colony was scraped off an NA culture plate with a sterile toothpick, and was resuspended in 4 μL sterile deionized water in an Eppendorf PCR tube as a DNA template. Amplifications were carried out in a final volume of 20 μL consisted of 12.4 μL sterile deionized water, 2 μL 10 × Taq-DNA polymerase buffer, forward and reverse primers at 0.25 μM each, dNTPs at 0.25 mM, 1 units Taq-DNA polymerase and 4 μL DNA template sample prepared above. The PCR products were resolved on 1.2 % agarose gels at 100 volts for about 35 min, stained with nucleic acid gel stain GelRed and photographed under UV light (BIO-RAD UNIVERSAL HOOD II).
Obtainment of the plasmid selection marker
As pXOCgx01 has no antibiotic selection markers used in our lab, a kanamycin resistance marker was inserted using EZ-Tn5™ < R6Kγori/KAN-2 > Insertion Kit (Cat. No. EZI011RK) according to the manufacturer’s instructions in vitro. The randomly inserted plasmids were transferred into EC100D pir+ by electroporation and clones harboring pXOCgx01::Tn5 were selected on LA amended with kanamycin. An EZ-Tn5™ < R6Kγori/KAN-2 > transposon-specific PCR-checking was established using primers Tn5-R6K-F/R (Additional file 1). Plasmids with Tn5 cassette from EC100D pir+ and the plasmid from the wild-type strain Xoc GX01 were extracted and digested with BamHI, followed by the contrastive analysis by 0.8 % agarose gel electrophoresis to make sure no fragments were trimmed in these transformants. The insertion site of the Tn5 cassette in pXOCgx01::Tn5 was determined through “Rescue Cloning” method. Briefly, plasmid DNA was extracted from EC100D pir+, and digested with one restriction endonuclease which no corresponding site exists in EZ-Tn5™ < R6Kγori/KAN-2 > Transposon like EcoRI. Then the digested DNA was purified and circularized by self-ligation. The mini-Tn5 cassette with the flanking DNA of the insertion site was transferred into EC100D pir+ by electroporation and clones were selected on LA amended with kanamycin. Positive clones were selected using primers Tn5-R6K-F/R as above and the mini-Tn5 cassette DNA was extracted by the alkaline lysis method and digested with the same enzyme as above and followed by electrophoresis analysis on 0.8 % agarose gel. Clones with different profiles which might be different insertion-site clones were chosen for sequencing using the Tn5 transposon-specific primers Tn5KAN2-F/Tn5R6KAN2-R (Additional file 1). Sequence analysis showed that one Tn5 cassette inserted in plasmid pXOCgx01 at the spacer region between XOCp0043 and XOCp0044. This clone was designated as pXOCgx01::Tn5.
Conjugal transfer of pXOCgx01
EC100D pir+/pXOCgx01::Tn5 was used as a donor to track conjugal transfer of pXOCgx01 to Xoo PXO99A. Donor and recipient strains were harvested in midlogarithmic phase and washed with 0.9 % saline solution and NB medium, mixed, and placed on NA medium plates without any antibiotics. After incubation at 30 °C for 24 h, cells were picked up to sterile Eppendorf tubes and suspended with 300 μL NB medium. The mixed cells were spread on NA medium amended with rifampin and kanamycin and incubated at 28 °C for 4 to 5 days. Putative transconjugants were examined with Tn5 cassette-specific primers Tn5-R6K-F/R, pXOCgx01-specific primers pXOC-virF/R, pXOC-res-F/R, pXOC-tra-F/R (see Additional file 1). Plasmid DNA was isolated from the putative transconjugants by the modified alkaline lysis method as above and digested with BamHI. Fragment profiles were examined with agarose gel electrophoresis.
Curing of plasmid pXOCgx01 from strain GX01
Kinds of approaches were adopted to cure plasmid pXOCgx01 from strain GX01. Xoc GX01 was grown at an elevated temperature (37 °C) in liquid NB medium, passaging every 24 h for a week. Cells were then diluted in NB medium and plated onto NA plates with rifampin. Single colonies (n = 500) were subsequently screened for the sequence of plasmid pXOCgx01 with the PCR assay as described above (use primers pXOC-virF/R, pXOC-res-F/R and pXOC-tra-F/R, see Additional file 1). And 10 colonies were selected to isolate plasmid DNA and be digested with BamHI for fragment analysis. Besides, GX01 was grown in NB medium with SDS (0.008 to 0.015 % final concentrations) at 28 °C, passage every 24 h for a week, and plasmid pXOCgx01 was checked as above. SDS and elevated temperature crossed method was also attempted. Other ways have been also conducted, such as acridine orange (1 μg/mL to 20 μg/mL final concentration) stress tests, and Xoc GX01 electrocompetent cells electric pulse tests (2-mm cuvette with a Bio-Rad Gene Pulser Xcell at 2000 to 3000 V for Time constant protocol, capacitance and resistivity were variable; 1000 to 2000 V for Square Wave Protocol, pulse length: 5 ms, number of pulses: 2, pulse interval: 5 s).
Some broad-host-range plasmids such as pLAFR6, pPH1JI, pBBad18K, pUFR034 and pBBR1MSC-5 were introduced into Xoc GX01 by electrotransformation in order to find an incompatibility plasmid and cure pXOCgx01 from Xoc GX01. The putative origin (coordinates 48,376–53,206 joined with coordinates 1–7175) was cyclized with the EZ-Tn5™ < R6Kγori/KAN-2 > transposon and introduced into Xoc GX01 by electrotransformation. Transformants were selected from NA plates with relative antibiotics, and positive clones were passaged every 24 h in 5 mL NB medium with relative antibiotics for a week. At each passage, aliquots of each culture were diluted and spread on NA plates with relative antibiotics. Single clones were screened by colony PCR described above to check the presence of the plasmid pXOCgx01.
Nucleotide sequence accession number
The complete sequence of plasmid pXOCgx01 has been deposited in GenBank under accession number KR071788.
Results
Identification and nucleotide sequencing of plasmid from Xoc strain GX01
An extrachromosomal DNA molecule was found in Xoc GX01 by comparing restriction fragment patterns of Xoc GX01 DNA samples extracted with different approaches. To determine its entire DNA sequence, the extrachromosomal DNA was extracted by alkaline lysis and digested with BamHI, EcoRI, PstI and SphI respectively, followed by being subcloned into pUC19 vector for sequencing. Meanwhile, whole-genome shotgun sequencing of Xoc GX01 was performed by using the Solexa sequencing method. By assembling the subclones’ sequencing and the genome sequencing data, followed by gap filling and PCR checking, a 53,206-bp circular DNA molecule was generated. The actual electrophoresis of the isolated extrachromosomal DNA molecule after digestion with BamHI was in accordance with the simulated result of the assembled circular molecule by using Vector NTI software (Fig. 1). Some plasmid-related protein genes, such as those for plasmid stability were predicted and the potential oriV and oriT were identified by sequence similarity searching pA506 and pXAC64 from Pseudomonas fluorescens A506 [35] and X. citri pv. citri 306 [36] respectively. These results indicated that the extrachromosomal DNA molecule from Xoc GX01 was a cryptic plasmid. And from the sequencing results and the plasmid profiles, we confirmed that there was only one type of plasmid in strain GX01, thus we designated this extrachromosomal DNA molecule as plasmid pXOCgx01. The copy number of pXOCgx01 was estimated to be four per cell, based on the Solexa sequencing data calculation, by dividing the coverage depth of the chromosome-related reads by that of the plasmid-related reads.
Fig. 1.
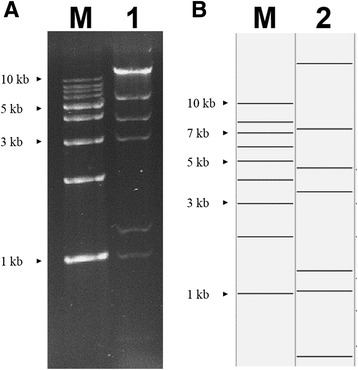
Restriction fragment patterns digested with BamHI of the isolated extrachromosomal DNA molecule from Xoc GX01. a the actual restriction enzyme electrophoresis (lane 1); b the simulated enzyme map (lane 2) by using Vector NTI software. M denotes DNA ladder marker (TianGen, 1 kb DNA Ladder)
General overview of the plasmid pXOCgx01
The total length of the indigenous plasmid pXOCgx01 is 53,206 bp, with an average G + C content 61.25 %, lower than that of the Xoc GX01 chromosome (64.08 %) and that of other xanthomonads genomes [7, 17, 24]. Of all the 64 open reading frames (ORFs), 28 were predicted to be transcribed from the leading replication strand (Fig. 2 and Additional file 2), and 37 were functionally assigned while 23 were predicted to encode conserved hypothetical proteins by homologous sequence search and domain characterization, whereas four ORFs could only be annotated as hypothetical proteins showing plasmid specificity. The average length of ORFs is 713 bp, similar to that of plasmids in other Xanthomonas [7]. One tRNA (tRNA-Ile) gene, with a length of 73 bp (coordinates 41,134–41,206), was identified using tRNAscan-SE. The virB and czcCBA loci were identified as well as plasmid replication and transportation protein genes. Unlike plasmids in other xanthomonads, no antibiotic resistance, copper resistance or type III effector related genes were identified in pXOCgx01 [7, 11, 12, 37]. Phylogenetic analysis of pXOCgx01 with the plasmids from GenBank reveals that the global similarity of pXOCgx01 with the vast majority plasmids is quite low, with less than 30 % sequence coverage. We found that only the plasmid pXAC64 from Xac 306 shares an identity of 96 % with 31 % sequence coverage against pXOCgx01 (Additional file 3).
Fig. 2.
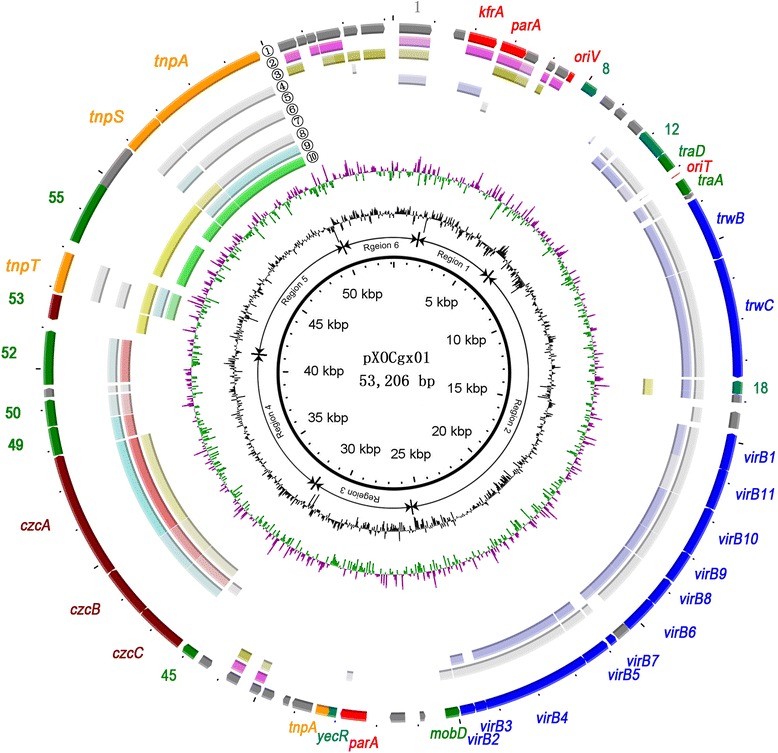
Circular map of plasmid pXOCgx01. The outer circle shows predicted coding sequences. Different colors represent different putative functions: gray, (conserved) hypothetical protein; red, intergenic regions with putative functions as the origin of transfer (oriT), origin of vegetative replication (oriV), and plasmid replication relative proteins as kfrA and parA proteins; green, transmembrane proteins and conjugal transfer proteins; blue, T4SS locus; maroon, metal resistance relative proteins, like czcABC locus and metal-binding proteins; teal, other proteins as CcgAII protein, putative PemK-like protein, plasmid stable inheritance protein, yecR. Circles 2 through 10 depict nine other plasmid or chromosome genomes owning conserved regions in pXOCgx01 as determined by Blastp (cutoff of 1e-5). Second circle, plasmid I from Xanthomonas campestris pv. campestris B1459; third circle, plasmid pla from Xanthomonas fuscans subsp. fuscans str. 4834-R; fourth circle, pXAC64 from Xanthomonas axonopodis pv. citri str. 306; fifth circle, pXCV38 from Xanthomonas campestris pv. vesicatoria str. 85–10; sixth circle, Pseudomonas aeruginosa UCBPP-PA14; seventh circle, Stenotrophomonas. maltophilia K279a; eighth circle, Pseudoxanthomonas spadix BD-a59; ninth circle, pXCV183 from Xanthomonas campestris pv. vesicatoria str. 85–10; tenth circle, Xanthomonas oryzae pv. oryzae PXO99A. Within each circle of the nine, the darkest color indicates nucleotide identity exceeding 70 % whereas the lightest color represents identity exceeding 40 %. Eleventh circle, G + C content. Twelfth circle, GC skew. The circular plasmid diagram was generated using BRIG
The mosaic structure of pXOCgx01 revealed by comparative and phylogenetic analyses
Comparative analyses and BLAST results of pXOCgx01 to GenBank showed that different parts of the plasmid have distinctly different similarities to other genomes (Additional file 4). Six DNA regions with obviously different genomic heterogeneities have been grouped based on G + C content, dinucleotide bias (δ*-differences) and BLAST results (Tables 2 and 3), outlining the mosaic structure of pXOCgx01. Of the six clusters, four have syntenic regions in the genomes of other organisms, but the other two have not. The longest cluster is 19,095 bp in length, which has a high identity to the virB locus on the plasmid pXAC64 of Xac 306 [15]. The second longest cluster is the czcABC locus containing 8 ORFs, which may represent one of the ancient DNA segments for heavy metal exclusion, identified basing on the phylogenetic analysis from gene-order data [38]. About one fourth of the plasmid genes have few significant homologs in GenBank based on the BLASTN search, especially the (conserved) hypothetical protein genes. The mosaic structure indicated that the generation of pXOCgx01 may be an event of multiple DNA rearrangements by horizontal gene transfers.
Table 2.
Plasmid DNA context features of pXOCgx01
| Features | Region I | Region II | Region III | Region IV | Region V | Region VI |
|---|---|---|---|---|---|---|
| Coordinates | 1492–6218 | 6219–25,313 | 25,314–31,747 | 31,748–41,053 | 41,054–49,841 | 49,842–1491 |
| Length (bp) | 4727 | 19,095 | 6434 | 9306 | 8788 | 4856 |
| GC (%) | 60.93 | 59.54 | 59.61 | 63.79 | 61.91 | 64.44 |
| δ*-differences | 88.58 | 59.23 | 66.91 | 65.39 | 86.15 | 88.51 |
| Functions | KrfA, ParA | oriT, T4SS | hypothetical | CzcCBA | Tn5044 | hypothetical |
δ*-differences (dinucleotide bias) indicate the cumulative dinucleotide differences between each region and the segment from dnaA to gyrB of chromosome of Xoc GX01
Table 3.
Dinucleotide bias (δ*-differences) between DNA regions of pXOCgx01
| Regions | Region I | Region II | Region III | Region IV | Region V | Region VI |
|---|---|---|---|---|---|---|
| Region I | 0 | 99.25 | 76.07 | 103.63 | 132.99 | 70.40 |
| Region II | 0 | 80.54 | 84.07 | 94.82 | 95.75 | |
| Region III | 0 | 79.73 | 74.62 | 72.29 | ||
| Region IV | 0 | 55.27 | 100.80 | |||
| Region V | 0 | 111.94 | ||||
| Region VI | 0 |
δ*-differences indicate the cumulative dinucleotide differences between each region
To obtain some insight into the evolution, unrooted phylogenetic trees were constructed from nucleic acid sequences of each region (Fig. 3, Additional file 5). From these phylogenetic trees, we could clearly see that different loci of pXOCgx01 derived from different species. For instance, the closest evolutionary relative with the kfrA locus was that of Xcc B1459 plasmid I; and the T4SS locus had the closest relationship with that of pXAC64 of Xac 306, while the Tn5044 locus with that of Xoo PXO99A. These results were in accordance with dinucleotide bias analysis above, supporting the mosaic structure of pXOCgx01.
Fig. 3.
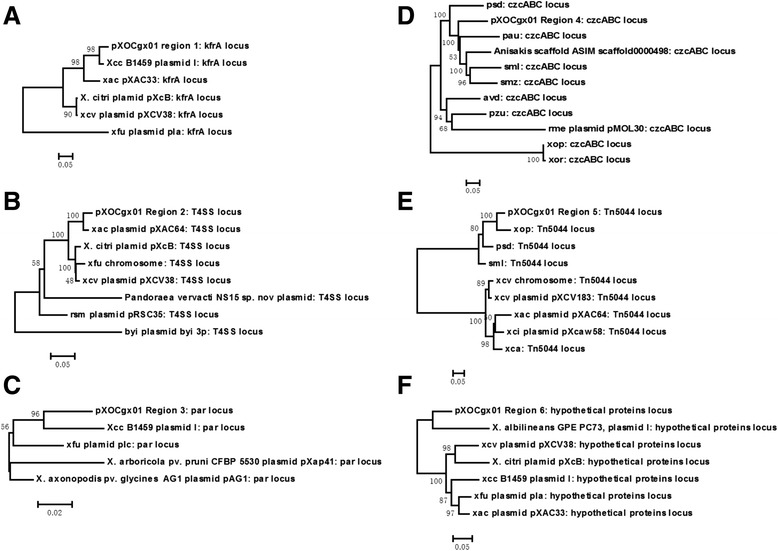
Unrooted phylogenetic tree (Neighbor-Joining) for six conserved regions in pXOCgx01. a. Region I; b. Region II; c. Region III; d. Region IV; e. Region V; f. Region VI. DNA sequences of other genomes were abstracted from Genbank. The specific locations are summarized in Additional file 5. Abbreviation of genome names: Xcc B1459 plasmid I: Xanthomonas campestris pv. campestris B1459 plasmid I; xfu plasmid pla: Xanthomonas fuscans subsp. fuscans str. 4834-R plasmid pla; xac plasmid pXAC33: Xanthomonas axonopodis pv. citri str. 306 plasmid pXAC33; xac plasmid pXAC64: Xanthomonas axonopodis pv. citri str. 306 plasmid pXAC64; xcv plasmid pXCV38: Xanthomonas campestris pv. vesicatoria str. 85–10 plasmid pXCV38; byi plasmid byi_3p: Burkholderia sp. YI23 plasmid byi_3p; rsm plasmid pRSC35: Ralstonia solanacearum CMR15 plasmid pRSC35; X. citri plasmid pXcB: Xanthomonas citri plasmid pXcB; avd: Azotobacter vinelandii CA6; pzu: Phenylobacterium zucineum HLK1; pau: Pseudomonas aeruginosa UCBPP-PA14; psd: Pseudoxanthomonas spadix BD-a59; smz: Stenotrophomonas maltophilia D457; sml: Stenotrophomonas maltophilia K279a; xop: Xanthomonas oryzae pv. oryzae PXO99A; xcv plasmid pXCV183: Xanthomonas campestris pv. vesicatoria plasmid pXCV183; xca: Xanthomonas campestris pv. campestris complete genome, strain B100; xci plasmid pXcaw58: Xanthomonas citri subsp. citri Aw12879 plasmid pXcaw58
Putative genes for type IV secretion system and conjugation
Gene annotation revealed that the virB locus of pXOCgx01 encodes almost the complete set of proteins for the Type IV secretion system (T4SS) [39], precisely the subgroup A of T4SS (T4ASS) which was identified on the Ti plasmid in Agrobacterium tumefaciens [40]. The comparative analysis showed that the distribution of virB loci differed significantly in xanthomonads (Additional file 6). By comparing with the T4ASS from A. tumefaciens, almost none of the xanthomonads have a typical virB7 homolog in their virB loci. Recently, Souza et al. [41] identified that XAC2622 in Xac 306 may have the function of VirB7, although XAC2622 does not exhibit any sequence similarity with the VirB7 family. Thus, we re-annotated the virB genes in xanthomonads (Additional file 6), according to the relevant researches [36, 41].
Two genes, traD and trwC, encoding a coupling protein and a ralaxase respectively, were found to be adjacent to the virB operon (Additional file 2). The TraD protein, sitting at the inner membrane in contact with the assembled pilus and its scaffold as well as the relaxosome-plasmid DNA complex, is supposed to perform an essential coupling function in conjugative type IV secretion systems [42]. TrwC, the relaxase in the relaxosome, is a DNA strand transferase which functions during the conjugative cell to cell DNA transfer. It binds to the origin of transfer (oriT) and melts the double helix [43].
Transposase and Tn3 family transposon
pXOCgx01 harbors two ORFs encoding an imperfect transposase (XOCp0038) and a transposase (XOCp0058, TnpA) with DDE motif respectively. The region including the transposase gene (tnpA) and two recombination genes, namely tnpS and tnpT, was identified as a Tn3-like transposon [44], and was designated as TnXocp1 in plasmid pXOCgx01. The genetic structure of tnpA-tnpS-tnpT can be found in both chromosomes and plasmids of some Gram-negative bacteria [44–46], in which tnpA and tnpS are closely linked. However, the gene composition between tnpS and tnpT is diverse in different bacteria (Fig. 4a), indicating a highly variable region.
Fig. 4.
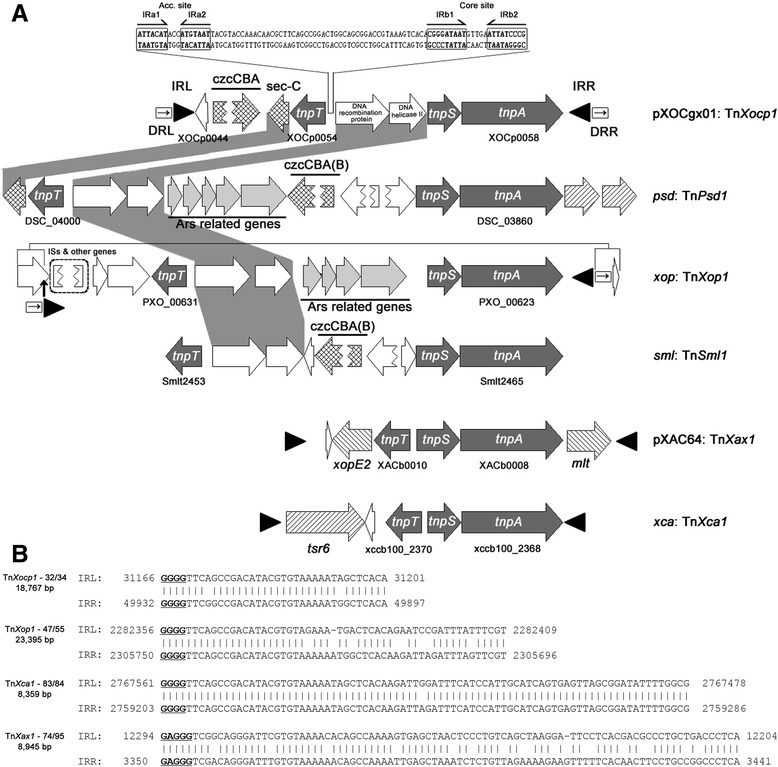
Genetic organizations of the TnXocp1 cassette and IRs sequence analysis. a Genetic organization of the TnXocp1 cassette located in plasmid pXOCgx01 and the alignment with TnXocp1-related structures in other genomes. Genes are indicated by different boxes with the direction of transcription shown by the arrowheads. Three core genes, tnpA, tnpS and tnpT, are shown in dark gray, and passenger genes related to czcCBA cluster in cross stripes, and genes related to arsenic resistance in pale gray, while other passenger genes in diagonal stripes or white boxes. The terminal inverted repeats (IRL and IRR) are shown as black triangles. The terminal direct repeats (DR) are shown as direct arrows in rectangle boxes. No IRs or DRs mean that there are no such sequences in those regions. Two segments with palindromes found in the rst region are supposed to be the Acc. site (17-bp IRa1 and IRa2 segment) for TnpT to bind and the core site (23-bp IRb1 and IRb2 segment) recognized by the TnpS recombinase. b IRs sequences identified from pXOCgx01 and other three tnpA-tnpS-tnpT cassettes. All of them are sharing high sequence similarities to TnXocp1 IRs and begin with GGGG except TnXax1, which begins with GAGG. Abbreviations for genomes with tnpA-tnpS-tnpT cassette: psd, Pseudoxanthomonas spadix BD-a59; xop, Xanthomonas oryzae pv. oryzae PXO99A; sml, Stenotrophomonas maltophilia K279a; pXAC64, Xanthomonas citri pv. citri 306 plasmid pXAC64; xca, Xanthomonas campestris pv. campestris B100
Recently, Ferreira et al. found that Tn3-like transposons, e.g., TnXax1, might play a major role in the spread of pathogenicity determinants [46]. TnXax1 found in plasmid pXAC64 of Xac 306 has a typical structure of Tn3 family structure, and similar genetic organizations were widely distributed in Xanthomonas species [46]. At the left and right ends of the TnXax1-related structures, different T3SE genes like xopE2, xopC or TALE (transcription activator-like effector) genes, and other passenger genes such as mlt, were found. The tnpA-tnpS-tnpT cassette is flanked by IRs (inverted repeats). Certain genes are flanked by the same IRs as that found in TnXax1 to form mobile insertion cassettes (MICs). This may serve as a DNA shuttle vehicle, carrying passenger genes and transmitting horizontally among genomes. Obviously, there are no genes between tnpS and tnpT in TnXax1 and TnXax1-related structures, which represent one type of Tn3-like transposon [46].
In pXOCgx01, we found that TnXocp1 carries passengers not only at left end of tnpT, but also in the intergenic region between tnpS and tnpT where the rst (resolution associated with TnpS and TnpT) region localizes [45]. The tnpS and tnpT promoters and two segments with palindromes, namely the Acc. site (17-bp IRa1 and IRa2 segments) for TnpT to bind and the core site (23-bp IRb1 and IRb2 segments) recognized by the TnpS recombinase were found in the functional rst region, similarly with the transposon Tn4651 described by Yano [45]. Similar structures were also found in X. oryzae pv. oryzae PXO99A, Stenotrophomonas maltophilia K279a, and Pseudoxanthomonas spadix BD-a59 (Fig. 4a), and were designated as TnXop1, TnSml1, and TnPsd1 respectively. In addition to the similar structure of tnpA-tnpS-tnpT, TnXocp1, TnXop1, TnSml1, and TnPsd1 carried two other allelic homologous genes, encoding DNA recombination protein and DNA helicase II, respectively. Moreover, in both plasmid pXOCgx01 and P. spadix strain BD-a59, a gene encoding sec-C metal-binding protein next to tnpT was found, and no other similar structure was found in other genomes. Although the czcCBA locus also exists in TnPsd1 and TnSml1, it differs from the locus in TnXocp1. Genes related to arsenic resistance are in both TnPsd1 and TnXop1 with a high similarity, but a 348-bp long ArsR family transcriptional regulator is found in TnPsd1 but not in TnXop1. The above information suggests that the TnPsd1 organization might represent one kind of ancestral and relatively intact transposons from which TnXocp1, TnXop1 and TnSml1 were formed by rearrangements.
Typical IR sequences were found in TnXocp1, TnXop1, TnXax1 and TnXca1, but not in TnPsd1 and TnSml1. The DR (directed repeat) sequences found in TnXocp1 and TnXop1 may suggest they are new insertions relative to the others. With a length of 18,767 bp, at both flanks of TnXocp1, IR sequences were found as shown in Fig. 4b. They are 34 bp long with 32 bp identical, shorter than that of TnXop1, TnXax1 and TnXca1. Both of TnXocp1 and TnXop1, 5-bp DR sequences outside IRs were found, GGATA for TnXocp1 and AAGGG for TnXop1. But no DR sequences were found flanking TnXax1 and TnXca1, and no IR sequences in TnPsd1 and TnSml1. Not like the TnXax1-like structures carrying passenger genes involved in pathogenicity, the TnXocp1-like structures harbor heavy-metal resistance clusters, which may help the bacteria adapt to the adverse environment with heavy metal stresses.
The TnpA of TnXocp1 shares 41.1 % protein sequence identity (with 98.9 % query coverage) with that of TnXax1, indicating that TnXocp1 may belong to a new type of transposon. We submitted TnXocp1 to the IS Finder database under the given name TnXo19. Genome analysis indicated that there are no Tn3 family transposons in the genome (chromosome) of Xoc BLS256 and draft chromosome of GX01, but there are typical MIC structures in both chromosomes. Whether the TnXocp1 can accelerate the transposition of the pathogenicity determinants in GX01 than those in BLS256 is noteworthy in future.
pXOCgx01 is a conjugative plasmid
Xoc GX01 has been tested to be susceptible to all the commonly used antibiotics in our lab, such as kanamycin, ampicillin, spectinomycin, chloramphenicol, gentamycin and tetracycline. Moreover, no antibiotic resistance genes were found in plasmid pXOCgx01, so an insertional derivative of pXOCgx01, namely pXOCgx01::Tn5, was generated to place a kanamycin resistance marker but without mutating any pXOCgx01 genes. An R6Kγ conditional origin (R6Kγori) was introduced into pXOCgx01 with the insertion of Tn5, so the derivative of plasmid pXOCgx01::Tn5 could replicate in E. coli EC100D pir+, which has a pir gene to express the Π protein. In order to find out whether plasmid pXOCgx01 could replicate in E. coli and Xanthomonas spp. or not, pXOCgx01::Tn5 was introduced into E. coli DH5α, Xoo PXO99A, Xcc 8004 and one plasmidless Xoc isolate by electroporation, and clones were selected on solid medium with kanamycin. The result indicated that pXOCgx01 could replicate in Xanthomonas spp., but not in E. coli DH5α.
Since pXOCgx01 appeared to carry virB locus encoding a T4SS, the transmissibility of the plasmid was investigated. EC100D pir+/pXOCgx01::Tn5 was used as a donor to track conjugal transfer of pXOCgx01 to Xoo PXO99A. Plasmid DNA was isolated from the putative transconjugants by the modified alkaline lysis method and digested with BamHI. Fragment profiles were examined with agarose gel electrophoresis. Plasmid profiles of the transconjugants were the same as that of EC100D pir+/pXOCgx01::Tn5 (data not shown). The pXOCgx01-specific PCR assay was done, and the evidence is shown as Fig. 5. From the plasmid profiles and PCR assay results, pXOCgx01 was experimentally determined to be self-transferable by conjugation, without transfer helper strains, from E. coli EC100D pir+ to Xoo PXO99A, indicating that pXOCgx01 is a conjugative plasmid.
Fig. 5.
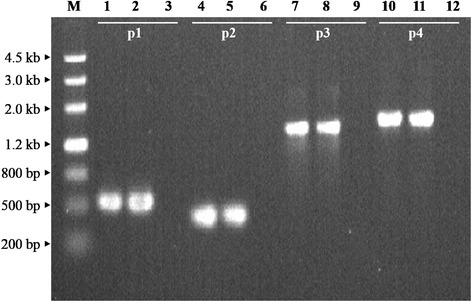
Evidence for conjugative transfer of pXOCgx01::Tn5 from EC100 pir + to Xoo PXO99A. PCR products electrophoresis analysis on 1.2 % agarose gel. M denotes marker III (TianGen). p1, 2, 3 and 4 denote products using primers Tn5-R6K-F/R, pXOC- tra -F/R, pXOC-virF/R and pXOC-res-F/R, respectively. Lane 1, 4, 7, 10, EC100 pir +/pXOCgx01::Tn5 gDNA as a template; lane 2, 5, 8, 11, Xoo PXO99A/pXOC::Tn5 gDNA as a template; lane 3, 6, 9, 12, Xoo PXO99A gDNA as a template
pXOCgx01 enhanced the heavy metal tolerance of Xoo PXO99A
The czcCBA locus on pXOCgx01 contains three Cobalt-zinc-cadmium resistance protein genes czcA, czcB, and czcC, the products of which constitute a membrane-bound protein complex catalyzing an energy-dependent efflux of the three metal cations, Co2+, Zn2+, and Cd2+. The archetype CzcCBA, an RND (resistance-nodulation-division) system for HME (heavy metal efflux), was first reported in plasmid pMOL30 of Cupriavidus metallidurans CH34, consisting of czcCBA and flanking regulatory genes [47]. Phylogenetic analysis showed that the czcCBA locus on pXOCgx01 shared a low sequence similarity against that in pMOL30, but high similarities to those in chromosomes of Stenotrophomonas maltophilia K279a, Dechlorosoma suillum PS, and Pseudomonas aeruginosa PA38182. It is noteworthy that there is one pair of direct repeat sequence at both ends of the pXOCgx01 czcCBA locus, indicating a recent horizontal gene transfer event. The BLAST results showed that the core czcCBA genes are conserved, but their flanking genes are variable in many other bacterial strains, in some cases only czcCBA or czcBA genes remaining (Fig. 6). It is also worth noting that the archetype pMOL30 czc or pXOCgx01 czcCBA ortholog genes are absent from the sequenced genomes of Xoo strains and Xoc BLS256, in which only a czcA gene was annotated, suggesting a series of genes trimming or rinsing during the evolution of czcCBA loci.
Fig. 6.
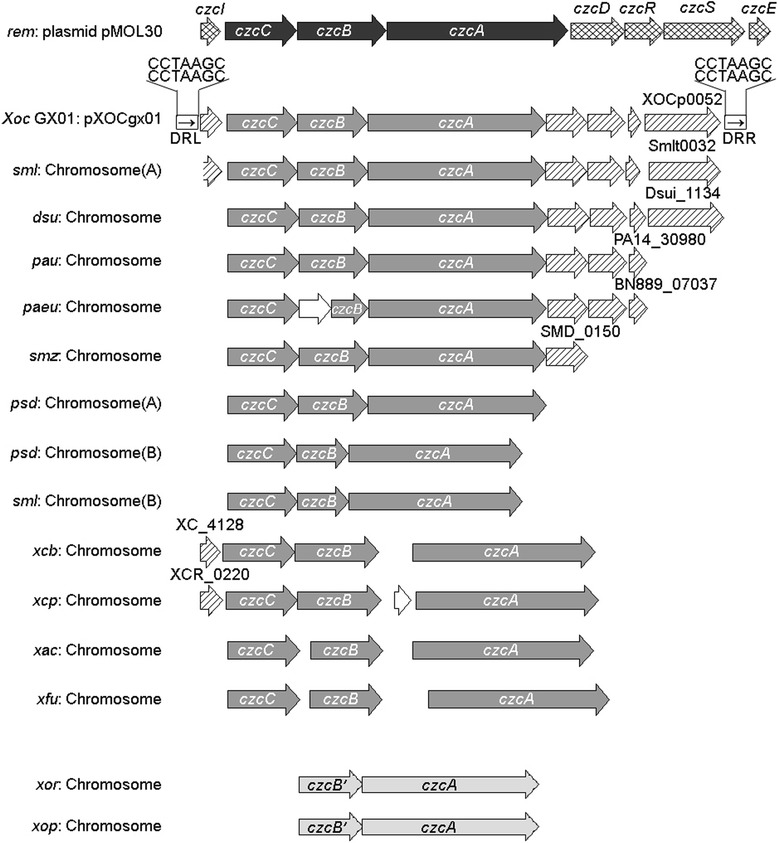
Genetic structure of the czcCBA system located in plasmid pXOCgx01 and alignment with other genomes. The core genes, czcA, czcB and czcC, are shown in gray. Genes of czcCBA cluster on plasmid pMOL30 were filled with different markers from pXOCgx01 denoting that those genes have a low similarity with pXOCgx01. Direct arrows in rectangle boxes at the flanking regions of czcCBA cluster on pXOCgx01 denote direct repeat sequences
To determine the function of pXOCgx01, we tested the strain Xoo PXO99A/pXOCgx01::Tn5 under the stresses of heavy metals and other chemicals. The results showed that the introduction of pXOCgx01::Tn5 into Xoo PXO99A could significantly enhance tolerances to Co2+, Zn2+, Cd2+ and Ni2+ (Fig. 7), but did not influence tolerances to Cu2+, Mn2+, Fe3+, organic phenol, SDS or H2O2, and showed no contribution to the adaptability under high osmotic pressure or different pH levels. Besides, the cell growth curve analysis showed no significant differences between Xoo PXO99A and the derivative harboring plasmid pXOCgx01 (data not shown). These plasmid-related phenotypes, namely tolerances to Co2+, Zn2+, Cd2+ and Ni2+, demonstrated the function of czcCBA locus. The rice field from which Xoc GX01 was isolated is near the Southern China lead-zinc mine districts and the background value of heavy metals is relatively high [48, 49]. The cadmium content in the arable layer (20 cm) of the reclaimed farmland near by the site where GX01 was isolated is 14.93 mg/kg in average (Gui-Rong Wu, personal communication). Thus, it is rational to explain that Xoc GX01 harboring the czcCBA locus on pXOCgx01.
Fig. 7.
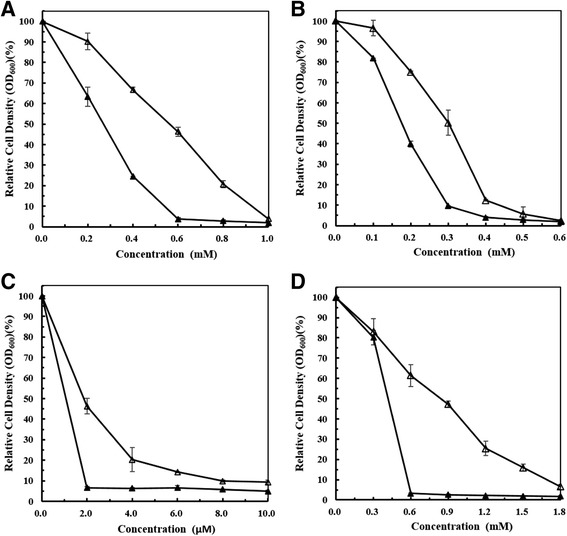
Co2+, Zn2+, Cd2+, Ni2+ tolerance analysis of Xoo PXO99A and the derivative strain in NB medium. ▲, wild-type strain Xoo PXO99A; ∆, strain Xoo PXO99A harboring pXOCgx01::Tn5. a CoCl2 tolerance analysis; b ZnSO4 tolerance analysis; c CdSO4 tolerance analysis; d NiCl2 tolerance analysis. Strains were inoculated into 10 mL NB medium with a final concentration of OD600 = 0.01, and incubated at 28 °C with shaking at 200 rpm. Bacterial growth was determined by measuring OD600 22 h after inoculation. Relative cell density means the percentage of each strain’s cell density at different heavy metal concentrations versus the cell density when no heavy metal added. Data presented are from a representative experiment; similar results were obtained in other two independent experiments
Curing of plasmid pXOCgx01 from Xoc GX01
To get a pXOCgx01-free derivative of strain GX01, all kinds of attempts, including elevated temperatures, the SDS-elevated temperature-crossed method, acridine orange stress tests, electrocompetent cells electric pulse tests, plasmid incompatibility tests (pLAFR6, pPH1JI, pBBad18K, pUFR034, pBBR1MSC-5), were adopted to cure plasmid pXOCgx01. By PCR assay and plasmid-isolation detection, no pXOCgx01-free derivatives were gotten. The putative origin linked with the EZ-Tn5™ < R6Kγori/KAN-2 > transposon was introduced into the wild-type strain GX01 by electroporation, but interestingly, the recombinant plasmid could coexist with pXOCgx01 in strain GX01 and showed no incompatibility. The failure to cure plasmids from their hosts is also found in X. arboricola pv. pruni CFBP 5530 harboring plasmid pXap41 [12]. The results may indicate a stability relationship between GX01 and the recalcitrant plasmid pXOCgx01, not just for surviving, but also for advantages in environmental adaptations.
Survey of plasmids in Xoc Chinese isolates
To examine the distribution of indigenous plasmids in Xoc Chinese isolates, we tested up to 257 Xoc strains isolated from Anhui (n = 79), Fujian (n = 13), Guangxi (n = 75), Guangdong (n = 22), Hainan (n = 58), Jiangsu (n = 1), Yunnan (n = 1) and Zhejiang (n = 8) provinces, using both the conventional plasmid isolation method and the kit method. Isolated plasmid DNA samples were digested with different restriction endonucleases and followed by agarose gel electrophoresis detections. We found that nine different isolates, of which seven isolated from Guangxi Province, one from Guangdong Province and one from Hainan Province, harbor at least one plasmid in each strain according to their plasmid profiles. The plasmid survey suggested that the presence of pXOCgx01 in Xoc GX01 was not an individual event and it is worthwhile studying more about plasmid functions in Xoc.
Discussion
Although plasmids have been considered to be widespread in bacteria, there is no any report about plasmids in Xoc strains. In this paper we reported an indigenous plasmid pXOCgx01 from a Xoc strain isolated from the central area of South China rice growing region, one of the putative cradles of rice Oryza sativa [26]. The plasmid, comprising six regions with distinct origins, has a chimeric structure, indicating that the generation of pXOCgx01 might be a result of multiple DNA rearrangement events. pXOCgx01 has been demonstrated to be a conjugative plasmid, displaying the biological function of the virB locus. The introduction of pXOCgx01 to Xoo PXO99A did significantly promote tolerances to certain heavy metals, but did not enhance the virulence. A plasmid survey indicated that at least 9 different plasmids exist in our Xoc Chinese isolates. As a model system for studying Xoc-rice interaction [1], it is of importance to assess the impact of indigenous plasmids of these phytopathogenic bacteria on metabolism, persistence and pathogenicity.
The virB locus in pXOCgx01 encodes a type IVA system (T4ASS). It has been proposed that T4SSs were evolved from preexisting sources, first into conjugation systems, and later into virulence systems [50]. Our results indicated that the pXOCgx01 T4SS might mediate the plasmid transferring, but not virulence. Similar results also obtained in other Xanthomonas species. El Yacoubi et al. [17] presented evidence that in planta the transfer of a 37 kb plasmid (pXcB) from X. aurantifolii to X. citri could occur via T4SS. In Xcc 8004, the T4SS deletion mutant displayed the same virulence as the wild type strain [51]. Recent studies showed that the T4SS is not induced during the infection process in X. citri, and is not involved in infection process in citrus, but may be very important in cell-to-cell communication [52]. These results at least suggested that T4SS is not the determinant of pathogenicity in some xanthomonads, unlike the T4SS in A. tumefaciens, Helicobacter pylori, and Legionella pneumophila [53].
Plasmid pXOCgx01 has a mosaic structure both on gene context and gene contents, showing a high recombination and heterogeneity. Transposases or recombinases catalyze DNA transpositions, which are involved in DNA rearrangements and horizontal gene transfers. pXOCgx01 harbors a tnpA-tnpS-tnpT locus which was found to be widely distributed in prokaryotic world, such as the important lab strain Xoo PXO99A, but absent from the American strains [54]. In some pathogenic bacteria, this kind of loci was found to be closely linked to virulence genes and avirulence genes, suggesting the pathogenic potential of Tn5044 [44]. Unlike TnXax1 in pXAC64 [46], TnXocp1 in plasmid pXOCgx01 carries no pathogenicity genes but genes involved in heavy metal tolerance.
Transition metals are essential micronutrients for healthy immune function for all living organisms, but a high level of these metals will potentiate toxicity to organisms, so transition metal ion homeostasis must be carefully regulated. To compete for limited metals and simultaneously to prevent metal toxicity within the host, pathogens have developed a series of metal regulatory, acquisition, and efflux systems [55]. Plasmid-mediated detoxification or resistance is always one of the major concerns in modern microbiology. Although plasmid pXOCgx01 were susceptible to the commonly used antibiotics tested in our lab, the introduction of pXOCgx01 into Xoo PXO99A could significantly increase the tolerance to heavy metals, indicating the function of czcCBA genes. The high heavy metal concentration of the region, from where Xoc GX01 was isolated, may provide a communal gene pool for strains [56] to adopt a CzcCBA-encoding plasmid for selective advantages.
Conclusions
In this study, we reported the identification and analysis of an indigenous plasmid, pXOCgx01, from Xoc GX01. pXOCgx01 is the first indigenous plasmid from Xoc, and the first completely sequenced plasmid from Xanthomonas oryzae species. pXOCgx01 has been demonstrated being a conjugative plasmid and can significantly enhance the tolerance of Xoo PXO99A to heavy metals. The virB genes, czc genes and the mobile insertion cassette on the pXOCgx01 are absent from other sequenced Xanthomonas oryzae strains. These results may be helpful for elucidation of adaptive evolution of Xoc.
Acknowledgment
This work was supported by the grants from the National Basic Research (973) Program of China (No. 2011CB100701), the National Natural Science Foundation of China (No. 31270139), and the Innovation Project of Guangxi Graduate Education (No. GXU11T31080).
Abbreviations
- Xoc
Xanthomonas oryzae pathovar oryzicola
- Xoo
Xanthomonas oryzae pathovar oryzae
- Xcc
Xanthomonas campestris pathovars campetris
- BLS
Bacterial leaf streak
- ORFs
Open reading frames
- IRs
Inverted repeats
- rst
Resolution associated with TnpS and TnpT
- DR
Directed repeat
- RND
Resistance-nodulation-division
- HME
Heavy metal efflux
- COG
Clusters of orthologous groups
- CDD
Conserved domain database
- TALEs
Transcription activator-like effectors
Additional files
Putative ORFs of pXOCgx01. (XLSX 22 kb)
The phylogenetic tree analysis of the whole sequence of plasmid pXOCgx01 with other plasmids. (JPEG 2166 kb)
The BLASTN alignment of plasmid pXOCgx01 with other whole plasmid and genome sequences. (TIFF 3082 kb)
Specific locations in corresponding genomes used in the phylogenetic tree. (DOCX 18 kb)
The comparison of type IVA genes of Xanthomonas with whole genome sequenced. (DOCX 19 kb)
Primers used in this study. (DOCX 14 kb)
Footnotes
Competing interests
The authors declare no conflicts of interest.
Authors’ contributions
YQH, JLT and XNN conceived and designed all of the study. XNN carried out the experiments, data analyses and drafted the manuscript. ZQW, HFZ, FW and KJL helped in plasmid sequencing, screening and curing and data analyses. GGX provided technical assistance in bioinformatics analyses. WJ helped in the study design and study implementation. YQH and XNN wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xiang-Na Niu, Email: niuxiangna@gmail.com.
Zhi-Qiong Wei, Email: 1148923010@qq.com.
Hai-Fan Zou, Email: 379382978@qq.com.
Gui-Gang Xie, Email: xie.guigang@gmail.com.
Feng Wu, Email: 895913834@qq.com.
Kang-Jia Li, Email: 1422886246@qq.com.
Wei Jiang, Email: weijiang@gxu.edu.cn.
Ji-Liang Tang, Phone: 867713239215, Email: jltang@gxu.edu.cn.
Yong-Qiang He, Phone: 867713239215, Email: yh634@cornell.edu.
References
- 1.Niño-Liu DO, Ronald PC, Bogdanove AJ. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol. 2006;7(5):303–24. doi: 10.1111/j.1364-3703.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 2.Tang D, Wu W, Li W, Lu H, Worland AJ. Mapping of QTLs conferring resistance to bacterial leaf streak in rice. Theor Appl Genet. 2000;101(1–2):286–91. doi: 10.1007/s001220051481. [DOI] [Google Scholar]
- 3.Zhao S, Poulin L, Rodriguez RL, Serna NF, Liu SY, Wonni I, et al. Development of a variable number of tandem repeats typing scheme for the bacterial rice pathogen Xanthomonas oryzae pv. oryzicola. Phytopathology. 2012;102(10):948–56. doi: 10.1094/PHYTO-04-12-0078-R. [DOI] [PubMed] [Google Scholar]
- 4.Wonni I, Cottyn B, Detemmerman L, Dao S, Ouedraogo L, Sarra S, et al. Analysis of Xanthomonas oryzae pv. oryzicola population in Mali and Burkina Faso reveals a high level of genetic and pathogenic diversity. Phytopathology. 2014;104(5):520–31. doi: 10.1094/PHYTO-07-13-0213-R. [DOI] [PubMed] [Google Scholar]
- 5.Kado CI. Origin and evolution of plasmids. Anton Leeuw Int J G. 1998;73(1):117–26. doi: 10.1023/A:1000652513822. [DOI] [PubMed] [Google Scholar]
- 6.Frost LS, Leplae R, Summers AO, Toussaint A. Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol. 2005;3(9):722–32. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- 7.Sundin GW. Genomic insights into the contribution of phytopathogenic bacterial plasmids to the evolutionary history of their hosts. Annu Rev Phytopathol. 2007;45:129–51. doi: 10.1146/annurev.phyto.45.062806.094317. [DOI] [PubMed] [Google Scholar]
- 8.Lazo GR, Gabriel DW. Conservation of plasmid DNA sequences and pathovar identification of strains of Xanthomonas campestris. Phytopathology. 1987;77:448–53. doi: 10.1094/Phyto-77-448. [DOI] [Google Scholar]
- 9.Xu G-W, Gonzalez CF. Plasmid, genomic, and bacteriocin diversity in U.S. strains of Xanthomonas campestris pv. oryzae. Phytopathology. 1991;81:628–31. doi: 10.1094/Phyto-81-628. [DOI] [Google Scholar]
- 10.Amuthan G, Mahadevan A. Plasmid and pathogenicity in Xanthomonas oryzae pathovar oryzae, the bacterial blight pathogen of Oryza sativa. J Appl Bacteriol. 1994;76(6):529–38. doi: 10.1111/j.1365-2672.1994.tb01649.x. [DOI] [Google Scholar]
- 11.Vivian A, Murillo J, Jackson RW. The roles of plasmids in phytopathogenic bacteria: mobile arsenals? Microbiology. 2001;147(Pt 4):763–80. doi: 10.1099/00221287-147-4-763. [DOI] [PubMed] [Google Scholar]
- 12.Pothier JF, Vorhölter FJ, Blom J, Goesmann A, Pühler A, Smits THM, et al. The ubiquitous plasmid pXap41 in the invasive phytopathogen Xanthomonas arboricola pv. pruni: complete sequence and comparative genomic analysis. FEMS Microbiol Lett. 2011;323(1):52–60. doi: 10.1111/j.1574-6968.2011.02352.x. [DOI] [PubMed] [Google Scholar]
- 13.Weng SF, Fan YF, Tseng YH, Lin JW. Sequence analysis of the small cryptic Xanthomonas campestris pv. vesicatoria plasmid pXV64 encoding a Rep protein similar to gene II protein of phage 12–2. Biochem Biophys Res Commun. 1997;231(1):121–5. doi: 10.1006/bbrc.1997.6058. [DOI] [PubMed] [Google Scholar]
- 14.Darrasse A, Carrere S, Barbe V, Boureau T, Arrieta-Ortiz ML, Bonneau S, et al. Genome sequence of Xanthomonas fuscans subsp. fuscans strain 4834-R reveals that flagellar motility is not a general feature of xanthomonads. BMC Genomics. 2013;14:761. doi: 10.1186/1471-2164-14-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva AC, Ferro JA, Reinach FC, Farah CS, Furlan LR, Quaggio RB, et al. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature. 2002;417(6887):459–63. doi: 10.1038/417459a. [DOI] [PubMed] [Google Scholar]
- 16.Kim JG, Choi S, Oh J, Moon JS, Hwang I. Comparative analysis of three indigenous plasmids from Xanthomonas axonopodis pv. glycines. Plasmid. 2006;56(2):79–87. doi: 10.1016/j.plasmid.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.El Yacoubi B, Brunings AM, Yuan Q, Shankar S, Gabriel DW. In planta horizontal transfer of a major pathogenicity effector gene. Appl Environ Microbiol. 2007;73(5):1612–21. doi: 10.1128/AEM.00261-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thieme F, Koebnik R, Bekel T, Berger C, Boch J, Buttner D, et al. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol. 2005;187(21):7254–66. doi: 10.1128/JB.187.21.7254-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieretti I, Royer M, Barbe V, Carrere S, Koebnik R, Cociancich S, et al. The complete genome sequence of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae. BMC Genomics. 2009;10:616. doi: 10.1186/1471-2164-10-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez C, Szurek B, Manceau C, Mathieu T, Sere Y, Verdier V. Molecular and pathotypic characterization of new Xanthomonas oryzae strains from West Africa. Mol Plant Microbe Interact. 2007;20(5):534–46. doi: 10.1094/MPMI-20-5-0534. [DOI] [PubMed] [Google Scholar]
- 21.Ji ZY, Zakria M, Zou LF, Xiong L, Li Z, Ji GH, et al. Genetic diversity of transcriptional activator-like effector genes in Chinese isolates of Xanthomonas oryzae pv. oryzicola. Phytopathology. 2014;104(7):672–82. doi: 10.1094/PHYTO-08-13-0232-R. [DOI] [PubMed] [Google Scholar]
- 22.Raymundo AK, Briones AM, Ardales EY, Perez MT, Fernandez LC, Leach JE, et al. Analysis of DNA polymorphism and virulence in Philippine strains of Xanthomonas oryzae pv. oryzicola. Plant Dis. 1999;83(5):434–40. doi: 10.1094/PDIS.1999.83.5.434. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Qian G, Yin F, Fan J, Zhai Z, Liu C, et al. Proteomic analysis of the regulatory function of DSF-dependent quorum sensing in Xanthomonas oryzae pv. oryzicola. Microb Pathog. 2011;50(1):48–55. doi: 10.1016/j.micpath.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanove AJ, Koebnik R, Lu H, Furutani A, Angiuoli SV, Patil PB, et al. Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J Bacteriol. 2011;193(19):5450–64. doi: 10.1128/JB.05262-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao YS, Wei XX, Gao HP, Niu XN, Cen ZL, Huang P, et al. Characterization of a Xanthomonas oryzae pv. oryzicola strain and the establishment of its genetic manipulation system (In Chinese) Genomics Appl Biol. 2011;30:1211–7. [Google Scholar]
- 26.Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, et al. A map of rice genome variation reveals the origin of cultivated rice. Nature. 2012;490(7421):497–501. doi: 10.1038/nature11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniels MJ, Barber CE, Turner PC, Sawczyc MK, Byrde RJ, Fielding AH. Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 1984;3(13):3323–8. doi: 10.1002/j.1460-2075.1984.tb02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Sullivan DJ, Klaenhammer TR. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol. 1993;59(8):2730–3. doi: 10.1128/aem.59.8.2730-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23(6):673–9. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besemer J, Lomsadze A, Borodovsky M. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001;29(12):2607–18. doi: 10.1093/nar/29.12.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Gao S, Lercher MJ, Hu S, Chen WH. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 2012;40(Web Server issue):W569–72. doi: 10.1093/nar/gks576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlin S, Campbell AM, Mrazek J. Comparative DNA analysis across diverse genomes. Annu Rev Genet. 1998;32:185–225. doi: 10.1146/annurev.genet.32.1.185. [DOI] [PubMed] [Google Scholar]
- 35.Stockwell VO, Davis EW, 2nd, Carey A, Shaffer BT, Mavrodi DV, Hassan KA, et al. pA506, a conjugative plasmid of the plant epiphyte Pseudomonas fluorescens A506. Appl Environ Microbiol. 2013;79(17):5272–82. doi: 10.1128/AEM.01354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alegria MC, Souza DP, Andrade MO, Docena C, Khater L, Ramos CHI, et al. Identification of new protein-protein interactions involving the products of the chromosome- and plasmid-encoded type IV secretion loci of the phytopathogen Xanthomonas axonopodis pv. citri. J Bacteriol. 2005;187(7):2315–25. doi: 10.1128/JB.187.7.2315-2325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang YN, Yuan QP, Gabriel DW. Watersoaking function(s) of XcmH1005 are redundantly encoded by members of the Xanthomonas avr/pth gene family. Mol Plant Microbe Interact. 1996;9(2):105–13. doi: 10.1094/MPMI-9-0105. [DOI] [Google Scholar]
- 38.Intorne AC, de Oliveira MV, de M Pereira L, de Souza Filho GA. Essential role of the czc determinant for cadmium, cobalt and zinc resistance in Gluconacetobacter diazotrophicus PAl 5. Int Microbiol. 2012;15(2):69–78. doi: 10.2436/20.1501.01.160. [DOI] [PubMed] [Google Scholar]
- 39.Christie PJ, Vogel JP. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 2000;8(8):354–60. doi: 10.1016/S0966-842X(00)01792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron C, Llosa M, Zhou S, Zambryski PC. VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens, is processed to a C-terminal secreted product, VirB1. J Bacteriol. 1997;179(4):1203–10. doi: 10.1128/jb.179.4.1203-1210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souza DP, Andrade MO, Alvarez-Martinez CE, Arantes GM, Farah CS, Salinas RK. A component of the Xanthomonadaceae type IV secretion system combines a VirB7 motif with a N0 domain found in outer membrane transport proteins. PLoS Pathog. 2011;7(5):e1002031. doi: 10.1371/journal.ppat.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beranek A, Zettl M, Lorenzoni K, Schauer A, Manhart M, Koraimann G. Thirty-eight C-terminal amino acids of the coupling protein TraD of the F-like conjugative resistance plasmid R1 are required and sufficient to confer binding to the substrate selector protein TraM. J Bacteriol. 2004;186(20):6999–7006. doi: 10.1128/JB.186.20.6999-7006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cesar CE, Llosa M. TrwC-mediated site-specific recombination is controlled by host factors altering local DNA topology. J Bacteriol. 2007;189(24):9037–43. doi: 10.1128/JB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kholodii G, Yurieva O, Mindlin S, Gorlenko Z, Rybochkin V, Nikiforov V. Tn5044, a novel Tn3 family transposon coding for temperature-sensitive mercury resistance. Res Microbiol. 2000;151(4):291–302. doi: 10.1016/S0923-2508(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 45.Yano H, Genka H, Ohtsubo Y, Nagata Y, Top EM, Tsuda M. Cointegrate-resolution of toluene-catabolic transposon Tn4651: determination of crossover site and the segment required for full resolution activity. Plasmid. 2013;69(1):24–35. doi: 10.1016/j.plasmid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira RM, de Oliveira AC, Moreira LM, Belasque J, Jr, Gourbeyre E, Siguier P, et al. A TALE of transposition: Tn3-like transposons play a major role in the spread of pathogenicity determinants of Xanthomonas citri and other xanthomonads. MBio. 2015;6(1):e02505–14. doi: 10.1128/mBio.02505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nies DH, Nies A, Chu L, Silver S. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc Natl Acad Sci U S A. 1989;86(19):7351–5. doi: 10.1073/pnas.86.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Probst A, Liao B. Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China) Sci Total Environ. 2005;339(1–3):153–66. doi: 10.1016/j.scitotenv.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang P, Lu H, Li Z, Zou B, McBride MB. Multiple exposure and effects assessment of heavy metals in the population near mining area in South China. PLoS One. 2014;9(4):e94484. doi: 10.1371/journal.pone.0094484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao TB, Saier MH. Conjugal type IV macromolecular transfer systems of Gram-negative bacteria: organismal distribution, structural constraints and evolutionary conclusions. Microbiology. 2001;147(Pt 12):3201–14. doi: 10.1099/00221287-147-12-3201. [DOI] [PubMed] [Google Scholar]
- 51.He YQ, Zhang L, Jiang BL, Zhang ZC, Xu RQ, Tang DJ, et al. Comparative and functional genomics reveals genetic diversity and determinants of host specificity among reference strains and a large collection of Chinese isolates of the phytopathogen Xanthomonas campestris pv. campestris. Genome Biol. 2007;8(10):R218. doi: 10.1186/gb-2007-8-10-r218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacob TR, de Laia ML, Moreira LM, Goncalves JF, Carvalho FM, Ferro MI, et al. Type IV secretion system is not involved in infection process in citrus. Int J Microbiol. 2014;2014:763575. doi: 10.1155/2014/763575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallden K, Rivera-Calzada A, Waksman G. Type IV secretion systems: versatility and diversity in function. Cell Microbiol. 2010;12(9):1203–12. doi: 10.1111/j.1462-5822.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Triplett LR, Hamilton JP, Buell CR, Tisserat NA, Verdier V, Zink F, et al. Genomic analysis of Xanthomonas oryzae isolates from rice grown in the United States reveals substantial divergence from known X. oryzae pathovars. Appl Environ Microbiol. 2011;77(12):3930–7. doi: 10.1128/AEM.00028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–56. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norman A, Hansen LH, Sorensen SJ. Conjugative plasmids: vessels of the communal gene pool. Philos Trans R Soc Lond B Biol Sci. 2009;364(1527):2275–89. doi: 10.1098/rstb.2009.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian W, Jia Y, Ren SX, He YQ, Feng JX, Lu LF, et al. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res. 2005;15(6):757–67. doi: 10.1101/gr.3378705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salzberg SL, Sommer DD, Schatz MC, Phillippy AM, Rabinowicz PD, Tsuge S, et al. Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics. 2008;9:204. doi: 10.1186/1471-2164-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]


