Abstract
Ischemic preconditioning (IPC) is a well-known phenomenon in which tissues are exposed to a brief period of ischemia prior to a longer ischemic event. This technique produces tissue tolerance to ischemia reperfusion injury (IRI). Currently, IPC’s mechanism of action is poorly understood. Using a porcine single kidney model, we performed remote IPC with renal IRI and evaluated the IPC mechanism of action. Following left nephrectomy, 15 female Yorkshire pigs were divided into three groups: no IPC and 90 minutes of warm ischemia (control), remote IPC immediately followed by 90 minutes of warm ischemia (rIPCe), and remote IPC with 90 minutes of warm ischemia performed 24 hours later (rIPCl). Differential gene expression analysis was performed using a porcine-specific microarray. The microarray analysis of porcine renal tissues identified 1,053 differentially expressed probes in preconditioned pigs. Among these, 179 genes had altered expression in both the rIPCe and rIPCl groups. The genes were largely related to oxidation reduction, apoptosis, and inflammatory response. In the rIPCl group, an additional 848 genes had altered expression levels. These genes were primarily related to immune response and inflammation, including those coding for cytokines and cytokine receptors and those that play roles in the complement system and coagulation cascade. In the complement system, the membrane attack complex was determined to be sublytic, because it colocalized with phosphorylated extracellular signal-regulated kinase. Furthermore, alpha 2 macroglobulin, tissue plasminogen activator, uterine plasmin trypsin inhibitor, and arginase-1 mRNA levels were elevated in the rIPCl group. These findings indicate that remote IPC produces renoprotective effects through multiple mechanisms, and these effects develop over a long timeframe rather than immediately following IPC.
Introduction
Ischemic preconditioning (IPC) is an effective method for protecting organs prior to ischemia reperfusion injury (IRI). Since the initial report of the IPC phenomenon, numerous studies have tried to incorporate IPC to protect various organs, including the heart, lung, small intestine, liver, brain, and kidney [1–6]. There are 2 types of IPC. Local IPC involves brief interruption of blood supply to the target organ alternated with brief episodes of reperfusion applied prior to prolonged lethal ischemia. In remote IPC, intermittent brief ischemia and reperfusion is applied to an organ (e.g., a limb or the intestine) distant to the target organ. For organs that are vulnerable to short-term ischemic injury, such as the brain, heart, or kidney, remote IPC is the preferred method [7–9].
Many studies have demonstrated renoprotective effects for remote IPC in renal IRI using rodent animal models [10]. Remote IPC can decrease blood urea nitrogen, creatinine, and malondialdehyde levels [11, 12]. In addition to these biomarkers, histological evidence supporting the renoprotective effects of remote IPC has been reported [13]. While encouraging, these results do not directly translate to the clinical application of remote IPC in humans, because human renal physiology is different from that of rodents [14]. While a few studies have investigated remote IPC in large animals [10, 15, 16], most large animal studies focused on local IPC [17–22]. To safely use IPC in human renal IRI, we first need to determine the most effective IPC protocol and understand its exact mechanism of action. Previous studies have not defined a mechanism of action for remote IPC. Several potential mechanisms have been suggested, including production of adenosine, bradykinins, opioids, antioxidants, humoral substances, and inflammatory molecules; however, there are no accurate and comprehensive reports regarding the remote IPC mechanism of action [23–27]. Recently, gene microarray technology has been used to analyze the molecular basis of many diseases. For example, genetic changes produced by renal IRI have been analyzed using microarrays [28]. However, microarray analysis of remote IPC tissues is limited, and, to our knowledge, there has been no analysis of the transcriptome of renal tissue after remote IPC [29, 30].
In our previous study, we concluded that remote IPC elicited fewer renal injury biomarkers following IRI; however, more animal studies deciphering the detailed remote IPC mechanism are needed before IPC can be incorporated into human surgeries [31]. In this study, we used a microarray analysis to evaluate the effect of the time between IPC and IRI in remote IPC and identify the exact mechanism of action for remote IPC in renal IRI using a porcine single kidney model.
Materials and Methods
Animals and design of experiments
The study was approved by the Institutional Animal Care and Use Committee, Yonsei University Health System in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institutes of Health and was conducted according to the principles of the Declaration of Helsinki. A total of 15 female Yorkshire pigs (20 weeks old; 35–38 kg, S. s. domesticus) were used (XP Bio Inc., Anseong-si, Korea). The animals were individually caged for 10 days before the start of the experiment and received standard laboratory food with free access to water. In a prospective design, pigs were allocated randomly to three experimental groups (Fig 1). Group 1 pigs (control, n = 5) underwent right renal ischemia without IPC. Group 2 pigs (rIPCe, n = 5) underwent remote IPC just before right renal ischemia, i.e., an early time window. Group 3 pigs (rIPCl, n = 5) underwent remote IPC with right renal ischemia 24 hours later, i.e., a late time window. All three groups underwent the same protocol for preoperative care, anesthesia, postoperative care, and sacrifice. Prior to anesthesia administration, the pigs were starved for 8 hours. Anesthesia was induced by intramuscular tiletamine-zolazepam (5 mg/kg) and xylazine (2 mg/kg). Following endotracheal intubation, anesthesia was maintained using controlled ventilation with 2% isoflurane at a respiratory rate of 15/min. All procedures were performed laparoscopically with standard 5-mm 3-port access by two surgeons (W.K.H. and Y.E.Y.). During the procedure, intraperitoneal pressure was maintained at 12 mmHg. To create the single kidney porcine model, we first performed a left nephrectomy. After a 1-week renal compensation period, we performed the IPCs and right renal ischemias, as appropriate, based upon the group assignment. We performed two cycles of IPC by clamping the right external iliac artery for 10 minutes followed by 10 minutes of reperfusion. We performed warm ischemia for the right kidney by clamping the right renal artery and vein together for 90 minutes. Antibiotic prophylaxis was administered using amoxicillin clavulanate (15 mg/kg/day), and meloxicam (0.2 mg/kg/day) was used as postoperative analgesia. Pigs were individually housed and had free access to water and feed. Every pig was sacrificed 72 hours postoperatively. Pigs were administered an intramuscular injection of ketamine (25 mg/kg) and xylazine (2 mg/kg) followed by an intravenous potassium chloride injection. Renal tissues were harvested immediately and stored in a -80°C freezer until the microarray analysis was performed.
Fig 1. Experimental protocol.
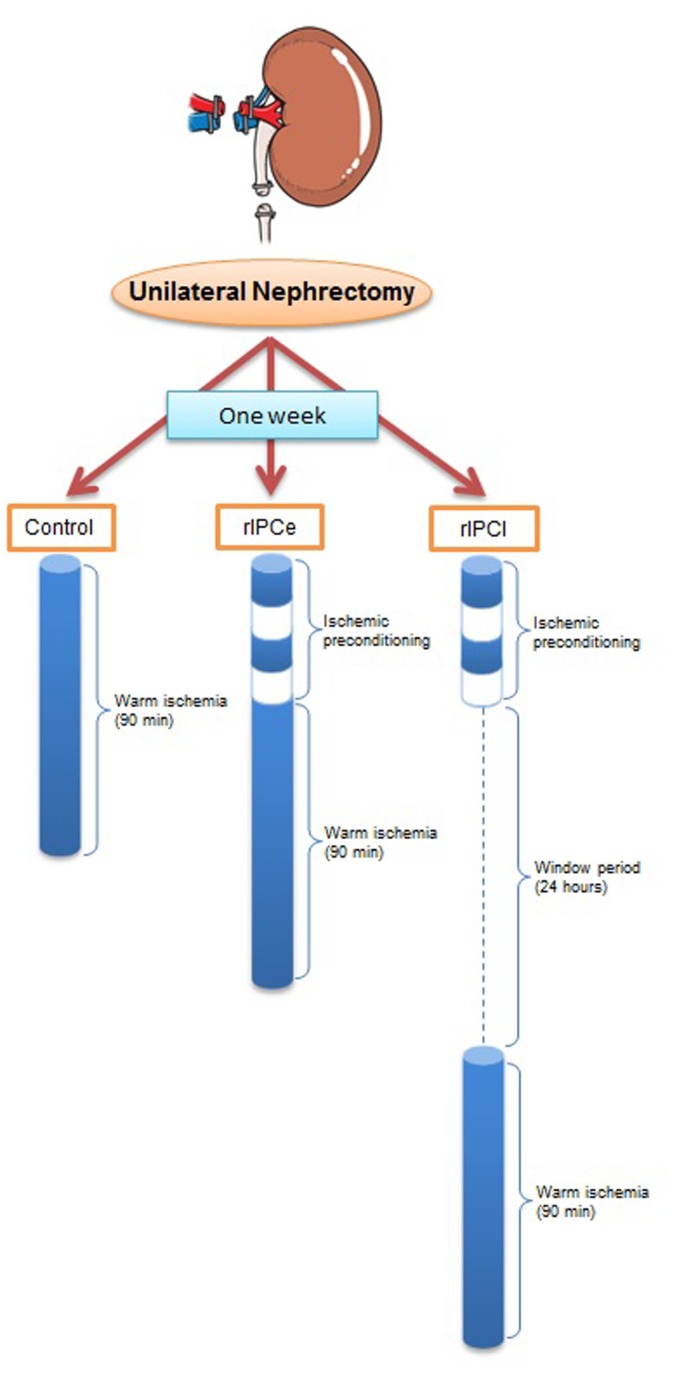
Blue cylinders represent the time zone of clamping and clear cylinders represent the declamping. Control, no IPC and 90 minutes of warm ischemia; rIPCe, 40 minutes of remote IPC immediately followed by 90 minutes of warm ischemia; and rIPCl, 40 minutes of remote IPC with 90 minutes of warm ischemia 24 hours later.
Microarray analysis
Total RNA was extracted from tissues using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol and purified with the RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA). RNA labeling and hybridization were performed using the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technologies, v6.5, Palo Alto, CA, USA). Briefly, 200 ng of total RNA from each sample was linearly amplified and labeled with Cy3-dCTP. The labeled cRNAs were hybridized onto the Porcine Gene Expression Microarray, 4×44K (Agilent Technologies). The hybridized array was immediately scanned with an Agilent Microarray Scanner (Agilent Technologies). Raw data were extracted using the Agilent Feature Extraction Software (Agilent Technologies, v11.0.1.1). The selected gProcessedSignal value was transformed logarithmically and normalized using the quantile method. The expression data were quantified as fold change, and the statistical significance was determined using an independent t-test. We adjusted the p-value to account for the false discovery rate using the Benjamini-Hochberg algorithm. A K-means clustering analysis was performed using the MacQueen algorithm to compare the expression profiles. Hierarchical cluster analysis was performed using complete linkage and Euclidean distance as a measure of similarity. Gene-enrichment and functional annotation analysis for significant probes was performed using DAVID (Database for Annotation, Visualization and Integrated Discovery v6.7, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA; http://david.abcc.ncifcrf.gov). All data analysis and differentially expressed gene visualization was performed using the R statistic software (R version 3.0.1, R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org).
Immunohistological staining and microscopic analysis
Kidneys were embedded in paraffin, and 2-μm sections were prepared for fluorescent immunostaining and immunohistochemical staining. Deparaffinized renal sections were fixed and incubated in PBS with 5% bovine serum albumin and 0.05% Tween 20 (PBST) at room temperature for 1 h in a humidified chamber to block non-specific binding sites. The sections were incubated overnight with the primary antibodies in PBST buffer with 3% BSA at 4°C. The primary antibodies used were anti-C3c-FITC (Abcam, Cambridge, MA, USA), mouse anti-membrane attack complex (MAC; Abcam), and rabbit anti-phosphorylated extracellular signal-regulated kinase (ERK) 1 and ERK2 (P-ERK; Cell Signaling Technology Inc., Danvers, MA, USA). After washing, the sections were incubated with fluorophore-conjugated secondary antibodies (anti-rabbit Alexa Fluor 488 and anti-mouse Alexa Fluor 594, Invitrogen Life Technologies) for 1 h at room temperature. The sections were mounted using Vectashield mounting medium with 4ʹ,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA). For immunohistochemical staining, the slides were pretreated in 0.01 M citric acid buffer (pH 6.0) for 10 minutes. After blocking, sections were incubated with primary antibody against P-ERK at 4°C overnight. After washing, sections were exposed to a streptavidin-horseradish-peroxidase complex for 30 minutes at 37°C and visualized with 3,3ʹ-diaminobenzidine.
Terminal Deoxynucleotidyl Transferase-mediated dUTP-X Nick End Labeling (TUNEL) staining
To detect apoptotic cells, TUNEL staining was performed using a TACS® 2 TdT-DAB In Situ Apoptosis Detection Kit (Trevigen, Inc., Gaithersburg, MD, USA) according to the manufacturer’s instructions. Kidneys were embedded in paraffin, and 2-μm sections were prepared for TUNEL staining. After deparaffinization, digestion with proteinase K (15 minutes), and quenching of endogenous peroxidase in 2% H2O2 (5 minutes), formalin-fixed renal sections were immersed in the TdT buffer and incubated with TdT, 1 mM Mn2+, and biotinylated dNTP at 37°C for 60 minutes. Then the sections were incubated with streptavidin–horseradish peroxidase at room temperature for 10 minutes and immersed in diaminobenzidine substrate for 5 minutes. The slides were then counterstained with 1% methyl green for 30 seconds.
Reverse transcriptase-polymerase chain reaction
Microarray results were validated using RT-PCR. Briefly, cDNA was synthesized from purified RNA using oligo dT-primers with SuperScriptTM II reverse transcriptase (Invitrogen Life Technologies) according to the supplier’s protocol. Primer sequences used in the study are listed in S1 Table. Equal volumes of amplified cDNAs were loaded into 1.5% agarose gels and separated by electrophoresis. The bands were identified using a Bio-Rad Gel Doc 2000 system with Bio-Rad TDS Quantity One Software.
Results
Microarray
All RNA samples had 260/280 ratios between 1.8–2.2, and agarose gel electrophoresis showed two clear bands for 28S and 18S rRNA. In the microarray, 43,603 probes were detected. Among those, 1,053 probes had significant intensity changes. The criteria for significance was |fold change| ≥2 and a p-value <0.05.
The K-mean hierarchal cluster analysis divided all 1,053 genes into two clusters (Fig 2). Cluster 1 consisted of 400 genes that were downregulated in rIPCe relative to the control group and downregulated further in rIPCl. Cluster 2 consisted of 653 genes that were upregulated in rIPCe relative to the control group and upregulated further in rIPCl.
Fig 2. Hierarchal cluster analysis of 1,053 altered genes.
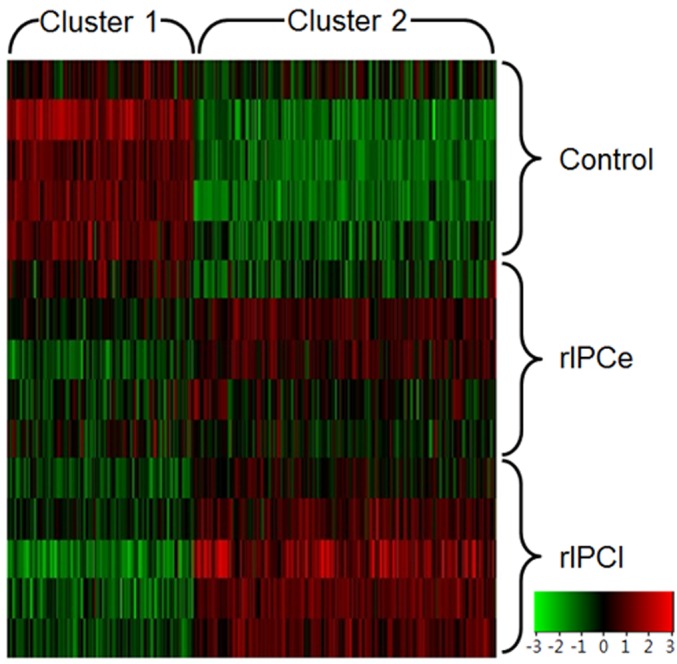
The genes were divided into two clusters via K-mean hierarchal cluster analysis. Cluster 1 genes are downregulated (green) in the rIPCe and rIPCl. Cluster 2 genes are upregulated in rIPCl. rIPCe, remote ischemic preconditioning immediately prior to ischemia (early); rIPCl, remote ischemic preconditioning followed by ischemia 24 hours later (late).
The rIPCe group had 198 genes with expression level changes; 96 genes were upregulated, and 102 genes were downregulated compared to those in the control group (Fig 3). The rIPCl group had 1,027 genes with expression level changes; 640 genes were upregulated, and 387 genes were downregulated. Fig 3 shows that nearly all genes with altered expression in the rIPCe group were also differentially expressed in the rIPCl group.
Fig 3. Venn diagrams of altered gene expression.
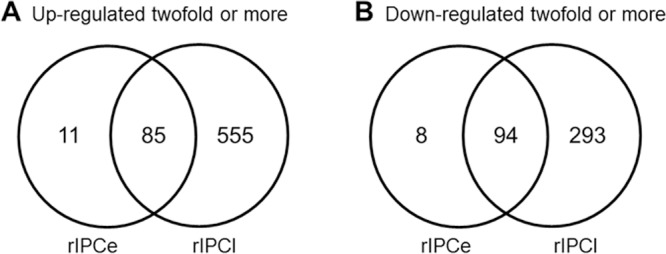
rIPCe, remote ischemic preconditioning immediately prior to ischemia (early); rIPCl, remote ischemic preconditioning followed by ischemia 24 hours later (late).
Altered gene expression shared by rIPCe and rIPCl
The rIPCe and rIPCl groups had 179 genes with similar expression levels; 85 genes were upregulated and 94 genes were downregulated compared to the control group genes. The similarly expressed genes were primarily related to oxidation, inflammation, macromolecule complex assembly, and apoptosis (S2 Table). Gene ontology enrichment analysis confirmed that some of the genes were related to oxidative reduction, inflammatory response, macromolecular complex assembly, and apoptosis. Gene ontology analysis also identified additional functions (Fig 4); however, these genes were not part of a specific pathway.
Fig 4. Major enriched gene ontology biological processes for the 179 genes with similar expression levels in the rIPCe and rIPCl groups.
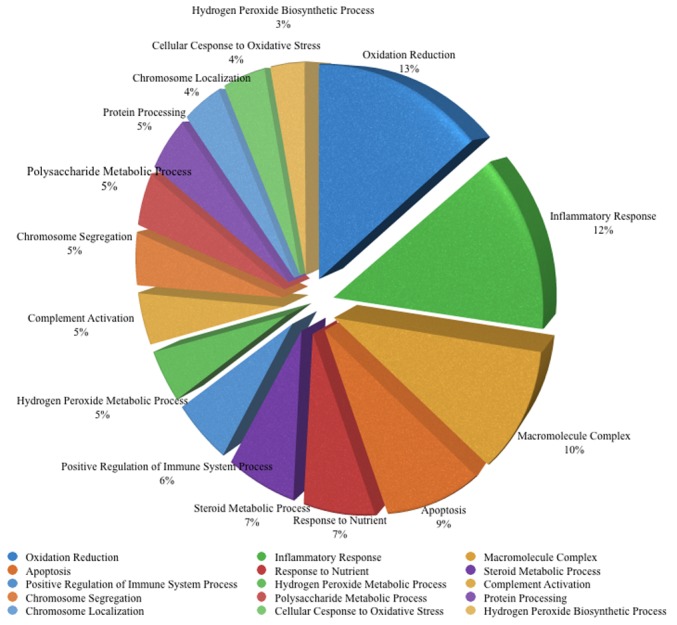
Similar gene ontology terms were excluded to avoid repetition. rIPCe, remote ischemic preconditioning immediately prior to ischemia (early); rIPCl, remote ischemic preconditioning 24 hours followed by ischemia 24 hours later (late).
Altered gene expression in the rIPCl group
Among the 1,053 genes in the rIPCl group with altered expression levels compared to the control group, 848 genes were altered in the rIPCl group only. Of those, 555 genes were upregulated, and 293 genes were downregulated. Gene ontology enrichment analysis showed that the rIPCl group genes with altered expression levels were related to immune response, response to wounding, proteolysis, and inflammation rather than apoptosis or oxidation (Fig 5).
Fig 5. Major enriched gene ontology biological processes for the 848 genes with altered expression levels in the rIPCl group only.
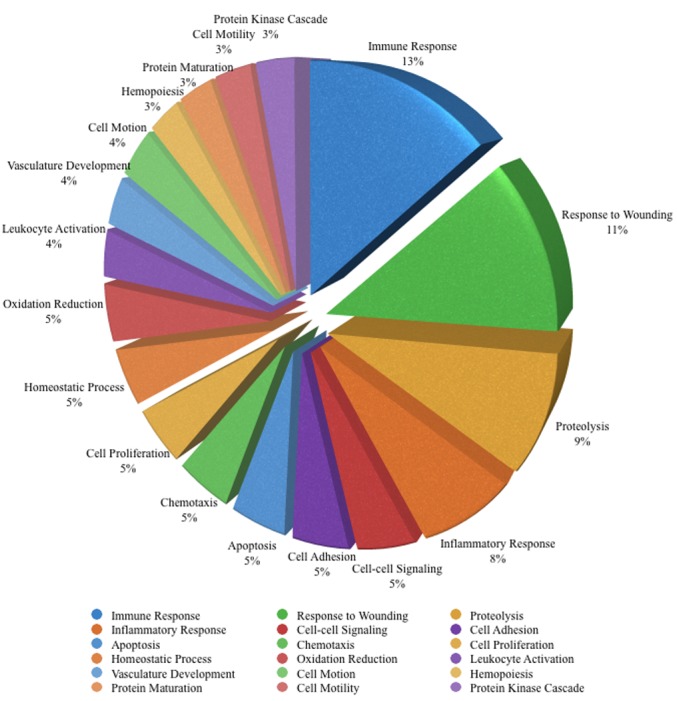
Similar gene ontology terms were excluded to avoid repetition. rIPCl, remote ischemic preconditioning followed by ischemia 24 hours later (late).
Functional classification of genes
We attempted to identify some potential IPC mechanisms with DAVID. When we examined the entire list of altered expression level genes, DAVID noted several enriched pathways (S3 Table), including the complement system, the coagulation cascade, and cytokines and cytokine receptors (Fig 6). The heatmap shows that most of these genes were upregulated in the rIPCl group (Fig 6).
Fig 6. Heatmap signature of suggested pathways by DAVID.
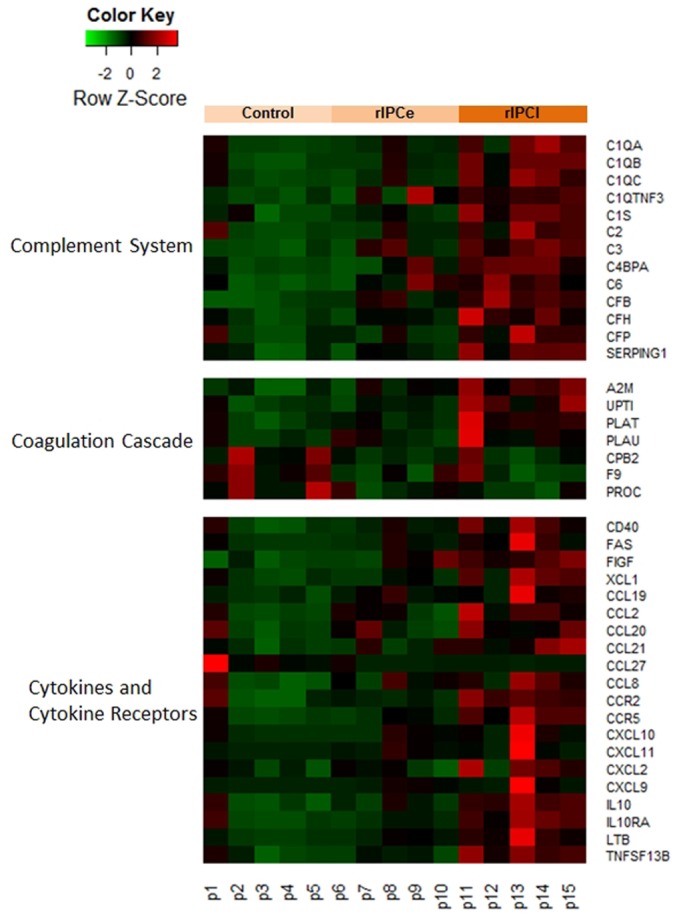
rIPCe, remote ischemic preconditioning immediately prior to ischemia (early); rIPCl, remote ischemic preconditioning followed by ischemia 24 hours later (late).
The complement system
We detected expression changes in many complement cascade genes in the rIPCl group compared to the control group (Fig 7). The classical pathway genes C1q, C1s, and C2 were upregulated. The alternative pathway genes C3 and factor B were also upregulated. However, complement inhibitors, including serpin peptidase inhibitor, clade G (C1 inhibitor), member 1 (SERPING1), C4b-binding protein (C4BP), and factor H, were also upregulated.
Fig 7. Genes in the complement system with altered expression levels.
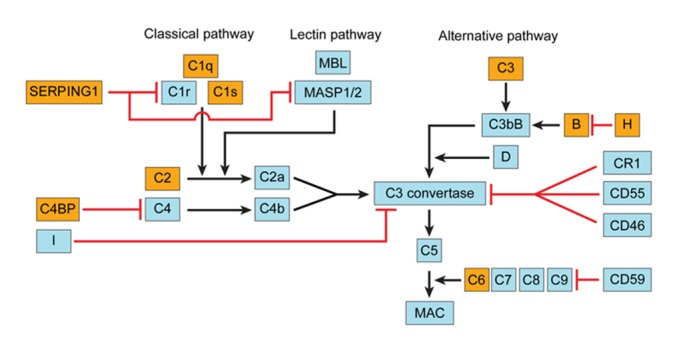
The yellow boxes indicate upregulated gene expression (fold change ≥2 and p < 0.05). The red lines indicate inhibition.
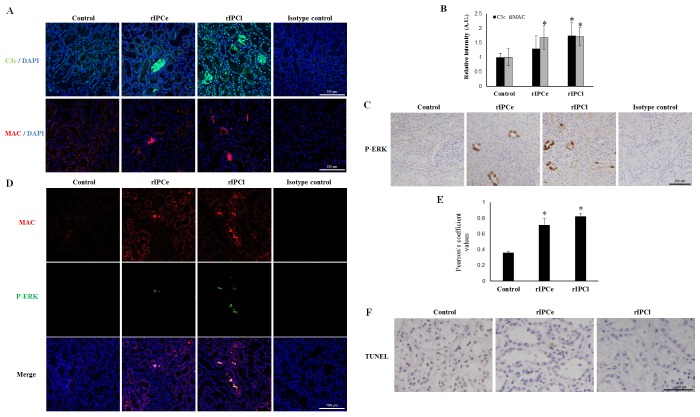
To confirm complement pathway activation, we performed immunofluorescent staining for C3c, a cleavage product of activated C3 and MAC, using a monoclonal antibody against neoantigen (Fig 8A). C3c and MAC deposition was observed in both the rIPCe and rIPCl groups. The relative fluorescence intensity of C3c and MAC was higher in the rIPCl group than that in the rIPCe group (Fig 8B). Because ERK is activated by sublytic MAC formation, we evaluated P-ERK levels in the renal tissues. The rIPCl group renal tubular cells were strongly positive for P-ERK staining, whereas control group renal tubular cells showed no staining (Fig 8C). MAC and P-ERK colocalization was also detected in the rIPCl group (Fig 8D), and the Pearson's coefficient for MAC and P-ERK colocalization in 12 fields was higher in the rIPCl group than in the control and rIPCe groups (Fig 8E). TUNEL staining revealed that the rIPCl group had fewer apoptotic cells in renal tubules compared with those in the control or rIPCe groups (Fig 8F).
Fig 8. Immunohistochemical analysis of pig renal tissue.
(A) C3c (green) and MAC (red) deposition was observed in confocal images of renal tissues, scale bar = 100 μm; DAPI, 4ʹ,6-diamidino-2-phenylindole (blue). (B) Relative fluorescence intensity of C3c and MAC staining was increased in rIPCe and rIPCl, *p < 0.05 vs. Control. (C) P-ERK IHC shows that ERK is activated in rIPCe and rIPCl, scale bar = 100 μm. (D) MAC (red) and P-ERK (green) colocalization was observed in confocal images of renal tissues, scale bar = 100 μm. (E) Pearson's coefficient for MAC and P-ERK colocalization (in 12 fields) was higher in rIPCe and rIPCl, *p < 0.05 vs. Control. (F) TUNEL staining showed fewer apoptotic renal tubular cells in the rIPCl group compared with Control, scale bar = 50 μm. MAC, membrane attack complex; P-ERK, phosphorylated extracellular signal-regulated kinase; rIPCe, remote ischemic preconditioning immediately prior to ischemia (early); rIPCl, remote ischemic preconditioning followed by ischemia 24 hours later (late); TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
The coagulation cascade
Many of the differentially expressed genes in the rIPCl group were involved in the coagulation cascade (Fig 9). Plasma thromboplastin antecedent (IX), protein C, and carboxypeptidase B2 (CPB2) were downregulated in the rIPCl group. In contrast, alpha 2 macroglobulin (A2M), tissue plasminogen activator (PLAT), and urokinase-type plasminogen activator (PLAU) were upregulated in the rIPCl group.
Fig 9. Genes in the coagulation cascade with altered expression levels.
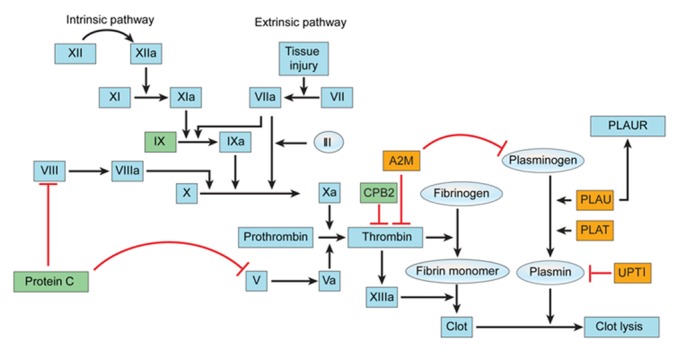
Yellow boxes indicate upregulated genes (fold change ≥2 and p < 0.05). Green boxes indicate downregulated genes (fold change ≤-2 and p < 0.05). Red lines indicate inhibition.
We used RT-PCR analysis to validate the gene expression changes detected during the microarray analysis. The mRNA levels of A2M, PLAT, and uterine plasmin trypsin inhibitor (UPTI) were elevated in both the rIPCe and rIPCl groups when compared with those in the control group (S1 Fig). While the A2M mRNA level in the rIPCl group was not upregulated to the same degree as that in the rIPCe group, the A2M expression levels in both groups were markedly elevated compared to that of the control group. These results were consistent with the microarray data. However, the RT-PCR analysis did not demonstrate any changes in the PLAU expression level in either the rIPCe or rIPCl groups, which contradicted the microarray data indicating that PLAU expression was upregulated in the rIPCl group (S1 Fig).
Cytokines and cytokine receptors
S4 Table lists the cytokines and cytokine receptors that the microarray analysis identified as differentially expressed in the rIPCe and rIPCl groups. Most of the cytokines and cytokine receptors were upregulated in the rIPCl group compared to those in the control group. We performed RT-PCR to validate the expression levels of interleukin 10 (IL-10) and transforming growth factor beta 1 (TGF-β1). The IL-10 and TGF-β1 mRNA levels were upregulated in both the rIPCe and rIPCl groups compared to those in the control group, confirming the microarray results (S1 Fig and S4 Table). The RT-PCR also verified that the M2 mononuclear phagocyte marker, arginase-1 (ARG-1) mRNA expression level was upregulated in both the rIPCe and rIPCl groups (S1 Fig).
Discussion
Renal IRI, which is caused by cardiopulmonary bypass, partial nephrectomy, and renal transplantation, is an emerging clinical problem, because acute renal injury can result in end-stage renal disease [32]. Chronic kidney disease increases the risk of death and cardiovascular morbidity, even when dialysis is unnecessary [33]. With this in mind, IPC may be an alternative strategy to preserve renal function after IRI.
In our previous study, we demonstrated that remote IPC with the late window group had lower urinary kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) levels than the control group following IRI [31]. Gardner et al. also demonstrated that remote IPC has a renoprotective effect in renal IRI in a porcine model [16]. Our results demonstrate that numerous gene expression levels were altered in the rIPC groups, especially in the rIPCl group. Hanto et al. verified that intraoperative administration of inhaled carbon monoxide reduces delayed graft function in a porcine delayed kidney transplantation model [34]. In that study, the authors hypothesized that the expression of antioxidant, proinflammatory, and reparative gene families improved renal function. Jun et al. performed a microarray study demonstrating a protective effect for IPC in a lung IRI model [29]. In their study, the differentially expressed genes had roles in inflammation, apoptosis, oxidation, antioxidation, and metabolism. While their study evaluated lung tissue rather than renal tissue, the microarray analysis results are similar to those from our present study, which provides additional evidence for our hypothesis that IPC affects numerous pathways rather than one specific pathway. Our microarray data suggest that IPC induces changes in the complement system, the coagulation cascade, and various cytokines and cytokine receptors.
The complement system
Of the three major complement pathways, the alternative and classical pathways are active in the kidney [35, 36]. A previous study confirmed that the complement system, especially the C3 protein, plays a major role in tissue damage in IRI [37]. In our study, the classical pathway proteins C1s, C1q, and C2 and the alternative pathway protein C3 were upregulated in the rIPCl group. However, inhibitors of these proteins (SERPING1, C4BP, and factor H) were also upregulated. In a kidney transplantation model, Castellano et al. demonstrated that C1 inhibitor infusion significantly reduced tubular damage and tubular epithelial cell apoptosis [38]. These authors concluded that classical and lectin pathway inhibition may provide an alternative therapeutic approach for renal IRI. Other studies demonstrated that a mutation in the factor H gene, a regulator of the alternative pathway, is a risk factor for some renal diseases, which supports the idea that complement inhibition has beneficial effect in IRI [39]. Factor H also has a major role in renal IRI, and a recombinant form of factor H reduces complement activation in the tubulointerstitium after renal IRI [40].
Our microarray data demonstrated upregulation of various complement cascade proteins and IRI-protective regulatory proteins, such as SERPING1 and Factor H. These results suggest that IPC may reduce IRI damage by neutralizing the complement cascade. We did detect increased C3c and MAC staining in rIPCe and rIPCl tissues; however, phosphorylated ERK colocalized with MAC. The colocalization data suggest that sublytic concentrations of MAC were present in the tissue [41]. Sublytic MAC concentrations reduce the infarction size in hearts [42]. Furthermore, sublytic MAC concentrations induce proliferation in specific cells, such as human aortic smooth muscle cells [43]. Sublytic doses of MAC desensitize cells and make them resistant to cell lysis through ERK activation [44]. Our TUNEL results demonstrated that there were fewer apoptotic cells in the rIPCl group than in the control and rIPCe groups, which agrees with previous studies.
The coagulation cascade
In our study, we stopped blood flow to produce renal IRI. As a result, coagulation cascade-related genes, such as PLAT, CPB2, and A2M, were differentially expressed in the rIPCl group. These data suggest that IPC may reduce ischemic injury by lysing blood clots. However, these genes may also regulate non-coagulation cascade pathways. Roelofs et al. showed that PLAT expression reduces neutrophil influx and preserves renal function during renal IRI using PLAT knockout mice [45]. Therefore, PLAT may also play an anti-inflammatory role in IPC. CPB2 mRNA expression was downregulated in the rIPCl group compared with the control group in our study. CPB2 is a thrombin-activated fibrinolysis inhibitor that eliminates fibrin carboxyl-terminal residues [46]. CPB2 downregulation may be beneficial during IRI, because reduced CPB2 expression increases fibrinolysis. The microarray analysis also showed that A2M expression increased in both the rIPCe and rIPCl groups (2.00- and 2.86-fold changes, respectively), which was verified by RT-PCR. A2M inhibits coagulation by inactivating thrombin and inhibits clot lysis by inactivating plasmin [47]. A2M also inhibits proteolysis by reducing protease activity [48]. Therefore, A2M may reduce ischemic injury following remote IPC by inhibiting coagulation and proteolysis.
Cytokines and cytokine receptors
CXCL10 and CXCL11 were upregulated in the rIPCe and rIPCl groups in our study. IFN-γ, which is a major macrophage activator, may upregulate the expression of chemokines, such as CXCL9, CXCL10, and CXCL11, in renal proximal tubular epithelial cells [49]. Fibronectin and fibrinogen can then bind to CXCL10 and CXCL11, and the interaction between fibronectin and CXCL11 potentiates wound repair [50]. The anti-inflammatory IL-10 was also upregulated in the rIPCl group. Administering exogenous IL-10 to normal rats stimulates mesangial cell growth, both in vitro and in vivo [51]. Mesangial cells are critical for maintaining renal function, because they provide structural support to the glomerulus and affect the glomerular filtration rate [52]. In addition, increased IL-10 levels decreased proteinuria and reduced interstitial fibrosis and glomerulosclerosis in a 5/6 nephrectomized rat model [53]. IL-10 also regulates the synthesis of cystatin C, which controls mesangial cell proliferation [54, 55]. Therefore, IL-10 may reduce ischemic injury by promoting mesangial cell proliferation and viability after remote IPC.
TGF-β, another anti-inflammatory cytokine, was upregulated in both rIPC groups compared with the control group. In the kidney, only mesangial cells secrete and activate TGF-β [56]. TGF-β can cause renal epithelial cell hypertrophy [57]. Mesangial cells initiate compensatory renal hypertrophy, even though tubular cells are the major players in the hypertrophic process [58]. Sinuani et al. verified that mesangial cells control the degree of compensatory tubular cell hypertrophy by regulating TGF-β-induced IL-10 expression levels [58]. IL-10 synthesis inhibition in mesangial cells results in a significant TGF-β expression reduction in remnant kidneys. Consequently, there is a 25% reduction in compensatory tubular cell hypertrophy. Taken together, these findings suggest that IPC may contribute to anti-inflammatory effects and tubular cell reproduction by altering IL-10 and TGF-β gene expression. To our knowledge, there are no reports on the roles of IL-10 or TGF-β in relation to the molecular mechanism of remote IPC. Further work is needed to verify the roles of IL-10 and TGF-β in remote IPC and to determine their roles in the kidney.
Conclusions
These results suggest that remote IPC produces renoprotective effects in renal IRI through multiple mechanisms rather than one specific pathway. Remote IPC produces expression changes in genes related to inflammatory cytokines and cytokine receptors, apoptosis, oxidation and antioxidation, ion channels and aquaporins, metabolism, and the cytoskeleton. Our data suggest that the complement system, the coagulation cascade, and various cytokines and cytokine receptors are the major mechanisms of remote IPC. The changes in these pathways were more pronounced when renal IRI was performed 24 hours after remote IPC rather than immediately afterwards. This study did not determine the optimal time frame to perform remote IPC prior to renal IRI; however, our results provide further evidence that remote IPC is renoprotective for renal IRI. Future studies will focus on elucidating the optimal time window for remote IPC and further defining the functional roles of the various genes identified in this study.
Supporting Information
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant (NRF-2012R1A1A1042968) funded by the Korean government (MEST).
Data Availability
All microarray analysis files are available from the NCBI GEO database (Accession: GSE72646).
Funding Statement
This work was supported by a National Research Foundation of Korea (NRF) grant (NRF-2012R1A1A1042968) funded by the Korea government (MEST).
References
- 1. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. [DOI] [PubMed] [Google Scholar]
- 2. Serafin A, Fernandez-Zabalegui L, Prats N, Wu ZY, Rosello-Catafau J, Peralta C. Ischemic preconditioning: tolerance to hepatic ischemia-reperfusion injury. Histology and histopathology. 2004;19:281–9. [DOI] [PubMed] [Google Scholar]
- 3. Takeshita M, Tani T, Harada S, Hayashi H, Itoh H, Tajima H, et al. Role of transcription factors in small intestinal ischemia-reperfusion injury and tolerance induced by ischemic preconditioning. Transplantation proceedings. 2010;42:3406–13. 10.1016/j.transproceed.2010.06.038 [DOI] [PubMed] [Google Scholar]
- 4. Simon C, Vara E, Garutti I, Gonzalez-Casaurran G, Azcarate L, Isea J, et al. Modulation of monocyte chemoattractant protein-1 expression by ischaemic preconditioning in a lung autotransplant model. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2012;41:933–9. [DOI] [PubMed] [Google Scholar]
- 5. Thompson JW, Dave KR, Young JI, Perez-Pinzon MA. Ischemic preconditioning alters the epigenetic profile of the brain from ischemic intolerance to ischemic tolerance. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2013;10:789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: from myth to a novel therapeutic option? Journal of the American Society of Nephrology: JASN. 2014;25:216–24. 10.1681/ASN.2013070708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J, Chen Y, Dong B, Kong W, Zhang J, Xue W, et al. Effect of remote ischaemic preconditioning on renal protection in patients undergoing laparoscopic partial nephrectomy: a 'blinded' randomised controlled trial. BJU international. 2013;112:74–80. 10.1111/bju.12004 [DOI] [PubMed] [Google Scholar]
- 8. Haji Mohd Yasin NA, Herbison P, Saxena P, Praporski S, Konstantinov IE. The role of remote ischemic preconditioning in organ protection after cardiac surgery: a meta-analysis. The Journal of surgical research. 2014;186:207–16. 10.1016/j.jss.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 9. Hu X, Lu Y, Zhang Y, Li Y, Jiang L. Remote ischemic preconditioning improves spatial learning and memory ability after focal cerebral ischemia-reperfusion in rats. Perfusion. 2013;28:546–51. 10.1177/0267659113487766 [DOI] [PubMed] [Google Scholar]
- 10. Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PloS one. 2012;7:e32296 10.1371/journal.pone.0032296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wever KE, Warle MC, Wagener FA, van der Hoorn JW, Masereeuw R, van der Vliet JA, et al. Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemia-reperfusion injury: the role of adenosine. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2011;26:3108–17. [DOI] [PubMed] [Google Scholar]
- 12. Lazaris AM, Maheras AN, Vasdekis SN, Karkaletsis KG, Charalambopoulos A, Kakisis JD, et al. Protective effect of remote ischemic preconditioning in renal ischemia/reperfusion injury, in a model of thoracoabdominal aorta approach. The Journal of surgical research. 2009;154:267–73. 10.1016/j.jss.2008.06.037 [DOI] [PubMed] [Google Scholar]
- 13. Ates E, Genc E, Erkasap N, Erkasap S, Akman S, Firat P, et al. Renal protection by brief liver ischemia in rats. Transplantation. 2002;74:1247–51. [DOI] [PubMed] [Google Scholar]
- 14. Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PS, Mizel D, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney international. 2010;77:519–26. 10.1038/ki.2009.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soendergaard P, Krogstrup NV, Secher NG, Ravlo K, Keller AK, Toennesen E, et al. Improved GFR and renal plasma perfusion following remote ischaemic conditioning in a porcine kidney transplantation model. Transplant international: official journal of the European Society for Organ Transplantation. 2012;25:1002–12. [DOI] [PubMed] [Google Scholar]
- 16. Gardner DS, Welham SJ, Dunford LJ, McCulloch TA, Hodi Z, Sleeman P, et al. Remote conditioning or erythropoietin before surgery primes kidneys to clear ischemia-reperfusion-damaged cells: a renoprotective mechanism? American journal of physiology Renal physiology. 2014;306:F873–84. 10.1152/ajprenal.00576.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kosieradzki M, Ametani M, Southard JH, Mangino MJ. Is ischemic preconditioning of the kidney clinically relevant? Surgery. 2003;133:81–90. [DOI] [PubMed] [Google Scholar]
- 18. Li FZ, Kimura S, Nishiyama A, Rahman M, Zhang GX, Abe Y. Ischemic preconditioning protects post-ischemic renal function in anesthetized dogs: role of adenosine and adenine nucleotides. Acta pharmacologica Sinica. 2005;26:851–9. [DOI] [PubMed] [Google Scholar]
- 19. Orvieto MA, Zorn KC, Mendiola FP, Gong EM, Lucioni A, Mikhail AA, et al. Ischemia preconditioning does not confer resilience to warm ischemia in a solitary porcine kidney model. Urology. 2007;69:984–7. [DOI] [PubMed] [Google Scholar]
- 20. Salehipour M, Khezri A, Monabbati A, Jalaeian H, Kroup M, Azizi V, et al. Ischemic preconditioning protects the dog kidney from ischemia-reperfusion injury. Urologia internationalis. 2007;79:328–31. [DOI] [PubMed] [Google Scholar]
- 21. Treska V, Molacek J, Kobr J, Racek J, Trefil L, Hes O. Ischemic training and immunosuppressive agents reduce the intensity of ischemic reperfusion injury after kidney transplantation. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation. 2006;4:439–44. [PubMed] [Google Scholar]
- 22. Hernandez DJ, Roberts WB, Miles-Thomas J, Magheli A, Saha S, Schaeffer EM, et al. Can ischemic preconditioning ameliorate renal ischemia-reperfusion injury in a single-kidney porcine model? Journal of endourology / Endourological Society. 2008;22:2531–6. [DOI] [PubMed] [Google Scholar]
- 23. Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–41. 10.1016/j.atherosclerosis.2008.10.029 [DOI] [PubMed] [Google Scholar]
- 24. Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock (Augusta, Ga). 1997;8:86–94. [DOI] [PubMed] [Google Scholar]
- 25. Das M, Das DK. Molecular mechanism of preconditioning. IUBMB life. 2008;60:199–203. 10.1002/iub.31 [DOI] [PubMed] [Google Scholar]
- 26. Surendra H, Diaz RJ, Harvey K, Tropak M, Callahan J, Hinek A, et al. Interaction of delta and kappa opioid receptors with adenosine A1 receptors mediates cardioprotection by remote ischemic preconditioning. Journal of molecular and cellular cardiology. 2013;60:142–50. 10.1016/j.yjmcc.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 27. Gross GJ, Baker JE, Moore J, Falck JR, Nithipatikom K. Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome P450 epoxygenase pathway in canine hearts. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2011;25:517–22. 10.1007/s10557-011-6321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basile DP, Fredrich K, Alausa M, Vio CP, Liang M, Rieder MR, et al. Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. American journal of physiology Renal physiology. 2005;288:F953–63. [DOI] [PubMed] [Google Scholar]
- 29. Jun N, Ke J, Gang C, Lin C, Jinsong L, Jianjun W. The protective effect of ischemic preconditioning associated with altered gene expression profiles in rat lung after reperfusion. The Journal of surgical research. 2011;168:281–93. 10.1016/j.jss.2009.10.020 [DOI] [PubMed] [Google Scholar]
- 30. Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiological genomics. 2004;19:143–50. [DOI] [PubMed] [Google Scholar]
- 31. Yoon YE, Lee KS, Choi KH, Kim KH, Yang SC, Han WK. Preconditioning strategies for kidney ischemia reperfusion injury: implications of the "time-window" in remote ischemic preconditioning. PloS one. 2015;10:e0124130 10.1371/journal.pone.0124130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. American journal of physiology Renal physiology. 2010;298:F1078–94. 10.1152/ajprenal.00017.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 34. Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, et al. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:2421–30. [DOI] [PubMed] [Google Scholar]
- 35. Passwell J, Schreiner GF, Nonaka M, Beuscher HU, Colten HR. Local extrahepatic expression of complement genes C3, factor B, C2, and C4 is increased in murine lupus nephritis. The Journal of clinical investigation. 1988;82:1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song D, Zhou W, Sheerin SH, Sacks SH. Compartmental localization of complement component transcripts in the normal human kidney. Nephron. 1998;78:15–22. [DOI] [PubMed] [Google Scholar]
- 37. Farrar CA, Asgari E, Schwaeble WJ, Sacks SH. Which pathways trigger the role of complement in ischaemia/reperfusion injury? Frontiers in immunology. 2012;3:341 10.3389/fimmu.2012.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castellano G, Melchiorre R, Loverre A, Ditonno P, Montinaro V, Rossini M, et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. The American journal of pathology. 2010;176:1648–59. 10.2353/ajpath.2010.090276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonomo JA, Palmer ND, Hicks PJ, Lea JP, Okusa MD, Langefeld CD, et al. Complement factor H gene associations with end-stage kidney disease in African Americans. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2014;29:1409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Renner B, Ferreira VP, Cortes C, Goldberg R, Ljubanovic D, Pangburn MK, et al. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney international. 2011;80:165–73. 10.1038/ki.2011.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nature medicine. 2011;17:1674–9. 10.1038/nm.2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanhehco EJ, Lee H, Lucchesi BR. Sublytic complement attack reduces infarct size in rabbit isolated hearts: evidence for C5a-mediated cardioprotection. Immunopharmacology. 2000;49:391–9. [DOI] [PubMed] [Google Scholar]
- 43. Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142:47–56. [DOI] [PubMed] [Google Scholar]
- 44. Kraus S, Seger R, Fishelson Z. Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis. Clinical and experimental immunology. 2001;123:366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roelofs JJ, Rouschop KM, Leemans JC, Claessen N, de Boer AM, Frederiks WM, et al. Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. Journal of the American Society of Nephrology: JASN. 2006;17:131–40. [DOI] [PubMed] [Google Scholar]
- 46. Boffa MB, Reid TS, Joo E, Nesheim ME, Koschinsky ML. Characterization of the gene encoding human TAFI (thrombin-activable fibrinolysis inhibitor; plasma procarboxypeptidase B). Biochemistry. 1999;38:6547–58. [DOI] [PubMed] [Google Scholar]
- 47. Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1992;6:3345–53. [DOI] [PubMed] [Google Scholar]
- 48. Wu K, Wang D, Feinman RD. Inhibition of proteases by alpha 2-macroglobulin. The role of lysyl amino groups of trypsin in covalent complex formation. The Journal of biological chemistry. 1981;256:10409–14. [PubMed] [Google Scholar]
- 49. Lin Q, Song Y, Zhu X, Yang S, Zheng J. [Expressions of CXCL9, CXCL10 and CXCL11 in renal tubular epithelial cells induced by IFN-gamma]. Xi bao yu fen zi mian yi xue za zhi = Chinese journal of cellular and molecular immunology. 2013;29:137–40. [PubMed] [Google Scholar]
- 50. Tortelli F, Pisano M, Briquez PS, Martino MM, Hubbell JA. Fibronectin binding modulates CXCL11 activity and facilitates wound healing. PloS one. 2013;8:e79610 10.1371/journal.pone.0079610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chadban SJ, Tesch GH, Foti R, Atkins RC, Nikolic-Paterson DJ. Interleukin-10 is a mesangial cell growth factor in vitro and in vivo. Laboratory investigation; a journal of technical methods and pathology. 1997;76:619–27. [PubMed] [Google Scholar]
- 52. Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiological reviews. 1998;78:723–44. [DOI] [PubMed] [Google Scholar]
- 53. Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, et al. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. Journal of the American Society of Nephrology: JASN. 2005;16:3651–60. [DOI] [PubMed] [Google Scholar]
- 54. Xu Y, Schnorrer P, Proietto A, Kowalski G, Febbraio MA, Acha-Orbea H, et al. IL-10 controls cystatin C synthesis and blood concentration in response to inflammation through regulation of IFN regulatory factor 8 expression. Journal of immunology (Baltimore, Md: 1950). 2011;186:3666–73. [DOI] [PubMed] [Google Scholar]
- 55. Tavera C, Leung-Tack J, Prevot D, Gensac MC, Martinez J, Fulcrand P, et al. Cystatin C secretion by rat glomerular mesangial cells: autocrine loop for in vitro growth-promoting activity. Biochemical and biophysical research communications. 1992;182:1082–8. [DOI] [PubMed] [Google Scholar]
- 56. Kitamura M, Suto T, Yokoo T, Shimizu F, Fine LG. Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. Journal of immunology (Baltimore, Md: 1950). 1996;156:2964–71. [PubMed] [Google Scholar]
- 57. Liu B, Preisig P. TGF-beta1-mediated hypertrophy involves inhibiting pRB phosphorylation by blocking activation of cyclin E kinase. The American journal of physiology. 1999;277:F186–94. [DOI] [PubMed] [Google Scholar]
- 58. Sinuani I, Averbukh Z, Gitelman I, Rapoport MJ, Sandbank J, Albeck M, et al. Mesangial cells initiate compensatory renal tubular hypertrophy via IL-10-induced TGF-beta secretion: effect of the immunomodulator AS101 on this process. American journal of physiology Renal physiology. 2006;291:F384–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All microarray analysis files are available from the NCBI GEO database (Accession: GSE72646).