Abstract
Bulliform phytoliths play an important role in researching rice origins as they can be used to distinguish between wild and domesticated rice. Rice bulliform phytoliths are characterized by numerous small shallow fish-scale decorations on the lateral side. Previous studies have shown that domesticated rice has a larger number of these decorations than wild rice and that the number of decorations ≥9 is a useful feature for identifying domesticated rice. However, this standard was established based on limited samples of modern rice plants. In this study, we analyzed soil samples from both wild and domesticated rice paddies. Results showed that, in wild rice soil samples, the proportion of bulliform phytoliths with ≥9 decorations was 17.46% ± 8.29%, while in domesticated rice soil samples, the corresponding proportion was 63.70% ± 9.22%. This suggests that the proportion of phytoliths with ≥9 decorations can be adopted as a criterion for discriminating between wild and domesticated rice in prehistoric soil. This indicator will be of significance in improving the application of fish-scale decorations to research into rice origins and the rice domestication process.
Introduction
Rice (Oryza sativa) is among the world’s most important and ancient domesticated crops [1]. There is still controversy regarding when rice cultivation and domestication started [2, 3]. Some researchers believe that rice was domesticated 9,000–10,000 years ago, based on evidence from archaeological rice fossils and rice DNA [4–10], while others argue that the process of rice domestication is only known to have begun with certainty 8,000–7,700 years ago, based on the study of unearthed rice spikelet bases [11, 12]. One cause of this disagreement is the lack of unified standards for distinguishing between wild and domesticated archaeological rice remains, with this remaining an urgent problem in the research of rice origins [13].
Traditionally, charred rice grains and spikelet bases from archaeological sites have been employed to identify wild/domesticated rice remains [4, 11, 12, 14]. Past research on rice grain morphology has shown that a wild rice grain is thinner and longer than a domesticated grain, which indicates that it has a greater length-width ratio (with a ratio boundary of 3.5) [15]. The spikelet bases of domesticated rice can be identified by their uneven profile, dimpled appearance, and less symmetrical scars. In contrast, wild-type rice spikelet bases typically have a straight profile at their base, and shattering results in a smooth and round abscission scar, with a small, distinct vascular pore [11, 16]. However, the application of these indictors to archaeological remains still leaves a degree of uncertainty [2]. Liu et al. [2] have proved that the size and shape of rice grains are not reliable identifiers for distinguishing domesticated grains from wild grains; in addition, charred remains and spikelet bases are heavily dependent on unique burial conditions and preservation processes.
To date, phytolith analysis has been a crucial method for identifying rice remains uncovered from archaeological sites and sediments. Rice plants produce three distinctive phytoliths: bilobate phytoliths from rice leaves and stems, double-peaked phytoliths from the rice husk, and bulliform phytoliths from the rice leaves [17]. The bilobate phytolith is typical of the Oryzoideae subfamily and contrasts with the characteristic features of Oryza plants [18]. However, measurement of bilobates does not enable discrimination of cultivated and wild Oryza species [19]. Double-peaked phytoliths can be used to distinguish domesticated rice from wild rice based on multivariate linear discriminant function analysis and three-dimensional measurements [19, 20]. However, very few double-peaked phytoliths have been found in prehistoric rice soil or sediments [21, 22].
Rice bulliform phytoliths are abundant and unique to the rice leaf, with the presence of fish-scale decorations on fan edges (bulliform phytoliths) [18]. Bulliform phytolith measurement is a method that could potentially be used to distinguish domesticated from wild rice. However, previous studies on rice plants and their relatives have suggested that bulliform measurement alone is unable to distinguish wild Oryza species from domesticated ones [19, 23–25].
Another method for discriminating bulliform phytoliths of wild and domesticated rice is based on the number of scales on fan edges. Fujiwara [26] was the first to find that fish-scale decorations are different in wild and domesticated rice. The scales of domesticated rice bulliform phytoliths are larger and have irregular shapes, while those of wild rice bulliform phytoliths look like a tortoise shell. However, this kind of qualitative discrimination is hard to apply to research in practice and it is thus important to explore quantitative standards.
Lu et al. [22] studied the number of fish-scale decorations on the rim of rice bulliform phytoliths from seven species of wild rice and six species of domesticated rice, finding that bulliform phytoliths of domesticated rice species generally had 8–14 fish-scale decorations, while those belonging to varieties of wild rice commonly had <9. It would thus seem that bulliform phytoliths with ≥9 decorations would be a useful standard for identifying domesticated species.
In practice, however, because of the overlap in the number of scale decorations between wild and domesticated rice species, a single bulliform phytolith is not sufficient to enable a distinction to be made between domesticated and wild rice species. Moreover, in locations where wild and domesticated forms of rice are likely to have overlapped, determining the precise source of fossil phytoliths recovered from sedimentary records can be problematic [23]. For these reasons, more clear specification is needed.
This study on domesticated and wild rice paddy surface soil suggests that the proportion of bulliform phytoliths with ≥9 decorations can be adopted as a criterion to discriminate between wild and domesticated rice.
Materials and Methods
Seventy-one samples of surface rice soil from south China, including from Hainan, Hu’nan, and Jiangxi Provinces, were collected for phytolith analysis (Fig 1). These included 29 surface soil samples from 9 wild rice fields, 30 surface soil samples from 24 modern rice paddies, and 12 soil samples from 9 other fields. Details of all samples are given in Table 1. All necessary permits for the described samples from Jiangxi Province were obtained from the Jiangxi Academy of Agricultural Sciences. No permits for other samples were needed.
Fig 1. Geographic locations of sample collection sites.
(map modified from Grass GIS; https://grass.osgeo.org/); red dots represent wild rice paddy soil samples, purple dots represent domesticated rice paddy soil samples, and blue dots represent other soil samples.
Table 1. List of samples used in the study.
| No. | Code | Source | Location | Longitude (°E) | Latitude (°N) |
|---|---|---|---|---|---|
| 1 | DY1 | Wild rice field | Dongxiang County, Jiangxi Province | 116.533 | 28.083 |
| 2 | DY2 | Wild rice field | Dongxiang County, Jiangxi Province | 116.533 | 28.1 |
| 3 | DY3 | Wild rice field | Dongxiang County, Jiangxi Province | 116.533 | 28.083 |
| 4 | HL-BT1 | Wild rice field | Wenchang City, Hainan Province | 110.681 | 19.787 |
| 5 | HL-BT2 | Wild rice field | Wenchang City, Hainan Province | 110.681 | 19.787 |
| 6 | TS-BT4 | Wild rice field | Wenchang City, Hainan Province | 110.694 | 19.727 |
| 7 | WN-2 | Wild rice field | Wanning City, Hainan Province | 110.411 | 18.741 |
| 8 | WN-4 | Wild rice field | Wanning City, Hainan Province | 110.411 | 18.741 |
| 9 | WN-5 | Wild rice field | Wanning City, Hainan Province | 110.411 | 18.741 |
| 10 | WN-BT6 | Wild rice field | Wanning City, Hainan Province | 110.411 | 18.741 |
| 11 | WN-BT7 | Wild rice field | Wanning City, Hainan Province | 110.411 | 18.741 |
| 12 | WN-BT8 | Wild rice field | Wanning City, Hainan Province | 110.411 | 18.741 |
| 13 | WN-BT9 | Wild rice field | Wanning City, Hainan Province | 110.411 | 18.741 |
| 14 | DXA | Wild rice field | Dongxiang County, Jiangxi Province | 116.528 | 28.108 |
| 15 | DXS-1 | Wild rice field | Dongxiang County, Jiangxi Province | 116.509 | 28.104 |
| 16 | DXS-2 | Wild rice field | Dongxiang County, Jiangxi Province | 116.509 | 28.104 |
| 17 | 10CL-B1 | Wild rice field | Chaling County, Hunan Province | 113.696 | 26.861 |
| 18 | 10CL-B3 | Wild rice field | Chaling County, Hunan Province | 113.696 | 26.861 |
| 19 | 10CL-B4 | Wild rice field | Chaling County, Hunan Province | 113.696 | 26.861 |
| 20 | 10CL-B5 | Wild rice field | Chaling County, Hunan Province | 113.697 | 26.861 |
| 21 | 10CL-B6 | Wild rice field | Chaling County, Hunan Province | 113.697 | 26.861 |
| 22 | 10CL-B7 | Wild rice field | Chaling County, Hunan Province | 113.696 | 26.862 |
| 23 | 10CL-B8 | Wild rice field | Chaling County, Hunan Province | 113.696 | 26.861 |
| 24 | 10CL-B9 | Wild rice field | Chaling County, Hunan Province | 113.697 | 26.861 |
| 25 | 10CL-B10 | Wild rice field | Chaling County, Hunan Province | 113.697 | 26.861 |
| 26 | 10CL-S4 | Wild rice field | Chaling County, Hunan Province | 113.696 | 26.861 |
| 27 | ZX-2 | Oryza officinalis field topsoil | Lingshui County, Hainan Province | 110.095 | 18.589 |
| 28 | ZX-3 | Edge of Oryza officinalis field | Lingshui County, Hainan Province | 110.095 | 18.589 |
| 29 | LHT-2 | Oryza granulate field topsoil | Sanya City, Hainan Province | 109.499 | 18.226 |
| 30 | JX01 | Domesticated rice paddy | Dongxiang County, Jiangxi Province | 116.533 | 28.117 |
| 31 | JX02 | Domesticated rice paddy | Yujiang County, Jiangxi Province | 116.783 | 28.25 |
| 32 | JX03 | Domesticated rice paddy | Jinxian County, Jiangxi Province | 116.283 | 28.333 |
| 33 | JX04 | Domesticated rice paddy | Gan County, Jiangxi Province | 115.133 | 26.15 |
| 34 | JX05 | Domesticated rice paddy | Gan County, Jiangxi Province | 115.133 | 26.15 |
| 35 | JX06 | Domesticated rice paddy | Nanchang County, Jiangxi Province | 115.9 | 28.4 |
| 36 | JX07 | Domesticated rice paddy | Fengcheng City, Jiangxi Province | 115.833 | 28.217 |
| 37 | JX08 | Domesticated rice paddy | Zhangshu City, Jiangxi Province | 115.517 | 28.083 |
| 38 | JX09 | Domesticated rice paddy | Xingan County, Jiangxi Province | 115.25 | 27.817 |
| 39 | JX10 | Domesticated rice paddy | Xiajiang County, Jiangxi Province | 115.117 | 27.583 |
| 40 | JX11 | Domesticated rice paddy | Jishui County, Jiangxi Province | 115.017 | 27.301 |
| 41 | JX12 | Domesticated rice paddy | Ji’an County, Jiangxi Province | 114.867 | 27 |
| 42 | JX13 | Domesticated rice paddy | Taihe County, Jiangxi Province | 114.9 | 26.85 |
| 43 | JX14 | Domesticated rice paddy | Xingguo County, Jiangxi Province | 115.267 | 26.483 |
| 44 | JX15 | Domesticated rice paddy | Ningdu County, Jiangxi Province | 115.85 | 26.367 |
| 45 | JX16 | Domesticated rice paddy | Shicheng County, Jiangxi Province | 116.3 | 26.433 |
| 46 | JX17 | Domesticated rice paddy | Guangchang County, Jiangxi Province | 116.333 | 26.717 |
| 47 | JX18 | Domesticated rice paddy | Guangchang County, Jiangxi Province | 116.35 | 26.883 |
| 48 | JX19 | Domesticated rice paddy | Nancheng County, Jiangxi Province | 116.617 | 27.567 |
| 49 | JX20 | Domesticated rice paddy | Fuzhou County, Jiangxi Province | 116.25 | 28.117 |
| 50 | HKML-1 | Domesticated rice paddy | Haikou City, Hainan Province | 110.498 | 19.918 |
| 51 | HL-OS1 | Domesticated rice paddy | Wenchang City, Hainan Province | 110.681 | 19.787 |
| 52 | TS-BT1 | Domesticated rice paddy | Wenchang City, Hainan Province | 110.694 | 19.727 |
| 53 | TS-BT2 | Domesticated rice paddy | Wenchang City, Hainan Province | 110.694 | 19.727 |
| 54 | TS-BT3 | Domesticated rice paddy | Wenchang City, Hainan Province | 110.694 | 19.727 |
| 55 | WN-BT1 | Domesticated rice paddy | Wanning City, Hainan Province | 110.411 | 18.741 |
| 56 | WN-BT2 | Domesticated rice paddy | Wanning City, Hainan Province | 110.411 | 18.741 |
| 57 | WN-BT3 | Domesticated rice paddy | Wanning City, Hainan Province | 110.411 | 18.741 |
| 58 | WN-BT4 | Domesticated rice paddy | Wanning City, Hainan Province | 110.411 | 18.741 |
| 59 | 10CL | Domesticated rice paddy | Chaling County, Hunan Province | 113.696 | 26.861 |
| 60 | ZX-4 | Natural topsoil | Lingshui County, Hainan Province | 110.095 | 18.589 |
| 61 | HKML-2 | Bushwood topsoil | Haikou City, Hainan Province | 110.498 | 19.918 |
| 62 | 10CL | Broad-leaved forest topsoil | Chaling County, Hunan Province | 113.696 | 26.861 |
| 63 | HL-OS2 | Edge of wild rice paddy | Wenchang City, Hainan Province | 110.681 | 19.787 |
| 64 | WN-BT5 | Edge of wild rice paddy | Wanning City, Hainan Province | 110.411 | 18.741 |
| 65 | WN-BT10 | Edge of wild rice paddy | Wanning City, Hainan Province | 110.411 | 18.741 |
| 66 | DXS | Edge of wild rice paddy | Dongxiang County, Jiangxi Province | 116.509 | 28.104 |
| 67 | 10CL-S1 | Soil around wild rice root | Chaling County, Hunan Province | 113.696 | 26.861 |
| 68 | 10CL-S2 | Soil around wild rice root | Chaling County, Hunan Province | 113.696 | 26.861 |
| 69 | 10CL-S3 | Soil around wild rice root | Chaling County, Hunan Province | 113.696 | 26.861 |
| 70 | 10CL-FB1 | Natural topsoil | Chaling County, Hunan Province | 113.696 | 26.861 |
| 71 | 10CL-FB2 | No wild rice area | Chaling County, Hunan Province | 113.696 | 26.861 |
Extraction of phytoliths from soil samples followed procedures described by Zhang [27], with slight modifications. Initially, 5 g of soil were weighed. Subsequently, 30% hydrogen peroxide (H2O2) and cold 15% hydrochloric acid (HCl) were added to each sample to remove organic matter and carbonates. The samples were then subjected to heavy liquid flotation using zinc bromide (ZnBr2, density 2.35 g/cm3) to separate phytoliths, with these mounted on a slide with Canada Balsam.
Phytolith counting and identification were performed using a Leica microscope with phase-contrast at 400X magnification. The bulliform phytolith selection criteria were modified from Wang & Lu [28] (both symmetric and asymmetric ones). Phytoliths <10 um in size were excluded because of the inability to clearly count decorations. For each rice bulliform phytolith, the number of fish-scale decorations around the edge of fan-shaped phytoliths (Fig 2) was counted. Each sample was scanned until 50 rice bulliform phytoliths were encountered [29]. In each case, the proportion of rice bulliform phytoliths with ≥9 fish-scale decorations was calculated.
Fig 2. Fish-scale decorations in rice bulliform phytoliths (Modified as per [22] [30]). a: wild rice plant and its growing environment; b: domesticated rice plant.
Results
Bulliform phytoliths in soil samples were mostly well preserved, enabling correct identification (Fig 3 and S1 Table). Abundant rice phytoliths (including bulliform phytoliths and bilobate phytoliths) were found, but these included almost no double-peaked phytoliths.
Fig 3. Bulliform phytoliths in sample rice (10 um).
a-c: bulliform phytoliths from wild rice fields with <9 fish-scale decorations; d: bulliform phytoliths from wild rice field with ≥9 fish-scale decorations; e-g: bulliform phytoliths from modern rice paddies with ≥9 fish-scale decorations; h: bulliform phytolith from modern rice paddies with <9 fish-scale decorations; i: bulliform phytolith from wild rice field; j: bulliform phytolith from modern rice paddy.
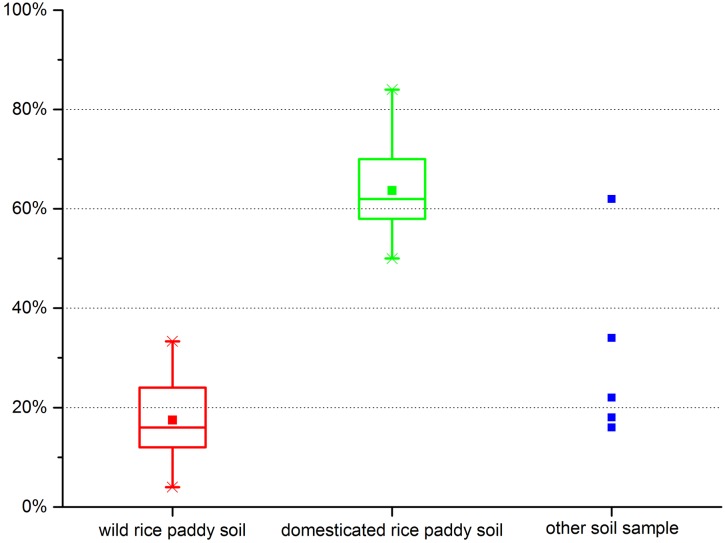
Bulliform phytoliths from wild rice field—Of all 29 wild rice soil samples, no rice bulliform phytoliths were found in samples Nos. 20, 25, and 29. In the other 26 wild rice soil samples, the highest proportion of bulliform phytoliths with ≥9 fish-scale decorations was 33.33% (No. 9), while the lowest proportion was 4% (Nos. 1 and 24); the average proportion in the 26 wild rice soil samples was 17.46% ± 8.29% (Fig 4, left).
Fig 4. The proportion of bulliform phytoliths with ≥9 fish-scale decorations in samples.
Bulliform phytoliths from domesticated rice paddies—Rice bulliform phytoliths were found in all 30 domesticated rice paddy soil samples. The highest proportion of bulliform phytoliths with ≥9 fish-scale decorations was 84% (No. 59), while the lowest proportion was 50% (No. 42); the average proportion in all 30 soil samples from domesticated rice paddies was 63.70% ± 9.22% (Fig 4, middle).
Bulliform phytoliths from other fields—Rice bulliform phytoliths were found in only five other field soil samples (Fig 4, right). Of these, the highest proportion of bulliform phytoliths with ≥9 fish-scale decorations was 62% (No. 63), while the lowest was 16% (No. 71). Although the five soil samples were neither from wild rice fields nor from domesticated rice paddies, their locations were very close to paddy or wild rice fields, so it is possible that these samples contained rice bulliform phytoliths because of soil tilling or other disturbance activities.
We also analyzed the proportion of bulliform phytoliths with ≥9 fish-scale decorations from different climatic regions. Jiangxi and Hunan Provinces are subtropical, while Hainan Province is located in the tropics. Table 2 shows the number of samples from different climatic regions. From Fig 5, we can see that in wild rice soil samples, the highest proportions of bulliform phytoliths with ≥9 fish-scale decorations were found in samples from the tropics, and the lowest proportions of bulliform phytoliths with ≥9 fish-scale decorations were found in samples from the subtropical region. Moreover, as shown from the analysis of the box height in Fig 5, the proportions from both wild and domesticated paddy soil samples from the tropics were more scattered than in the case of samples from the subtropical region.
Table 2. Number of samples from different climatic regions.
| subtropical region | tropical region | |
|---|---|---|
| wild rice field soil sample | 16 | 13 |
| domesticated rice paddy soil sample | 21 | 9 |
Fig 5. The proportion of bulliform phytoliths with ≥9 fish-scale decorations from different climatic regions.
WS: wild rice field soil in subtropical region; WT: wild rice field soil in tropical region. DS: domesticated rice paddy soil in subtropical region; DT: domesticated rice paddy soil in tropical region.
Discussion
From our study, it is evident that the indicator—proportion of bulliform phytoliths with ≥9 fish-scale decorations—can be used to clearly discriminate wild and domesticated rice. We therefore believe that when the proportion of bulliform phytoliths with ≥9 fish-scale decorations is higher than 63.70% ± 9.22%, the sample can be regarded as domesticated rice; when the proportion of bulliform phytoliths with ≥9 fish-scale decorations is less than 17.46% ± 8.29%, the sample can be classified as wild rice.
Previous studies have often employed morphometric parameters (length, width and b/a—ratio of length of handle to fan) of bulliform phytoliths to analyze ancient rice remains [31–38]. Some researchers have established discriminant equations to differentiate between japonica and indica rice [39–41]. However, Wang et al. verified these discriminant equations and found that wild rice was always misjudged; for this reason, when using discriminant equations to determine the origin of archaeological samples, extreme caution is required [28]. Moreover, Gu et al. found that three-dimensional morphological features of bulliform phytoliths from Oryza sativa are scattered, with significant overlap of this species with its relatives. Due to this wide overlap, bulliform phytolith measurement alone cannot be used to distinguish wild Oryza species from domesticated ones [19].
The fish-scale decoration features in single bulliform phytolith have shown great potential in previous studies [22, 27, 42–45]. However, use of these features in single bulliform phytoliths to distinguish wild/domesticated rice remains controversial. One important reason is that the number of fish-scale decorations overlaps across species, so that it is not possible to use a single bulliform phytolith to classify rice properties.
In order to improve the validity of this method, in this study we examined at least 50 phytoliths from each sample to calculate the proportion of bulliform phytoliths with ≥9 fish-scale decorations. This method can help avoid the uncertainty inherent in single phytolith analysis. Besides, bulliform phytoliths are abundant in the genus Oryza and previous research has shown that the highest silica percentage is present in the leaf blade [46]. It is therefore feasible to discriminate wild and domesticated rice through the number of fish-scale decorations found around the bulliform phytolith.
Bulliform cells (motor cells), situated in the upper epidermis of leaf blades [47], are water storage mechanisms and play a role in mature leaf rolling and/or folding in case of water stress [48, 49]. During periods of excessive water loss, the bulliform cells became flaccid and enable the leaf to roll in order to maintain water; under sufficient water conditions, bulliform cells are filled with water and expand, and the blade thus flattens [50]. Leaf rolling can affect light interception of the base and enhance the ability to resist water stress [50, 51].
Bulliform cells are closely associated with adjacent colorless cells [52]. Their morphology, combined with that of enlarged colorless cells, has been used as a taxonomic characteristic [53]. The colorless cells are smaller than the bulliform cells, translucent, voluminous, highly vacuolized, and arranged in uniseriate columns connecting the abaxial epidermis and bulliform cells [52]. The colorless cells are variable in shape and size [53]. Fish-scale decorations on the bulliform phytolith are comprised of cavities squeezed by colorless cells [28] and leaf curling will increase the number of cavities. Wild rice usually grows in swampy conditions [54], where water is abundant (Fig 2, left), and so leaves curl less; on the contrary, domesticated rice leaves are erect and distant from water (Fig 2, right), and so the leaves need to curl repeatedly to hold water. This might explain why bulliform phytoliths of domesticated rice have more fish-scale decorations than those of wild rice.
In our study, the proportion of bulliform phytoliths with ≥9 fish-scale decorations in samples from different climatic regions was analyzed. We found that in subtropical and tropical regions, the proportion of bulliform phytoliths with ≥9 fish-scale decorations in wild rice field soil samples was notably different, while the difference was smaller in the case of domesticated rice paddy samples; it is unclear whether the difference in the former case is climate-related. All domesticated rice paddy samples collected in the study came from southern China; however, since northern China is also a main rice growing area, we hope to study more soil samples from northern China in future.
Admittedly, the discrimination of wild and domesticated bulliform phytoliths might be influenced by other factors, such as erosion and dissolution of bulliform phytoliths [55], making it more difficult to precisely count the number of fish-scale decorations. In addition, hybridization of wild rice and domesticated rice species would also affect the ability to discriminate between wild/domesticated rice. We, therefore, recommend further research on wild-domesticated hybridization, which should help understand the variation in decorations and document cultivation and domestication.
Conclusion
This study systematically analyzed differences in bulliform phytolith fish-scale decoration numbers between domesticated rice paddy soil and wild rice field soil in South China. Results showed that, in domesticated rice soil, the proportion of bulliform phytoliths with ≥9 fish-scale decorations was higher than 63.70% ± 9.22%, while in wild rice soil, the proportion was less than 17.46% ± 8.29%. The study therefore indicates that the proportion of bulliform phytoliths with ≥9 fish-scale decorations can be used to successfully discriminate between wild and domesticated rice. This provides significant insights for research into rice origin and domestication.
Supporting Information
(XLSX)
Acknowledgments
We thank Zhang J. P., Xu D.K. for helpful discussions. We also thank Xu E.Q., Yao L.N., and Kang L. for assistance in producing several of the figures. We would like to thank Editage http://online.editage.cn/ for English language editing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Natural Science Foundation of China (Grant No. 41230104), the National Science and Technology Major Project of China (Grant No. 2015CB953801), the “Strategic Priority Research Program: Climate Change, Carbon Budget and Relevant Issues” of the Chinese Academy of Sciences (Grant No. XDA05130604), the National Key Technology R&D Program of China (Grant No. 2013BAK08B02) and National Natural Science Foundation of China (Grant No. 41461043).
References
- 1. Callaway E. Domestication: The birth of rice. Nature. 2014; 514(7524): S58–S9. [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Lee G-A, Jiang L, Zhang J. Evidence for the early beginning (c. 9000 cal. BP) of rice domestication in China: a response. The Holocene. 2007; 17(8): 1059–68. [Google Scholar]
- 3. Archaeologists T. Recipe for rice domestication required millennia. Science. 2007; 29: 1830. [DOI] [PubMed] [Google Scholar]
- 4. Zheng Y, Sun G, Chen X. Characteristics of the short rachillae of rice from archaeological sites dating to 7000 years ago. Chinese Science Bulletin. 2007; 52(12): 1654–60. [Google Scholar]
- 5. Zhijun Z. The middle Yangtze region in China is one place where rice was domesticated: phytolith evidence from the Diaotonghuan cave, northern Jiangxi. Antiquity. 1998; 72(278): 885–97. [Google Scholar]
- 6. Higham C, Higham C. The origins and dispersal of rice cultivation. Antiquity. 1998; 72(278): 867–77. [Google Scholar]
- 7. Jiang L, Liu L. New evidence for the origins of sedentism and rice domestication in the Lower Yangzi River, China. Antiquity. 2006; 80(308): 355–61. [Google Scholar]
- 8. Gong Z, Chen H, Yuan D, Zhao Y, Wu Y, Zhang G. The temporal and spatial distribution of ancient rice in China and its implications. Chinese Science Bulletin. 2007; 52(5): 562–7. [Google Scholar]
- 9. Molina J, Sikora M, Garud N, Flowers JM, Rubinstein S, Reynolds A, et al. Molecular evidence for a single evolutionary origin of domesticated rice. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108(20): 8351–6. doi: 10.1073/pnas.1104686108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng Y, Shi H, Qi X-b, Xiao C-j, Zhong H, Run-lin ZM, et al. The ADH1B Arg47His polymorphism in East Asian populations and expansion of rice domestication in history. BMC evolutionary biology. 2010; 10(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuller DQ, Qin L, Zheng Y, Zhao Z, Chen X, Hosoya LA, et al. The domestication process and domestication rate in rice: spikelet bases from the Lower Yangtze. Science. 2009;323(5921):1607–10. doi: 10.1126/science.1166605 [DOI] [PubMed] [Google Scholar]
- 12. Fuller DQ, Harvey E, Qin L. Presumed domestication? Evidence for wild rice cultivation and domestication in the fifth millennium BC of the Lower Yangtze region. Antiquity. 2007; 81(312): 316–31. [Google Scholar]
- 13. Lu L. The problem of identification between wild and cultivated rice in archaeological sites. Relics From South. 2009; (3): 72–4. [Google Scholar]
- 14. Zhang W, Yuan J. A preliminary study on the ancient rice excavated from Yuchanyan, Dao xian, Hunan Province. Acta Agronomica Sinica. 1998; 24(4): 416–20 [Google Scholar]
- 15. Wang X, Sun C, Cai H, Zhang J. Origin and differentiation of Chinese Cultivated Rice. Chinese Science Bulletin. 1998; (22): 2354–63. [Google Scholar]
- 16. Li CB, Zhou AL, Sang T. Rice domestication by reducing shattering. Science. 2006; 311(5769): 1936–9. [DOI] [PubMed] [Google Scholar]
- 17. Lu H, Wu N, Liu B. Recognition of rice phytoliths In: Pinilla A, Juan-Tresserras J, Machado MJ, editors. Monografias del Centro de Ciencias Medioambientales. Madrid; 1997. p.159–74. [Google Scholar]
- 18. Wang Y, Lu H. The Study of Phytolith and Its Application. Beijing: China Ocean Press, 1993. [Google Scholar]
- 19. Gu Y, Zhao Z, Pearsall DM. Phytolith morphology research on wild and domesticated rice species in East Asia. Quaternary International. 2013; 287: 141–8. [Google Scholar]
- 20. Zhao Z, Pearsall D, Benfer R, Piperno D. Distinguishing rice (Oryza sativa poaceae) from wild Oryza species through phytolith analysis, II Finalized method. Economic Botany. 1998; 52(2): 134–45. [Google Scholar]
- 21. Huan X, Li Q, Ma Z, Jiang L, Yang X. Fan-shaped phytoliths reveal the process of rice domestication at Shangshan Site, Zhejiang Province. Quaternary Sciences. 2014; 34(1): 106–13. [Google Scholar]
- 22. Lu H, Liu Z, Wu N, Berne S, Saito Y, Liu B, et al. Rice domestication and climatic change: phytolith evidence from East China. Boreas. 2002; 31(4): 378–85. [Google Scholar]
- 23. Pearsall DM, Piperno DR, Dinan EH, Umlauf M, Zhao Z, Benfer RA Jr. Distinguishing rice (Oryza sativa Poaceae) from wild Oryza species through phytolith analysis: results of preliminary research. Economic Botany. 1995; 49(2): 183–96. [Google Scholar]
- 24. Ma X, Fang J. Silicas in leaves of eight wild rice species. Acta Botanica Boreali-Occidentalia Sinica. 2007; (8): 1531–6. [Google Scholar]
- 25. Zhang W, Wang L. Phytoliths in leaves of 7 Oryza species. Journal of China Agricultural University. 1998; (3): 21–5. [Google Scholar]
- 26. Fujiwara H. Fundamental studies of plant opal analysis (1): On the silica bodies of motor cell of rice plants and their relatives, and the method of quantitative analysis. Archaeology and Nature Science. 1976; 9: 15–29. [Google Scholar]
- 27. Zhang J, Lu H, Wu N, Li F, Yang X, Wang W, et al. Phytolith evidence for rice cultivation and spread in Mid-Late Neolithic archaeological sites in central North China. Boreas. 2010; 39(3): 592–602. [Google Scholar]
- 28. Wang C, Lu H. Research progress of fan-shaped phytolith of rice ang relevant issues. Quaternary Sciences. 2012; (02):269–81. [Google Scholar]
- 29. Wang C, Udatsu T, Fujiwara H, Tang L, Zou J. Morphological characteristics of silica bodies from motor cells in rice (Oryza sativa L.) and their differences between indica and japonica . Jiangsu Journal of Agricultural Sciences. 1997; 13(3): 129–38. [Google Scholar]
- 30. Fukiwara H, Kaner S. Research into the history of rice cultivation using plant opal analysis. MASCA research papers in science and archaeology. 1993; 10: 147–58. [Google Scholar]
- 31. Chen B, Zhang J, Lu H. Discovery and significance of rice phytolith in Jiahu Neolithic Site,Henan Province. Chinese Science Bulletin. 1995; 40(4): 339–42. [Google Scholar]
- 32. Lu H, Wu N, Wang Y. Identification of fan-shaped phytolith of rice and its application in archaeology. Archaeology. 1996; (4): 82–6. [Google Scholar]
- 33. Zheng Y, Fujiwara H, You X, Yu W, Liu B, Ding J, et al. Morphological characters of plant opals from motor cells of rice in the Neolithic Age of the Taihu Region. Chinese Journal of Rice Science. 1999; (1): 25–30. [Google Scholar]
- 34. Zheng Y, Jiang L. Remains of ancient rice unearthed from the Shang-Shan and their significance. Archaeology. 2007; 9: 19–25. [Google Scholar]
- 35. Zheng Y, Jiang L, Zheng J. Study on the remains of ancient rice from Kuahuqiao Site in Zhejiang Province. Chinese Rice Science. 2004; 18(2): 119–24. [Google Scholar]
- 36. Zheng Y, Sun Z, Wang C. Morphological characteristics of plant opal from motor cells of rice in paddy fields soil Chinese Rice Research Newsletter. RICE SCIENCE. 2000; 8(3): 9–11. [Google Scholar]
- 37. Zou J, Tang L, Wang C. On the origin of cultivated Keng rice(Oryza Sativa L. subsp. Japonica). Scientia Agricultural Sinica. 1998;31(5): 75–81. [Google Scholar]
- 38. Wang C, Zhang M. Further research of the primitive rice cultivation remains in Longqiuzhuang Site, Gaoyou. Agricultural Archaeology. 1998; (1):172–81. [Google Scholar]
- 39. Wang C, Udatsu T, Fujiwara H. Relationship between motor cell silica body shape and grain morphological/physiological traits for discriminating indica and japonica rice in China. Japanese Journal of Breeding. 1996; 46: 61–6. [Google Scholar]
- 40. Gu H. An overview of the methods distinguishing the rice phytolith between Oryza Sativa subsp. Hsien and Oryza Sativa subsp. Keng at archaeological sites In: The Institute of Archaeology of Hunan Province ed. Journal of Hunan Archaeology (Vol.8). Changsha:Yuelu Publishing House, 2009. p. 268–276. [Google Scholar]
- 41. Sato YI, Fujiwara H, Udatsu T. Morphological differences in silica body derived from motor cell of indica and japonica in rice. Japanese Journal of Breeding. 1990; 40(4): 495–504. [Google Scholar]
- 42. Jin G, Yan S, Udatsu T, Lan Y, Wang C, Tong P. Neolithic rice paddy from the Zhaojiazhuang site, Shandong, China. Chinese Science Bulletin. 2007; 52(24): 3376–84. [Google Scholar]
- 43. Li X, Zhou X, Zhang H, Zhou J, Shang X, Dodson J. The record of cultivated rice from archaeobiological evidence in northwestern China 5000 years ago. Chinese Science Bulletin. 2007; 52(10): 1372–8. [Google Scholar]
- 44. Saxena A, Prasad V, Singh I, Chauhan M, Hasan R. On the Holocene record of phytoliths of wild and cultivated rice from Ganga Plain: evidence for rice-based agriculture. Current Science. 2006; 90(11): 1547–52. [Google Scholar]
- 45. Tewari R, Srivastava R, Saraswat K, Singh I, Singh K. Early farming at Lahuradewa. Pragdhara. 2008; 18: 347–73. [Google Scholar]
- 46. Chauhan DK, Tripathi DK, Kumar D, Kumar Y. Diversity, Distribution and Frequency Based Attributes of Phytolith in Arundo donax L. International Journal of Innovations in Biological and Chemical Sciences. 2011; 1: 22–7. [Google Scholar]
- 47. Qu B, Zhu M, Chen X, Zhang C, Xu Y, Ding W, et al. Morphological charater of bulliform cells in 22 species of Poaceae. Acta Botanica Borealioccidentalla Sinica. 2010; 30(08): 1595–1601. [Google Scholar]
- 48. Jane WN, Chiang S-HT. Morphology and development of bulliform cells in around-formosana hack. Taiwania. 1991;36(1):85–97. [Google Scholar]
- 49. Vecchia F, El Asmar T, Calamassi R, Rascio N, Vazzana C. Morphological and ultrastructural aspects of dehydration and rehydration in leaves of Sporobolus stapfianus. Plant Growth Regulation. 1998; 24(3): 219–28. [Google Scholar]
- 50. Wang Y. The bulliform cells of Poaceae. Biology Teaching. 2005; 30(11): 7–9. [Google Scholar]
- 51. Parry DW, Smithson F. Silicification of bulliform cells in grasses. 1958. [Google Scholar]
- 52. Alvarez JM, Rocha JF, Machado SR. Bulliform cells in Loudetiopsis chrysothrix (Nees) Conert and Tristachya leiostachya Nees (Poaceae): structure in relation to function. Brazilian Archives of Biology and Technology. 2008. [Google Scholar]
- 53. Ellis R. A procedure for standardizing comparative leaf anatomy in the Poaceae. I. The leaf-blade as viewed in transverse section. Bothalia. 1976; 12(1): 65–109. [Google Scholar]
- 54. Dong Y, Liu X, Zheng D. Crops and their wild relatives in China: food crops. Beijing: China Agriculture Press; 2006. [Google Scholar]
- 55. Piperno D. Phytoliths: a comprehensive guide for archaeologists and paleoecologists. California: AltaMira; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.