Abstract
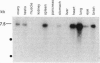
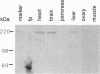
The complete amino acid sequence of Xenopus laevis nonmuscle myosin heavy chain B (MHC-B) has been deduced from overlapping cDNA clones isolated from an XTC cell library. RNA blots of various developmental stages, adult tissues, and XTC cells detect a single transcript of 7.5 kb which is expressed at similar levels throughout development. MHC-B mRNA was detected in XTC cells, heart, lung, spleen, and brain, at lower levels in ovary, testis, pancreas, stomach, liver, and eye, but not in kidney and skeletal muscle. Protein expression in adult tissues, as detected by immunoblot analysis, correlates well with mRNA expression. In chickens and humans, a fraction of the mRNA encoding the MHC-B isoform was found previously to contain a 10-amino acid insert at amino acid 211 near the ATP-binding site. As reported elsewhere, in the chicken this insert-bearing isoform is nervous system-specific. The Xenopus sequence shows a 16-amino acid insertion at the same position; 7 of 16 residues are identical to those in the chicken and human insertion, and these identical residues include a consensus target sequence for cyclin-p34cdc2 kinase. In contrast to chicken, all frog tissues and embryonic stages tested contained the insert-bearing form, and no evidence for a non-insert-bearing MHC-B isoform was found in Xenopus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B., Wright C. V., De Robertis E. M., Cho K. W. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991 Jul 12;253(5016):194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- Cheney R. E., Mooseker M. S. Unconventional myosins. Curr Opin Cell Biol. 1992 Feb;4(1):27–35. doi: 10.1016/0955-0674(92)90055-h. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesel R., Dawid I. B. cDNA cloning and developmental expression of fibroblast growth factor receptors from Xenopus laevis. Mol Cell Biol. 1991 May;11(5):2481–2488. doi: 10.1128/mcb.11.5.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., De Lozanne A., Spudich J. A. Structure and function of the cytoskeleton of a Dictyostelium myosin-defective mutant. J Cell Biol. 1990 Feb;110(2):367–378. doi: 10.1083/jcb.110.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P. J., Richter K., Dawid I. B. A nervous system-specific isotype of the beta subunit of Na+,K(+)-ATPase expressed during early development of Xenopus laevis. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9088–9092. doi: 10.1073/pnas.87.23.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J. D., Kobel H. R. Genetics of Xenopus laevis. Methods Cell Biol. 1991;36:19–34. doi: 10.1016/s0091-679x(08)60270-8. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Adelstein R. S. Chicken nonmuscle myosin heavy chains: differential expression of two mRNAs and evidence for two different polypeptides. J Cell Biol. 1991 Mar;112(5):915–924. doi: 10.1083/jcb.112.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Hammer J. A., 3rd Myosins of nonmuscle cells. Annu Rev Biophys Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- LaFlamme S. E., Dawid I. B. XK endo B is preferentially expressed in several induced embryonic tissues during the development of Xenopus laevis. Differentiation. 1990 Mar;43(1):1–9. doi: 10.1111/j.1432-0436.1990.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Doberstein S. K., Zot H. G. Myosin-I. Annu Rev Physiol. 1991;53:653–681. doi: 10.1146/annurev.ph.53.030191.003253. [DOI] [PubMed] [Google Scholar]
- Radice G. P., Malacinski G. M. Expression of myosin heavy chain transcripts during Xenopus laevis development. Dev Biol. 1989 Jun;133(2):562–568. doi: 10.1016/0012-1606(89)90058-4. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Richter K., Grunz H., Dawid I. B. Gene expression in the embryonic nervous system of Xenopus laevis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8086–8090. doi: 10.1073/pnas.85.21.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991 Feb;3(1):98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Shohet R. V., Conti M. A., Kawamoto S., Preston Y. A., Brill D. A., Adelstein R. S. Cloning of the cDNA encoding the myosin heavy chain of a vertebrate cellular myosin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7726–7730. doi: 10.1073/pnas.86.20.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Wang M., McBride O. W., Kawamoto S., Yamakawa K., Gdula D., Adelstein R. S., Weir L. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ Res. 1991 Aug;69(2):530–539. doi: 10.1161/01.res.69.2.530. [DOI] [PubMed] [Google Scholar]
- Tafuri S. R., Wolffe A. P. Xenopus Y-box transcription factors: molecular cloning, functional analysis and developmental regulation. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9028–9032. doi: 10.1073/pnas.87.22.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Kawamoto S., Adelstein R. S. Evidence for inserted sequences in the head region of nonmuscle myosin specific to the nervous system. Cloning of the cDNA encoding the myosin heavy chain-B isoform of vertebrate nonmuscle myosin. J Biol Chem. 1992 Sep 5;267(25):17864–17871. [PubMed] [Google Scholar]
- Watts F. Z., Shiels G., Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987 Nov;6(11):3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. E., Pesacreta T. C., Kiehart D. P. Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development. 1991 Jan;111(1):1–14. doi: 10.1242/dev.111.1.1. [DOI] [PubMed] [Google Scholar]
- de Bold A. J. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985 Nov 15;230(4727):767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]