Abstract
VopK, a type III effector protein, has been implicated in the pathogenesis of Vibrio cholerae strains belonging to diverse serogroups. Ectopic expression of this protein exhibits strong toxicity in yeast model system. In order to map critical residues in VopK, we scanned the primary sequence guided by available data on various toxins and effector proteins. Our in silico analysis of VopK indicated the presence of predicted MCF1-SHE (SHxxxE) serine peptidase domain at the C-terminus region of the protein. Substitution of each of the predicted catalytic triad residues namely Ser314, His353 and Glu357 with alanine resulted in recombinant VopK proteins varying in lethality as evaluated in yeast model system. We observed that replacement of glutamate357 to alanine causes complete loss in toxicity while substitutions of serine314 and histidine353 with alanine exhibited partial loss in toxicity without affecting the stability of variants. In addition, replacement of another conserved serine residue at position 354 (S354) within predicted S314H353E357 did not affect toxicity of VopK. In essence, combined in silico and site directed mutagenesis, we have identified critical amino acids contributing to the lethal activity of VopK in yeast model system.
Introduction
Vibiro cholerae, causative agent of the life threatening diarrheal disease, cholera, possesses a multitude of virulence associated factors whose collective action aids the organism in becoming a successful pathogen. Of late, the type III secretion system (T3SS) and several effector proteins which are delivered by this specialized transport machinery have been implicated in the pathogenesis of Vibrio cholerae strains belonging to diverse serogroups [1]. In recent years, much progress has been made in characterizing various T3SS effector proteins of Vibrio cholerae. Of these, VopF has received much of the attention. By employing yeast, tissue culture and animal model systems, many crucial facts on the structural and functional aspects of VopF have been garnered [2–7]. In addition to VopF, the existence of additional 11 effector proteins has been revealed in the T3SS island of Vibrio cholerae strain AM-19226 [5]. Of these, VopX and VopK exhibit strong lethality in yeast model system [5]. Using yeast model system, VopX has been shown to interact with components of the cell wall integrity mitogen-activated protein kinase (MAPK) pathway. Interestingly, VopK (locus number A33_1699) shares homology with Mcf (makes caterpillars floppy), an insecticidal toxin of Photorhabdus luminescens and it is also known as McfV [8].
In recent years, T3SS and effector proteins (T3Es) delivered by such export machinery directly into host cytosol have consumed much attention. As a consequence, a great deal of work has been attributed to gain structural and functional insights of numerous effector proteins. There is now compelling evidence how evolution has incorporated eukaryotic motif and domains to many effector proteins and toxins [9, 10], thereby enabling pathogens to usurp host cellular machinery by employing such specialized virulence factors.
Until now, limited information is available in the literature about the structural and functional aspects of VopK. In this work, we wished to investigate the presence of a functional unit and evaluate its contribution to the toxic activity of VopK. VopK shows some homology (29% identity, 45% positives) with the 136-amino acid region preceding the BH3 domain in Mcf, an insecticidal toxin [8]. Recently, Aravind and co-workers have identified a novel serine peptidase catalytic centre otherwise known as MCF1-SHE domain in Mcf toxin [9]. As illustrated, the MCF1-SHE catalytic core comprises two kinked helices, which are associated with a conserved serine and a HxxxE motif [9]. Incidentally, this domain is frequently observed at the extreme C-terminus of many polymorphic toxins (e.g entomotoxins from Pseudomonas fluorescens) as well as secreted proteins from various intracellular and extracelluar pathogens, further potentiating its role in the toxic activity of such toxins [9, 11]. Armed with the available information, we scanned the primary sequence of VopK. Our in-silico analyses predicted a MCF1-SHE domain and catalytic triad residues (Ser314/His353/Glu357) in VopK. In addition, we also identified another conserved serine residue at position 354 within the predicted SHE (Ser314/His353/Glu357) domain. Combined site-directed mutagenesis and yeast model system helped us to evaluate the contribution of these residues within the predicted MCF1-SHE domain of VopK where substitution of glutamate with alanine at position 357 (Glu357Ala) completely jeopardized the function whereas substitutions of serine and histidine at positions 314 and 353 respectively resulted a partial loss of toxicity of this effector protein. Replacement of serine at position 354 with alanine did not affect the functionality of VopK.
Materials and Methods
Bacterial strains and media
The strains and plasmids used in this study are listed in Table 1. Escherichia coli Nova blue (Novagen) was used for general cloning purpose. The E. coli strain was propagated at 37°C in liquid with agitation or on solid (1.5% agar) in Luria Broth unless mentioned otherwise. Escherichia coli BL21 (DE3) (Novagen) was used for the over expression of protein. Saccharomyces cerevisiae strain BY4741 (MATa; his3Δ 1; leu2Δ 0; met15Δ 0; ura3Δ 0) used in this study (Table 1) was grown in YPD (1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) glucose) broth or agar (2%).
Table 1. Strains and Plasmids used in this study.
| Strains/Plasmids | Genotype/Description | Source |
|---|---|---|
| E. coli Strains | ||
| BL21 (DE3) | E.coli B, F - ampT lon, with a λ prophage carrying the T7 RNA polymerase | Novagen |
| NovaBlue | E. coli K-12, recA endA, lacIq,lacY | Novagen |
| Saccharomyces cerevisiae strains | ||
| BY4741 | MATa; his3Δ 1; leu2Δ 0; met15Δ 0; ura3Δ 0 | This study |
| BY4741—LEU | BY4741 + pESC-LEU vector, leu2 selection | This study |
| BY4741—LEU-VopK | BY4741 + pESC-LEU-VopK, LEU2 selection | This study |
| BY4741—LEU-VopKS314A | BY4741 + pESC-LEU-VopKS314A, LEU2 selection | This study |
| BY4741—LEU-VopK H353A | BY4741 + pESC-LEU-VopKH353A, LEU2 selection | This study |
| BY4741—LEU-VopK E357A | BY4741 + pESC-LEU-VopKE357A, LEU2 selection | This study |
| BY4741—H10 | BY4741 + pGMH10 vector, HIS3 selection | This study |
| BY4741—H10—VopK | BY4741 + pGMH10—VopK, HIS3 selection | This study |
| BY4741—H10—VopKS314A | BY4741 + pGMH10—VopKS314A, HIS3 selection | This study |
| BY4741—H10—VopKH353A | BY4741 + pGMH10—VopKH353A, HIS3 selection | This study |
| BY4741—H10—VopKE357A | BY4741 + pGMH10—VopKE357A, HIS3 selection | This study |
| BY4741—H10—VopKS354A | BY4741 + pGMH10—VopKS354A, HIS3 selection | This study |
| Plasmids | ||
| pESC-LEU | LEU2 selection | Agilent Technologies |
| pGMH10 | HIS3 selection | RIKEN |
| pET15b | Apr, N-terminal 6His-tag expression vector | Novagen |
| pESC-LEU-VopK | 1.275 kb VopK gene fragment amplified from genomic DNA with XhoI—NheI VopK primers by PCR and cloned into pESC—LEU which was digested with same restriction enzymes | This study |
| pGMH10-VopK | 1.275 kb VopK gene fragment amplified from genomic DNA with BamHI-SmaI VopK primers by PCR and cloned into pGMH10 which was digested with same restriction enzymes | This study |
| pESC-LEU-VopKS314A | VopK harboring A in place of S at position 314 | This study |
| pESCL-EU-VopK H353A | VopK harboring A in place of H at position 353 | This study |
| pESC-LEU-VopK E357A | VopK harboring A in place of E at position 357 | This study |
| pGMH10-VopKS314A | VopK harboring A in place of S at position 314 | This study |
| pGMH10-VopKH353A | VopK harboring A in place of H at position 353 | This study |
| pGMH10-VopKE357A | VopK harboring A in place of E at position 357 | This study |
| pGMH10-VopKS354A | VopK harboring A in place of S at position 354 | This study |
Construction of strains and alanine substitution mutagenesis of putative SHE catalytic triad of VopK
The vopK gene was amplified from the genomic DNA of Vibrio cholerae strain SC110 using the primers XhoI VopK and NheI VopK. After verifying the fragment by sequencing, it was cloned into the XhoI /NheI sites of pESC-LEU (Agilent Technologies) under the control of the GAL1 promoter. According to manufacturer’s guidelines, ORF cloned in this vector will be expressed as a tagged protein with an N-terminal Myc-tag. Positive clones were further confirmed by sequencing and maintained in E. coli strain NovaBlue cells. The selected construct, designated as pESC-LEU-VopK, was transformed into S. cerevisiae strain BY4741. The resultant strain was marked as BY4741- LEU-VopK.
The recombinant plasmid pESC-LEU-VopK was used as a template for alanine mutagenesis reactions. The alanine substitution of S314H353E357 residues was performed using Stratagene one step mutagenesis kit. All the constructs were sequenced in their entirety to confirm the clones and the desired mutations at the corresponding positions. The defined plasmids were further transformed into BY4741 to generate a series of recombinant S. cerevisiae strains (Table 1). In addition, we have also cloned wild type VopK and alanine variants of S314H353E357S354 in BamHI /SmaI sites of pGMH10, low copy number vector (RIKEN) under the control of the GAL1 promoter (Table 1). According to the manufacturer’s guidelines, ORF cloned in this vector will also be expressed as a tagged protein with a C-terminal Myc-tag. Positive clones were further confirmed by sequencing and maintained in E. coli strain NovaBlue cells. The desired construct, designated as pGMH10-VopK, was retransformed into S. cerevisiae strain BY4741. The resultant strain was marked as BY4741-H10-VopK.
Purification and proteolytic assay of VopK
VopK protein was purified by Ni2+-nitrilotriacetic acid (Ni2+-NTA) chromatography by following a published protocol [12]. The wild-type gene was cloned into the NdeI-BamHI site of the pET15b vector (Novagen) to generate an N-terminal His6-VopK fusion protein. The clone was confirmed by sequencing and transformed into E. coli BL21 (DE3). After induction with 0.4mM IPTG (isopropyl1-thio-β-D-galactopyranoside), VopK protein was purified through Qiagen Ni2+-NTA column. The purified protein was dialyzed overnight in a solution of buffer A containing 10mM Tris, pH 7.9, 100 mM KCl, 0.1mM EDTA, 0.1mM DTT, 5% glycerol. Protease assay was done essentially as described earlier [13].
Yeast growth assay
The wild type and the corresponding alanine derivatives of S314H353E357S354 residues of VopK were transformed into S. cerevisiae strain BY4741. The transformed yeast strains (Table 1) were grown in synthetic selective medium (SC) containing 0.17% (w/v) yeast nitrogen base without amino acids, 0.5% (w/v) ammonium sulphate, 2% (w/v) glucose and was supplemented with appropriate amino acids and nucleic acid bases. SCGal and SCRaf were SC media containing 2% (w/v) galactose or raffinose, respectively instead of glucose. All the recombinant yeast strains were subjected to galactose induction experiment essentially as described previously [4] with modification [4, 14]. Briefly, overnight cultures of all recombinant strains in SCRaf medium were diluted and grown again in SCRaf medium at 30°C to exponential phase till OD600 was 0.8–1.0. Effect of the expression of VopK and alanine derivatives on the growth of S. cerevisiae BY4741 was examined by spotting equal number of cells onto SC and SCGal plates lacking the corresponding auxotrophic markers to maintain the plasmids. Growth was monitored after 60-70h at 30°C and photographed accordingly. Liquid growth assay was done as described earlier [7, 15]. Briefly, overnight cultures of recombinant yeast strains harboring wild type and alanine variants of VopK grown in raffinose were diluted back to an OD600 of 0.05 in 25 ml of indicated media with appropriate amino acids supplementation. The absorbance was measured at 600nm for stipulated period.
Preparation of Yeast Extracts and Immunoblot Analysis
The immunoblot analysis was essentially done by adopting a published protocol [7]. All recombinant yeast cells were grown in SCRaf medium at 30°C till mid-log phase. The cultures were then diluted directly in induction media (SCGal). After 6 hours of induction, cultures were pelleted in a refrigerated centrifuge. Cells were lysed in 150μl of breaking buffer (50mM Tris-HCl (pH 7.5), 10% glycerol, 1% Triton X-100, 0.1% SDS, 150mM NaCl, 50mM NaF, 1mM Sodium Orthovandate, 50mM β-Glycerol phosphate, 5mM EDTA, 1mM phenylmethylsulfonylfluoride and the 1X protease inhibitor cocktail by vigorous vortex with 0.3-mm glass beads (Sigma)). Cell extracts were separated from glass beads and cell debris, collected in a new centrifuge tube by centrifugation, and further clarified by a 13,000 x g spin for 15 min at 4°C. The protein concentration of the supernatants was measured by BCA Protein assay kit (Pierce). Equal concentration of protein samples were fractionated by SDS-polyacrylamide gel electrophoresis using 12% polyacrylamide gels and transferred to Immobilon—P Transfer Membrane (Millipore). Membranes were probed with either anti-myc monoclonal antibody (Thermo Scientific) or anti-actin monoclonal antibody (Millipore). The primary antibody was detected using a horseradish peroxidase conjugated anti-rabbit antibody with the Millipore detection system.
Results and Discussion
Expression of VopK is lethal to yeast model system
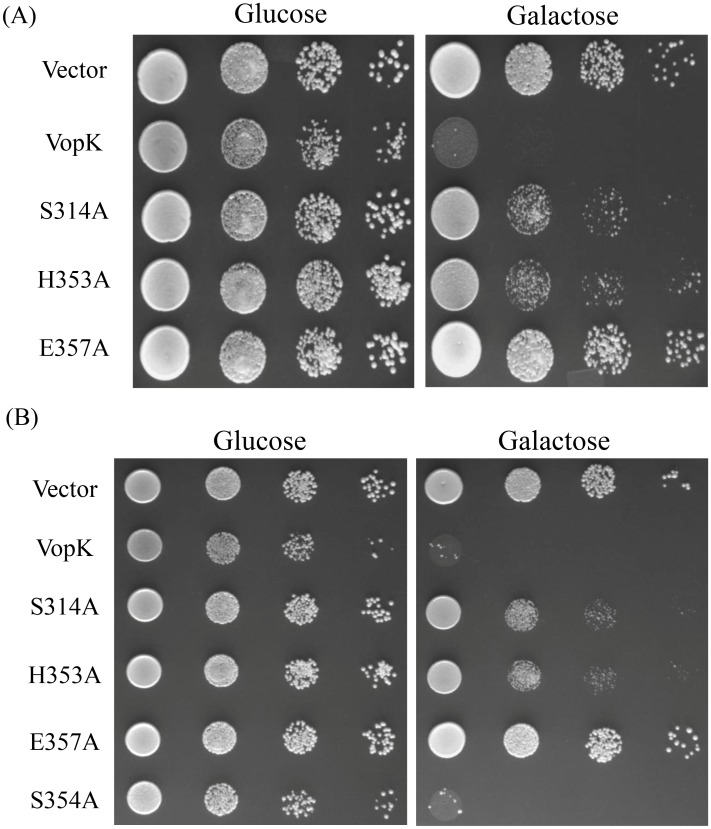
There is now a growing appreciation on the utility of Saccharomyces cerevisiae, the budding yeast as a non-mammalian model system to identify and evaluate functionality of diverse arrays of virulence factors [14, 16–21]. Previously, our group has also exploited the utility of this yeast model system for gathering functional insights of another Vibrio cholerae effector protein [4, 7]. To examine the lethality of VopK, we expressed VopK ectopically under the control of GAL promoter in S. cerevisiae strain BY4741 from pESC-LEU, a high copy number vector (Table 1). Yeast cells transformed with pESC-LEU-VopK (Table 1) yielded a strong lethal phenotype under inducing condition (Fig 1A). Our spotting data is also in congruence with previous observation made by Dziejman and colleagues [5]. We also observed weak growth inhibition under repressing (glucose) condition (Fig 1A). This can be explained in terms of leaky expression from GAL1 promoter of pESC-LEU vector. The leaky expression problem associated with GAL1/10 promoter from different vectors has also been documented in earlier studies [20–22].
Fig 1. Yeast growth inhibition assay.
The growth of yeast strain BY4741 expressing wild type and mutant proteins of VopK under the control of GAL1 promoter from pESC-LEU (A) or pGMH10 (B). Cells transformed with empty vector alone were used as a negative control. The indicated strains were grown overnight in non-inducing selective synthetic medium (SCraf) containing 2% raffinose as the carbon source. Equal number of cells were spotted at 10° and three serial dilutions of 10−2, 10−3 and 10−4 (left to right) on SCGlu (left panel, repression) and SCGal (induction) agar. The data shown are representative of three independent experiments.
Earlier studies have clearly demonstrated that low level expression of effector proteins increases the specificity while high level expression promotes sensitivity of inhibition in yeast model system [20]. As evidenced, several effector proteins exhibit toxicity only when expressed at high level [20]. In some cases, it is also surmised that high level expression may result in non-specific toxic effects [23]. We wanted to ascertain whether low level expression of VopK maintains similar toxicity as presented in the preceding section, the gene encoding wild type VopK was cloned in pGMH10, a low copy number vector (Table 1). Yeast cells transformed with pGMH10-VopK (Table 1) yielded a lethal phenotype only in inducing condition (Fig 1B).
Identification of a predicted MCF1-SHE domain in VopK
The recent flurry of articles has produced a rich harvest of data that exudes how diverse bacterial effector proteins have evolved with eukaryotic domains and motifs, thus enabling pathogens to hijack and evade host surveillance system [10]. To evaluate whether VopK (McfV) contains any functional unit contributing to its lethality, we carried out sequence analysis with PSI-BLAST (default parameters) using VopK as query. Subsequently, a multiple alignment of VopK with polymorphic toxins and T3SS effector proteins reveals the presence of an MCF1-SHE domain in VopK (Fig 2). The predicted MCF1-SHE domain in VopK has conserved Ser314, His353 and Glu357 residues where His353 and Glu357 form the characteristic HxxxE motif (Fig 2). Further analysis of secondary structure also confirms that the positional distribution of these residues is similar to that in known MCF1-SHE domain containing toxins and effector proteins (Fig 2, [9]). We also noticed a glycine residue (Gly 315) in close proximity to the serine residue (Ser 314) (Fig 2), a criterion to satisfy the presence of a serine protease catalytic triad [24].
Fig 2. Multiple sequence alignment of MCF1-SHE domain proteins.
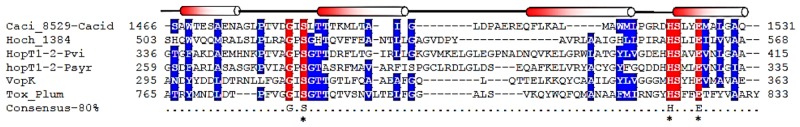
Alignment of VopK along with other polymorphic toxins and effector proteins as carried out using T-coffee [36]. The conserved and predicted SHE motif is marked with star (*). Identical and similar regions are shown highlighted as red and blue, respectively. Helices in the secondary structure of VopK as predicted by PSI-PRED [37, 38] are shown as cylinders (red and white gradient) along with coils as lines (black). Secondary elements of VopK are overlapping with those of other proteins as shown by Arvind and colleagues [9]. Accession numbers are as follows: ACU77352,Caci_8529 [Catenulispora acidiphila DSM 44928]; ACY13938, Hoch_1384 [Haliangium ochraceum DSM 14365]; EEA94476, HopT1-2 [Pseudovibrio sp. JE062]; EEB62143, hopT1-2 [Pseudomonas syringae pv. tomato T1]; EDN16474, VopK A33_1699 [Vibrio cholerae AM-19226]; AAM88787, Toxin protein [Photorhabdus luminescens].
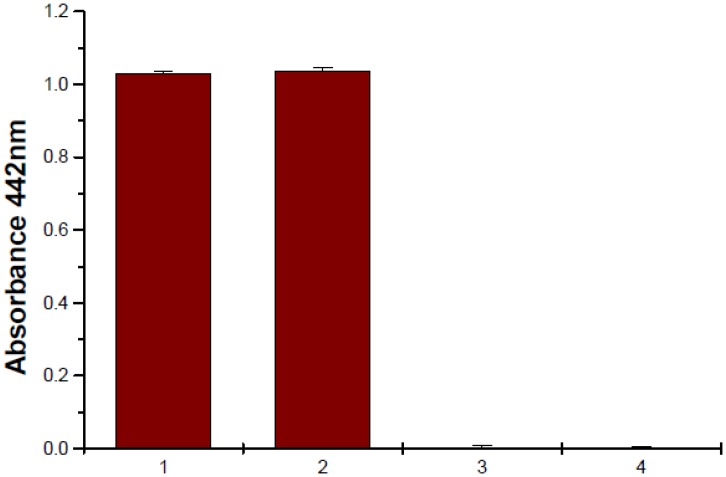
As demonstrated in the preceding section that VopK harbors catalytic triads further prompted us to evaluate protease activity of the protein under in-vitro condition. In this regard, recombinant VopK was purified by Ni+2 –nitriloacetic acid (Ni-NTA) chromatography and subjected to protease assay using azocasein as substrate as described elsewhere [13]. Contrary to our expectation, no protease activity was detected with VopK under this condition (Fig 3). Similar observation was also noticed by Nimchuck and colleagues where HopX family effector proteins despite of having strong homology and conservation of PNGase-like catalytic triad residues failed to exhibit any detectable enzymatic activity on commonly tested substrates under in vitro condition [25]. It is also evidenced from other studies that numerous type III effecotrs belonging to family of cysteine proteases and protein kinases become enzymatically active under in vitro condition only in the presence of an in vivo host target or such enzymes require processing to become active or both [11, 26–29]. Thus far, we have surmised that an MCF1-SHE serine peptidase domain has been predicted and the related catalytic triad residues (Ser314/His353/Glu357) have been identified in VopK. Based on our in vitro protease assay, we concluded that protease activity of VopK may require the presence of in vivo substrates and or host activators.
Fig 3. Protease assay.
Purified VopK protein of different amounts was subjected to proteolytic activity using azocasein as a substrate. Proteinase K treated as a control (1, 2). 1: proteinase K 50μg; 2: proteinase K 100μg; 3: VopK 50μg; 4: VopK 100μg
Role of predicted Ser314/His353/Glu357 catalytic triad residues in the lethality of VopK by employing yeast as a model system
To determine the significance of S314H353E357 residues in the functioning of VopK, the selected amino acids were substituted with alanine residues and the corresponding alanine variants were transformed into S. cerevisiae strain BY4741. The recombinant yeast strains were subjected to solid agar spotting assay on SC and SCGal. The growth of each recombinant yeast strain in the dilution spotting assay is used as a marker to evaluate the extent of functional alteration of each VopK- SHE alanine variant. This was done according to the published protocol [7]. For example, any recombinant yeast strain with a VopK variant protein that exhibits growth at the highest dilution as well as scores equal to or greater than 70% of growth restoration as compared to vector control (calculated on the basis of mean area density as compared to vector control) is considered as demonstrating full restoration of growth. Conversely, this indicates complete loss of lethality in the corresponding VopK variant protein harbored by that recombinant yeast strain. If percentage of growth restoration falls within the range of 30–70%, it is considered to display partial restoration of growth, which further suggests partial loss in toxicity of corresponding VopK variant protein. Our data suggested that the substitution of glutamate357 with alanine resulted in complete loss of toxicity of VopK while replacements of histidine353 and serine314 with alanine showed partial loss of lethality as evaluated by growth of recombinant yeast strains according to the parameters described earlier (Fig 1A, [7]).
To investigate whether alanine variants of SHE triads (Ser314Ala/His353Ala/Glu357Ala) of VopK exert similar toxicity as presented in the preceding section (Fig 1A) under low level expression, we reconstructed recombinant clones of SHE alanine variants in pGMH10, a low copy number vector (Table 1). All recombinants clones in this vector were subsequently transformed into S. cerevisiae strain BY4741 and subjected to solid agar spotting assay. We observed similar pattern of growth where VopK exhibited its strong lethality while substitution of glutamate at position 357 with alanine completely abrogated toxicity of VopK-E357A variant and both VopK-S314 and VopK-H353 maintained partial toxicity as enumerated by published method (Fig 1B, [7]).
As documented, certain effectors exhibit an altered toxicity in solid and liquid growth assay conditions in yeast model system [18, 20, 30]. To evaluate, we subjected our recombinant yeast strains harboring wild type and alanine derivatives (Ser314Ala/His353Ala/Glu357Ala) of VopK cloned in both low copy (pGMH10) and high copy (pESC-LEU) vectors in liquid growth assay as described elsewhere [7, 15]. In case of recombinant yeast strains harboring wild type and alanine mutants of pGMH10 vector background, we observed an agreement between solid and liquid growth assay results where VopK showed its strong lethality and VopK-E357A variant was non-lethal. On the other hand, VopK-S314A and VopK-H353A maintained partial loss in toxicity (Figs 1B and 4A). In case of recombinant yeast strains carrying wild type and alanine variants of pESC-LEU vector background, we noticed that data are congruent for VopK and VopK-E357A variant in both solid and liquid growth conditions (Figs 1A and 4B). But partial loss in toxicity of VopK-S314A and VopK-H353A are not comparable under these conditions. In liquid growth assay, VopK-S314A and VopK-H353A exhibited little more lethality than that of in solid agar spotting (Figs 1A and 4B). The discrepancy of lethality pattern of VopK-S314A and VopK-H353A variants from pESC-LEU background (high copy number vector) in solid and liquid growth conditions may be explained in terms level of expression under different growth conditions as evidenced earlier [18, 20, 30].
Fig 4. Liquid growth assay.
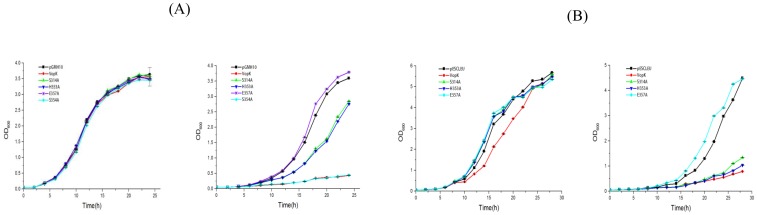
Exponential growth curves demonstrate the effect of wild type and mutant proteins of VopK from pGMH10 (A) or pESC-LEU (B) on BY4741. Cultures were grown in SC media (uninduced, Left panel) and SCGal media (induced, Right panel). OD600 of three technical replicates were tracked. Error bars depict the standard deviation from the mean.
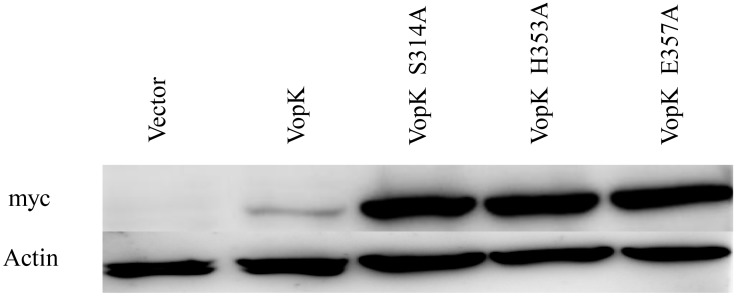
It could be surmised that the loss of lethality (complete and partial) in various VopK alanine congeners (Ser314Ala/His353Ala/Glu357Ala) could be a result of instability of recombinant proteins under in vivo condition. To ensure the in vivo stability of all alanine congeners of VopK, western blot analysis of recombinant yeast strains harboring wild type and SHE alanine variants (Ser314Ala/His353Ala/Glu357Ala) of VopK in pESC-LEU vector was carried out by using anti-myc antibody as described previously [7]. Our western blot data clearly indicated that all the alanine derivatives such as VopK-S314A, VopK-H353A and VopK-E357A were stable under in vivo condition (Fig 5). It should be noted that the stability of wild type protein is relatively less than the corresponding alanine variants. Similar observation has also been recorded earlier where mutants were found more stable than wild type [14, 22, 25].
Fig 5. Western blot analysis.
Expression of the myc tagged proteins form high copy vector confirmed by immunoblot analysis of whole cell lysates harvested after galactose induction, probed with anti-myc monoclonal antibody. Anti-actin antibodies were used as a loading control (lower panel).
Examine the importance of another conserved serine residue at position 354 in the lethality of VopK
Interestingly, there is another conserved serine at position 354 within predicted S314H353E357 region (Fig 2). To ascertain the contribution of serine354 residue into the toxicity of VopK, alanine substitution mutagenesis was carried out using pGMH10-VopK as a template and the functionality of corresponding alanine variant was examined in yeast model system. We found that toxicity of VopK-S354A variant remains unaltered as compared to wild type VopK protein, thereby suggesting no contribution of serine at position 354 in the lethality of VopK. Solid agar spotting result was further corroborated by liquid growth assay where similar pattern of growth inhibition was observed (Fig 4A). This data further bolster the importance of serine at position 314 in contributing to the lethality of VopK (Fig 1B).
In essence, present study reveals i) the identification of a predicted MCF1-SHE domain and related catalytic triads in VopK; ii) The lack of in vitro protease activity can be explained in terms of absence of proper in vivo substrates or host factors; iii) Alanine substitution mutagenesis underscores the importance of S314H353E357 in the lethality of VopK in yeast model system where glutamate at position 357 largely contributes in VopK function. The alanine derivative of another conserved serine (Ser354) within the predicted SHE domain did not affect the toxicity of VopK.
Multiple lines of converging evidences clearly underpin how evolution contributes to microbial pathogenesis by incorporating unique functional domains and motifs in several bacterial virulence determinants [10, 31]. Integration of unique domains and motifs endows the effector proteins with diverse biochemical activities including (de-) phosphorylation, (de-) ubiquitinylation, adenylate cyclase, AMPylation, ribosylation, lyase reactivity, O-GlcNAcylation and proteolysis [32]. There is now a growing understanding on the evolution of several effector proteins (YopJ, YopT, VopA, AvrA, SseL etc) and polymorphic toxins (such as MCF1, FitD and HopT1-1) as proteases where catalytic triad residues have been evidenced to play a key role in the function of such effectors and large toxins [9–11]. As mentioned in the preceding section, several polymorphic toxins share a novel serine peptidase domain named as MCF1-SHE domain having a catalytic core that comprises serine, histidine and glutamate residues [9]. It is noteworthy to mention that each residue in the catalytic core functions differently in various proteases. For example, chymotrypsin, trypsin and subtilisin contain Ser/His/Asp triad where the contribution of serine and histidine residues is different from that of the aspartic acid toward catalysis [33, 34]. In case of aspartyl dipeptidase protease of Salmonella typhimurium, a variation of conventional Ser/His/Asp triad is noticed where aspartate residue is replaced with glutamate [35]. Interestingly, mutagenesis of glutamate residue results in a 100-fold drop in activity whereas mutagenesis of aspartate residue in case of the conventional triad (Ser/His/Asp) results in 10,000-fold reduction in activity [33, 34]. In case of VopK, we observed a complete loss of toxicity in VopK-E357A as compared to other alanine derivatives (e.g VopK-S314A and VopK-H353A), thus indicating differential contribution of triad residues in the functionality of VopK as evaluated by employing yeast model system. In addition, the yeast model of VopK will definitely help to identify cellular pathway (s) targeted by this effector protein which can further be evaluated in a proper mammalian host system. Such model is also useful to screen small molecule inhibitors against this effector molecule. Additional studies are necessary to address these issues.
Acknowledgments
We thank Deepak Bhatt and members of our laboratory for running sequencing facility and giving critical suggestions respectively.
Data Availability
All relevant data are within the paper.
Funding Statement
This work is supported by the grants from the institutional projects OLP-80, supra institutional project (SIP-BSC0210E) and GENESIS-CSIR. Leela Krishna Bankapalli and Rahul Chandra Mishra acknowledge the Department of Biotechnology (DBT India) and Council of Scientific and Industrial (CSIR-India) Research (project UNSEEN-CSIR) respectively for their fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dziejman M, Serruto D, Tam VC, Sturtevant D, Diraphat P, Faruque SM, et al. (2005) Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci USA 102: 3465–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tam VC, Serruto D, Dziejman M, Brieher W, Mekalanos JJ (2007) A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe 1: 95–107. [DOI] [PubMed] [Google Scholar]
- 3. Tam VC, Suzuki M, Coughlin M, Saslowsky D, Biswas K, Lencer WI, et al. (2010) Functional analysis of VopF activity required for colonization in Vibrio cholerae . mBio 1: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tripathi R, Singh Naorem S, Dureja C, Haldar S, Mondal AK, Raychaudhuri S (2010) VopF, a type III effector protein from a non-O1, non-O139 Vibrio cholerae strain, demonstrates toxicity in a Saccharomyces cerevisiae model. J Med Microbiol 59: 17–24. 10.1099/jmm.0.012336-0 [DOI] [PubMed] [Google Scholar]
- 5. Alam A, Miller KA, Chaand M, Butler JS, Dziejman M (2011) Identification of Vibrio cholerae type III secretion system effector proteins. Infect Immun 79: 1728–1740. 10.1128/IAI.01194-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pernier J, Orban J, Avvaru BS, Jegou A, Romet-Lemonne G, Guichard B, et al. (2013) Dimeric WH2 domains in Vibrio VopF promote actin filament barbed-end uncapping and assisted elongation. Nat Struct Mol Biol 20: 1069–1076. 10.1038/nsmb.2639 [DOI] [PubMed] [Google Scholar]
- 7. Tripathi R, Kaithwas V, Dureja C, Raychaudhuri S (2013) Alanine-scanning mutagenesis of WH2 domains of VopF reveals residues important for conferring lethality in a Saccharomyces cerevisiae model. Gene 525: 116–123. 10.1016/j.gene.2013.04.071 [DOI] [PubMed] [Google Scholar]
- 8. Shin OS, Tam VC, Suzuki M, Ritchie JM, Bronson RT, Waldor MK, et al. Type III secretion is essential for the rapidly fatal diarrheal disease caused by non-O1, non-O139 Vibrio cholerae . mBio 2011, 2: e00106–00111. 10.1128/mBio.00106-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang D, De Souza FR, Anantharaman V, Iyer LM, Aravind L (2012) Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct 7: 1–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dean P (2011) Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol Rev 1–26. [DOI] [PubMed] [Google Scholar]
- 11. Agarwar S, Agarwal S, Biancucci M, Satchell KJF (2015) Induced autoprocessing fo the cytopathic Makes Caterpillars Floppy-like effector domain of the Vibrio vulnificus MARTX toxin. Cell Microbiol, April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dongre M, Singh NS, Dureja C, Pedda N, Solanki AK, Ashish, et al. (2011) Evidence on how a conserved glycine in the hinge region of HapR regulates its DNA binding ability: lessons from a natural variant. J Biol Chem 286 (17):15043–9. 10.1074/jbc.M110.209346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parente JA, Salem-Izacc SM, Santana JM, Pereira M, Borges CL, Bailao AM, et al. (2010) A secreted serine protease of Paracoccidioides brasiliensis and its interactions with fungal proteins. BMC Microbiol 10: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calder T, Kinch LN, Fernandez J, Salomon D, Grishin NV, Orth K (2014) Vibrio type III effector VPA1380 is related to the cysteine protease domain of large bacterial toxins. PLos One 9: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trosky JE, Mukherjee S, Burdette DL, Roberts M, McCarter L, Siegel RM, et al. (2004). Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus . J Biol Chem. 279, 51953–51957. [DOI] [PubMed] [Google Scholar]
- 16. Valdivia RH (2004) Modeling the function of bacterial virulence factors in Saccharomyces cerevisiae . Eukaryotic Cell 3: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sisko JL, Spaeth K, Kumar Y, Valdivia RH (2006) Multifunctional analysis of Chlamydia-specific genes in a yeast expression system. Mol Microbiol 60:51–66. [DOI] [PubMed] [Google Scholar]
- 18. Kramer RW, Slagowski NL, Eze NA, Giddings KS, Morrison MF, Siggers KA, et al. (2007) Yeast functional genomic screens lead to identification of a role for a bacterial effector in innate immunity regulation. Plos Pathog 3: 0179–0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siggers KA, Lesser CF (2008) The Yeast Saccharomyces cerevisiae: a versatile model system for the identification and characterization of bacterial virulence proteins. Cell Host Microbe 4: 8–15. 10.1016/j.chom.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slagowski NL, Kramer RW, Morrison MF, LaBaer J, Lesser CF (2008) A functional genomic yeast screen to identify pathogenic bacterial proteins. PLos Pathog 4: 0096–0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stirling FR, Evans TJ (2006) Effects of the type III secreted pseudomonal toxin ExoS in the yeast Saccharomyces cerevisiae . Microbiology 152: 2273–2285. [DOI] [PubMed] [Google Scholar]
- 22. Belyi Y, Tartakovskaya D, Tais A, Fitzke E, Tzivelekidis T, Jank T, et al. (2012) elongation Factor 1A is the target of growth inhibition in yeast caused by Legionella pneumophila glucosyltransferase Lgt1. J Biol Chem 287: 26029–26037. 10.1074/jbc.M112.372672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salomon D, Bosis E, Dar D, Nachman I, Sessa G (2012). Expression of Pseudomonas syringe type III effectors in yeast under stress conditions reveals that HopX1 attenuates activation of the high osmolarity glycerol MAP kinase pathway. Microbiol 158: 2859–2869. [DOI] [PubMed] [Google Scholar]
- 24. Gupta V, Udaya Prakash NA, Lakshmi V, Boopathy R, Jeyekanthan J, Velmurugan D, et al. (2010) Recognition of active and inactive catalytic triads: A template based approach. Intl J Biol Macro 46: 317–323. [DOI] [PubMed] [Google Scholar]
- 25. Nimchuk ZL, Fisher EJ, Desveaux D, Chang JH, Dangl JL (2007) The Hopx (AvrPphE) family of Pseudomonas syringae type III effectors require a catalytic triad and a novel N-terminal domain for function. Mol Plant-Microbe Interact 20: 346–357. [DOI] [PubMed] [Google Scholar]
- 26. Juris SJ, Rudolph AE, Huddler D, Orth K, Dixon JE (2000) A distinctive role for the Yersinia protein kinase:Actin binding, kinase activation, and cytoskeleton disruption. Proc Natl Acad Sci 97:9431–9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Axtell MJ, Chisholm ST, Dahlbeck D, Staskawicz BJ (2003) Genetic and molecular evidence that the Pseudomonas syringe type III effector protein AvrRpt2 is a cysteine protease. Mol Microbiol 49: 1537–1546. [DOI] [PubMed] [Google Scholar]
- 28. Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes R (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301: 1230–1233. [DOI] [PubMed] [Google Scholar]
- 29. Coaker G, Falick A, Staskawica B (2005) Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science 308:548–550. [DOI] [PubMed] [Google Scholar]
- 30. Irena A, Biancucci M, Satchell JFK (2014) Cytotoxicity of the Vibrio vulnificus MARTX toxin effector DUF5 is linked to the C2A subdomain. Proteins [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salomon D, Orth K (2013) What pathogens have taught us about posttranslational modifications. Cell Host Microbe 14: 269–279. 10.1016/j.chom.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruter C, Hardwidge PR (2014) ‘Drugs from Bugs’: bacterial effector proteins as promising biological (immune-) therapeutics. FEMS Microbiol Rev 351:126–132. [DOI] [PubMed] [Google Scholar]
- 33. Ekici DO, Paetzel M, Dalbey ER (2008) Unconventional serine proteases: Variations on the catalytic Ser/His/Asp triad configuration. Prot Sci 17: 2023–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carter P, Wells JA (1988) Dissecting the catalytic triad of a serine protease. Nature 332: 564–568. [DOI] [PubMed] [Google Scholar]
- 35. Hakansson K, Wang AH, Miller CG (2000) The structure of aspartyl dipeptidase reveals a unique fold with a Ser-His-Glu catalytic triad. Proc Natl Acad Sci USA 19: 14097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Notredame C, Higgins DG, Heringa J (2000) T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol 302(1):205–17. [DOI] [PubMed] [Google Scholar]
- 37. Jones DT (1999) Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292: 195–202. [DOI] [PubMed] [Google Scholar]
- 38. Buchan DWA, Minneci F, Nugent TCO, Bryson K, Jones DT (2013) Scalable web services for the PSIPRED protein analysis workbench. Nucleic Acid Res 41: W340–W348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.